Pituitary–Adrenal Axis and Peripheral Immune Cell Profile in Long COVID
Abstract
1. Introduction
2. Materials and Methods
2.1. Inclusion Criteria for Patients
2.2. Health-Related Quality-of-Life Tests
2.3. Adrenal Function
2.4. Flow Cytometry
2.5. Cytokine Assessment
2.6. Statistical Analysis
3. Results
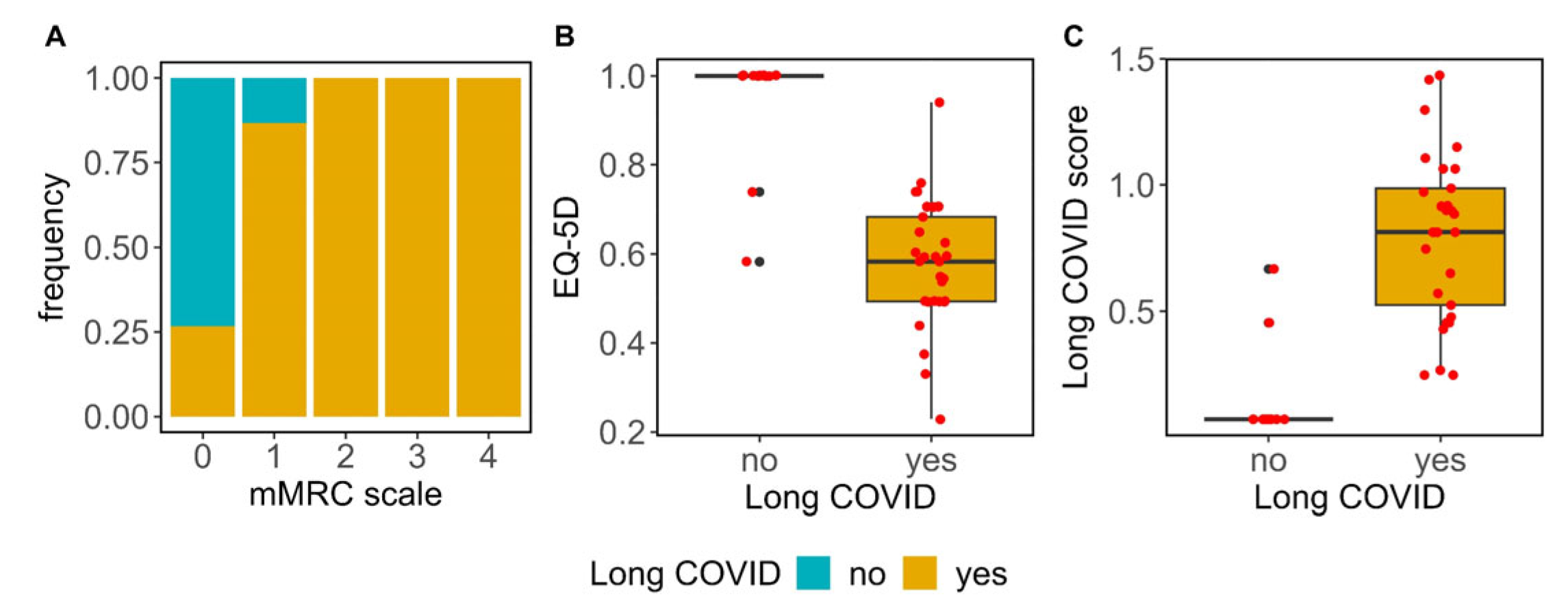
3.1. Long COVID Assessment
3.2. Preclinical and Acute COVID-19 Profiles
3.3. Symptom Evaluation and Quality of Life
3.4. Vaccination and Seropositivity for SARS-CoV2, CMV and EBV Antibodies
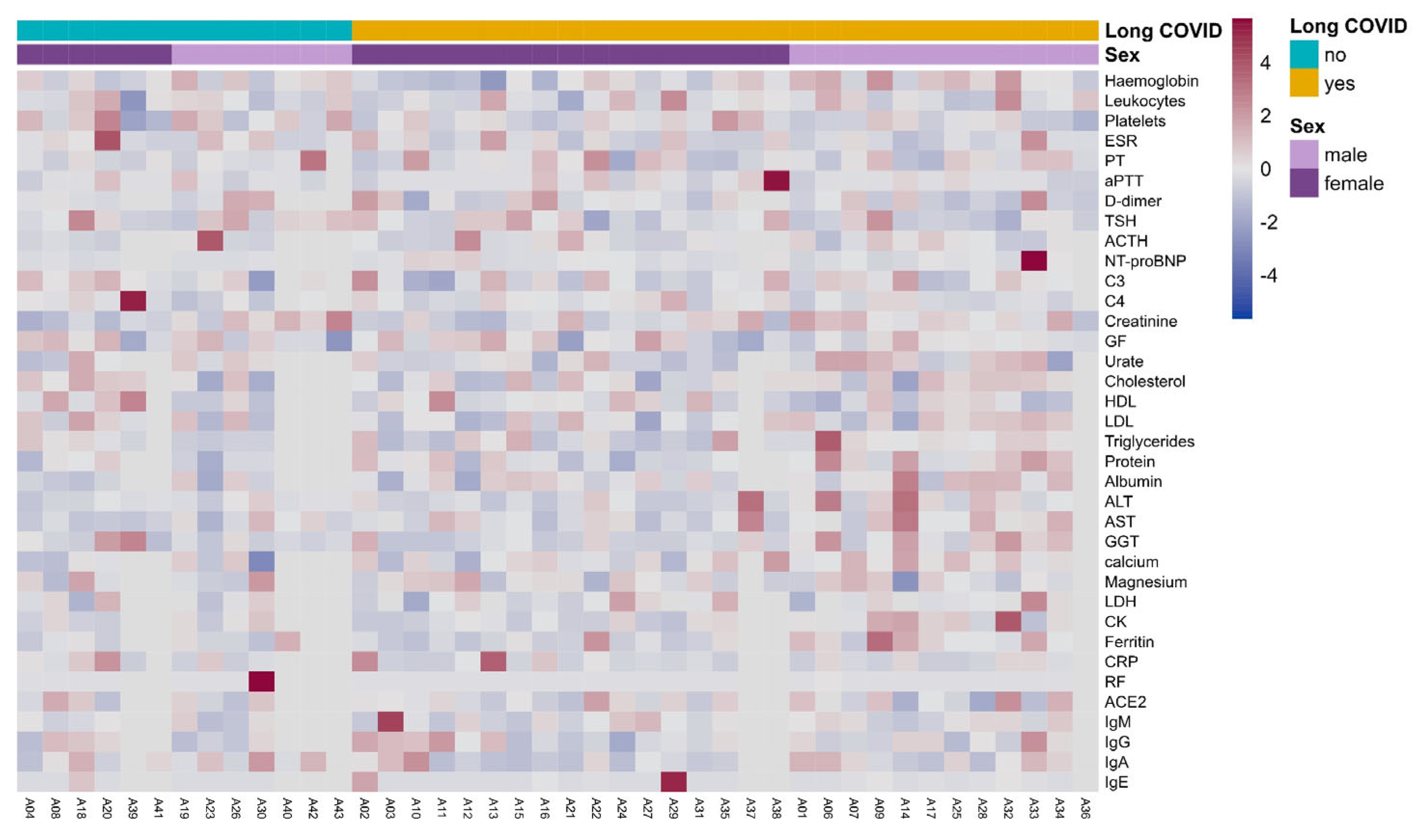
3.5. Clinical Laboratory Parameters in Long COVID
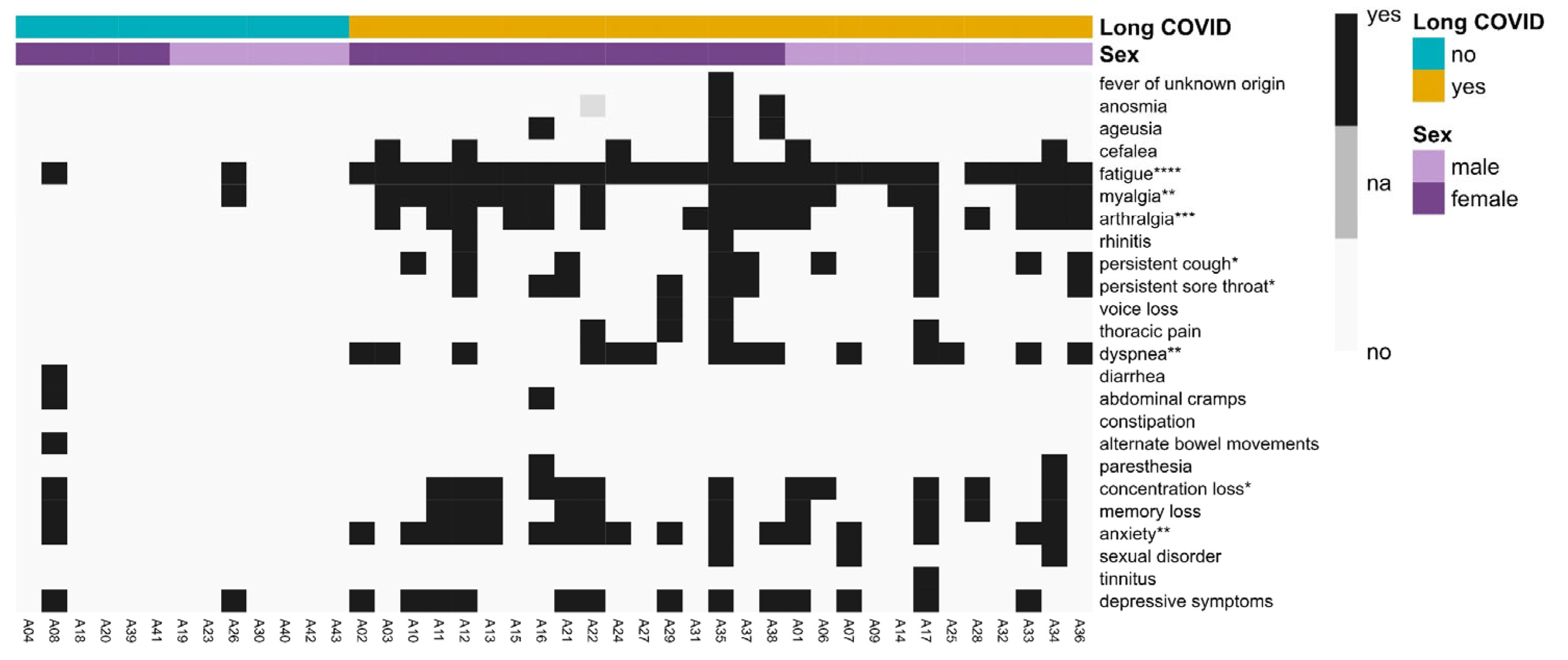
3.6. Long COVID Symptomatology
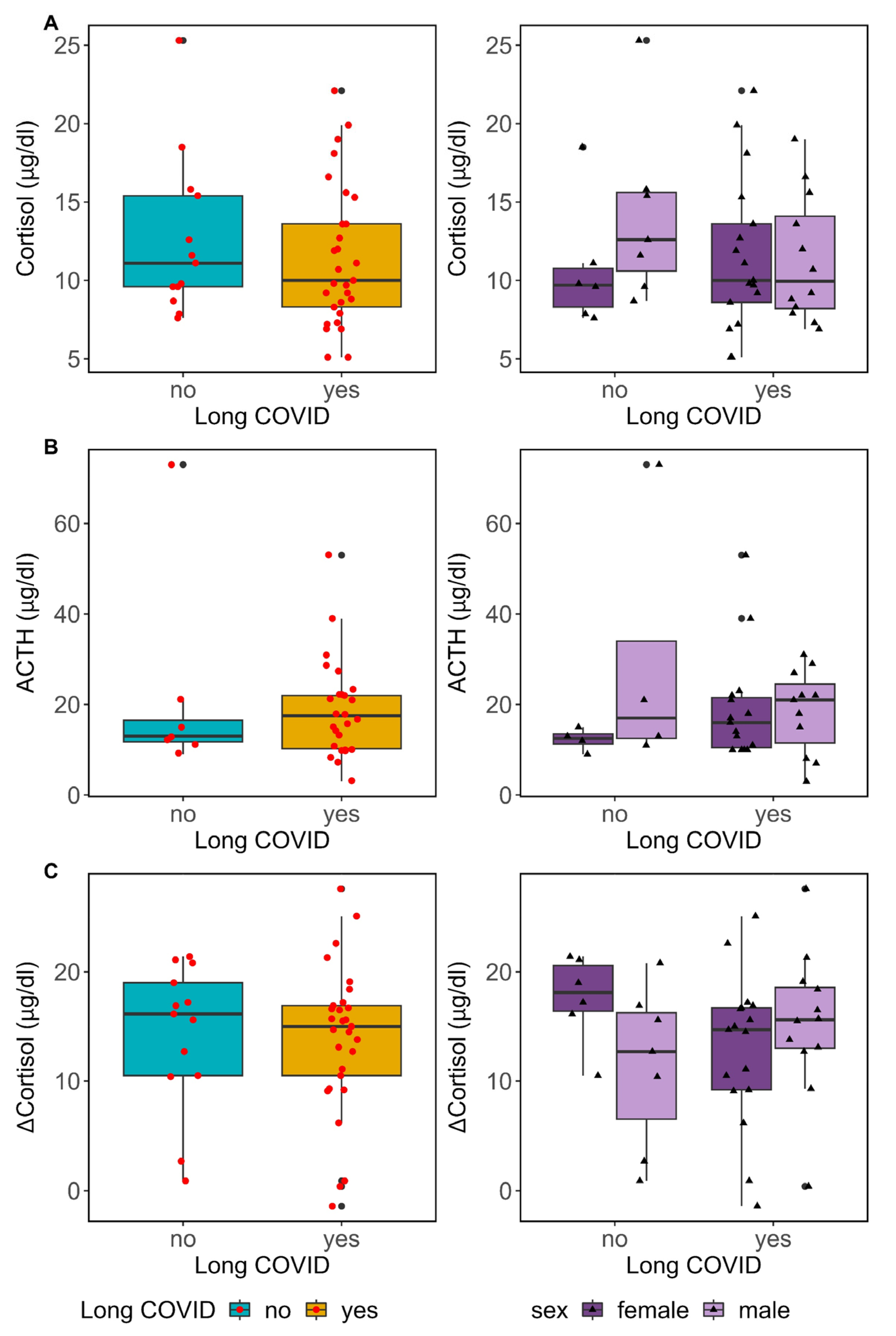
3.7. Pituitary–Adrenal Axis Function in Long COVID
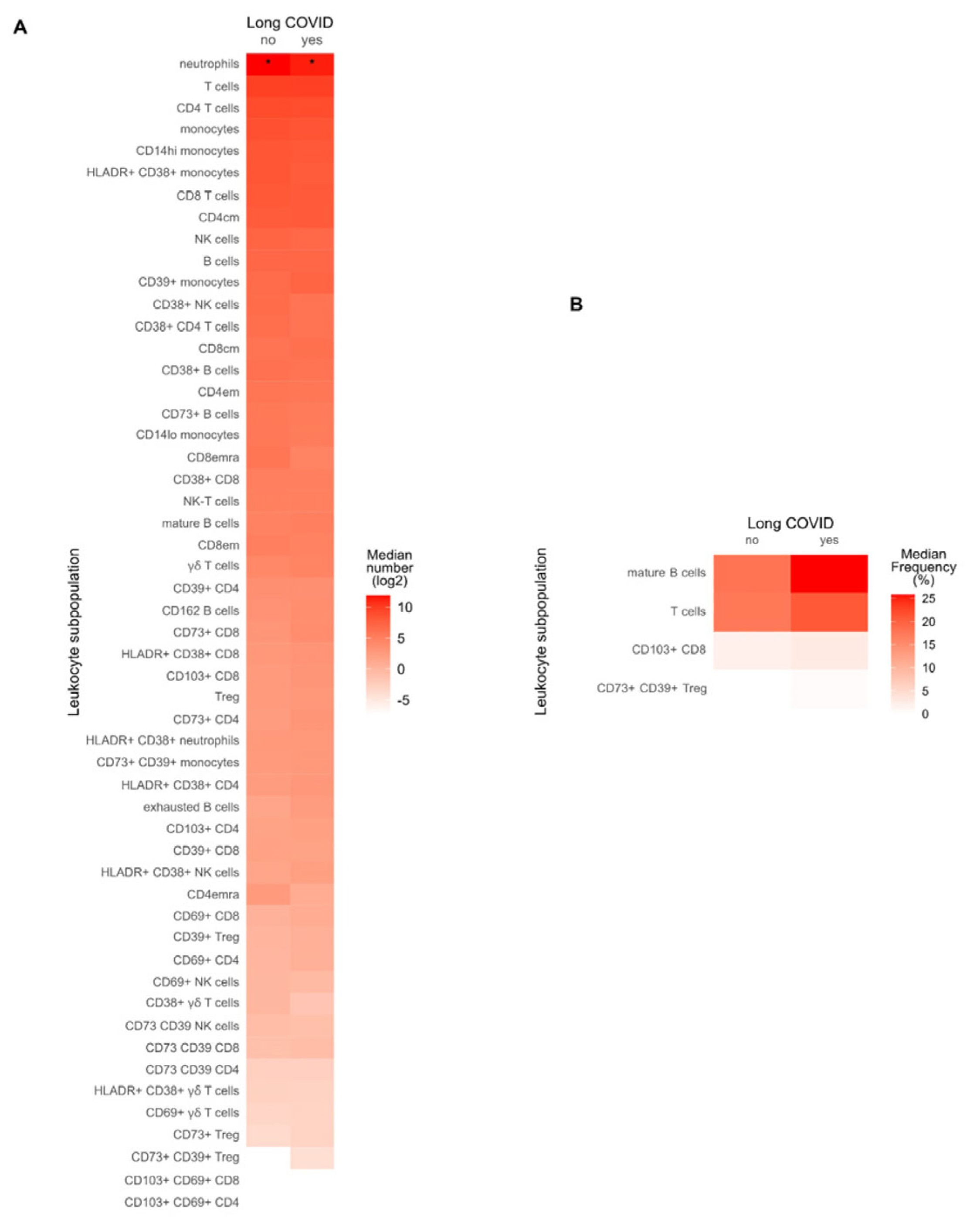
3.8. Immune Profile in Long COVID Patients
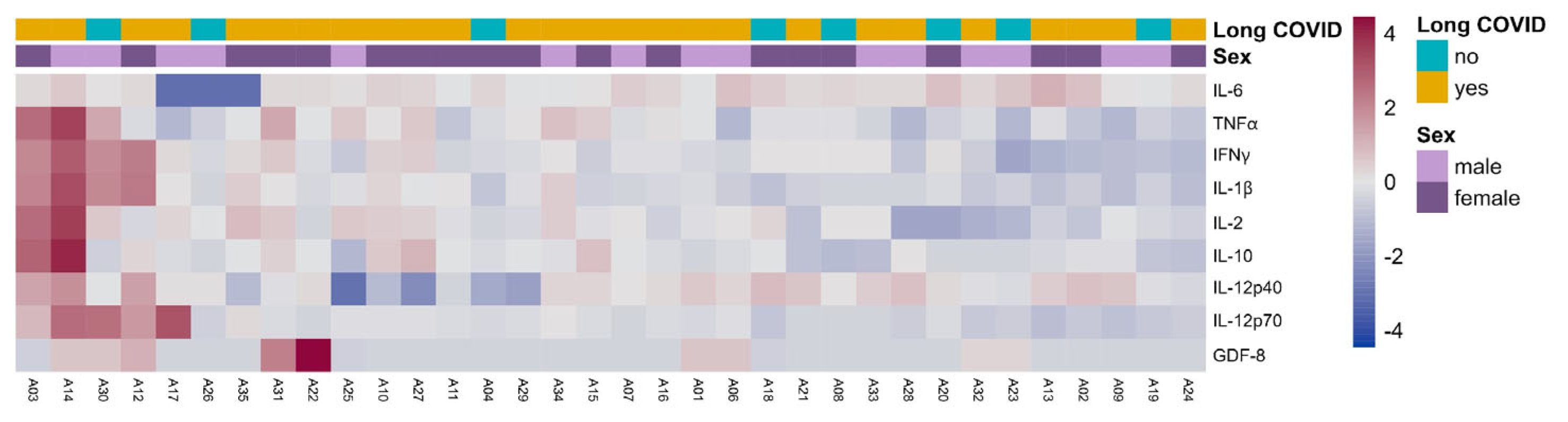
3.9. Cytokine Evaluation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Al-Aly, Z.; Xie, Y.; Bowe, B. High-dimensional characterization of post-acute sequelae of COVID-19. Nature 2021, 594, 259–264. [Google Scholar] [CrossRef]
- Davis, H.E.; McCorkell, L.; Vogel, J.M.; Topol, E.J. Long COVID: Major findings, mechanisms and recommendations. Nat. Rev. Microbiol. 2023, 21, 133–146. [Google Scholar] [CrossRef]
- Bowe, B.; Xie, Y.; Al-Aly, Z. Postacute sequelae of COVID-19 at 2 years. Nat. Med. 2023, 29, 2347–2357. [Google Scholar] [CrossRef]
- Tansey, C.M.; Louie, M.; Loeb, M.; Gold, W.L.; Muller, M.P.; de Jager, J.; Cameron, J.I.; Tomlinson, G.; Mazzulli, T.; Walmsley, S.L.; et al. One-Year Outcomes and Health Care Utilization in Survivors of Severe Acute Respiratory Syndrome. Arch. Intern. Med. 2007, 167, 1312–1320. [Google Scholar] [CrossRef]
- Moldofsky, H.; Patcai, J. Chronic widespread musculoskeletal pain, fatigue, depression and disordered sleep in chronic post-SARS syndrome; a case-controlled study. BMC Neurol. 2011, 11, 37. [Google Scholar] [CrossRef]
- Cortinovis, M.; Perico, N.; Remuzzi, G. Long-term follow-up of recovered patients with COVID-19. Lancet 2021, 397, 173–175. [Google Scholar] [CrossRef] [PubMed]
- Patient-Led Research Collaborative. Report: What Does COVID-19 Recovery Actually Look Like?—Patient Led Research Collaborative 2020. Available online: https://patientresearchcovid19.com/research/report-1/ (accessed on 30 November 2023).
- Wang, Z.; Yang, L. Post-acute Sequelae of SARS-CoV-2 Infection: A Neglected Public Health Issue. Front. Public Health 2022, 10, 908757. [Google Scholar] [CrossRef] [PubMed]
- Marshall, M. The lasting misery of coronavirus long-haulers. Nature 2020, 585, 339–341. [Google Scholar] [CrossRef] [PubMed]
- Belluck, P. Covid Survivors with Long-Term Symptoms Need Urgent Attention, Experts Say. N Y Times 2021. Available online: https://www.nytimes.com/2021/01/08/health/Covid-survivors-longterm.html (accessed on 21 January 2021).
- Groff, D.; Sun, A.; Ssentongo, A.E.; Ba, D.M.; Parsons, N.; Poudel, G.R.; Lekoubou, A.; Oh, J.S.; Ericson, J.E.; Ssentongo, P.; et al. Short-term and Long-term Rates of Postacute Sequelae of SARS-CoV-2 Infection: A Systematic Review. JAMA Netw. Open 2021, 4, e2128568. [Google Scholar] [CrossRef] [PubMed]
- Jiang, D.H.; Roy, D.J.; Gu, B.J.; Hassett, L.C.; McCoy, R.G. Postacute Sequelae of Severe Acute Respiratory Syndrome Coronavirus 2 Infection: A State-of-the-Art Review. JACC Basic Transl. Sci. 2021, 6, 796–811. [Google Scholar] [CrossRef] [PubMed]
- Parker, A.M.; Brigham, E.; Connolly, B.; McPeake, J.; Agranovich, A.V.; Kenes, M.T.; Casey, K.; Reynolds, C.; Schmidt, K.F.R.; Kim, S.Y.; et al. Addressing the post-acute sequelae of SARS-CoV-2 infection: A multidisciplinary model of care. Lancet Respir. Med. 2021, 9, 1328–1341. [Google Scholar] [CrossRef]
- Tenforde, M.W.; Kim, S.S.; Lindsell, C.J.; Rose, E.B.; Shapiro, N.I.; Files, D.C.; Gibbs, K.W.; Erickson, H.L.; Steingrub, J.S.; Smithline, H.A.; et al. Symptom Duration and Risk Factors for Delayed Return to Usual Health Among Outpatients with COVID-19 in a Multistate Health Care Systems Network. Morb. Mortal. Wkly. Rep. 2020, 69, 993–998. [Google Scholar] [CrossRef]
- Bergwerk, M.; Gonen, T.; Lustig, Y.; Amit, S.; Lipsitch, M.; Cohen, C.; Mandelboim, M.; Levin, E.G.; Rubin, C.; Indenbaum, V.; et al. Covid-19 Breakthrough Infections in Vaccinated Health Care Workers. N. Engl. J. Med. 2021, 385, 1474–1484. [Google Scholar] [CrossRef] [PubMed]
- Antonelli, M.; Capdevila Pujol, J.; Spector, T.D.; Ourselin, S.; Steves, C.J. Risk of long COVID associated with delta versus omicron variants of SARS-CoV-2. Lancet 2022, 399, 2264. [Google Scholar] [CrossRef]
- Xie, Y.; Choi, T.; Al-Aly, Z. Association of Treatment with Nirmatrelvir and the Risk of Post–COVID-19 Condition. JAMA Intern. Med. 2023, 183, 554–564. [Google Scholar] [CrossRef] [PubMed]
- Al-Aly, Z.; Bowe, B.; Xie, Y. Long COVID after breakthrough SARS-CoV-2 infection. Nat. Med. 2022, 28, 1461–1467. [Google Scholar] [CrossRef]
- Davis, H.E.; Assaf, G.S.; McCorkell, L.; Wei, H.; Low, R.J.; Re’Em, Y.; Redfield, S.; Austin, J.P.; Akrami, A. Characterizing long COVID in an international cohort: 7 months of symptoms and their impact. EClinicalMedicine 2021, 38, 101019. [Google Scholar] [CrossRef] [PubMed]
- Agergaard, J.; Gunst, J.D.; Schiøttz-Christensen, B.; Østergaard, L.; Wejse, C. Long-term prognosis at 1.5 years after infection with wild-type strain of SARS-CoV-2 and Alpha, Delta, as well as Omicron variants. Int. J. Infect. Dis. 2023, 137, 126–133. [Google Scholar] [CrossRef]
- Bildirici, Y.; Metintas, S.; Ucan, A.; Ak, G.; Guvenc, S.E.; Metintas, M. Post-COVID-19 syndrome in a cohort of hospitalized COVID-19 patients. Eur. Rev. Med. Pharmacol. Sci. 2023, 27, 8291–8300. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Huang, C.; Gu, X.; Wang, Y.; Li, X.; Liu, M.; Wang, Q.; Xu, J.; Wang, Y.; Dai, H.; et al. 3-year outcomes of discharged survivors of COVID-19 following the SARS-CoV-2 omicron (B.1.1.529) wave in 2022 in China: A longitudinal cohort study. Lancet Respir. Med. 2023, 12, 55–66. [Google Scholar] [CrossRef]
- Doykov, I.; Hällqvist, J.; Gilmour, K.C.; Grandjean, L.; Mills, K.; Heywood, W.E. ‘The long tail of COVID-19′—The detection of a prolonged inflammatory response after a SARS-CoV-2 infection in asymptomatic and mildly affected patients. F1000Research 2020, 9, 1349. [Google Scholar] [CrossRef]
- Nalbandian, A.; Sehgal, K.; Gupta, A.; Madhavan, M.V.; McGroder, C.; Stevens, J.S.; Cook, J.R.; Nordvig, A.S.; Shalev, D.; Sehrawat, T.S.; et al. Post-acute COVID-19 syndrome. Nat. Med. 2021, 27, 601–615. [Google Scholar] [CrossRef]
- Klein, J.; Wood, J.; Jaycox, J.R.; Dhodapkar, R.M.; Lu, P.; Gehlhausen, J.R.; Tabachnikova, A.; Greene, K.; Tabacof, L.; Malik, A.A.; et al. Distinguishing features of long COVID identified through immune profiling. Nature 2023, 623, 139–148. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.; Yuan, D.; Chen, D.G.; Ng, R.H.; Wang, K.; Choi, J.; Li, S.; Hong, S.; Zhang, R.; Xie, J.; et al. Multiple early factors anticipate post-acute COVID-19 sequelae. Cell 2022, 185, 881–895.e20. [Google Scholar] [CrossRef] [PubMed]
- Dani, M.; Dirksen, A.; Taraborrelli, P.; Torocastro, M.; Panagopoulos, D.; Sutton, R.; Lim, P.B. Autonomic dysfunction in ‘long COVID’: Rationale, physiology and management strategies. Clin. Med. 2021, 21, e63–e67. [Google Scholar] [CrossRef] [PubMed]
- Wong, A.C.; Devason, A.S.; Umana, I.C.; Cox, T.O.; Dohnalová, L.; Litichevskiy, L.; Perla, J.; Lundgren, P.; Etwebi, Z.; Izzo, L.T.; et al. Serotonin reduction in post-acute sequelae of viral infection. Cell 2023, 186, 4851–4867.e20. [Google Scholar] [CrossRef] [PubMed]
- Mancini, D.M.; Brunjes, D.L.; Lala, A.; Trivieri, M.G.; Contreras, J.P.; Natelson, B.H. Use of Cardiopulmonary Stress Testing for Patients with Unexplained Dyspnea Post– Coronavirus Disease. JACC Heart Fail. 2021, 9, 927–937. [Google Scholar] [CrossRef]
- Guarnieri, J.W.; Dybas, J.M.; Fazelinia, H.; Kim, M.S.; Frere, J.; Zhang, Y.; Albrecht, Y.S.; Murdock, D.G.; Angelin, A.; Singh, L.N.; et al. Core mitochondrial genes are down-regulated during SARS-CoV-2 infection of rodent and human hosts. Sci. Transl. Med. 2023, 15, eabq1533. [Google Scholar] [CrossRef]
- Mandarano, A.H.; Maya, J.; Giloteaux, L.; Peterson, D.L.; Maynard, M.; Gottschalk, C.G.; Hanson, M.R. Myalgic encephalomyelitis/chronic fatigue syndrome patients exhibit altered T cell metabolism and cytokine associations. J. Clin. Investig. 2020, 130, 1491–1505. [Google Scholar] [CrossRef]
- Cheong, J.-G.; Ravishankar, A.; Sharma, S.; Parkhurst, C.N.; Grassmann, S.A.; Wingert, C.K.; Laurent, P.; Ma, S.; Paddock, L.; Miranda, I.C.; et al. Epigenetic memory of coronavirus infection in innate immune cells and their progenitors. Cell 2023, 186, 3882–3902.e24. [Google Scholar] [CrossRef]
- Klok, F.A.; Boon, G.J.; Barco, S.; Endres, M.; Geelhoed, J.M.; Knauss, S.; Rezek, S.A.; Spruit, M.A.; Vehreschild, J.; Siegerink, B. The Post-COVID-19 Functional Status scale: A tool to measure functional status over time after COVID-19. Eur. Respir. J. 2020, 56, 2001494. [Google Scholar] [CrossRef] [PubMed]
- Herdman, M.; Badia, X.; Berra, S. EuroQol-5D: A simple alternative for measuring health-related quality of life in primary care. Aten Primaria 2001, 28, 425–430. [Google Scholar] [CrossRef]
- WHO COVID-19 Dashboard. Spain: WHO Coronavirus Disease (COVID-19) Dashboard with Vaccination Data 2020. Available online: https://covid19.who.int (accessed on 30 November 2023).
- Troyano-Hernáez, P.; Reinosa, R.; Holguín, A. Evolution of SARS-CoV-2 in Spain during the First Two Years of the Pandemic: Circulating Variants, Amino Acid Conservation, and Genetic Variability in Structural, Non-Structural, and Accessory Proteins. Int. J. Mol. Sci. 2022, 23, 6394. [Google Scholar] [CrossRef] [PubMed]
- Swank, Z.; Senussi, Y.; Manickas-Hill, Z.; Yu, X.G.; Li, J.Z.; Alter, G.; Walt, D.R. Persistent Circulating Severe Acute Respiratory Syndrome Coronavirus 2 Spike Is Associated with Post-acute Coronavirus Disease 2019 Sequelae. Clin. Infect. Dis. 2022, 76, e487–e490. [Google Scholar] [CrossRef] [PubMed]
- Ceccato, F.; Selmin, E.; Antonelli, G.; Barbot, M.; Daniele, A.; Boscaro, M.; Plebani, M.; Scaroni, C. Low-dose short synacthen test with salivary cortisol in patients with suspected central adrenal insufficiency. Endocr. Connect. 2021, 10, 1189–1199. [Google Scholar] [CrossRef] [PubMed]
- Thaler, L.M.; Blevins, L.S. The Low Dose (1-µg) Adrenocorticotropin Stimulation Test in the Evaluation of Patients with Suspected Central Adrenal Insufficiency. J. Clin. Endocrinol. Metab. 1998, 83, 2726–2729. [Google Scholar] [CrossRef][Green Version]
- Gandhi, P.G.; Shah, N.S.; Khandelwal, A.G.; Chauhan, P.; Menon, P.S. Evaluation of low dose ACTH stimulation test in suspected secondary adrenocortical insufficiency. J. Postgrad. Med. 2002, 48, 280–282. [Google Scholar]
- Sharma, A.; Lazarus, K.; Papadopoulou, D.; Prabhudev, H.; Tan, T.; Meeran, K.; Choudhury, S. Optimising prednisolone or prednisone replacement in adrenal insufficiency. Endocr. Connect. 2023, 12, e230097. [Google Scholar] [CrossRef]
- Alijotas-Reig, J.; Anunciación-Llunell, A.; Morales-Pérez, S.; Trapé, J.; Esteve-Valverde, E.; Miro-Mur, F. Thrombosis and Hyperinflammation in COVID-19 Acute Phase Are Related to Anti-Phosphatidylserine and Anti-Phosphatidylinositol Antibody Positivity. Biomedicines 2023, 11, 2301. [Google Scholar] [CrossRef]
- Blomberg, B.; Mohn, K.G.-I.; Brokstad, K.A.; Zhou, F.; Linchausen, D.W.; Hansen, B.-A.; Lartey, S.; Onyango, T.B.; Kuwelker, K.; Sævik, M.; et al. Long COVID in a prospective cohort of home-isolated patients. Nat. Med. 2021, 27, 1607–1613. [Google Scholar] [CrossRef]
| Long COVID | |||
|---|---|---|---|
| Variables | No (13) | Yes (29) | p-Value 1 |
| Age (years) | 50 [8] | 53 [18] | 0.64 |
| Gender (female) | 6 (46.15) | 17 (58.62) | 0.52 |
| HTA | 1 (7.69) | 5 (17.24) | 0.65 |
| Dyslipidaemia | 1 (7.69) | 5 (17.24) | 0.65 |
| DM2 | 0 (0) | 5 (17.24) | 0.30 |
| Previous smoker | 3 (23.08) | 5 (17.24) | 0.69 |
| BMI > 30 | 1 (7.69) | 2 (6.9) | 1.00 |
| Lung disease | 1 (7.69) | 1 (3.45) | 0.53 |
| AIDs | 0 (0) | 2 (6.9) | 1.00 |
| Chronic fatigue | 0 (0) | 1 (3.45) | 1.00 |
| Fibromyalgia | 0 (0) | 1 (3.45) | 1.00 |
| Chemical sensitivity | 0 (0) | 1 (3.45) | 1.00 |
| Complications in acute phase | |||
| Hospitalization | 7 (53.85) | 17 (58.62) | 1.00 |
| Bacterial lung coinfection | 2 (15.38) | 1 (3.45) | 0.22 |
| Respiratory bacterial sepsis | 1 (7.69) | 1 (3.45) | 0.53 |
| Other bacterial infection | 0 (0) | 1 (3.57) | 1.00 |
| Pulmonary thromboembolism | 1 (7.69) | 2 (6.9) | 1.00 |
| Treatment | |||
| No treatment | 0 (0) | 2 (6.9) | 1.00 |
| Paracetamol | 1 (7.69) | 3 (10.34) | 1.00 |
| NSAIDs | 1 (7.69) | 7 (24.14) | 0.40 |
| Dexamethasone | 7 (53.85) | 16 (55.17) | 1.00 |
| Heparin | 7 (53.85) | 17 (58.62) | 1.00 |
| Tociluzumab | 1 (7.69) | 7 (24.14) | 0.40 |
| Remdesivir | 0 (0) | 2 (6.9) | 1.00 |
| Antibiotics | 2 (15.38) | 2 (6.9) | 0.58 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alijotas-Reig, J.; Anunciacion-Llunell, A.; Esteve-Valverde, E.; Morales-Pérez, S.; Rivero-Santana, S.; Trapé, J.; González-García, L.; Ruiz, D.; Marques-Soares, J.; Miro-Mur, F. Pituitary–Adrenal Axis and Peripheral Immune Cell Profile in Long COVID. Biomedicines 2024, 12, 581. https://doi.org/10.3390/biomedicines12030581
Alijotas-Reig J, Anunciacion-Llunell A, Esteve-Valverde E, Morales-Pérez S, Rivero-Santana S, Trapé J, González-García L, Ruiz D, Marques-Soares J, Miro-Mur F. Pituitary–Adrenal Axis and Peripheral Immune Cell Profile in Long COVID. Biomedicines. 2024; 12(3):581. https://doi.org/10.3390/biomedicines12030581
Chicago/Turabian StyleAlijotas-Reig, Jaume, Ariadna Anunciacion-Llunell, Enrique Esteve-Valverde, Stephanie Morales-Pérez, Sergio Rivero-Santana, Jaume Trapé, Laura González-García, Domingo Ruiz, Joana Marques-Soares, and Francesc Miro-Mur. 2024. "Pituitary–Adrenal Axis and Peripheral Immune Cell Profile in Long COVID" Biomedicines 12, no. 3: 581. https://doi.org/10.3390/biomedicines12030581
APA StyleAlijotas-Reig, J., Anunciacion-Llunell, A., Esteve-Valverde, E., Morales-Pérez, S., Rivero-Santana, S., Trapé, J., González-García, L., Ruiz, D., Marques-Soares, J., & Miro-Mur, F. (2024). Pituitary–Adrenal Axis and Peripheral Immune Cell Profile in Long COVID. Biomedicines, 12(3), 581. https://doi.org/10.3390/biomedicines12030581






