Polyamine Catabolism and Its Role in Renal Injury and Fibrosis in Mice Subjected to Repeated Low-Dose Cisplatin Treatment
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Generation and Genotyping of Sat1- and Smox-KO Mice
2.3. Mouse RLCD
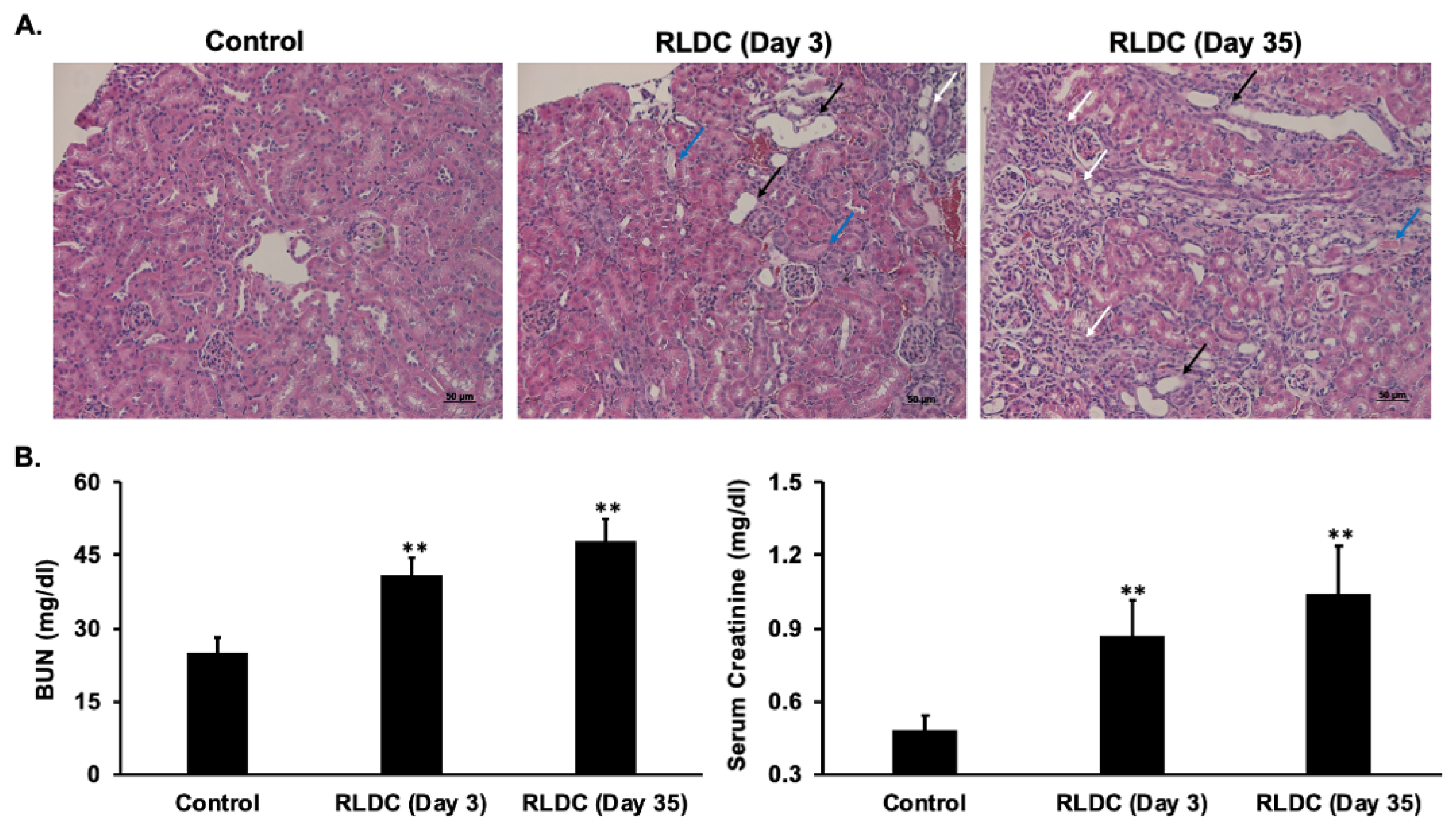
2.4. Assessment of Renal Function
2.5. Histopathology, Immunofluorescence Microscopy, and Immunohistochemical Examination of Kidneys
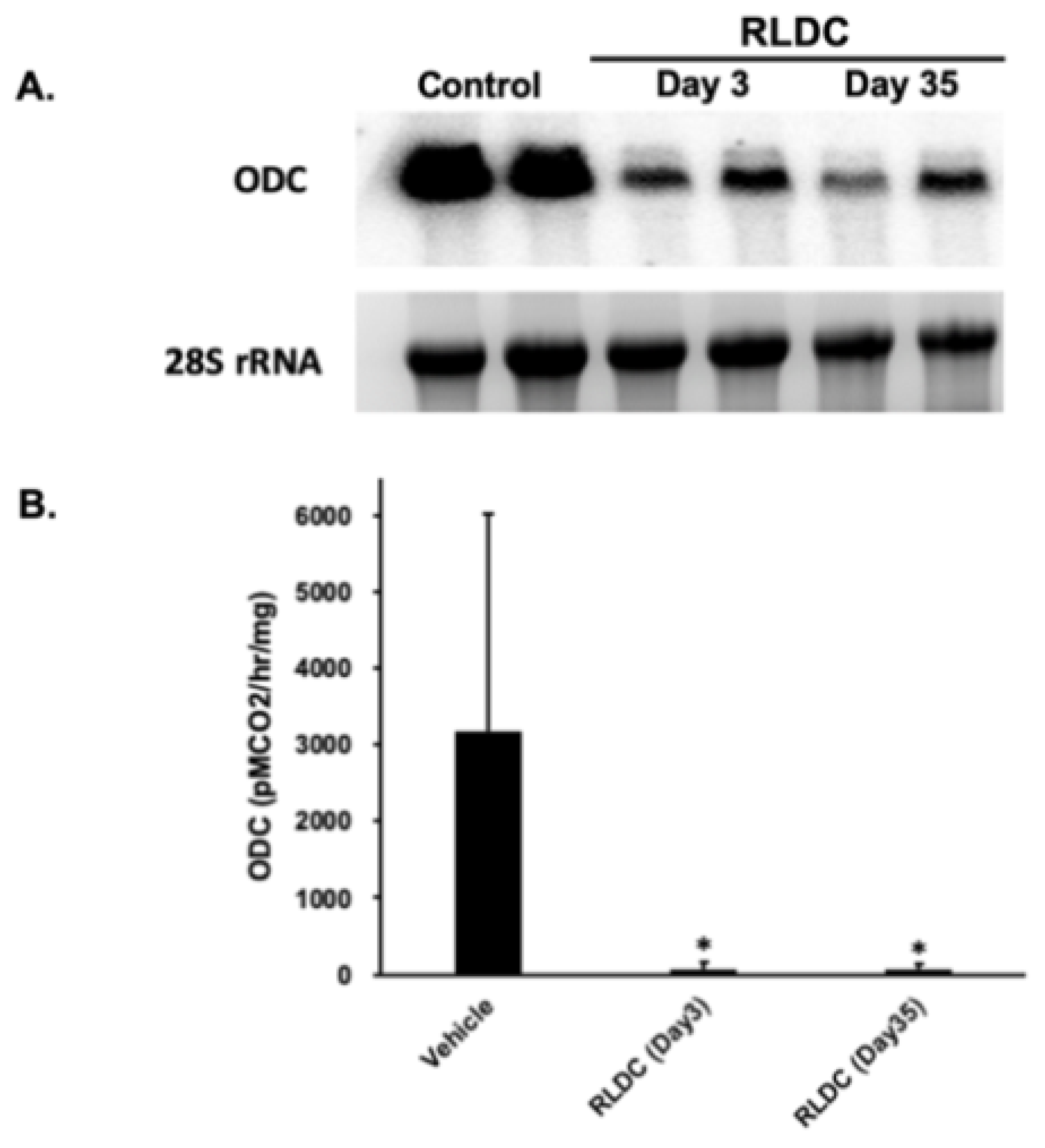
2.6. Measurement of Kidney Polyamine and ODC Levels
2.7. RNA Extraction and Northern Blot Analysis
2.8. RNA-Seq Analysis
2.9. Statistical Analysis
3. Results
3.1. RLDC Treatment Leads to Early and Persistent Renal Injury
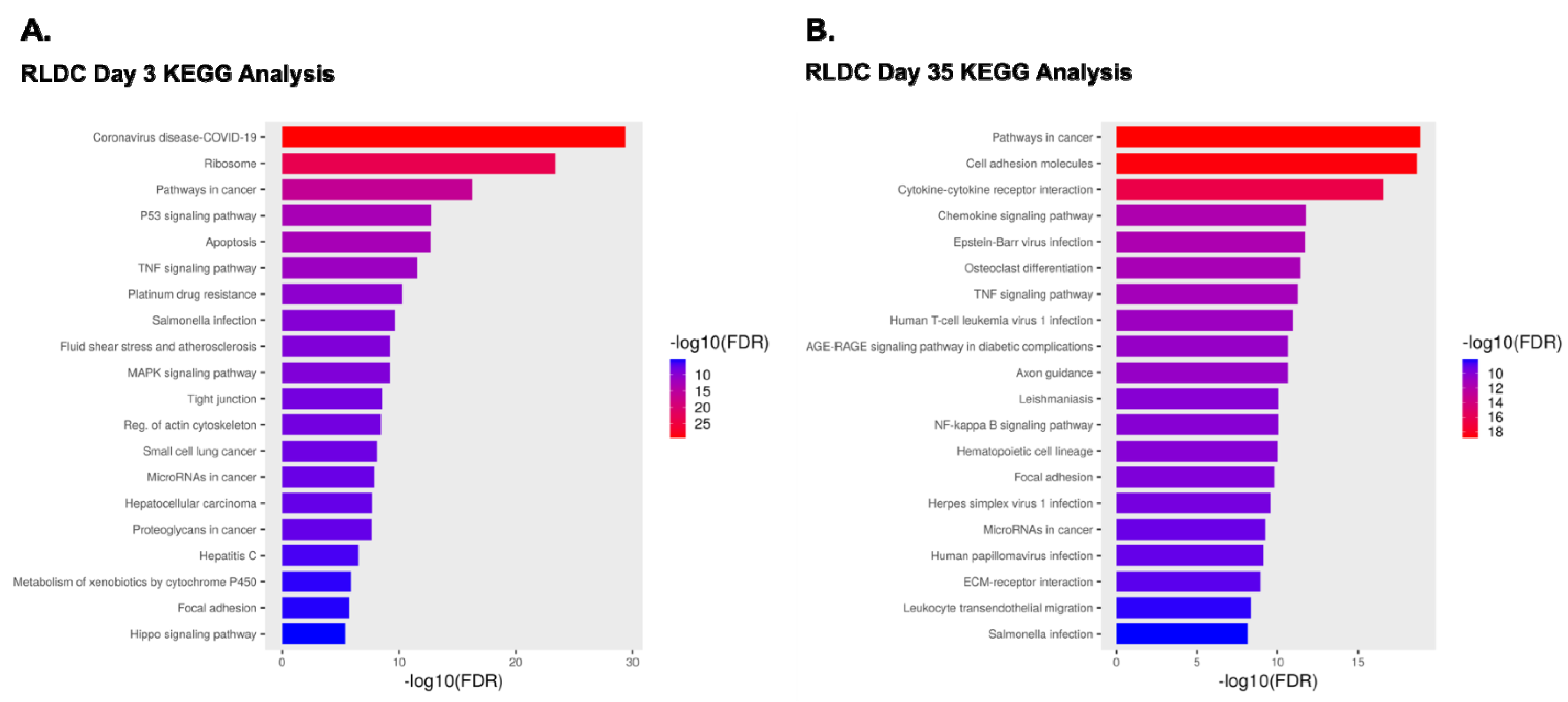
3.2. Comparison of Transcriptomes of Control Mice and Mice Subjected to RLDC
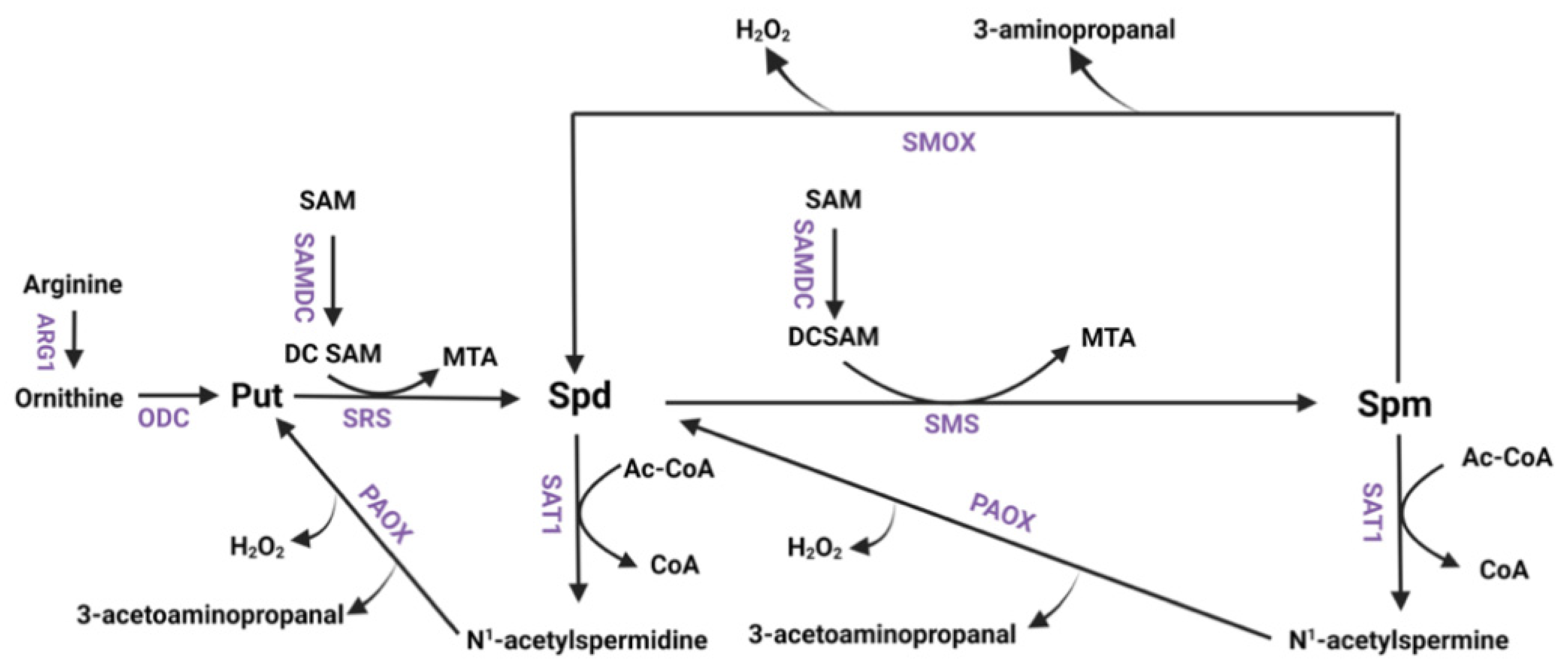
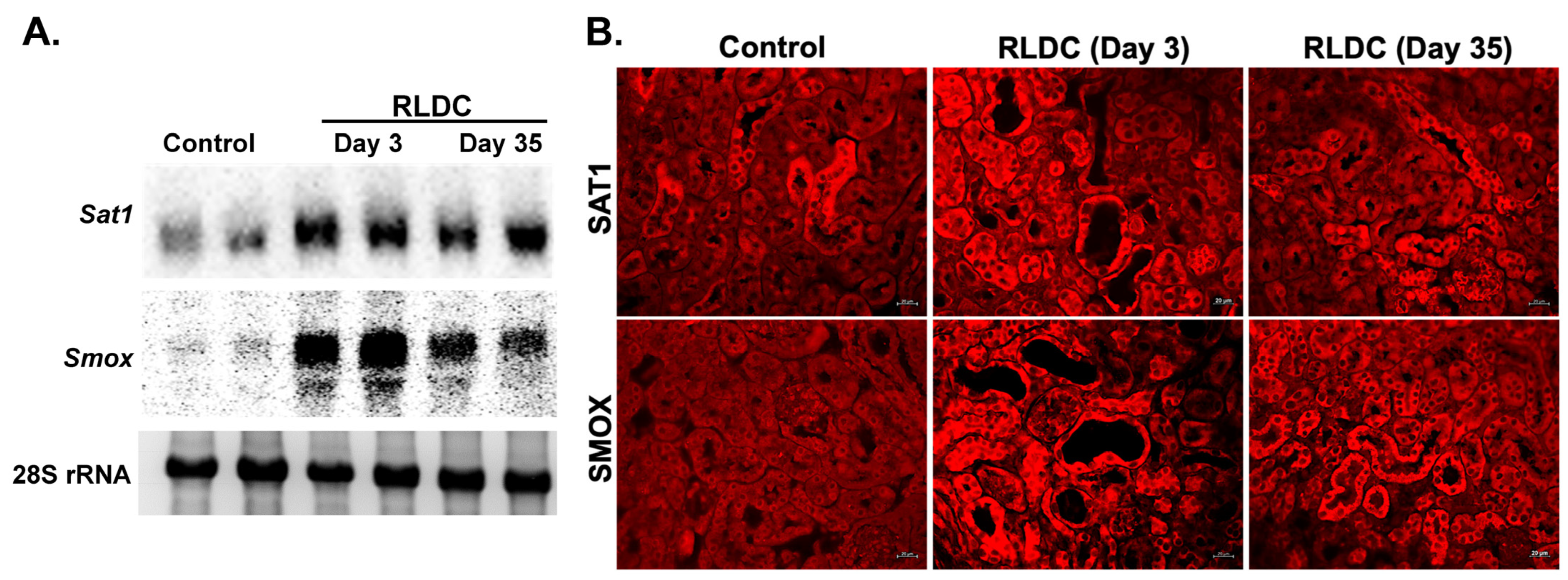
3.3. Examination of the Expression of Polyamine Pathway Enzymes in RLDC Mice
3.4. Comparison of Renal Polyamine Levels in Control and RLDC Mice
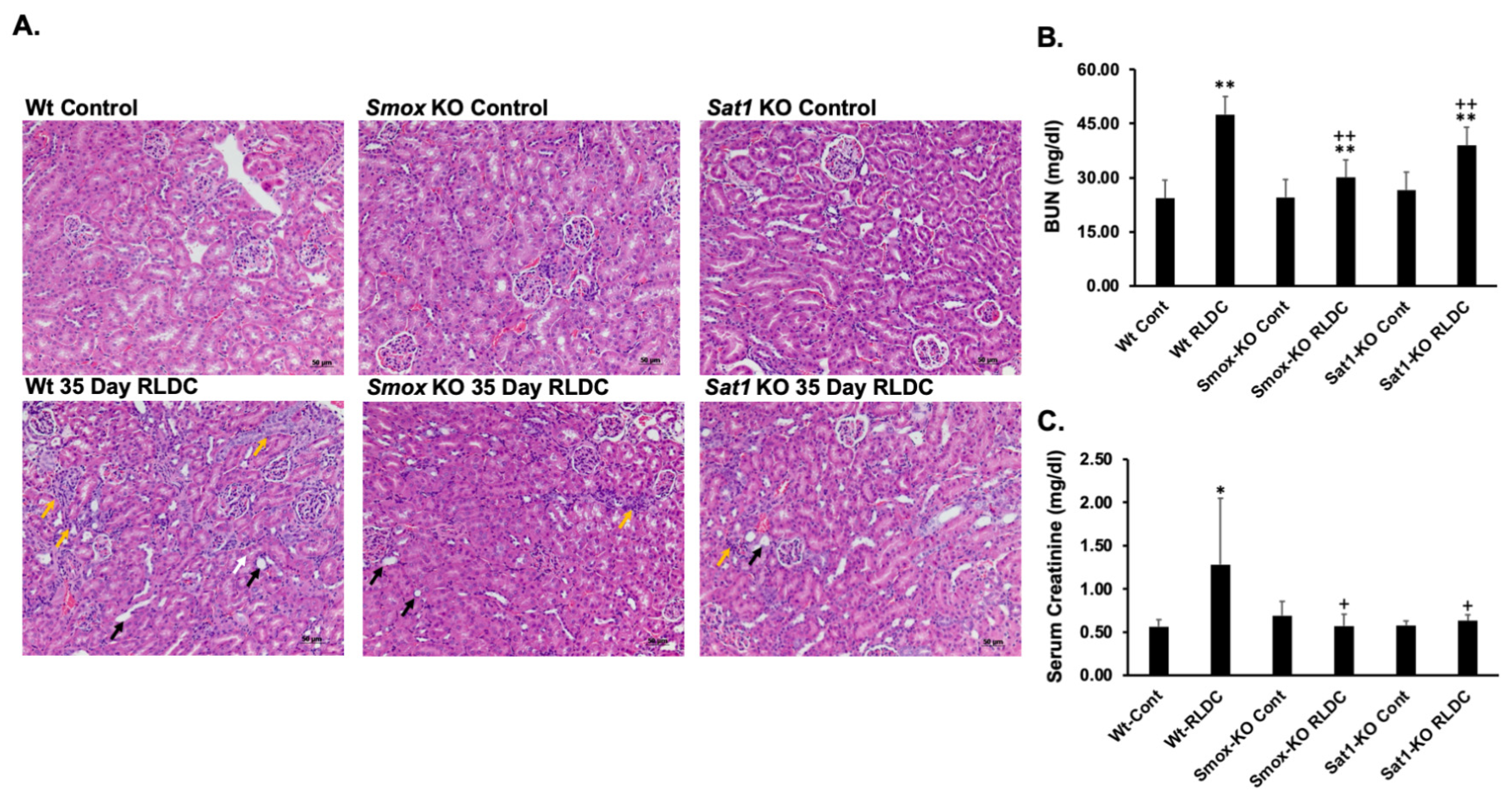
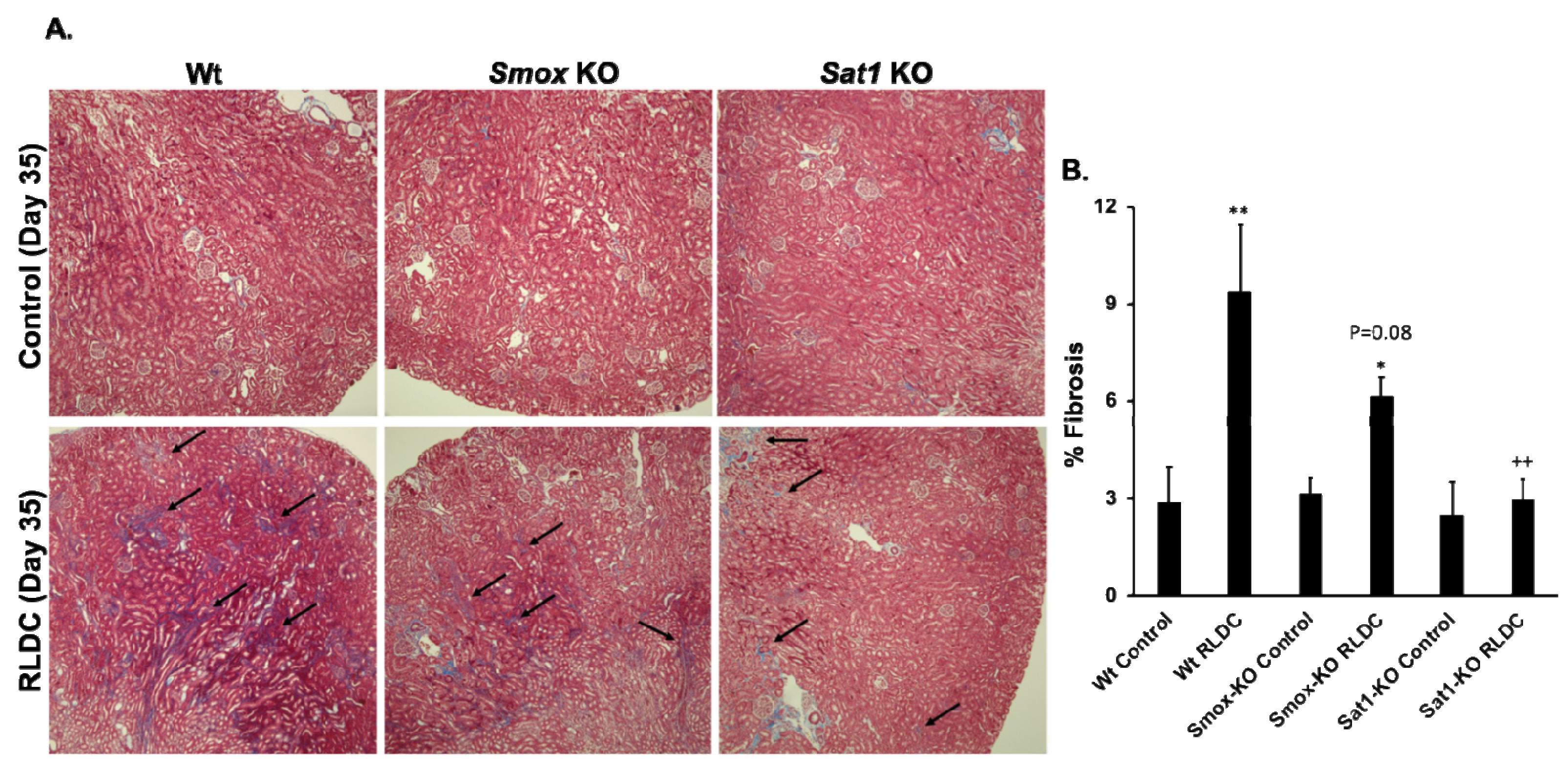
3.5. Ablation of Sat1 or Smox Reduces the Severity of RLDC-Induced Renal Injury and Fibrosis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ozkok, A.; Edelstein, C.L. Pathophysiology of cisplatin-induced acute kidney injury. Biomed. Res. Int. 2014, 2014, 967826. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Pabla, N.; Tang, C.; He, L.; Dong, Z. DNA damage response in cisplatin-induced nephrotoxicity. Arch. Toxicol. 2015, 89, 2197–2205. [Google Scholar] [CrossRef] [PubMed]
- Miller, R.P.; Tadagavadi, R.K.; Ramesh, G.; Reeves, W.B. Mechanisms of Cisplatin nephrotoxicity. Toxins 2010, 2, 2490–2518. [Google Scholar] [CrossRef] [PubMed]
- Santos, N.; Ferreira, R.S.; Santos, A.C.D. Overview of cisplatin-induced neurotoxicity and ototoxicity, and the protective agents. Food Chem. Toxicol. 2020, 136, 111079. [Google Scholar] [CrossRef] [PubMed]
- Pabla, N.; Dong, Z. Cisplatin nephrotoxicity: Mechanisms and renoprotective strategies. Kidney Int. 2008, 73, 994–1007. [Google Scholar] [CrossRef]
- Nakanishi, S.; Cleveland, J.L. Polyamine Homeostasis in Development and Disease. Med. Sci. 2021, 9, 28. [Google Scholar] [CrossRef] [PubMed]
- Hasan, R.; Alam, M.K.; Ali, R. Polyamine induced Z-conformation of native calf thymus DNA. FEBS Lett. 1995, 368, 27–30. [Google Scholar] [CrossRef] [PubMed]
- Igarashi, K.; Kashiwagi, K. Polyamines: Mysterious modulators of cellular functions. Biochem. Biophys. Res. Commun. 2000, 271, 559–564. [Google Scholar] [CrossRef]
- Janne, J.; Alhonen, L.; Leinonen, P. Polyamines: From molecular biology to clinical applications. Ann. Med. 1991, 23, 241–259. [Google Scholar] [CrossRef]
- Marton, L.J.; Pegg, A.E. Polyamines as targets for therapeutic intervention. Annu. Rev. Pharmacol. Toxicol. 1995, 35, 55–91. [Google Scholar] [CrossRef]
- Murray Stewart, T.; Dunston, T.T.; Woster, P.M.; Casero, R.A., Jr. Polyamine catabolism and oxidative damage. J. Biol. Chem. 2018, 293, 18736–18745. [Google Scholar] [CrossRef] [PubMed]
- Abdulhussein, A.A.; Wallace, H.M. Polyamines and membrane transporters. Amino Acids 2014, 46, 655–660. [Google Scholar] [CrossRef] [PubMed]
- Pegg, A.E.; McCann, P.P. Polyamine metabolism and function. Am. J. Physiol. 1982, 243, C212–C221. [Google Scholar] [CrossRef] [PubMed]
- Soda, K. Overview of Polyamines as Nutrients for Human Healthy Long Life and Effect of Increased Polyamine Intake on DNA Methylation. Cells 2022, 11, 164. [Google Scholar] [CrossRef] [PubMed]
- Zahedi, K.; Barone, S.; Soleimani, M. Polyamines and Their Metabolism: From the Maintenance of Physiological Homeostasis to the Mediation of Disease. Med. Sci. 2022, 10, 38. [Google Scholar] [CrossRef] [PubMed]
- Minois, N.; Carmona-Gutierrez, D.; Madeo, F. Polyamines in aging and disease. Aging 2011, 3, 716–732. [Google Scholar] [CrossRef]
- Sagar, N.A.; Tarafdar, S.; Agarwal, S.; Tarafdar, A.; Sharma, S. Polyamines: Functions, Metabolism, and Role in Human Disease Management. Med. Sci. 2021, 9, 44. [Google Scholar] [CrossRef]
- Xuan, M.; Gu, X.; Li, S.; Huang, D.; Xue, C.; He, Y. Polyamines: Their significance for maintaining health and contributing to disease. Cell Commun. Signal. 2023, 21, 384. [Google Scholar] [CrossRef]
- Pegg, A.E. Toxicity of polyamines and their metabolic products. Chem. Res. Toxicol. 2013, 26, 1782–1800. [Google Scholar] [CrossRef]
- Seiler, N. Catabolism of polyamines. Amino Acids 2004, 26, 217–233. [Google Scholar] [CrossRef] [PubMed]
- Pledgie, A.; Huang, Y.; Hacker, A.; Zhang, Z.; Woster, P.M.; Davidson, N.E.; Casero, R.A., Jr. Spermine oxidase SMO(PAOh1), Not N1-acetylpolyamine oxidase PAO, is the primary source of cytotoxic H2O2 in polyamine analogue-treated human breast cancer cell lines. J. Biol. Chem. 2005, 280, 39843–39851. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Devereux, W.; Woster, P.M.; Stewart, T.M.; Hacker, A.; Casero, R.A., Jr. Cloning and characterization of a human polyamine oxidase that is inducible by polyamine analogue exposure. Cancer Res. 2001, 61, 5370–5373. [Google Scholar] [PubMed]
- Cohen, S.S. A Guide to the Polyamines; Oxford University Press: New York, NY, USA, 1998. [Google Scholar]
- Boya, P.; Gonzalez-Polo, R.A.; Poncet, D.; Andreau, K.; Vieira, H.L.; Roumier, T.; Perfettini, J.L.; Kroemer, G. Mitochondrial membrane permeabilization is a critical step of lysosome-initiated apoptosis induced by hydroxychloroquine. Oncogene 2003, 22, 3927–3936. [Google Scholar] [CrossRef] [PubMed]
- Boya, P.; Kroemer, G. Lysosomal membrane permeabilization in cell death. Oncogene 2008, 27, 6434–6451. [Google Scholar] [CrossRef] [PubMed]
- Brunk, U.T.; Zhang, H.; Dalen, H.; Ollinger, K. Exposure of cells to nonlethal concentrations of hydrogen peroxide induces degeneration-repair mechanisms involving lysosomal destabilization. Free Radic. Biol. Med. 1995, 19, 813–822. [Google Scholar] [CrossRef]
- Brunk, U.T.; Zhang, H.; Roberg, K.; Ollinger, K. Lethal hydrogen peroxide toxicity involves lysosomal iron-catalyzed reactions with membrane damage. Redox Rep. 1995, 1, 267–277. [Google Scholar] [CrossRef]
- Mandal, S.; Mandal, A.; Park, M.H. Depletion of the polyamines spermidine and spermine by overexpression of spermidine/spermine N(1)-acetyltransferase 1 (SAT1) leads to mitochondria-mediated apoptosis in mammalian cells. Biochem. J. 2015, 468, 435–447. [Google Scholar] [CrossRef]
- Mandic, A.; Hansson, J.; Linder, S.; Shoshan, M.C. Cisplatin induces endoplasmic reticulum stress and nucleus-independent apoptotic signaling. J. Biol. Chem. 2003, 278, 9100–9106. [Google Scholar] [CrossRef]
- Wang, Z.; Zahedi, K.; Barone, S.; Tehrani, K.; Rabb, H.; Matlin, K.; Casero, R.A.; Soleimani, M. Overexpression of SSAT in kidney cells recapitulates various phenotypic aspects of kidney ischemia-reperfusion injury. J. Am. Soc. Nephrol. 2004, 15, 1844–1852. [Google Scholar] [CrossRef]
- Zahedi, K.; Barone, S.; Wang, Y.; Murray-Stewart, T.; Roy-Chaudhury, P.; Smith, R.D.; Casero, R.A., Jr.; Soleimani, M. Proximal tubule epithelial cell specific ablation of the spermidine/spermine N1-acetyltransferase gene reduces the severity of renal ischemia/reperfusion injury. PLoS ONE 2014, 9, e110161. [Google Scholar] [CrossRef] [PubMed]
- Zahedi, K.; Bissler, J.J.; Wang, Z.; Josyula, A.; Lu, L.; Diegelman, P.; Kisiel, N.; Porter, C.W.; Soleimani, M. Spermidine/spermine N1-acetyltransferase overexpression in kidney epithelial cells disrupts polyamine homeostasis, leads to DNA damage, and causes G2 arrest. Am. J. Physiol. Cell Physiol. 2007, 292, C1204–C1215. [Google Scholar] [CrossRef]
- Chaturvedi, R.; de Sablet, T.; Asim, M.; Piazuelo, M.B.; Barry, D.P.; Verriere, T.G.; Sierra, J.C.; Hardbower, D.M.; Delgado, A.G.; Schneider, B.G.; et al. Increased Helicobacter pylori-associated gastric cancer risk in the Andean region of Colombia is mediated by spermine oxidase. Oncogene 2015, 34, 3429–3440. [Google Scholar] [CrossRef]
- Goodwin, A.C.; Destefano Shields, C.E.; Wu, S.; Huso, D.L.; Wu, X.; Murray-Stewart, T.R.; Hacker-Prietz, A.; Rabizadeh, S.; Woster, P.M.; Sears, C.L.; et al. Polyamine catabolism contributes to enterotoxigenic Bacteroides fragilis-induced colon tumorigenesis. Proc. Natl. Acad. Sci. USA 2011, 108, 15354–15359. [Google Scholar] [CrossRef] [PubMed]
- Zahedi, K.; Huttinger, F.; Morrison, R.; Murray-Stewart, T.; Casero, R.A.; Strauss, K.I. Polyamine catabolism is enhanced after traumatic brain injury. J. Neurotrauma 2010, 27, 515–525. [Google Scholar] [CrossRef] [PubMed]
- Zahedi, K.; Lentsch, A.B.; Okaya, T.; Barone, S.; Sakai, N.; Witte, D.P.; Arend, L.J.; Alhonen, L.; Jell, J.; Janne, J.; et al. Spermidine/spermine-N1-acetyltransferase ablation protects against liver and kidney ischemia-reperfusion injury in mice. Am. J. Physiol. Gastrointest. Liver Physiol. 2009, 296, G899–G909. [Google Scholar] [CrossRef] [PubMed]
- Zahedi, K.; Wang, Z.; Barone, S.; Prada, A.E.; Kelly, C.N.; Casero, R.A.; Yokota, N.; Porter, C.W.; Rabb, H.; Soleimani, M. Expression of SSAT, a novel biomarker of tubular cell damage, increases in kidney ischemia-reperfusion injury. Am. J. Physiol. Renal Physiol. 2003, 284, F1046–F1055. [Google Scholar] [CrossRef] [PubMed]
- Golab, F.; Kadkhodaee, M.; Zahmatkesh, M.; Hedayati, M.; Arab, H.; Schuster, R.; Zahedi, K.; Lentsch, A.B.; Soleimani, M. Ischemic and non-ischemic acute kidney injury cause hepatic damage. Kidney Int. 2009, 75, 783–792. [Google Scholar] [CrossRef] [PubMed]
- Igarashi, K.; Ueda, S.; Yoshida, K.; Kashiwagi, K. Polyamines in renal failure. Amino Acids 2006, 31, 477–483. [Google Scholar] [CrossRef] [PubMed]
- Ivanova, S.; Batliwalla, F.; Mocco, J.; Kiss, S.; Huang, J.; Mack, W.; Coon, A.; Eaton, J.W.; Al-Abed, Y.; Gregersen, P.K.; et al. Neuroprotection in cerebral ischemia by neutralization of 3-aminopropanal. Proc. Natl. Acad. Sci. USA 2002, 99, 5579–5584. [Google Scholar] [CrossRef]
- Rider, J.E.; Hacker, A.; Mackintosh, C.A.; Pegg, A.E.; Woster, P.M.; Casero, R.A., Jr. Spermine and spermidine mediate protection against oxidative damage caused by hydrogen peroxide. Amino Acids 2007, 33, 231–240. [Google Scholar] [CrossRef]
- Zahedi, K.; Barone, S.; Destefano-Shields, C.; Brooks, M.; Murray-Stewart, T.; Dunworth, M.; Li, W.; Doherty, J.R.; Hall, M.A.; Smith, R.D.; et al. Activation of endoplasmic reticulum stress response by enhanced polyamine catabolism is important in the mediation of cisplatin-induced acute kidney injury. PLoS ONE 2017, 12, e0184570. [Google Scholar] [CrossRef] [PubMed]
- Zahedi, K.; Barone, S.L.; Xu, J.; Steinbergs, N.; Schuster, R.; Lentsch, A.B.; Amlal, H.; Wang, J.; Casero, R.A., Jr.; Soleimani, M. Hepatocyte-specific ablation of spermine/spermidine-N1-acetyltransferase gene reduces the severity of CCl4-induced acute liver injury. Am. J. Physiol. Gastrointest. Liver Physiol. 2012, 303, G546–G560. [Google Scholar] [CrossRef] [PubMed]
- Fukushima, K.; Futatsugi, A.; Maekawa, M.; Naito, S.; Okada, A.; Sugioka, N. Comparison of cisplatin-induced nephrotoxicity between single-dose and split-dose administration to rats. Biomed. Pharmacother. 2022, 147, 112619. [Google Scholar] [CrossRef] [PubMed]
- Perse, M. Cisplatin Mouse Models: Treatment, Toxicity and Translatability. Biomedicines 2021, 9, 1406. [Google Scholar] [CrossRef]
- Sharp, C.N.; Siskind, L.J. Developing better mouse models to study cisplatin-induced kidney injury. Am. J. Physiol. Renal Physiol. 2017, 313, F835–F841. [Google Scholar] [CrossRef] [PubMed]
- Sharp, C.N.; Doll, M.A.; Dupre, T.V.; Shah, P.P.; Subathra, M.; Siow, D.; Arteel, G.E.; Megyesi, J.; Beverly, L.J.; Siskind, L.J. Repeated administration of low-dose cisplatin in mice induces fibrosis. Am. J. Physiol. Renal Physiol. 2016, 310, F560–F568. [Google Scholar] [CrossRef] [PubMed]
- Sharp, C.N.; Doll, M.A.; Megyesi, J.; Oropilla, G.B.; Beverly, L.J.; Siskind, L.J. Subclinical kidney injury induced by repeated cisplatin administration results in progressive chronic kidney disease. Am. J. Physiol. Renal Physiol. 2018, 315, F161–F172. [Google Scholar] [CrossRef]
- Jell, J.; Merali, S.; Hensen, M.L.; Mazurchuk, R.; Spernyak, J.A.; Diegelman, P.; Kisiel, N.D.; Barrero, C.; Deeb, K.K.; Alhonen, L.; et al. Genetically altered expression of spermidine/spermine N1-acetyltransferase affects fat metabolism in mice via acetyl-CoA. J. Biol. Chem. 2007, 282, 8404–8413. [Google Scholar] [CrossRef]
- Niiranen, K.; Pietila, M.; Pirttila, T.J.; Jarvinen, A.; Halmekyto, M.; Korhonen, V.P.; Keinanen, T.A.; Alhonen, L.; Janne, J. Targeted disruption of spermidine/spermine N1-acetyltransferase gene in mouse embryonic stem cells. Effects on polyamine homeostasis and sensitivity to polyamine analogues. J. Biol. Chem. 2002, 277, 25323–25328. [Google Scholar] [CrossRef]
- Kabra, P.M.; Lee, H.K.; Lubich, W.P.; Marton, L.J. Solid-phase extraction and determination of dansyl derivatives of unconjugated and acetylated polyamines by reversed-phase liquid chromatography: Improved separation systems for polyamines in cerebrospinal fluid, urine and tissue. J. Chromatogr. 1986, 380, 19–32. [Google Scholar] [CrossRef]
- Porter, C.W.; Ganis, B.; Libby, P.R.; Bergeron, R.J. Correlations between polyamine analogue-induced increases in spermidine/spermine N1-acetyltransferase activity, polyamine pool depletion, and growth inhibition in human melanoma cell lines. Cancer Res. 1991, 51, 3715–3720. [Google Scholar]
- Seely, J.E.; Pegg, A.E. Changes in mouse kidney ornithine decarboxylase activity are brought about by changes in the amount of enzyme protein as measured by radioimmunoassay. J. Biol. Chem. 1983, 258, 2496–2500. [Google Scholar] [CrossRef]
- Seely, J.E.; Pegg, A.E. Ornithine decarboxylase (mouse kidney). Methods Enzymol. 1983, 94, 158–161. [Google Scholar] [CrossRef]
- Zheng, M.; Hu, Z.; Wang, Y.; Wang, C.; Zhong, C.; Cui, W.; You, J.; Gao, B.; Sun, X.; La, L. Zhen Wu decoction represses renal fibrosis by invigorating tubular NRF2 and TFAM to fuel mitochondrial bioenergetics. Phytomedicine 2023, 108, 154495. [Google Scholar] [CrossRef]
- Cervelli, M.; Bellavia, G.; D’Amelio, M.; Cavallucci, V.; Moreno, S.; Berger, J.; Nardacci, R.; Marcoli, M.; Maura, G.; Piacentini, M.; et al. A New Transgenic Mouse Model for Studying the Neurotoxicity of Spermine Oxidase Dosage in the Response to Excitotoxic Injury. PLoS ONE 2013, 8, e64810. [Google Scholar] [CrossRef]
- Sierra, J.C.; Piazuelo, M.B.; Luis, P.B.; Barry, D.P.; Allaman, M.M.; Asim, M.; Sebrell, T.A.; Finley, J.L.; Rose, K.L.; Hill, S.; et al. Spermine oxidase mediates Helicobacter pylori-induced gastric inflammation, DNA damage, and carcinogenic signaling. Oncogene 2020, 39, 4465–4474. [Google Scholar] [CrossRef]
- Zahedi, K.; Barone, S.; Kramer, D.L.; Amlal, H.; Alhonen, L.; Janne, J.; Porter, C.W.; Soleimani, M. The role of spermidine/spermine N1-acetyltransferase in endotoxin-induced acute kidney injury. Am. J. Physiol. Cell Physiol. 2010, 299, C164–C174. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.; Hu, X.; Ding, H.F.; Zhang, M.; Huo, Y.; Dong, Z. Single-Nucleus Transcriptional Profiling of Chronic Kidney Disease after Cisplatin Nephrotoxicity. Am. J. Pathol. 2022, 192, 613–628. [Google Scholar] [CrossRef] [PubMed]
- Sears, S.M.; Vega, A.A.; Kurlawala, Z.; Oropilla, G.B.; Krueger, A.; Shah, P.P.; Doll, M.A.; Miller, R.; Beverly, L.J.; Siskind, L.J. F4/80(hi) Resident Macrophages Contribute to Cisplatin-Induced Renal Fibrosis. Kidney360 2022, 3, 818–833. [Google Scholar] [CrossRef] [PubMed]
- Soda, K.; Uemura, T.; Sanayama, H.; Igarashi, K.; Fukui, T. Polyamine-Rich Diet Elevates Blood Spermine Levels and Inhibits Pro-Inflammatory Status: An Interventional Study. Med. Sci. 2021, 9, 22. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Caragine, T.; Wang, H.; Cohen, P.S.; Botchkina, G.; Soda, K.; Bianchi, M.; Ulrich, P.; Cerami, A.; Sherry, B.; et al. Spermine inhibits proinflammatory cytokine synthesis in human mononuclear cells: A counterregulatory mechanism that restrains the immune response. J. Exp. Med. 1997, 185, 1759–1768. [Google Scholar] [CrossRef] [PubMed]
- Ivanova, S.; Botchkina, G.I.; Al-Abed, Y.; Meistrell, M., 3rd; Batliwalla, F.; Dubinsky, J.M.; Iadecola, C.; Wang, H.; Gregersen, P.K.; Eaton, J.W.; et al. Cerebral ischemia enhances polyamine oxidation: Identification of enzymatically formed 3-aminopropanal as an endogenous mediator of neuronal and glial cell death. J. Exp. Med. 1998, 188, 327–340. [Google Scholar] [CrossRef]
- Kaissling, B.; Lehir, M.; Kriz, W. Renal epithelial injury and fibrosis. Biochim. Biophys. Acta 2013, 1832, 931–939. [Google Scholar] [CrossRef]
- Nath, K.A. Tubulointerstitial changes as a major determinant in the progression of renal damage. Am. J. Kidney Dis. 1992, 20, 1–17. [Google Scholar] [CrossRef]
- Qi, R.; Yang, C. Renal tubular epithelial cells: The neglected mediator of tubulointerstitial fibrosis after injury. Cell Death Dis. 2018, 9, 1126. [Google Scholar] [CrossRef]
- Zahedi, K.; Barone, S.; Soleimani, M. Polyamine Catabolism in Acute Kidney Injury. Int. J. Mol. Sci. 2019, 20, 4790. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Xu, C.; Gao, X.; Yao, Q. Platinum-based drugs for cancer therapy and anti-tumor strategies. Theranostics 2022, 12, 2115–2132. [Google Scholar] [CrossRef] [PubMed]
- Pabla, N.; Dong, G.; Jiang, M.; Huang, S.; Kumar, M.V.; Messing, R.O.; Dong, Z. Inhibition of PKCdelta reduces cisplatin-induced nephrotoxicity without blocking chemotherapeutic efficacy in mouse models of cancer. J. Clin. Investig. 2011, 121, 2709–2722. [Google Scholar] [CrossRef]
- Santoso, J.T.; Lucci, J.A., 3rd; Coleman, R.L.; Schafer, I.; Hannigan, E.V. Saline, mannitol, and furosemide hydration in acute cisplatin nephrotoxicity: A randomized trial. Cancer Chemother. Pharmacol. 2003, 52, 13–18. [Google Scholar] [CrossRef]
- Ramesh, G.; Reeves, W.B. TNF-alpha mediates chemokine and cytokine expression and renal injury in cisplatin nephrotoxicity. J. Clin. Investig. 2002, 110, 835–842. [Google Scholar] [CrossRef]
- Tang, C.; Ma, Z.; Zhu, J.; Liu, Z.; Liu, Y.; Liu, Y.; Cai, J.; Dong, Z. P53 in kidney injury and repair: Mechanism and therapeutic potentials. Pharmacol. Ther. 2019, 195, 5–12. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Ramesh, G.; Uematsu, S.; Akira, S.; Reeves, W.B. TLR4 signaling mediates inflammation and tissue injury in nephrotoxicity. J. Am. Soc. Nephrol. 2008, 19, 923–932. [Google Scholar] [CrossRef] [PubMed]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zahedi, K.; Barone, S.; Brooks, M.; Stewart, T.M.; Foley, J.R.; Nwafor, A.; Casero, R.A., Jr.; Soleimani, M. Polyamine Catabolism and Its Role in Renal Injury and Fibrosis in Mice Subjected to Repeated Low-Dose Cisplatin Treatment. Biomedicines 2024, 12, 640. https://doi.org/10.3390/biomedicines12030640
Zahedi K, Barone S, Brooks M, Stewart TM, Foley JR, Nwafor A, Casero RA Jr., Soleimani M. Polyamine Catabolism and Its Role in Renal Injury and Fibrosis in Mice Subjected to Repeated Low-Dose Cisplatin Treatment. Biomedicines. 2024; 12(3):640. https://doi.org/10.3390/biomedicines12030640
Chicago/Turabian StyleZahedi, Kamyar, Sharon Barone, Marybeth Brooks, Tracy Murray Stewart, Jackson R. Foley, Ashley Nwafor, Robert A. Casero, Jr., and Manoocher Soleimani. 2024. "Polyamine Catabolism and Its Role in Renal Injury and Fibrosis in Mice Subjected to Repeated Low-Dose Cisplatin Treatment" Biomedicines 12, no. 3: 640. https://doi.org/10.3390/biomedicines12030640
APA StyleZahedi, K., Barone, S., Brooks, M., Stewart, T. M., Foley, J. R., Nwafor, A., Casero, R. A., Jr., & Soleimani, M. (2024). Polyamine Catabolism and Its Role in Renal Injury and Fibrosis in Mice Subjected to Repeated Low-Dose Cisplatin Treatment. Biomedicines, 12(3), 640. https://doi.org/10.3390/biomedicines12030640






