Immunological Similarities and Differences between Post-COVID-19 Lung Sequelae and Idiopathic Pulmonary Fibrosis
Abstract
1. Introduction
2. Material and Methods
2.1. Study Population
2.2. Peripheral Blood Mononuclear Cells
2.3. Gating Strategy
2.4. Lung Function Tests
2.5. Statistical Analysis
3. Results
3.1. Study Population
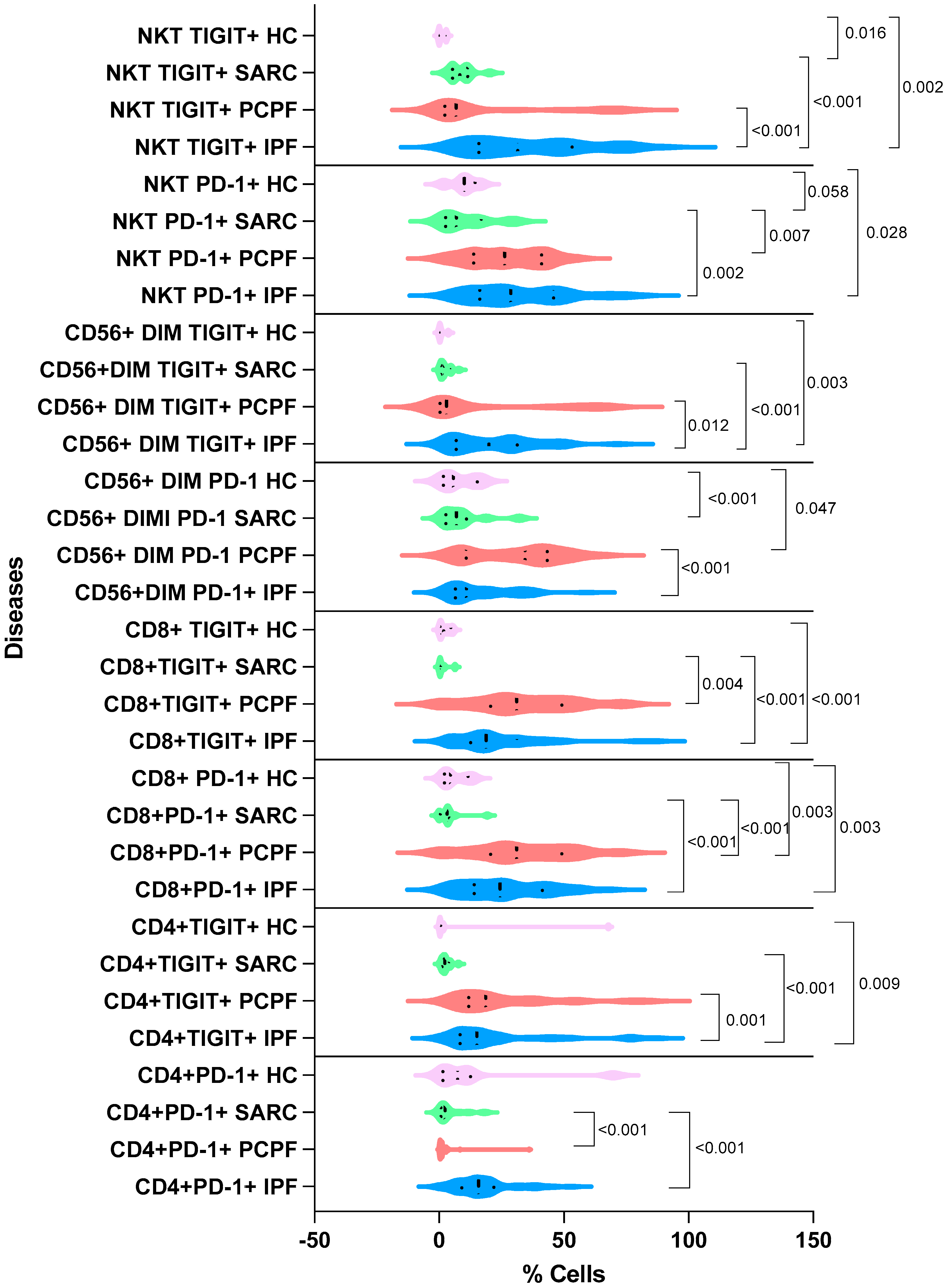
3.2. Immunological Findings
3.3. Lung Function Tests
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Mutsaers, S.E.; Miles, T.; Prêle, C.M.; Hoyne, G.F. Emerging role of immune cells as drivers of pulmonary fibrosis. Pharmacol. Ther. 2023, 252, 108562. [Google Scholar] [CrossRef] [PubMed]
- Desai, O.; Winkler, J.; Minasyan, M.; Herzog, E.L. The Role of Immune and Inflammatory Cells in Idiopathic Pulmonary Fibrosis. Front. Med. 2018, 5, 43. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, I.E.; Greiffo, F.R.; Frankenberger, M.; Bandres, J.; Heinzelmann, K.; Neurohr, C.; Hatz, R.; Hartl, D.; Behr, J.; Eickelberg, O. Peripheral blood myeloid-derived suppressor cells reflect disease status in idiopathic pulmonary fibrosis. Eur. Respir. J. 2016, 48, 1171–1183. [Google Scholar] [CrossRef] [PubMed]
- Patrucco, F.; Solidoro, P.; Gavelli, F.; Apostolo, D.; Bellan, M. Idiopathic Pulmonary Fibrosis and Post-COVID-19 Lung Fibrosis: Links and Risks. Microorganisms 2023, 11, 895. [Google Scholar] [CrossRef] [PubMed]
- Deng, L.; Huang, T.; Zhang, L. T cells in idiopathic pulmonary fibrosis: Crucial but controversial. Cell Death Discov. 2023, 9, 62. [Google Scholar] [CrossRef] [PubMed]
- Koh, C.-H.; Lee, S.; Kwak, M.; Kim, B.-S.; Chung, Y. CD8 T-cell subsets: Heterogeneity, functions, and therapeutic potential. Exp. Mol. Med. 2023, 55, 2287–2299. [Google Scholar] [CrossRef] [PubMed]
- Croft, M.; Carter, L.; Swain, S.L.; Dutton, R.W. Generation of polarized antigen-specific CD8 effector populations: Reciprocal action of interleukin (IL)-4 and IL-12 in promoting type 2 versus type 1 cytokine profiles. J. Exp. Med. 1994, 180, 1715–1728. [Google Scholar] [CrossRef] [PubMed]
- Bergantini, L.; d’Alessandro, M.; Gangi, S.; Bianchi, F.; Cameli, P.; Perea, B.; Meocci, M.; Fabbri, G.; Marrucci, S.; Ederbali, M.; et al. Predictive Role of Cytokine and Adipokine Panel in Hospitalized COVID-19 Patients: Evaluation of Disease Severity, Survival and Lung Sequelae. Int. J. Mol. Sci. 2023, 24, 12994. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Qu, Y.; Hao, C.; Yao, W. PD-1/PD-L1 axis in organ fibrosis. Front. Immunol. 2023, 14, 1145682. [Google Scholar] [CrossRef] [PubMed]
- Ge, Z.; Peppelenbosch, M.P.; Sprengers, D.; Kwekkeboom, J. TIGIT, the Next Step Towards Successful Combination Immune Checkpoint Therapy in Cancer. Front. Immunol. 2021, 12, 699895. [Google Scholar] [CrossRef] [PubMed]
- Galati, D.; De Martino, M.; Trotta, A.; Rea, G.; Bruzzese, D.; Cicchitto, G.; Stanziola, A.A.; Napolitano, M.; Sanduzzi, A.; Bocchino, M. Peripheral depletion of NK cells and imbalance of the Treg/Th17 axis in idiopathic pulmonary fibrosis patients. Cytokine 2014, 66, 119–126. [Google Scholar] [CrossRef] [PubMed]
- Achaiah, A.; Rathnapala, A.; Pereira, A.; Bothwell, H.; Dwivedi, K.; Barker, R.; Iotchkova, V.; Benamore, R.; Hoyles, R.K.; Ho, L.-P. Neutrophil lymphocyte ratio as an indicator for disease progression in Idiopathic Pulmonary Fibrosis. BMJ Open Respir. Res. 2022, 9, e001202. [Google Scholar] [CrossRef] [PubMed]
- Celada, L.J.; Kropski, J.A.; Herazo-Maya, J.D.; Luo, W.; Creecy, A.; Abad, A.T.; Chioma, O.S.; Lee, G.; Hassell, N.E.; Shaginurova, G.I.; et al. PD-1 up-regulation on CD4+ T cells promotes pulmonary fibrosis through STAT3-mediated IL-17A and TGF-β1 production. Sci. Transl. Med. 2018, 10, eaar8356. [Google Scholar] [CrossRef] [PubMed]
- Anderson, A.C.; Joller, N.; Kuchroo, V.K. Lag-3, Tim-3, and TIGIT: Co-inhibitory Receptors with Specialized Functions in Immune Regulation. Immunity 2016, 44, 989–1004. [Google Scholar] [CrossRef] [PubMed]
- Rha, M.-S.; Shin, E.-C. Activation or exhaustion of CD8+ T cells in patients with COVID-19. Cell. Mol. Immunol. 2021, 18, 2325–2333. [Google Scholar] [CrossRef] [PubMed]
- d’Alessandro, M.; Bergantini, L.; Mezzasalma, F.; Cavallaro, D.; Gangi, S.; Baglioni, S.; Armati, M.; Abbritti, M.; Cattelan, S.; Cameli, P.; et al. Immune-Checkpoint Expression on CD4, CD8 and NK Cells in Blood, Bronchoalveolar Lavage and Lymph Nodes of Sarcoidosis. Mol. Diagn. Ther. 2022, 26, 437–449. [Google Scholar] [CrossRef] [PubMed]
- Bergantini, L.; d’Alessandro, M.; Cameli, P.; Otranto, A.; Finco, T.; Curatola, G.; Sestini, P.; Bargagli, E. Prognostic role of NK cell percentages in bronchoalveolar lavage from patients with different fibrotic interstitial lung diseases. Clin. Immunol. 2021, 230, 108827. [Google Scholar] [CrossRef] [PubMed]
- Esen, F.; Deniz, G.; Aktas, E.C. PD-1, CTLA-4, LAG-3, and TIGIT: The roles of immune checkpoint receptors on the regulation of human NK cell phenotype and functions. Immunol. Lett. 2021, 240, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Quatrini, L.; Mariotti, F.R.; Munari, E.; Tumino, N.; Vacca, P.; Moretta, L. The Immune Checkpoint PD-1 in Natural Killer Cells: Expression, Function and Targeting in Tumour Immunotherapy. Cancers 2020, 12, 3285. [Google Scholar] [CrossRef] [PubMed]
- Hasan, M.F.; Croom-Perez, T.J.; Oyer, J.L.; Dieffenthaller, T.A.; Robles-Carrillo, L.D.; Eloriaga, J.E.; Kumar, S.; Andersen, B.W.; Copik, A.J. TIGIT Expression on Activated NK Cells Correlates with Greater Anti-Tumor Activity but Promotes Functional Decline upon Lung Cancer Exposure: Implications for Adoptive Cell Therapy and TIGIT-Targeted Therapies. Cancers 2023, 15, 2712. [Google Scholar] [CrossRef] [PubMed]
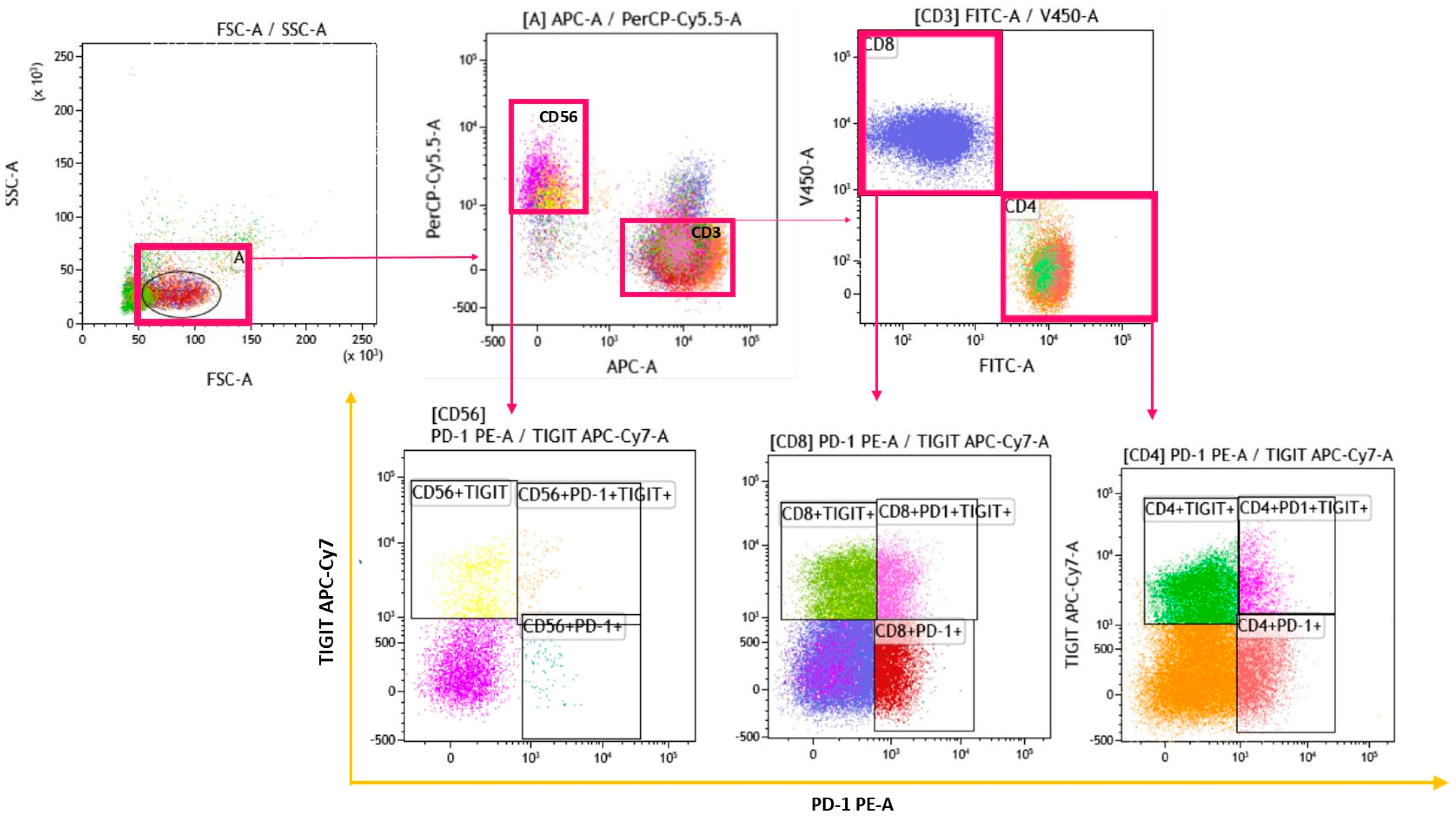


| Cluster of Differentiation (CD-) | Clone | Fluorochrome | Company |
|---|---|---|---|
| CD3 | OKT3 | APC | BioLegend (San Diego, CA, USA) |
| CD4 | SK3 | FITC | Becton Dickinson (Franklin Lakes, NJ, USA) |
| CD8 | SK1 | BV421 | BioLegend |
| PD-1 | PE | PD1.3.1.3 | Miltenyi Biotec (Bergisch Gladbach, Germay) |
| TIGIT | APC-Cy7 | A15153G | BioLegend |
| CD56 | 5.1H11 | PerCP/Cy5.5 | BioLegend |
| Clinical and Demographic Parameters | IPF (n = 48) | PCPF (n = 55) | Sarcoidosis (n = 12) | HC (n = 10) |
|---|---|---|---|---|
| Sex (F/M) | 13/35 | 23/29 | 6/6 | 5/5 |
| Age (median) | 73 ± 8.11 | 75 ± 8.24 | 56 ± 6.76 | 69 ± 15.9 |
| Smoking status (never/former) | 41/7 | 52/3 | 5/7 | 10/0 |
| Lung function test parameters (median and ±standard deviation): | ||||
| FEV1% | 76.5 ± 20.42 | 94 ± 16.07 | 104 ± 10.09 | |
| FVC% | 73 ± 19.61 | 107 ± 10.98 | 110 ± 13.87 | |
| DLCO% | 45.5 ± 33.26 | 60 ± 12.35 | 76 ± 18.52 | |
| Radiological findings | UIP (n = 48) | Fibrotic inter- or intralobular thickening (n = 55)/Air trapping (n = 43)/Groundglass (n = 40) | Scadding stage II (n = 12) |
| Cell Percentages | IPF (n = 48) | PCPF (n = 55) | Sarcoidosis (n = 12) | HCs (n = 10) | p Value |
|---|---|---|---|---|---|
| CD3 | 49.0 ± 26.0 | 51.1 ± 22.3 | 41.3 ± 22.8 | 55.8 ± 7.58 | 0.561 |
| CD4 | 41.8 ± 17.8 | 55.2 ± 18.6 | 63.8 ± 14.4 | 46.9 ± 12.2 | <0.001 |
| CD8 | 49.3 ± 18.5 | 28.4 ± 17.5 | 22.5 ± 12.8 | 17.4 ± 5.14 | <0.001 |
| CD4+PD-1+ | 17.9 ± 11.7 | 24.7 ± 19.9 | 4.37 ± 5.52 | 14.1 ± 23.0 | <0.001 |
| CD4+TIGIT+ | 22.3 ± 21.3 | 15.6 ± 23.2 | 2.73 ± 2.02 | 9.09 ± 23.7 | <0.001 |
| CD8+PD-1+ | 26.5 ± 16.9 | 33.5 ± 19.6 | 4.04 ± 5.16 | 6.25 ± 5.05 | <0.001 |
| CD8+TIGIT+ | 23.4 ± 17.5 | 30.3 ± 29.2 | 1.61 ± 2.30 | 2.40 ± 2.17 | <0.001 |
| CD56 | 0.922 ± 2.70 | 1.27 ± 1.38 | 11.3 ± 5.88 | 7.23 ± 10.9 | <0.001 |
| CD56+PD-1+ | 14.1 ± 20.9 | 12.6 ± 20.4 | 0.394 ± 1.11 | 7.32 ± 10.0 | <0.001 |
| CD56+TIGIT+ | 28.6 ± 24.3 | 22.9 ± 27.7 | 5.82 ± 6.13 | 7.73 ± 9.25 | 0.001 |
| NK | 45.8 ± 23.9 | 37.3 ± 19.0 | 46.1 ± 19.2 | 21.6 ± 22.3 | 0.044 |
| CD56bright | 3.33 ± 8.48 | 2.25 ± 3.49 | 4.70 ± 6.24 | 8.42 ± 3.35 | 0.029 |
| CD56dim | 95.7 ± 9.30 | 96.7 ± 14.1 | 92.2 ± 5.34 | 81.7 ± 6.53 | 0.005 |
| CD56brightPD-1+ | 0.965 ± 6.65 | 1.36 ± 13.7 | 1.34 ± 0.879 | 0.360 ± 1.51 | 0.328 |
| CD56brightTIGIT+ | 0.665 ± 5.92 | 0.480 ± 2.79 | 0.395 ± 0.561 | 0.0600 ± 1.58 | 0.076 |
| CD56dimPD-1+ | 10.9 ± 13.2 | 34.4 ± 18.1 | 6.93 ± 8.88 | 5.73 ± 7.18 | <0.001 |
| CD56dimTIGIT+ | 19.9 ± 16.8 | 2.92 ± 25.7 | 1.94 ± 2.36 | 0.240 ± 1.50 | <0.001 |
| NKT-like cells | 6.33 ± 6.94 | 7.97 ± 6.33 | 10.00 ± 14.9 | 0.180 ± 2.65 | 0.030 |
| NKT PD-1+ | 28.6 ± 19.4 | 26.2 ± 14.9 | 6.93 ± 10.3 | 10.0 ± 5.43 | <0.001 |
| NKT TIGIT+ | 31.5 ± 23.7 | 6.83 ± 26.5 | 8.22 ± 4.81 | 0.00 ± 1.50 | <0.001 |
| Double Positive Cell Percentages | IPF (n = 48) | PCPF (n = 55) | SARCOIDOSIS (n = 12) | HCs (n = 10) |
|---|---|---|---|---|
| CD4+PD-1+TIGIT+ | 6.21 ± 5.97 a | 8.67 ± 14.6 | 4.01 ± 9.83 | 0.0187 ± 0.0236 |
| CD8+PD-1+TIGIT+ | 7.12 ± 8.12 b | 17.9 ± 20.3 c | 0.0709 ± 0.235 | 0.374 ± 0.716 |
| CD56+PD-1+TIGIT+ | 15.7 ± 23.2 d | 10.5 ± 21.9 | 3.39 ± 6.64 | 1.61 ± 2.80 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gangi, S.; Bergantini, L.; Cameli, P.; Paggi, I.; Spalletti, M.; Mezzasalma, F.; Bargagli, E.; d’Alessandro, M. Immunological Similarities and Differences between Post-COVID-19 Lung Sequelae and Idiopathic Pulmonary Fibrosis. Biomedicines 2024, 12, 630. https://doi.org/10.3390/biomedicines12030630
Gangi S, Bergantini L, Cameli P, Paggi I, Spalletti M, Mezzasalma F, Bargagli E, d’Alessandro M. Immunological Similarities and Differences between Post-COVID-19 Lung Sequelae and Idiopathic Pulmonary Fibrosis. Biomedicines. 2024; 12(3):630. https://doi.org/10.3390/biomedicines12030630
Chicago/Turabian StyleGangi, Sara, Laura Bergantini, Paolo Cameli, Irene Paggi, Marco Spalletti, Fabrizio Mezzasalma, Elena Bargagli, and Miriana d’Alessandro. 2024. "Immunological Similarities and Differences between Post-COVID-19 Lung Sequelae and Idiopathic Pulmonary Fibrosis" Biomedicines 12, no. 3: 630. https://doi.org/10.3390/biomedicines12030630
APA StyleGangi, S., Bergantini, L., Cameli, P., Paggi, I., Spalletti, M., Mezzasalma, F., Bargagli, E., & d’Alessandro, M. (2024). Immunological Similarities and Differences between Post-COVID-19 Lung Sequelae and Idiopathic Pulmonary Fibrosis. Biomedicines, 12(3), 630. https://doi.org/10.3390/biomedicines12030630









