Antiproliferative Activity of N-Acylhydrazone Derivative on Hepatocellular Carcinoma Cells Involves Transcriptional Regulation of Genes Required for G2/M Transition
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Lines and Culture Cell Conditions
2.2. Synthesis of the N-Acylhydrazone Derivatives
2.3. Cell Viability—Sulforhodamine B (SRB) Colorimetric Assay
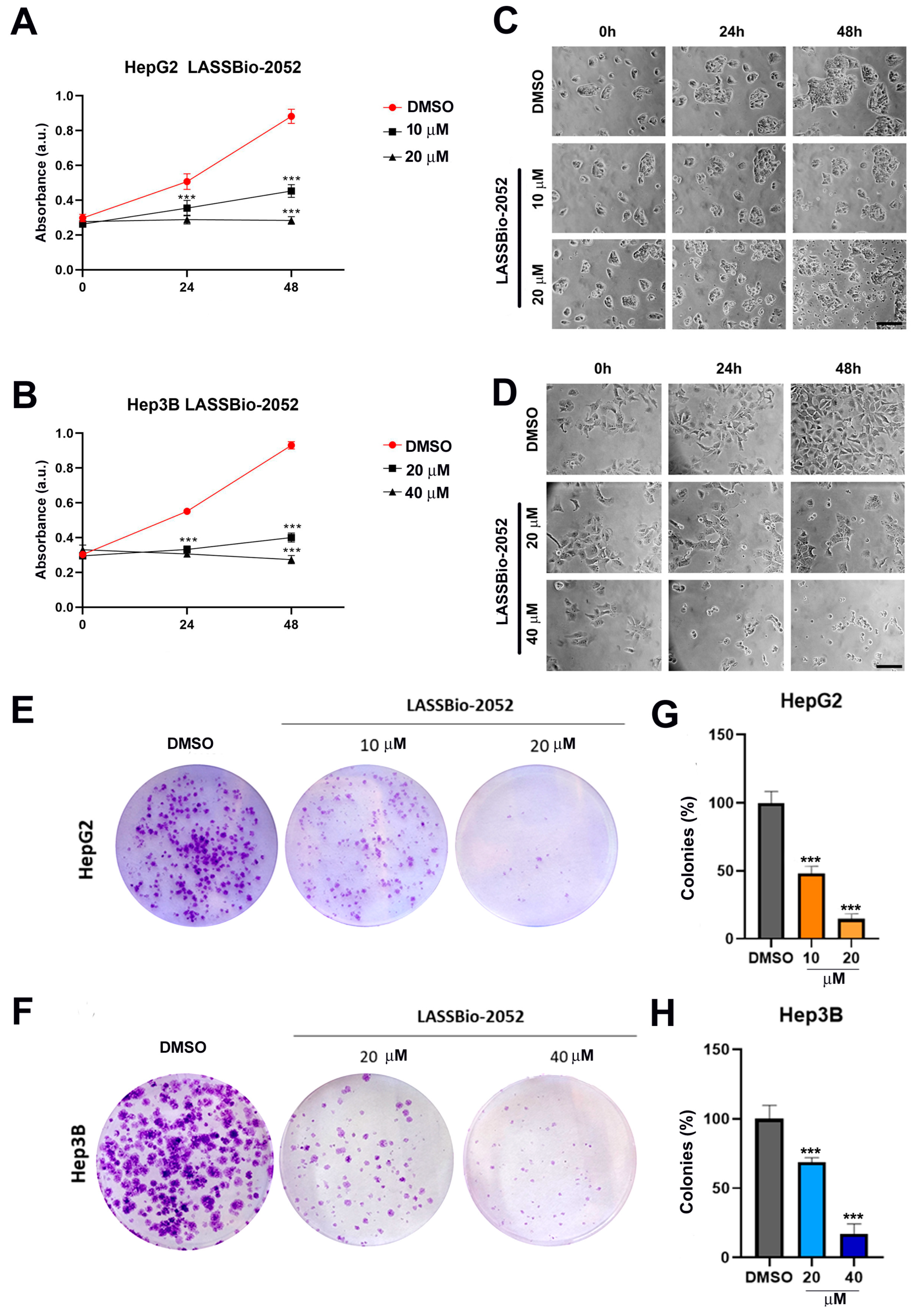
2.4. Clonogenic Assay
2.5. Cell Cycle Analysis
2.6. Gene Expression Profile Determined by qPCR
2.7. Apoptosis Evaluation
2.8. Survival Analysis of HCC Patients
2.9. Statistical Analysis
3. Results
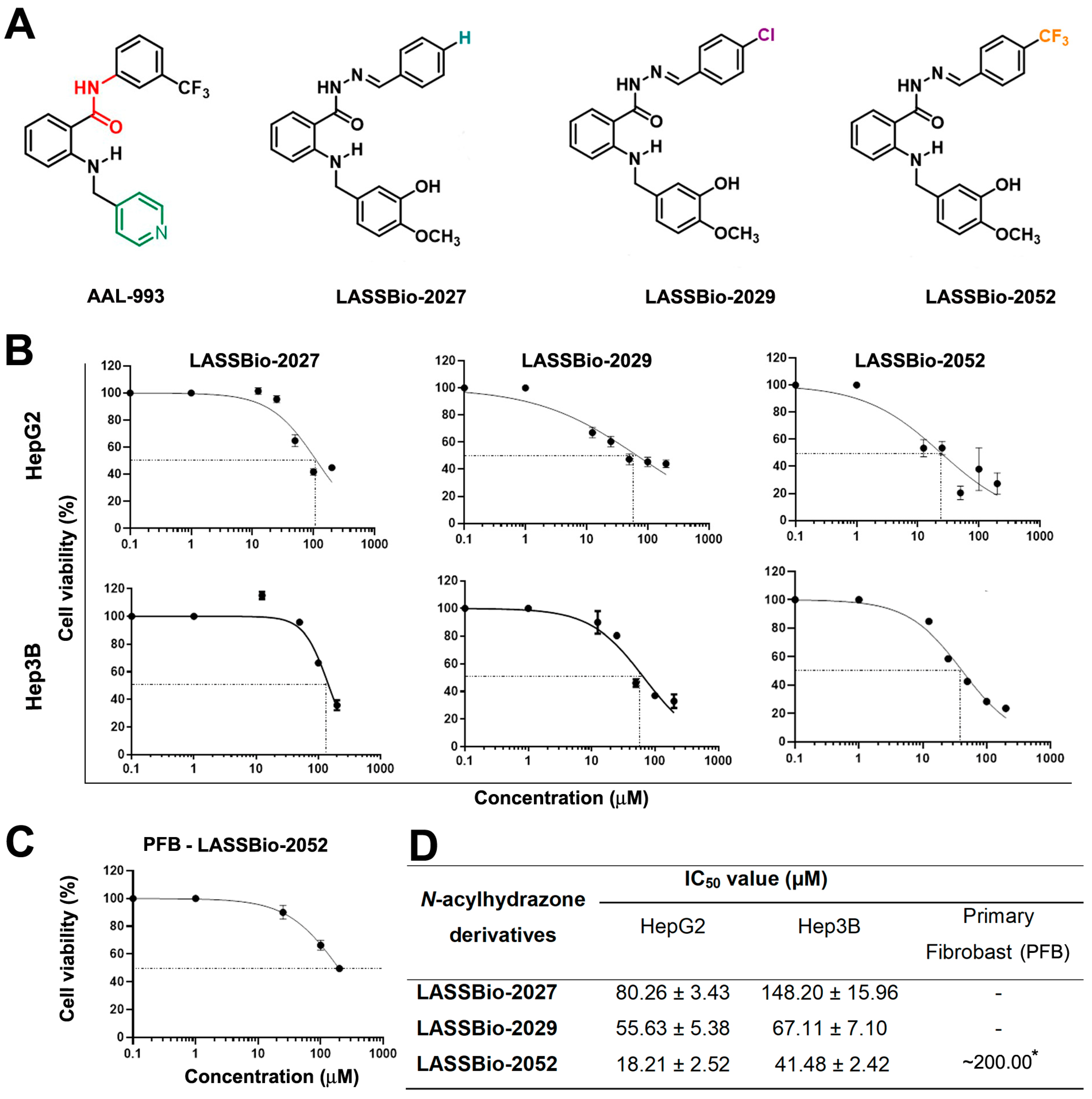
3.1. LASSBio-2052 Has Antiproliferative Activity on Hepatocarcinoma Cells
3.2. LASSBio-2052 Inhibits Cell Cycle Progression in Hepatocarcinoma Cells
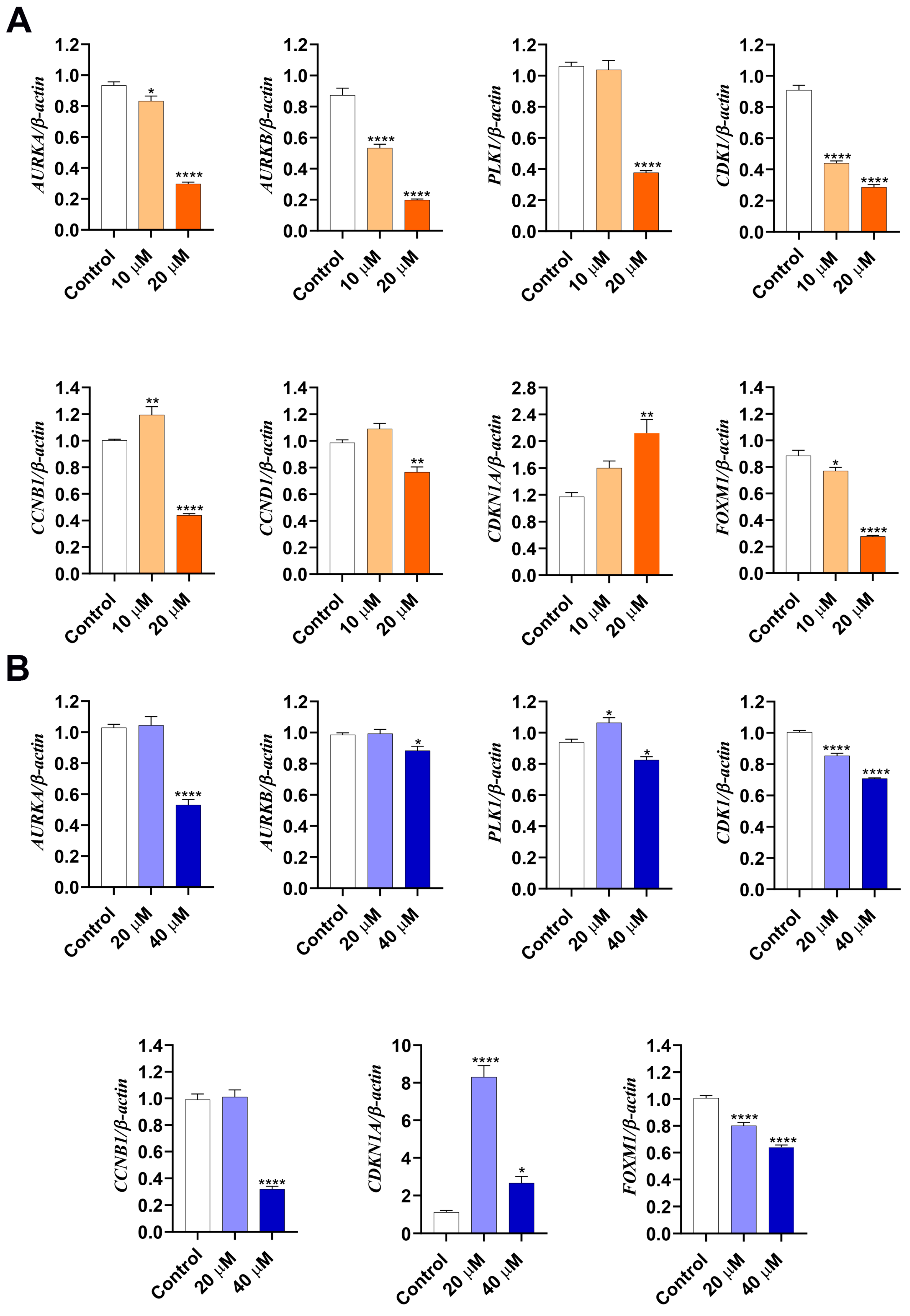
3.3. LASSBio-2052 Modulates Expression Profiles of Regulators of Cell Cycle
3.4. Cytotoxic Activity of LASSBio-2052 on HCC Cells Involves Apoptosis Induction
3.5. Gene Expression Changes Induced by LASSBio-2052 Are Associated with the Better Overall Survival of HCC Patients
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA A Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Villanueva, A. Hepatocellular Carcinoma. N. Engl. J. Med. 2019, 380, 1450–1462. [Google Scholar] [CrossRef] [PubMed]
- McGlynn, K.A.; Petrick, J.L.; El-Serag, H.B. Epidemiology of Hepatocellular Carcinoma. Hepatology 2021, 73 (Suppl. S1), 4–13. [Google Scholar] [CrossRef] [PubMed]
- Tümen, D.; Heumann, P.; Gülow, K.; Demirci, C.-N.; Cosma, L.-S.; Müller, M.; Kandulski, A. Pathogenesis and Current Treatment Strategies of Hepatocellular Carcinoma. Biomedicines 2022, 10, 3202. [Google Scholar] [CrossRef]
- Montironi, C.; Montal, R.; Llovet, J.M. New Drugs Effective in the Systemic Treatment of Hepatocellular Carcinoma. Clin. Liver Dis. 2019, 14, 56–61. [Google Scholar] [CrossRef]
- Chagas, A.L.; Mattos, A.A.D.; Carrilho, F.J.; Bittencourt, P.L.; Vezozzo, D.C.P.; Horvat, N.; Rocha, M.D.S.; Alves, V.A.F.; Coral, G.P.; Alvares-Da-Silva, M.R.; et al. Brazilian society of hepatology updated recommendations for diagnosis and treatment of hepatocellular carcinoma. Arq. Gastroenterol. 2020, 57, 1–20. [Google Scholar] [CrossRef]
- Lapenna, S.; Giordano, A. Cell Cycle Kinases as Therapeutic Targets for Cancer. Nat. Rev. Drug Discov. 2009, 8, 547–566. [Google Scholar] [CrossRef]
- Bolanos-Garcia, V.M. Aurora Kinases. Int. J. Biochem. Cell Biol. 2005, 37, 1572–1577. [Google Scholar] [CrossRef]
- Borah, N.A.; Reddy, M.M. Aurora Kinase B Inhibition: A Potential Therapeutic Strategy for Cancer. Molecules 2021, 26, 1981. [Google Scholar] [CrossRef]
- Hole, S.; Pedersen, A.M.; Lykkesfeldt, A.E.; Yde, C.W. Aurora Kinase A and B as New Treatment Targets in Aromatase Inhibitor-Resistant Breast Cancer Cells. Breast Cancer Res. Treat. 2015, 149, 715–726. [Google Scholar] [CrossRef] [PubMed]
- Matthews, H.K.; Bertoli, C.; De Bruin, R.A.M. Cell Cycle Control in Cancer. Nat. Rev. Mol. Cell Biol. 2022, 23, 74–88. [Google Scholar] [CrossRef] [PubMed]
- Ducreux, M.; Abou-Alfa, G.K.; Bekaii-Saab, T.; Berlin, J.; Cervantes, A.; de Baere, T.; Eng, C.; Galle, P.; Gill, S.; Gruenberger, T.; et al. The Management of Hepatocellular Carcinoma. Current Expert Opinion and Recommendations Derived from the 24th ESMO/World Congress on Gastrointestinal Cancer, Barcelona, 2022. ESMO Open 2023, 8, 101567. [Google Scholar] [CrossRef] [PubMed]
- Connell, L.C.; Harding, J.J.; Shia, J.; Abou-Alfa, G.K. Combined Intrahepatic Cholangiocarcinoma and Hepatocellular Carcinoma. Chin. Clin. Oncol. 2016, 5, 66. [Google Scholar] [CrossRef] [PubMed]
- Pauli, F.P.; Martins, J.R.; Paschoalin, T.; Ionta, M.; Barbosa, M.L.C.; Barreiro, E.J. Novel VEGFR-2 Inhibitors with an N-acylhydrazone Scaffold. Arch. Der Pharm. 2020, 353, 2000130. [Google Scholar] [CrossRef] [PubMed]
- Qiu, G.-H.; Xie, X.; Xu, F.; Shi, X.; Wang, Y.; Deng, L. Distinctive Pharmacological Differences between Liver Cancer Cell Lines HepG2 and Hep3B. Cytotechnology 2015, 67, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Skehan, P.; Storeng, R.; Scudiero, D.; Monks, A.; McMahon, J.; Vistica, D.; Warren, J.T.; Bokesch, H.; Kenney, S.; Boyd, M.R. New Colorimetric Cytotoxicity Assay for Anticancer-Drug Screening. JNCI J. Natl. Cancer Inst. 1990, 82, 1107–1112. [Google Scholar] [CrossRef] [PubMed]
- Franken, N.A.P.; Rodermond, H.M.; Stap, J.; Haveman, J.; van Bree, C. Clonogenic Assay of Cells in Vitro. Nat. Protoc. 2006, 1, 2315–2319. [Google Scholar] [CrossRef] [PubMed]
- Melo, M.L.; Fonseca, R.; Pauli, F.; Zavan, B.; Hanemann, J.A.C.; Miyazawa, M.; Caixeta, E.S.; Nacif, J.L.M.; Aissa, A.F.; Barreiro, E.J.; et al. N-Acylhydrazone Derivative Modulates Cell Cycle Regulators Promoting Mitosis Arrest and Apoptosis in Estrogen Positive MCF-7 Breast Cancer Cells. Toxicol. Vitr. 2023, 93, 105686. [Google Scholar] [CrossRef] [PubMed]
- Pfaffl, M.W. A New Mathematical Model for Relative Quantification in Real-Time RT-PCR. Nucleic Acids Res. 2001, 29, e45. [Google Scholar] [CrossRef]
- Kassambara, A.; Kosinski, M.; Biecek, P.; Fabian, S. Drawing Survival Curves Using “ggplot2”, [R Package Survminer Version 0.4.9]; 2021. Available online: https://cran.r-project.org/web/packages/survminer/index.html (accessed on 7 November 2023).
- R Core Team. R: A Language and Environment for Statistical Computing, R version 4.1.0 (2021-05-18); R Foundation for Statistical Computing: Vienna, Austria, 2020; Available online: https://www.R-project.org/ (accessed on 7 November 2023).
- Ramos, M.; Geistlinger, L.; Oh, S.; Schiffer, L.; Azhar, R.; Kodali, H.; de Bruijn, I.; Gao, J.; Carey, V.J.; Morgan, M.; et al. Multiomic Integration of Public Oncology Databases in Bioconductor. JCO Clin. Cancer Inform. 2020, 4, 958–971. [Google Scholar] [CrossRef]
- Kalathil, D.; John, S.; Nair, A.S. FOXM1 and Cancer: Faulty Cellular Signaling Derails Homeostasis. Front. Oncol. 2021, 10, 626836. [Google Scholar] [CrossRef]
- Nair, A.S.; Singh, A.K.; Kumar, A.; Kumar, S.; Sukumaran, S.; Koyiparambath, V.P.; Pappachen, L.K.; Rangarajan, T.M.; Kim, H.; Mathew, B. FDA-Approved Trifluoromethyl Group-Containing Drugs: A Review of 20 Years. Processes 2022, 10, 2054. [Google Scholar] [CrossRef]
- Arzumanian, V.A.; Kiseleva, O.I.; Poverennaya, E.V. The Curious Case of the HepG2 Cell Line: 40 Years of Expertise. Int. J. Mol. Sci. 2021, 22, 13135. [Google Scholar] [CrossRef] [PubMed]
- Levrero, M.; Zucman-Rossi, J. Mechanisms of HBV-Induced Hepatocellular Carcinoma. J. Hepatol. 2016, 64, S84–S101. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Luo, L.; Chen, W.; Xu, H.-X.; Chen, F.; Chen, L.-Z.; Zeng, W.-T.; Chen, J.-S.; Huang, X.-H. MicroRNA-24 Increases Hepatocellular Carcinoma Cell Metastasis and Invasion by Targeting P53: miR-24 Targeted P53. Biomed. Pharmacother. 2016, 84, 1113–1118. [Google Scholar] [CrossRef]
- Pollutri, D.; Gramantieri, L.; Bolondi, L.; Fornari, F. TP53/MicroRNA Interplay in Hepatocellular Carcinoma. Int. J. Mol. Sci. 2016, 17, 2029. [Google Scholar] [CrossRef] [PubMed]
- Müller-Coan, B.G.; Caetano, B.F.R.; Pagano, J.S.; Elgui De Oliveira, D. Cancer Progression Goes Viral: The Role of Oncoviruses in Aggressiveness of Malignancies. Trends Cancer 2018, 4, 485–498. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Sun, Q.; Wang, X. PLK1, A Potential Target for Cancer Therapy. Transl. Oncol. 2017, 10, 22–32. [Google Scholar] [CrossRef] [PubMed]
- Willems, E.; Dedobbeleer, M.; Digregorio, M.; Lombard, A.; Lumapat, P.N.; Rogister, B. The Functional Diversity of Aurora Kinases: A Comprehensive Review. Cell Div. 2018, 13, 7. [Google Scholar] [CrossRef]
- Zhao, X.; Yan, H.; Yan, X.; Chen, Z.; Zhuo, R. A Novel Prognostic Four-Gene Signature of Breast Cancer Identified by Integrated Bioinformatics Analysis. Dis. Markers 2022, 2022, 5925982. [Google Scholar] [CrossRef] [PubMed]
- Islam, B.; Yu, H.-Y.; Duan, T.-Q.; Pan, J.; Li, M.; Zhang, R.-Q.; Masroor, M.; Huang, J.-F. Cell Cycle Kinases (AUKA, CDK1, PLK1) Are Prognostic Biomarkers and Correlated with Tumor-Infiltrating Leukocytes in HBV Related HCC. J. Biomol. Struct. Dyn. 2023, 41, 11845–11861. [Google Scholar] [CrossRef] [PubMed]
- Su, W.-L.; Chuang, S.-C.; Wang, Y.-C.; Chen, L.-A.; Huang, J.-W.; Chang, W.-T.; Wang, S.-N.; Lee, K.-T.; Lin, C.-S.; Kuo, K.-K. Expression of FOXM1 and Aurora-A Predicts Prognosis and Sorafenib Efficacy in Patients with Hepatocellular Carcinoma. Cancer Biomark. 2020, 28, 341–350. [Google Scholar] [CrossRef] [PubMed]
- Ahn, J.Y.; Chung, E.Y.; Kwun, H.J.; Jang, K.L. Transcriptional Repression of P21waf1 Promoter by Hepatitis B Virus X Protein via a P53-Independent Pathway. Gene 2001, 275, 163–168. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.; Zhou, N.; Wang, L.; Zeng, Q.; Tang, H. Long Noncoding RNA GATA3-AS1 Promotes Cell Proliferation and Metastasis in Hepatocellular Carcinoma by Suppression of PTEN, CDKN1A, and TP53. Can. J. Gastroenterol. Hepatol. 2019, 2019, 1389653. [Google Scholar] [CrossRef] [PubMed]
- Xie, M.; Sun, M.; Zhu, Y.; Xia, R.; Liu, Y.; Ding, J.; Ma, H.; He, X.; Zhang, Z.; Liu, Z.; et al. Long Noncoding RNA HOXA-AS2 Promotes Gastric Cancer Proliferation by Epigenetically Silencing P21/PLK3/DDIT3 Expression. Oncotarget 2015, 6, 33587–33601. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.A.; Khan, P.; Ahmad, A.; Fatima, M.; Nasser, M.W. FOXM1: A Small Fox That Makes More Tracks for Cancer Progression and Metastasis. Semin. Cancer Biol. 2023, 92, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Hu, G.; Yan, Z.; Zhang, C.; Cheng, M.; Yan, Y.; Wang, Y.; Deng, L.; Lu, Q.; Luo, S. FOXM1 Promotes Hepatocellular Carcinoma Progression by Regulating KIF4A Expression. J. Exp. Clin. Cancer Res. 2019, 38, 188. [Google Scholar] [CrossRef]
- Li, R.; Okada, H.; Yamashita, T.; Nio, K.; Chen, H.; Li, Y.; Shimakami, T.; Takatori, H.; Arai, K.; Sakai, Y.; et al. FOXM1 Is a Novel Molecular Target of AFP-Positive Hepatocellular Carcinoma Abrogated by Proteasome Inhibition. Int. J. Mol. Sci. 2022, 23, 8305. [Google Scholar] [CrossRef] [PubMed]
- Wang, I.-C.; Chen, Y.-J.; Hughes, D.; Petrovic, V.; Major, M.L.; Park, H.J.; Tan, Y.; Ackerson, T.; Costa, R.H. Forkhead Box M1 Regulates the Transcriptional Network of Genes Essential for Mitotic Progression and Genes Encoding the SCF (Skp2-Cks1) Ubiquitin Ligase. Mol. Cell Biol. 2005, 25, 10875–10894. [Google Scholar] [CrossRef] [PubMed]
- Chai, N.; Xie, H.; Yin, J.; Sa, K.; Guo, Y.; Wang, M.; Liu, J.; Zhang, X.; Zhang, X.; Yin, H.; et al. FOXM1 Promotes Proliferation in Human Hepatocellular Carcinoma Cells by Transcriptional Activation of CCNB1. Biochem. Biophys. Res. Commun. 2018, 500, 924–929. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Andrade, A.A.R.; Pauli, F.; Pressete, C.G.; Zavan, B.; Hanemann, J.A.C.; Miyazawa, M.; Fonseca, R.; Caixeta, E.S.; Nacif, J.L.M.; Aissa, A.F.; et al. Antiproliferative Activity of N-Acylhydrazone Derivative on Hepatocellular Carcinoma Cells Involves Transcriptional Regulation of Genes Required for G2/M Transition. Biomedicines 2024, 12, 892. https://doi.org/10.3390/biomedicines12040892
Andrade AAR, Pauli F, Pressete CG, Zavan B, Hanemann JAC, Miyazawa M, Fonseca R, Caixeta ES, Nacif JLM, Aissa AF, et al. Antiproliferative Activity of N-Acylhydrazone Derivative on Hepatocellular Carcinoma Cells Involves Transcriptional Regulation of Genes Required for G2/M Transition. Biomedicines. 2024; 12(4):892. https://doi.org/10.3390/biomedicines12040892
Chicago/Turabian StyleAndrade, Amanda Aparecida Ribeiro, Fernanda Pauli, Carolina Girotto Pressete, Bruno Zavan, João Adolfo Costa Hanemann, Marta Miyazawa, Rafael Fonseca, Ester Siqueira Caixeta, Julia Louise Moreira Nacif, Alexandre Ferro Aissa, and et al. 2024. "Antiproliferative Activity of N-Acylhydrazone Derivative on Hepatocellular Carcinoma Cells Involves Transcriptional Regulation of Genes Required for G2/M Transition" Biomedicines 12, no. 4: 892. https://doi.org/10.3390/biomedicines12040892
APA StyleAndrade, A. A. R., Pauli, F., Pressete, C. G., Zavan, B., Hanemann, J. A. C., Miyazawa, M., Fonseca, R., Caixeta, E. S., Nacif, J. L. M., Aissa, A. F., Barreiro, E. J., & Ionta, M. (2024). Antiproliferative Activity of N-Acylhydrazone Derivative on Hepatocellular Carcinoma Cells Involves Transcriptional Regulation of Genes Required for G2/M Transition. Biomedicines, 12(4), 892. https://doi.org/10.3390/biomedicines12040892





