Trace Amine-Associated Receptors’ Role in Immune System Functions
Abstract
1. Introduction
2. The History of TAARs’ Discovery
3. Expression Profile of TAARs and Immune Function
4. Immune Function of TAARs in Lymphoid Cells
4.1. B-Lymphocytes
4.2. T-Lymphocytes
4.3. NK Cells
5. Immune Function of TAARs in Myeloid Cells
5.1. Monocytes and Macrophages
5.2. Polymorphonuclear Leukocytes
5.3. Microglia
6. Immunity Pathophysiology of TAARs
| Immunological Role | Receptor | Expression | Biological Function | References |
|---|---|---|---|---|
| Antibacterial immunity | TAAR1 | - | The TAAR1 agonist tyramine intensifies the adhesion and invasion of E. durans in the human large intestine epithelium. | [59] |
| TAAR8 | Astrocytes | TAAR8 transcription in astroglial cells intensifies after the effect of lipopolysaccharide. | [57] | |
| TAAR1 TAAR2 | Granulocytes | The effect of TAAR agonists stimulates the chemosensory migration of polymorphonuclear leukocytes. | [34] | |
| Antiviral immunity | TAAR1 | Peripheral mononuclear blood cells (PBMC). | HIV1 infection activates TAAR1 in PBMCs, the activation is intensified during the preliminary effect of amphetamine. | [38] |
| Bronchial asthma | TAAR6 | - | The presence of single-nucleotide polymorphisms of the TAAR6 gene affects the results of treating bronchial asthma patients. | [42] |
| Fibromyalgia | TAAR1 | - | TAAR1 gene polymorphism may be interlinked to the risk of developing fibromyalgia. | [62] |
| Inflammatory bowel diseases | TAAR2 TAAR5 TAAR9 | Large intestine epitheliocytes | Elevated TAAR expression was found in the large intestine wall cells of patients with Crohn’s disease. | [58,60,61] |
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gainetdinov, R.R.; Hoener, M.C.; Berry, M.D. Trace Amines and Their Receptors. Pharmacol. Rev. 2018, 70, 549–620. [Google Scholar] [CrossRef]
- Karovicová, J.; Kohajdová, Z.; Simko, P.; Lukácová, D. Using capillary isotachophoresis for the determination of biogenic amines and D-isocitric acid in food products. Nahrung 2003, 47, 188–190. [Google Scholar] [CrossRef]
- Wimbiscus, M.; Kostenko, O.; Malone, D. MAO inhibitors: Risks, benefits, and lore. Clevel. Clin. J. Med. 2010, 77, 859–882. [Google Scholar] [CrossRef]
- Berry, M.D.; Gainetdinov, R.R.; Hoener, M.C.; Shahid, M. Pharmacology of Human Trace Amine-Associated Receptors: Therapeutic Opportunities and Challenges. Pharmacol. Ther. 2017, 180, 161–180. [Google Scholar] [CrossRef]
- Grandy, D.K. Trace amine-associated receptor 1-Family archetype or iconoclast. Pharmacol. Ther. 2007, 116, 355–390. [Google Scholar] [CrossRef]
- Berry, M.D. The potential of trace amines and their receptors for treating neurological and psychiatric diseases. Rev. Recent Clin. Trials 2007, 2, 3–19. [Google Scholar] [CrossRef]
- Espinoza, S.; Sukhanov, I.; Efimova, E.V.; Kozlova, A.; Antonova, K.A.; Illiano, P.; Leo, D.; Merkulyeva, N.; Kalinina, D.; Musienko, P.; et al. Trace Amine-Associated Receptor 5 Provides Olfactory Input Into Limbic Brain Areas and Modulates Emotional Behaviors and Serotonin Transmission. Front. Mol. Neurosci. 2020, 13, 18. [Google Scholar] [CrossRef]
- Efimova, E.V.; Kozlova, A.A.; Razenkova, V.; Katolikova, N.V.; Antonova, K.A.; Sotnikova, T.D.; Merkulyeva, N.S.; Veshchitskii, A.S.; Kalinina, D.S.; Korzhevskii, D.E.; et al. Increased dopamine transmission and adult neurogenesis in trace amine-associated receptor 5 (TAAR5) knockout mice. Neuropharmacology 2021, 182, 108373. [Google Scholar] [CrossRef]
- Efimova, E.V.; Kuvarzin, S.R.; Mor, M.S.; Katolikova, N.V.; Shemiakova, T.S.; Razenkova, V.; Ptukha, M.; Kozlova, A.A.; Murtazina, R.Z.; Smirnova, D.; et al. Trace Amine-Associated Receptor 2 Is Expressed in the Limbic Brain Areas and Is Involved in Dopamine Regulation and Adult Neurogenesis. Front. Behav. Neurosci. 2022, 16, 847410. [Google Scholar] [CrossRef]
- Vaganova, A.N.; Shemyakova, T.S.; Lenskaia, K.V.; Rodionov, R.N.; Steenblock, C.; Gainetdinov, R.R. Trace Amine-Associated Receptors and Monoamine-Mediated Regulation of Insulin Secretion in Pancreatic Islets. Biomolecules 2023, 13, 1618. [Google Scholar] [CrossRef]
- Nelson, D.A.; Tolbert, M.D.; Singh, S.J.; Bost, K.L. Expression of neuronal trace amine-associated receptor (Taar) mRNAs in leukocytes. J. Neuroimmunol. 2007, 192, 21–30. [Google Scholar] [CrossRef]
- Fleischer, L.M.; Somaiya, R.D.; Miller, G.M. Review and Meta-Analyses of TAAR1 Expression in the Immune System and Cancers. Front. Pharmacol. 2018, 9, 683. [Google Scholar] [CrossRef]
- Vogelsang, T.L.R.; Vattai, A.; Schmoeckel, E.; Kaltofen, T.; Chelariu-Raicu, A.; Zheng, M.; Mahner, S.; Mayr, D.; Jeschke, U.; Trillsch, F. Trace Amine-Associated Receptor 1 (TAAR1) Is a Positive Prognosticator for Epithelial Ovarian Cancer. Int. J. Mol. Sci. 2021, 22, 8479. [Google Scholar] [CrossRef]
- Vaganova, A.N.; Maslennikova, D.D.; Konstantinova, V.V.; Kanov, E.V.; Gainetdinov, R.R. The Expression of Trace Amine-Associated Receptors (TAARs) in Breast Cancer Is Coincident with the Expression of Neuroactive Ligand-Receptor Systems and Depends on Tumor Intrinsic Subtype. Biomolecules 2023, 13, 1361. [Google Scholar] [CrossRef]
- Borowsky, B.; Adham, N.; Jones, K.A.; Raddatz, R.; Artymyshyn, R.; Ogozalek, K.L.; Durkin, M.M.; Lakhlani, P.P.; Bonini, J.A.; Pathirana, S.; et al. Trace amines: Identification of a family of mammalian G protein-coupled receptors. Proc. Natl. Acad. Sci. USA 2001, 98, 8966–8971. [Google Scholar] [CrossRef]
- Bunzow, J.R.; Sonders, M.S.; Arttamangkul, S.; Harrison, L.M.; Zhang, G.; Quigley, D.I.; Darland, T.; Suchland, K.L.; Pasumamula, S.; Kennedy, J.L.; et al. Amphetamine, 3,4-methylenedioxymethamphetamine, lysergic acid diethylamide, and metabolites of the catecholamine neurotransmitters are agonists of a rat trace amine receptor. Mol. Pharmacol. 2001, 60, 1181–1188. [Google Scholar] [CrossRef]
- Lindemann, L.; Ebeling, M.; Kratochwil, N.A.; Bunzow, J.R.; Grandy, D.K.; Hoener, M.C. Trace amine-associated receptors form structurally and functionally distinct subfamilies of novel G protein-coupled receptors. Genomics 2005, 85, 372–385. [Google Scholar] [CrossRef]
- Eyun, S.I.; Moriyama, H.; Hoffmann, F.G.; Moriyama, E.N. Molecular Evolution and Functional Divergence of Trace Amine-Associated Receptors. PLoS ONE 2016, 11, e0151023. [Google Scholar] [CrossRef]
- Gloriam, D.E.; Bjarnadóttir, T.K.; Schiöth, H.B.; Fredriksson, R. High species variation within the repertoire of trace amine receptors. Ann. N. Y. Acad. Sci. 2005, 1040, 323–327. [Google Scholar] [CrossRef]
- Lindemann, L.; Meyer, C.A.; Jeanneau, K.; Bradaia, A.; Ozmen, L.; Bluethmann, H.; Bettler, B.; Wettstein, J.G.; Borroni, E.; Moreau, J.L.; et al. Trace amine-associated receptor 1 modulates dopaminergic activity. J. Pharmacol. Exp. Ther. 2008, 324, 948–956. [Google Scholar] [CrossRef]
- Dinter, J.; Mühlhaus, J.; Wienchol, C.L.; Yi, C.X.; Nürnberg, D.; Morin, S.; Grüters, A.; Köhrle, J.; Schöneberg, T.; Tschöp, M.; et al. Inverse agonistic action of 3-iodothyronamine at the human trace amine-associated receptor 5. PLoS ONE 2015, 10, e0117774. [Google Scholar] [CrossRef]
- Espinoza, S.; Lignani, G.; Caffino, L.; Maggi, S.; Sukhanov, I.; Leo, D.; Mus, L.; Emanuele, M.; Ronzitti, G.; Harmeier, A.; et al. TAAR1 Modulates Cortical Glutamate NMDA Receptor Function. Neuropsychopharmacology 2015, 40, 2217–2227. [Google Scholar] [CrossRef]
- Liu, J.F.; Seaman, R.; Siemian, J.N.; Bhimani, R.; Johnson, B.; Zhang, Y.; Zhu, Q.; Hoener, M.C.; Park, J.; Dietz, D.M.; et al. Role of trace amine-associated receptor 1 in nicotine’s behavioral and neurochemical effects. Neuropsychopharmacology 2018, 43, 2435–2444. [Google Scholar] [CrossRef]
- Ferragud, A.; Howell, A.D.; Moore, C.F.; Ta, T.L.; Hoener, M.C.; Sabino, V.; Cottone, P. The Trace Amine-Associated Receptor 1 Agonist RO5256390 Blocks Compulsive, Binge-like Eating in Rats. Neuropsychopharmacology 2017, 42, 1458–1470. [Google Scholar] [CrossRef]
- Martin, C.R.; Osadchiy, V.; Kalani, A.; Mayer, E.A. The Brain-Gut-Microbiome Axis. Cell. Mol. Gastroenterol. Hepatol. 2018, 6, 133–148. [Google Scholar] [CrossRef]
- Bugda Gwilt, K.; González, D.; Olliffe, N.; Oller, H.; Hoffing, R.; Puzan, M.; El Aidy, S.; Miller, G. Actions of Trace Amines in the Brain-Gut-Microbiome Axis via Trace Amine-Associated Receptor-1 (TAAR1). Cell. Mol. Neurobiol. 2020, 40, 191–201. [Google Scholar] [CrossRef]
- Christian, S.L.; Berry, M.D. Trace Amine-Associated Receptors as Novel Therapeutic Targets for Immunomodulatory Disorders. Front. Pharmacol. 2018, 9, 680. [Google Scholar] [CrossRef]
- Simmler, L.D.; Buchy, D.; Chaboz, S.; Hoener, M.C.; Liechti, M.E. In Vitro Characterization of Psychoactive Substances at Rat, Mouse, and Human Trace Amine-Associated Receptor 1. J. Pharmacol. Exp. Ther. 2016, 357, 134–144. [Google Scholar] [CrossRef]
- Hu, L.A.; Zhou, T.; Ahn, J.; Wang, S.; Zhou, J.; Hu, Y.; Liu, Q. Human and mouse trace amine-associated receptor 1 have distinct pharmacology towards endogenous monoamines and imidazoline receptor ligands. Biochem. J. 2009, 424, 39–45. [Google Scholar] [CrossRef]
- Sukhanov, I.; Espinoza, S.; Yakovlev, D.S.; Hoener, M.C.; Sotnikova, T.D.; Gainetdinov, R.R. TAAR1-dependent effects of apomorphine in mice. Int. J. Neuropsychopharmacol. 2014, 17, 1683–1693. [Google Scholar] [CrossRef]
- Liu, X.; Grandy, D.K.; Janowsky, A. Ractopamine, a livestock feed additive, is a full agonist at trace amine-associated receptor 1. J. Pharmacol. Exp. Ther. 2014, 350, 124–129. [Google Scholar] [CrossRef]
- Bradaia, A.; Trube, G.; Stalder, H.; Norcross, R.D.; Ozmen, L.; Wettstein, J.G.; Pinard, A.; Buchy, D.; Gassmann, M.; Hoener, M.C.; et al. The selective antagonist EPPTB reveals TAAR1-mediated regulatory mechanisms in dopaminergic neurons of the mesolimbic system. Proc. Natl. Acad. Sci. USA 2009, 106, 20081–20086. [Google Scholar] [CrossRef]
- Stalder, H.; Hoener, M.C.; Norcross, R.D. Selective antagonists of mouse trace amine-associated receptor 1 (mTAAR1): Discovery of EPPTB (RO5212773). Bioorg. Med. Chem. Lett. 2011, 21, 1227–1231. [Google Scholar] [CrossRef]
- Babusyte, A.; Kotthoff, M.; Fiedler, J.; Krautwurst, D. Biogenic amines activate blood leukocytes via trace amine-associated receptors TAAR1 and TAAR2. J. Leukoc. Biol. 2013, 93, 387–394. [Google Scholar] [CrossRef]
- Andersen, G.; Krautwurst, D. Trace Amine-Associated Receptors in the Cellular Immune System. In Trace Amines and Neurological Disorders; Academic Press: Cambridge, MA, USA, 2016; pp. 97–105. [Google Scholar] [CrossRef]
- Kovács, T.; Mikó, E.; Vida, A.; Sebő, E.; Toth, J.; Csonka, T.; Boratkó, A.; Ujlaki, G.; Lente, G.; Kovács, P.; et al. Cadaverine, a metabolite of the microbiome, reduces breast cancer aggressiveness through trace amino acid receptors. Sci. Rep. 2019, 9, 1300. [Google Scholar] [CrossRef]
- Zhang, X.Q.; Li, J.T.; Si, T.M.; Su, Y.A. Research progress on the immunomodulatory effects and mechanisms of trace amine-associated receptor 1. Sheng Li Xue Bao 2023, 75, 248–254. [Google Scholar]
- Sriram, U.; Cenna, J.M.; Haldar, B.; Fernandes, N.C.; Razmpour, R.; Fan, S.; Ramirez, S.H.; Potula, R. Methamphetamine induces trace amine-associated receptor 1 (TAAR1) expression in human T lymphocytes: Role in immunomodulation. J. Leukoc. Biol. 2016, 99, 213–223. [Google Scholar] [CrossRef]
- Polini, B.; Ricardi, C.; Bertolini, A.; Carnicelli, V.; Rutigliano, G.; Saponaro, F.; Zucchi, R.; Chiellini, G. T1AM/TAAR1 System Reduces Inflammatory Response and β-Amyloid Toxicity in Human Microglial HMC3 Cell Line. Int. J. Mol. Sci. 2023, 24, 11569. [Google Scholar] [CrossRef]
- Wasik, A.M.; Millan, M.J.; Scanlan, T.; Barnes, N.M.; Gordon, J. Evidence for functional trace amine associated receptor-1 in normal and malignant B cells. Leuk. Res. 2012, 36, 245–249. [Google Scholar] [CrossRef]
- D’Andrea, G.; Terrazzino, S.; Fortin, D.; Farruggio, A.; Rinaldi, L.; Leon, A. HPLC electrochemical detection of trace amines in human plasma and platelets and expression of mRNA transcripts of trace amine receptors in circulating leukocytes. Neurosci. Lett. 2003, 346, 89–92. [Google Scholar] [CrossRef]
- Chang, H.S.; Heo, J.S.; Shin, S.W.; Bae, D.J.; Song, H.J.; Jun, J.A.; Kim, J.D.; Park, J.S.; Park, B.L.; Shin, H.D.; et al. Association between TAAR6 polymorphisms and airway responsiveness to inhaled corticosteroids in asthmatic patients. Pharmacogenet. Genom. 2015, 25, 334–342. [Google Scholar] [CrossRef] [PubMed]
- Panas, M.W.; Xie, Z.; Panas, H.N.; Hoener, M.C.; Vallender, E.J.; Miller, G.M. Trace amine associated receptor 1 signaling in activated lymphocytes. J. Neuroimmune Pharmacol. 2012, 7, 866–876. [Google Scholar] [CrossRef] [PubMed]
- Samanta, M.; Iwakiri, D.; Kanda, T.; Imaizumi, T.; Takada, K. EB virus-encoded RNAs are recognized by RIG-I and activate signaling to induce type I IFN. EMBO J. 2006, 25, 4207–4214. [Google Scholar] [CrossRef] [PubMed]
- Luckey, C.J.; Bhattacharya, D.; Goldrath, A.W.; Weissman, I.L.; Benoist, C.; Mathis, D. Memory T and memory B cells share a transcriptional program of self-renewal with long-term hematopoietic stem cells. Proc. Natl. Acad. Sci. USA 2006, 103, 3304–3309. [Google Scholar] [CrossRef] [PubMed]
- Barnes, P.J. Pathophysiology of allergic inflammation. Immunol. Rev. 2011, 242, 31–50. [Google Scholar] [CrossRef] [PubMed]
- Potula, R.; Hawkins, B.J.; Cenna, J.M.; Fan, S.; Dykstra, H.; Ramirez, S.H.; Morsey, B.; Brodie, M.R.; Persidsky, Y. Methamphetamine causes mitrochondrial oxidative damage in human T lymphocytes leading to functional impairment. J. Immunol. 2010, 185, 2867–2876. [Google Scholar] [CrossRef] [PubMed]
- Terunuma, H.; Deng, X.; Dewan, Z.; Fujimoto, S.; Yamamoto, N. Potential role of NK cells in the induction of immune responses: Implications for NK cell-based immunotherapy for cancers and viral infections. Int. Rev. Immunol. 2008, 27, 93–110. [Google Scholar] [CrossRef] [PubMed]
- Mogensen, T.H. Pathogen recognition and inflammatory signaling in innate immune defenses. Clin. Microbiol. Rev. 2009, 22, 240–273. [Google Scholar] [CrossRef] [PubMed]
- Duan, T.; Du, Y.; Xing, C.; Wang, H.Y.; Wang, R.F. Toll-Like Receptor Signaling and Its Role in Cell-Mediated Immunity. Front. Immunol. 2022, 13, 812774. [Google Scholar] [CrossRef]
- Barnes, D.A.; Hoener, M.C.; Moore, C.S.; Berry, M.D. TAAR1 Regulates Purinergic-induced TNF Secretion from Peripheral, But Not CNS-resident, Macrophages. J. Neuroimmune Pharmacol. 2023, 18, 100–111. [Google Scholar] [CrossRef]
- Bugda Gwilt, K.; Olliffe, N.; Hoffing, R.A.; Miller, G.M. Trace amine associated receptor 1 (TAAR1) expression and modulation of inflammatory cytokine production in mouse bone marrow-derived macrophages: A novel mechanism for inflammation in ulcerative colitis. Immunopharmacol. Immunotoxicol. 2019, 41, 577–585. [Google Scholar] [CrossRef] [PubMed]
- Amulic, B.; Cazalet, C.; Hayes, G.L.; Metzler, K.D.; Zychlinsky, A. Neutrophil function: From mechanisms to disease. Annu. Rev. Immunol. 2012, 30, 459–489. [Google Scholar] [CrossRef] [PubMed]
- Lattin, J.E.; Schroder, K.; Su, A.I.; Walker, J.R.; Zhang, J.; Wiltshire, T.; Saijo, K.; Glass, C.K.; Hume, D.A.; Kellie, S.; et al. Expression analysis of G Protein-Coupled Receptors in mouse macrophages. Immunome Res. 2008, 4, 5. [Google Scholar] [CrossRef] [PubMed]
- Geoffrey, R.; Jia, S.; Kwitek, A.E.; Woodliff, J.; Ghosh, S.; Lernmark, A.; Wang, X.; Hessner, M.J. Evidence of a functional role for mast cells in the development of type 1 diabetes mellitus in the BioBreeding rat. J. Immunol. 2006, 177, 7275–7286. [Google Scholar] [CrossRef]
- Cisneros, I.E.; Ghorpade, A. HIV-1, methamphetamine and astrocyte glutamate regulation: Combined excitotoxic implications for neuro-AIDS. Curr. HIV Res. 2012, 10, 392–406. [Google Scholar] [CrossRef] [PubMed]
- D’Andrea, G.; D’Arrigo, A.; Facchinetti, F.; Del Giudice, E.; Colavito, D.; Bernardini, D.; Leon, A. Octopamine, unlike other trace amines, inhibits responses of astroglia-enriched cultures to lipopolysaccharide via a β-adrenoreceptor-mediated mechanism. Neurosci. Lett. 2012, 517, 36–40. [Google Scholar] [CrossRef] [PubMed]
- Taquet, N.; Philippe, C.; Reimund, J.-M.; Muller, C.D. Inflammatory bowel disease G-protein coupled receptors (GPCRs) expression profiling with microfluidic cards. In Crohn’s Disease; Books on Demand GmbH: Norderstedt, Germany, 2012; pp. 59–86. [Google Scholar] [CrossRef]
- Fernández de Palencia, P.; Fernández, M.; Mohedano, M.L.; Ladero, V.; Quevedo, C.; Alvarez, M.A.; López, P. Role of tyramine synthesis by food-borne Enterococcus durans in adaptation to the gastrointestinal tract environment. Appl. Environ. Microbiol. 2011, 77, 699–702. [Google Scholar] [CrossRef] [PubMed]
- Jansson, J.; Willing, B.; Lucio, M.; Fekete, A.; Dicksved, J.; Halfvarson, J.; Tysk, C.; Schmitt-Kopplin, P. Metabolomics reveals metabolic biomarkers of Crohn’s disease. PLoS ONE 2009, 4, e6386. [Google Scholar] [CrossRef] [PubMed]
- Zhukov, I.S.; Vaganova, A.N.; Murtazina, R.Z.; Alferova, L.S.; Ermolenko, E.I.; Gainetdinov, R.R. Gut Microbiota Alterations in Trace Amine-Associated Receptor 9 (TAAR9) Knockout Rats. Biomolecules 2022, 12, 1823. [Google Scholar] [CrossRef]
- Smith, S.B.; Maixner, D.W.; Fillingim, R.B.; Slade, G.; Gracely, R.H.; Ambrose, K.; Zaykin, D.V.; Hyde, C.; John, S.; Tan, K.; et al. Large candidate gene association study reveals genetic risk factors and therapeutic targets for fibromyalgia. Arthritis Rheum. 2012, 64, 584–593. [Google Scholar] [CrossRef]
- Barnes, D.A.; Galloway, D.A.; Hoener, M.C.; Berry, M.D.; Moore, C.S. TAAR1 Expression in Human Macrophages and Brain Tissue: A Potential Novel Facet of MS Neuroinflammation. Int. J. Mol. Sci. 2021, 22, 11576. [Google Scholar] [CrossRef] [PubMed]
| Receptor | Expression in Human Immune Cell Populations | Known Ligands | Biological Function | References |
|---|---|---|---|---|
| TAAR1 | Peripheral mononuclear cells, B-lymphocytes, T-lymphocytes, polymorphonuclear neutrophils, monocyte, NK-cells | β-Phenylethylamine (PEA)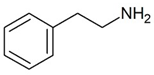 | The joint effect of β-phenylethylamine and IL-4-stimulated IgE synthesis. The chemosensory migration of polymorphonuclear leukocytes towards TAAR agonists. Possible joint effect with TAAR2 due to heterodimerization. | [34] |
| TAAR1 | Methamphetamine (METH)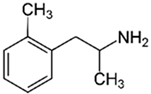 | The elevated concentration of intracellular calcium, active forms of oxygen. Stimulation of the differentiation of Th0 into Th2, reduced production of IL-2, intensified production of IL-6. | [34,37,38] | |
| TAAR1 | Microglia | 3-iodothyronamine (T1AM)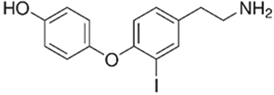 | T1AM is capable of reducing the amyloid-beta (Aβ)-stimulated TNFα and LPS’s inflammatory response on part of microglia through the inhibition of the release of pro-inflammatory factors (IL-6, TNFα, NF-kB, MCP1, and MIP1), stimulating the release of anti-inflammatory mediators (IL-10) | [39] |
| TAAR1 | Peripheral mononuclear cells, B-lymphocytes, T-lymphocytes, polymorphonuclear neutrophils, monocyte, NK-cells | Tyramine (TYR)  3-iodothyronamine (T1AM)  | The chemosensory migration of polymorphonuclear leukocytes towards TAAR agonists. | [34,40] |
| TAAR2 | Peripheral mononuclear cells, B-lymphocytes, T-lymphocytes, polymorphonuclear neutrophils, monocyte, NK-cells | β-Phenylethylamine (PEA) | – | [34] |
| TAAR5 | B-lymphocytes, T-lymphocytes, polymorphonuclear neutrophils, monocytes, NK-cells | Trimethylamine (TMA)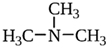 Derivative of choline 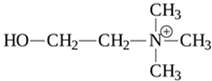 | – | [11,34] |
| TAAR6 | B-lymphocytes, T-lymphocytes, polymorphonuclear neutrophils, monocytes, NK-cells | Potent ligands have not yet been identified Weak activity: N-methylpiperdine 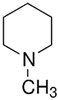 | – | [36] |
| TAAR8 | mRNA expression in leucocytes is controversial | Potent ligands have not yet been identified Weak activity: N-methylpiperdine 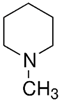 Cadaverine  | – | [34,36,41] |
| TAAR9 | B-lymphocytes, T-lymphocytes, polymorphonuclear neutrophils, monocytes, NK-cells | Potent ligands have not yet been identified Weak activity: N-methylpiperdine  Cadaverine 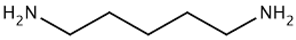 | – | [36] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moiseenko, V.I.; Apryatina, V.A.; Gainetdinov, R.R.; Apryatin, S.A. Trace Amine-Associated Receptors’ Role in Immune System Functions. Biomedicines 2024, 12, 893. https://doi.org/10.3390/biomedicines12040893
Moiseenko VI, Apryatina VA, Gainetdinov RR, Apryatin SA. Trace Amine-Associated Receptors’ Role in Immune System Functions. Biomedicines. 2024; 12(4):893. https://doi.org/10.3390/biomedicines12040893
Chicago/Turabian StyleMoiseenko, Vyacheslav I., Vera A. Apryatina, Raul R. Gainetdinov, and Sergey A. Apryatin. 2024. "Trace Amine-Associated Receptors’ Role in Immune System Functions" Biomedicines 12, no. 4: 893. https://doi.org/10.3390/biomedicines12040893
APA StyleMoiseenko, V. I., Apryatina, V. A., Gainetdinov, R. R., & Apryatin, S. A. (2024). Trace Amine-Associated Receptors’ Role in Immune System Functions. Biomedicines, 12(4), 893. https://doi.org/10.3390/biomedicines12040893






