Rift Valley Fever Virus: An Overview of the Current Status of Diagnostics
Abstract
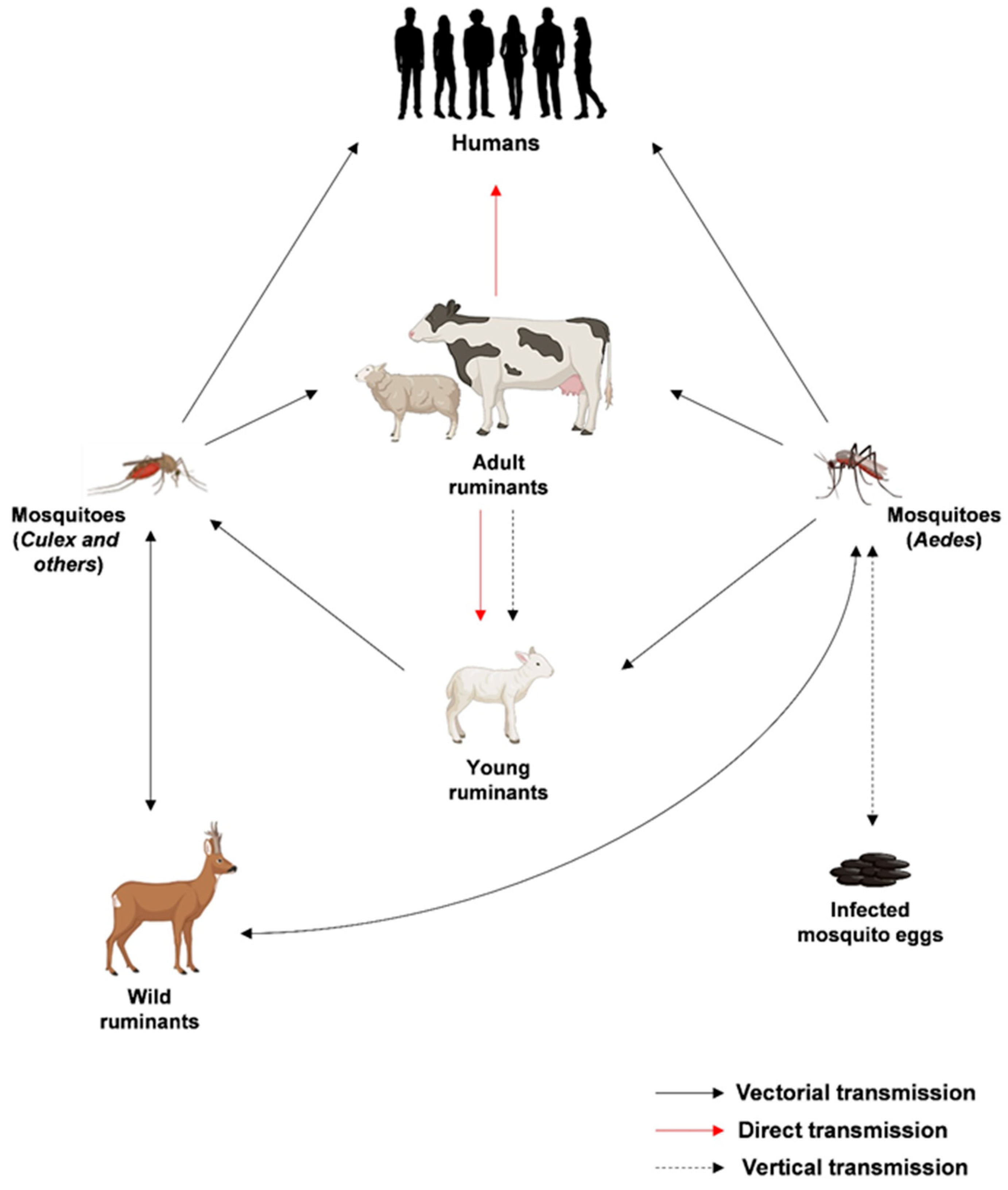
1. Introduction
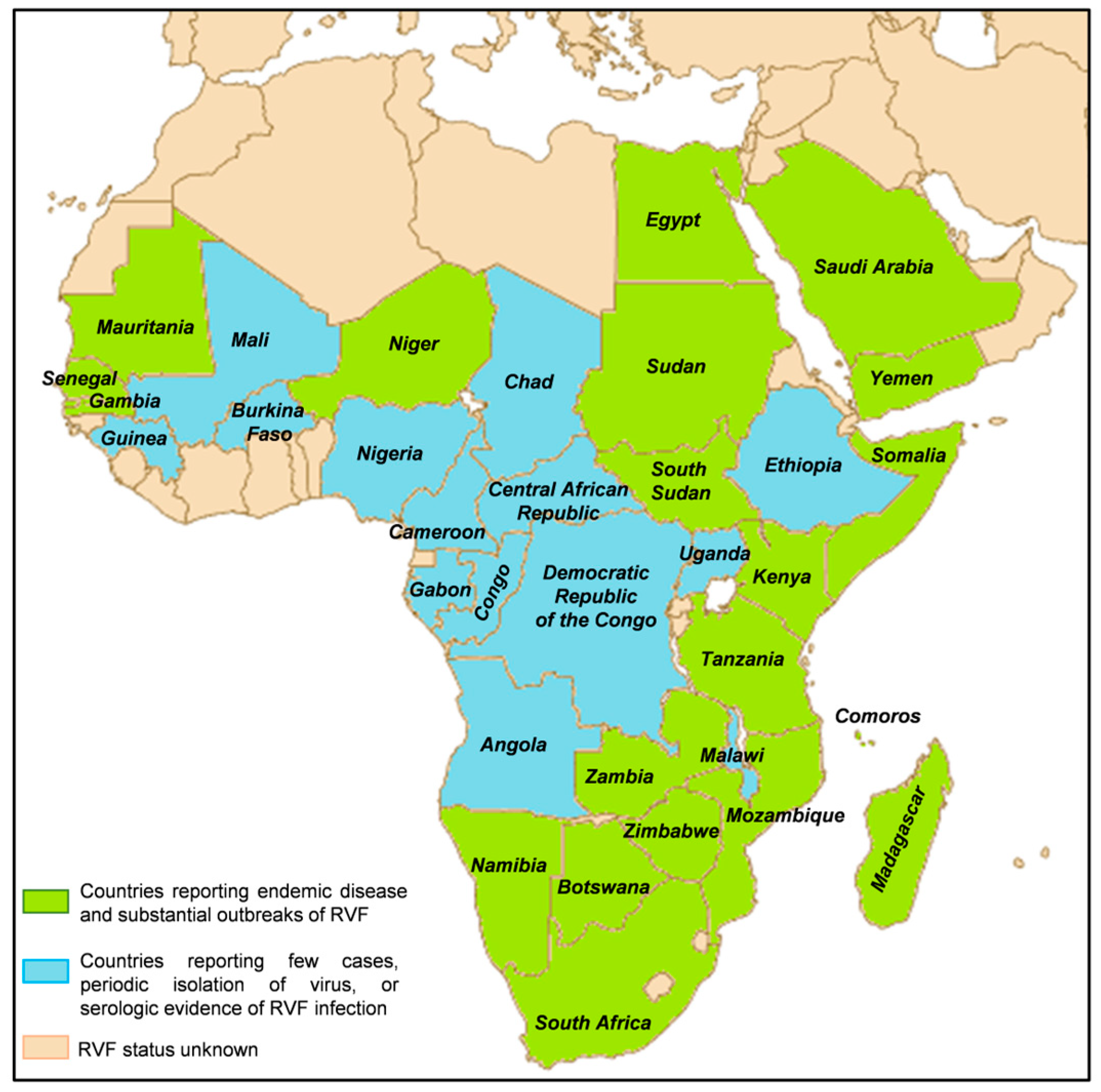
2. Epidemiology
3. Diagnosis
3.1. Molecular Diagnostics
| Test | Test Type | Virus Detected | Target Gene | Biological Matrix | Reference Assay | Sample Size | Sensitivity | Specificity | Author |
|---|---|---|---|---|---|---|---|---|---|
| RVFV RT-nested PCR | Homemade one-step RT-PCR nested method | RVFV different strains (Gabek forest, Gordil, saint Floris, Arumwot, Belterra, ArD38661, AnD100286, MP 12) | NS coding region of S segment | Virus produced in Vero E6 cells; serum from infected mice | Virus isolation method | ND | 0.5 pfu/reaction | Nd | Sall et al., 2001 [45] |
| RVFV quantitative real-time PCR | qRT-PCR with fluorescent signal from probes for quality control | RVFV; MP12, ZH501, ZH548, ArD38661, 74 HB59 strains | NS coding region of S segment | Virus produced in Vero E6 cells; serum from infected mice | ND | ND | 50–100 copies/reaction | No amplification with Toscana, Icoraci, and Belterra closely related phlebovirus | Garcia et al., 2001 [46] |
| RT-Real-time PCR | 5′ nuclease technology on a light cycler instrument | RVFV | G2 gene | Synthetic RNA | ND | ND | 2835 geq/mL | no cross-reactivity with other HCV, HBV, HSV1, CMV, Modoc virus, Mycobacterium tuberculosis, Mycobacterium leprae, Borrelia spp., Leptospira spp., Neisseria spp., Plasmodium spp., Leishmania spp. | Drosten et al., 2002 [48] |
| RT-Real-time PCR homemade | Fluorescent nested PCR TaqMan assay | RVFV | S segment | Synthetic RNA | ND | ND | 100 copies/reaction | SFNV cell culture | Weidmann et al., 2008 [47] |
| Real-time qRT-PCR homemade | qRT-PCR with fluorescent reporter dye detected at each PCR cycle | RVFV | G2 gene | Plasma of suspected patients with HVF | 272 RVFV confirmed cases | 2ND | 100 infectious particles/mL | IgM anti RVFV positive sera 100% | Njenga et al., 2009 [49] |
| RT-LAMP homemade | Reverse transcription-loop-mediated isothermal amplification with a vertical | RVFV | L segment | Serum samples | TaqMan Real Time | 64 | Whole blood: LLOD: 10 copies RNA/reaction | No cross reactivity with phleboviruses; flaviviruses and chikungunja virus | Peyrefitte et al., 2008 [50] |
| RT-LAMP homemade | Reverse transcription-loop-mediated isothermal amplification with a vertical | RVFV | L segment | Bleed samples from sheep (n = 20), human plasma from suspected cases (n = 65); 3 liver, kidney, serum from animals | Whole blood: LLOD: 10 copies/ reaction | Six African phleboviruses and unrelated arbovirus did not give cross reactivity | Le Roux et al., 2009 [51] | ||
| RT-LAMP homemade | Reverse transcription-loop-mediated isothermal amplification | RVFV | S segment | Synthetic RNA | Real-time RT-PCR | ND | whole blood: LLOD: 1.94 copies/microliters within 60 min | No cross reactivity with JEV, H3N2 influenza virus, EBOV, MARV | Han et al., 2020 [52] |
| RT-LAMP homemade | Reverse transcription-loop-mediated isothermal amplification | RVFV | M segment | Blood samples | Real-time RT-PCR | 130 | 98.36% sensitivity | 100%; no cross-reactivity with PPR and capripox viruses | Wekesa et al., 2023 [54] |
| Isothermal recombinase polymerase amplification (RPA) | Isothermal exponential nucleic acid amplification and detection method | RVFV | S segment | Synthetic RNA | ND | ND | 19RNA molecules/reaction | No cross reactivity with Yersinia pestis, Francisella tularensis, Bacillus antracis, vaccinia virus, Ebola virus, Marburg virus, Crimean–Congo virus and phleboviruses | Euler et al., 2012 [53] |
| RT-qPCR genotyping assay | One step RT-qPCR for typing different strains of RVFV, melting curve to identify different strains of RVFV | RVFV | L, M, S segments | ND | Sanger sequencing | ND | Balaraman et al., 2023 [56] | ||
| BioT DNA multiplex PCR-enzyme hybridization assay | Multiplex RT-PCR | RVFV | GP2 gene | 196 swabs, 45 skin swabs,15 serum, 7 sputum | ND | 260 clinical samples | 105–106 copies/mL with nucleic acid extraction | No cross reactivity with Influenza A, EBV, CMV, RSV A, ADV C, human metapneumovirus | He et al., 2009 [55] |
| Oligonucleotide microarray | Microarray | RVFV | GP gene | Culture samples | Real-time PCR | 60 | 100% | Yao et al., 2021 [57] | |
| Real-time qRT-PCR commercial | qRT-PCR with fluorescent reporter dye detected at each PCR cycle | RVFV | 0.89 copies/μL | cross-reactivity with flavivirus, Marburg virus, and Ebola virus | [61] |
3.2. Serological Diagnosis
- Virus neutralization test (VNT)
- Indirect immunofluorescent assay (IFA)
- IgG and IgM antibody enzyme-linked immunosorbent assay (ELISA)
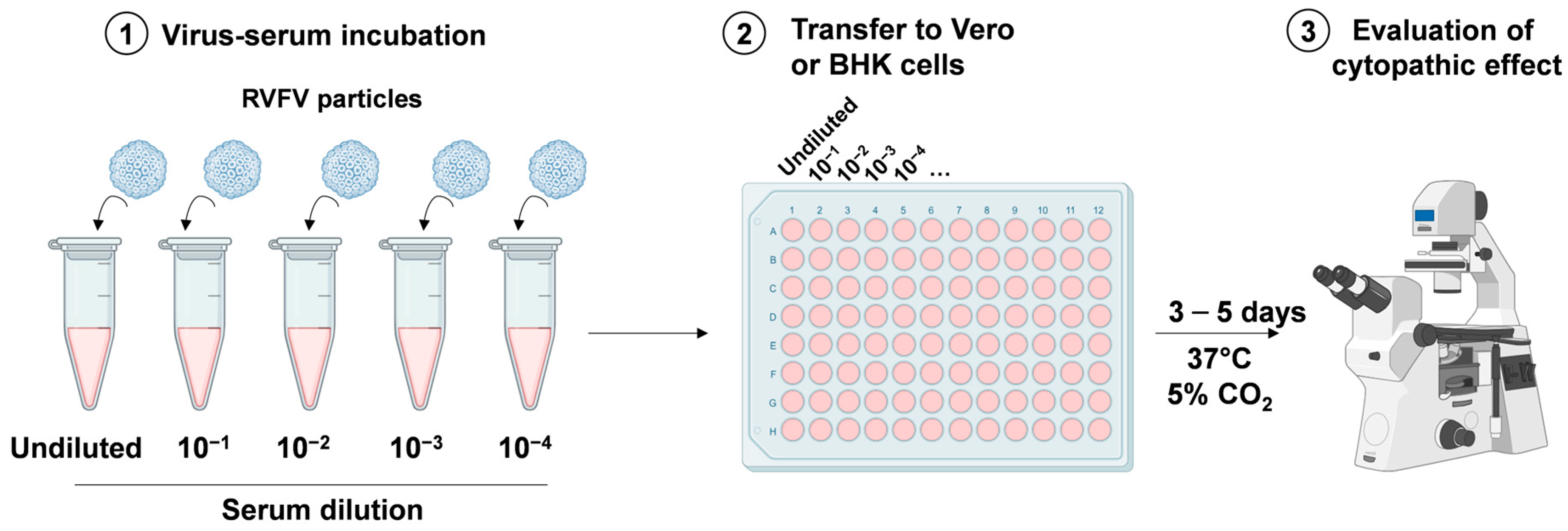
3.2.1. Virus Neutralization Test Assay
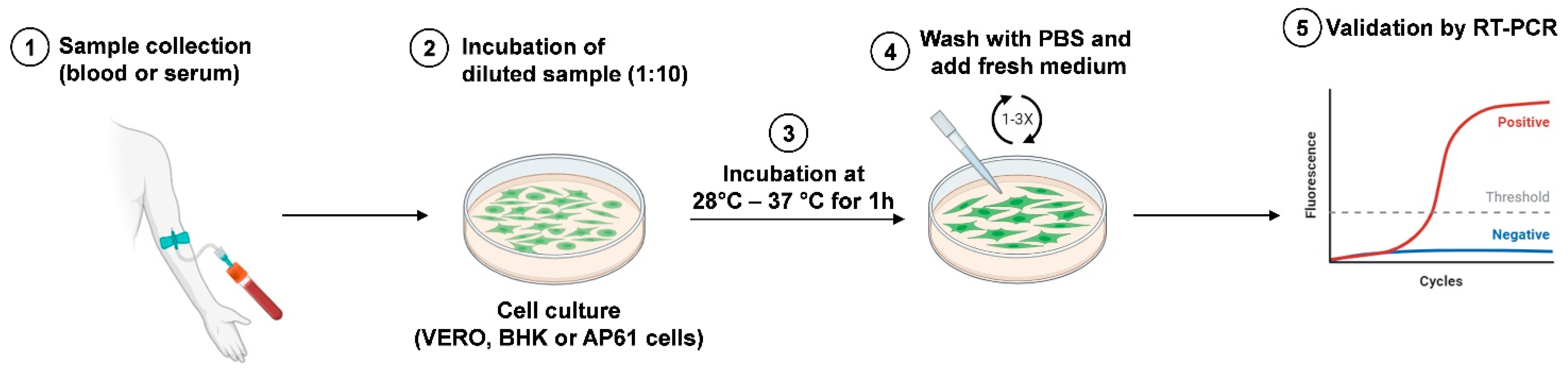
3.2.2. Viral Isolation
3.2.3. Indirect Immunofluorescent Assay
3.2.4. ELISA Assay
4. Surveillance in Humans and Animals
5. Discussion
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jonkmans, N.; D’Acremont, V.; Flahault, A. Scoping Future Outbreaks: A Scoping Review on the Outbreak Prediction of the WHO Blueprint List of Priority Diseases. BMJ Glob. Health 2021, 6, e006623. [Google Scholar] [CrossRef] [PubMed]
- Daubney, R.; Hudson, J.R.; Garnham, P.C. Enzootic Hepatitis or Rift Valley Fever. An Undescribed Virus Disease of Sheep Cattle and Man from East Africa. J. Pathol. Bacteriol. 1931, 34, 545–579. [Google Scholar] [CrossRef]
- Ikegami, T.; Makino, S. The Pathogenesis of Rift Valley Fever. Viruses 2011, 3, 493–519. [Google Scholar] [CrossRef] [PubMed]
- Gaudreault, N.N.; Indran, S.V.; Balaraman, V.; Wilson, W.C.; Richt, J.A. Molecular Aspects of Rift Valley Fever Virus and the Emergence of Reassortants. Virus Genes 2019, 55, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Saluzzo, J.F.; Smith, J.F. Use of Reassortant Viruses to Map Attenuating and Temperature-Sensitive Mutations of the Rift Valley Fever Virus MP-12 Vaccine. Vaccine 1990, 8, 369–375. [Google Scholar] [CrossRef] [PubMed]
- Ly, H.J.; Lokugamage, N.; Nishiyama, S.; Ikegami, T. Risk Analysis of Inter-Species Reassortment through a Rift Valley Fever Phlebovirus MP-12 Vaccine Strain. PLoS ONE 2017, 12, e0185194. [Google Scholar] [CrossRef]
- Pepin, M.; Bouloy, M.; Bird, B.H.; Kemp, A.; Paweska, J. Rift Valley Fever Virus (Bunyaviridae: Phlebovirus): An Update on Pathogenesis, Molecular Epidemiology, Vectors, Diagnostics and Prevention. Vet. Res. 2010, 41, 61. [Google Scholar] [CrossRef]
- Wright, D.; Kortekaas, J.; Bowden, T.A.; Warimwe, G.M. Rift Valley Fever: Biology and Epidemiology. J. Gen. Virol. 2019, 100, 1187–1199. [Google Scholar] [CrossRef]
- Wright, D.; Allen, E.R.; Clark, M.H.A.; Gitonga, J.N.; Karanja, H.K.; Hulswit, R.J.G.; Taylor, I.; Biswas, S.; Marshall, J.; Mwololo, D.; et al. Naturally Acquired Rift Valley Fever Virus Neutralizing Antibodies Predominantly Target the Gn Glycoprotein. iScience 2020, 23, 101669. [Google Scholar] [CrossRef]
- Quellec, J.; Pédarrieu, A.; Piro-Mégy, C.; Barthelemy, J.; Simonin, Y.; Salinas, S.; Cêtre-Sossah, C. Rift Valley Fever Virus Modulates Apoptosis and Immune Response during Infection of Human Astrocytes. Emerg. Microbes Infect. 2023, 12, 2207672. [Google Scholar] [CrossRef]
- Wichgers Schreur, P.J.; Kortekaas, J. Single-Molecule FISH Reveals Non-Selective Packaging of Rift Valley Fever Virus Genome Segments. PLoS Pathog. 2016, 12, e1005800. [Google Scholar] [CrossRef]
- World Health Organization. Rift Valley Fever. Available online: https://www.who.int/news-room/fact-sheets/detail/rift-valley-fever (accessed on 28 December 2023).
- Alrajhi, A.A.; Al-Semari, A.; Al-Watban, J. Rift Valley Fever Encephalitis. Emerg. Infect. Dis. 2004, 10, 554–555. [Google Scholar] [CrossRef]
- Nayak, N.; Mishra, M. Drosophila Melanogaster as a Model to Understand the Mechanisms of Infection Mediated Neuroinflammation in Neurodegenerative Diseases. J. Integr. Neurosci. 2022, 21, 66. [Google Scholar] [CrossRef]
- Evans, A.; Gakuya, F.; Paweska, J.T.; Rostal, M.; Akoolo, L.; Van Vuren, P.J.; Manyibe, T.; Macharia, J.M.; Ksiazek, T.G.; Feikin, D.R.; et al. Prevalence of Antibodies against Rift Valley Fever Virus in Kenyan Wildlife. Epidemiol. Infect. 2008, 136, 1261–1269. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, S.S.; Alvarez, J.; Bicout, D.J.; Calistri, P.; Depner, K.; Drewe, J.A.; Garin-Bastuji, B.; Rojas, J.L.G.; Schmidt, C.G.; Michel, V.; et al. Rift Valley Fever—Epidemiological Update and Risk of Introduction into Europe. EFSA J. Eur. Food Saf. Auth. 2020, 18, e06041. [Google Scholar] [CrossRef] [PubMed]
- Hartman, A. Rift Valley Fever. Clin. Lab. Med. 2017, 37, 285–301. [Google Scholar] [CrossRef]
- Paweska, J.T. Rift Valley Fever. Rev. Sci. Tech. OIE 2015, 34, 375–389. [Google Scholar] [CrossRef] [PubMed]
- Davies, F.G. The Historical and Recent Impact of Rift Valley Fever in Africa. Am. J. Trop. Med. Hyg. 2010, 83, 73–74. [Google Scholar] [CrossRef]
- Lumley, S.; Horton, D.L.; Hernandez-Triana, L.L.M.; Johnson, N.; Fooks, A.R.; Hewson, R. Rift Valley Fever Virus: Strategies for Maintenance, Survival and Vertical Transmission in Mosquitoes. J. Gen. Virol. 2017, 98, 875–887. [Google Scholar] [CrossRef]
- Linthicum, K.J.; Britch, S.C.; Anyamba, A. Rift Valley Fever: An Emerging Mosquito-Borne Disease. Annu. Rev. Entomol. 2016, 61, 395–415. [Google Scholar] [CrossRef]
- Ratovonjato, J.; Olive, M.-M.; Tantely, L.M.; Andrianaivolambo, L.; Tata, E.; Razainirina, J.; Jeanmaire, E.; Reynes, J.-M.; Elissa, N. Detection, Isolation, and Genetic Characterization of Rift Valley Fever Virus from Anopheles (Anopheles) Coustani, Anopheles (Anopheles) Squamosus, and Culex (Culex) Antennatus of the Haute Matsiatra Region, Madagascar. Vector-Borne Zoonotic Dis. 2011, 11, 753–759. [Google Scholar] [CrossRef]
- Sang, R.C.; Ahmed, O.; Faye, O.; Kelly, C.L.H.; Yahaya, A.A.; Mmadi, I.; Toilibou, A.; Sergon, K.; Brown, J.; Agata, N.; et al. Entomologic Investigations of a Chikungunya Virus Epidemic in the Union of the Comoros, 2005. Am. J. Trop. Med. Hyg. 2008, 78, 77–82. [Google Scholar] [CrossRef]
- Food and Agriculture Organization of the United Nations. Rift Valley Fever Surveillance. Available online: https://www.fao.org/3/i8475en/I8475EN.pdf (accessed on 18 November 2023).
- Gerdes, G.H. Rift Valley Fever. Rev. Sci. Tech. OIE 2004, 23, 613–623. [Google Scholar] [CrossRef]
- Adam, I.; Karsany, M.S. Case Report: Rift Valley Fever with Vertical Transmission in a Pregnant Sudanese Woman. J. Med. Virol. 2008, 80, 929. [Google Scholar] [CrossRef]
- Antonis, A.F.G.; Kortekaas, J.; Kant, J.; Vloet, R.P.M.; Vogel-Brink, A.; Stockhofe, N.; Moormann, R.J.M. Vertical Transmission of Rift Valley Fever Virus Without Detectable Maternal Viremia. Vector-Borne Zoonotic Dis. 2013, 13, 601–606. [Google Scholar] [CrossRef] [PubMed]
- Soti, V.; Tran, A.; Degenne, P.; Chevalier, V.; Lo Seen, D.; Thiongane, Y.; Diallo, M.; Guégan, J.-F.; Fontenille, D. Combining Hydrology and Mosquito Population Models to Identify the Drivers of Rift Valley Fever Emergence in Semi-Arid Regions of West Africa. PLoS Negl. Trop. Dis. 2012, 6, e1795. [Google Scholar] [CrossRef]
- Caminade, C.; Ndione, J.; Diallo, M.; MacLeod, D.; Faye, O.; Ba, Y.; Dia, I.; Morse, A. Rift Valley Fever Outbreaks in Mauritania and Related Environmental Conditions. Int. J. Environ. Res. Public Health 2014, 11, 903–918. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Efficacy Trials of Rift Valley Fever Vaccines and Therapeutics. Guidance on Clinical Trial Design. Meeting Report. 2019. Available online: https://cdn.who.int/media/docs/default-source/blue-print/rift-valley-fever-blueprint-trial-design-meeting-report-2019.pdf?sfvrsn=ee74e0fb_3 (accessed on 5 December 2023).
- European Centre for Disease Prevention and Control. Facts about Rift Valley Fever. Available online: https://www.ecdc.europa.eu/en/rift-valley-fever/facts (accessed on 25 August 2023).
- Grossi-Soyster, E.N.; Banda, T.; Teng, C.Y.; Muchiri, E.M.; Mungai, P.L.; Mutuku, F.M.; Gildengorin, G.; Kitron, U.; King, C.H.; Desiree Labeaud, A. Rift Valley Fever Seroprevalence in Coastal Kenya. Am. J. Trop. Med. Hyg. 2017, 97, 115–120. [Google Scholar] [CrossRef]
- Memish, Z.A.; Masri, M.A.; Anderson, B.D.; Heil, G.L.; Merrill, H.R.; Khan, S.U.; Alsahly, A.; Gray, G.C. Elevated Antibodies against Rift Valley Fever Virus among Humans with Exposure to Ruminants in Saudi Arabia. Am. J. Trop. Med. Hyg. 2015, 92, 739–743. [Google Scholar] [CrossRef] [PubMed]
- Bosworth, A.; Ghabbari, T.; Dowall, S.; Varghese, A.; Fares, W.; Hewson, R.; Zhioua, E.; Chakroun, M.; Tiouiri, H.; Ben Jemaa, M.; et al. Serologic Evidence of Exposure to Rift Valley Fever Virus Detected in Tunisia. New Microbes New Infect. 2016, 9, 1–7. [Google Scholar] [CrossRef]
- Tezcan-Ulger, S.; Kurnaz, N.; Ulger, M.; Aslan, G.; Emekdas, G. Serological Evidence of Rift Valley Fever Virus among Humans in Mersin Province of Turkey. J. Vector Borne Dis. 2019, 56, 373–379. [Google Scholar] [CrossRef]
- Lapa, D.; Specchiarello, E.; Francalancia, M.; Girardi, E.; Maggi, F.; Garbuglia, A.R. Detection of Anti-Rift Valley Fever Virus Antibodies in Serum Samples of Patients with Suspected Arbovirus Infection. Microorganisms 2023, 11, 2081. [Google Scholar] [CrossRef]
- Britch, S.C.; Binepal, Y.S.; Ruder, M.G.; Kariithi, H.M.; Linthicum, K.J.; Anyamba, A.; Small, J.L.; Tucker, C.J.; Ateya, L.O.; Oriko, A.A.; et al. Rift Valley Fever Risk Map Model and Seroprevalence in Selected Wild Ungulates and Camels from Kenya. PLoS ONE 2013, 8, e66626. [Google Scholar] [CrossRef]
- Kwaśnik, M.; Rożek, W.; Rola, J. Rift Valley Fever—A Growing Threat to Humans and Animals. J. Vet. Res. 2021, 65, 7–14. [Google Scholar] [CrossRef]
- Zouaghi, K.; Bouattour, A.; Aounallah, H.; Surtees, R.; Krause, E.; Michel, J.; Mamlouk, A.; Nitsche, A.; M’ghirbi, Y. First Serological Evidence of Crimean-Congo Hemorrhagic Fever Virus and Rift Valley Fever Virus in Ruminants in Tunisia. Pathogens 2021, 10, 769. [Google Scholar] [CrossRef] [PubMed]
- Shoemaker, T.; Boulianne, C.; Vincent, M.J.; Pezzanite, L.; Al-Qahtani, M.M.; Al-Mazrou, Y.; Khan, A.S.; Rollin, P.E.; Swanepoel, R.; Ksiazek, T.G.; et al. Genetic Analysis of Viruses Associated with Emergence of Rift Valley Fever in Saudi Arabia and Yemen, 2000–2001. Emerg. Infect. Dis. 2002, 8, 1415–1420. [Google Scholar] [CrossRef] [PubMed]
- European Centre for Disease Prevention and Control (eCDC). Rift Valley Fever. Annual Epidemiological Report for 2019; ECDC: Stockholm, Sweden, 2021; Available online: https://www.ecdc.europa.eu/sites/default/files/documents/AER-Rift-Valley-fever-2019_0.pdf (accessed on 10 February 2024).
- Gossner, C.M.; Hallmaier-Wacker, L.; Briet, O.; Haussig, J.M.; de Valk, H.; Wijermans, A.; Bakonyi, T.; Madubuko, T.; Frank, C.; Noel, H.; et al. Arthropod-Borne Diseases among Travellers Arriving in Europe from Africa, 2015 to 2019. Euro Surveill. Bull. Eur. Sur Mal. Transm. Eur. Commun. Dis. Bull. 2023, 28, 2200270. [Google Scholar] [CrossRef]
- Chevalier, V. Relevance of Rift Valley Fever to Public Health in the European Union. Clin. Microbiol. Infect. Off. Publ. Eur. Soc. Clin. Microbiol. Infect. Dis. 2013, 19, 705–708. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention (CDC). RVF Distribution Map. Available online: https://www.cdc.gov/vhf/rvf/outbreaks/distribution-map.html#print (accessed on 14 January 2024).
- Sall, A.A.; Thonnon, J.; Sene, O.K.; Fall, A.; Ndiaye, M.; Baudez, B.; Mathiot, C.; Bouloy, M. Single-Tube and Nested Reverse Transcriptase-Polymerase Chain Reaction for Detection of Rift Valley Fever Virus in Human and Animal Sera. J. Virol. Methods 2001, 91, 85–92. [Google Scholar] [CrossRef]
- Garcia, S.; Crance, J.M.; Billecocq, A.; Peinnequin, A.; Jouan, A.; Bouloy, M.; Garin, D. Quantitative Real-Time PCR Detection of Rift Valley Fever Virus and Its Application to Evaluation of Antiviral Compounds. J. Clin. Microbiol. 2001, 39, 4456–4461. [Google Scholar] [CrossRef][Green Version]
- Weidmann, M.; Sanchez-Seco, M.P.; Sall, A.A.; Ly, P.O.; Thiongane, Y.; Lô, M.M.; Schley, H.; Hufert, F.T. Rapid Detection of Important Human Pathogenic Phleboviruses. J. Clin. Virol. Off. Publ. Pan Am. Soc. Clin. Virol. 2008, 41, 138–142. [Google Scholar] [CrossRef]
- Drosten, C.; Göttig, S.; Schilling, S.; Asper, M.; Panning, M.; Schmitz, H.; Günther, S. Rapid Detection and Quantification of RNA of Ebola and Marburg Viruses, Lassa Virus, Crimean-Congo Hemorrhagic Fever Virus, Rift Valley Fever Virus, Dengue Virus, and Yellow Fever Virus by Real-Time Reverse Transcription-PCR. J. Clin. Microbiol. 2002, 40, 2323–2330. [Google Scholar] [CrossRef] [PubMed]
- Njenga, M.K.; Paweska, J.; Wanjala, R.; Rao, C.Y.; Weiner, M.; Omballa, V.; Luman, E.T.; Mutonga, D.; Sharif, S.; Panning, M.; et al. Using a Field Quantitative Real-Time PCR Test to Rapidly Identify Highly Viremic Rift Valley Fever Cases. J. Clin. Microbiol. 2009, 47, 1166–1171. [Google Scholar] [CrossRef]
- Peyrefitte, C.N.; Boubis, L.; Coudrier, D.; Bouloy, M.; Grandadam, M.; Tolou, H.J.; Plumet, S. Real-Time Reverse-Transcription Loop-Mediated Isothermal Amplification for Rapid Detection of Rift Valley Fever Virus. J. Clin. Microbiol. 2008, 46, 3653–3659. [Google Scholar] [CrossRef] [PubMed]
- Le Roux, C.A.; Kubo, T.; Grobbelaar, A.A.; van Vuren, P.J.; Weyer, J.; Nel, L.H.; Swanepoel, R.; Morita, K.; Paweska, J.T. Development and Evaluation of a Real-Time Reverse Transcription-Loop-Mediated Isothermal Amplification Assay for Rapid Detection of Rift Valley Fever Virus in Clinical Specimens. J. Clin. Microbiol. 2009, 47, 645–651. [Google Scholar] [CrossRef] [PubMed]
- Han, Q.; Zhang, S.; Liu, D.; Yan, F.; Wang, H.; Huang, P.; Bi, J.; Jin, H.; Feng, N.; Cao, Z.; et al. Development of a Visible Reverse Transcription-Loop-Mediated Isothermal Amplification Assay for the Detection of Rift Valley Fever Virus. Front. Microbiol. 2020, 11, 590732. [Google Scholar] [CrossRef]
- Euler, M.; Wang, Y.; Nentwich, O.; Piepenburg, O.; Hufert, F.T.; Weidmann, M. Recombinase Polymerase Amplification Assay for Rapid Detection of Rift Valley Fever Virus. J. Clin. Virol. Off. Publ. Pan Am. Soc. Clin. Virol. 2012, 54, 308–312. [Google Scholar] [CrossRef]
- Wekesa, F.; Wamalwa, M.; Oduor, R.; Binepal, Y.; Ateya, L.; Okumu, N.; M’kwenda, A.; Masaba, C.; Mukhaye, E. Development and Validation of Rapid Colorimetric Reverse Transcription Loop-Mediated Isothermal Amplification for Detection of Rift Valley Fever Virus. Adv. Virol. 2023, 2023, 1863980. [Google Scholar] [CrossRef]
- He, J.; Kraft, A.J.; Fan, J.; Van Dyke, M.; Wang, L.; Bose, M.E.; Khanna, M.; Metallo, J.A.; Henrickson, K.J. Simultaneous Detection of CDC Category “A” DNA and RNA Bioterrorism Agents by Use of Multiplex PCR & RT-PCR Enzyme Hybridization Assays. Viruses 2009, 1, 441–459. [Google Scholar] [CrossRef]
- Balaraman, V.; Gaudreault, N.N.; Trujillo, J.D.; Indran, S.V.; Wilson, W.C.; Richt, J.A. RT-qPCR Genotyping Assays for Differentiating Rift Valley Fever Phlebovirus Strains. J. Virol. Methods 2023, 315, 114693. [Google Scholar] [CrossRef]
- Yao, W.; Yang, Z.; Lou, X.; Mao, H.; Yan, H.; Zhang, Y. Simultaneous Detection of Ebola Virus and Pathogens Associated with Hemorrhagic Fever by an Oligonucleotide Microarray. Front. Microbiol. 2021, 12, 713372. [Google Scholar] [CrossRef]
- Venter, M.; Zaayman, D.; Van Niekerk, S.; Stivaktas, V.; Goolab, S.; Weyer, J.; Paweska, J.T.; Swanepoel, R. Macroarray Assay for Differential Diagnosis of Meningoencephalitis in Southern Africa. J. Clin. Virol. 2014, 60, 50–56. [Google Scholar] [CrossRef]
- Sardi, S.I.; Somasekar, S.; Naccache, S.N.; Bandeira, A.C.; Tauro, L.B.; Campos, G.S.; Chiu, C.Y. Coinfections of Zika and Chikungunya Viruses in Bahia, Brazil, Identified by Metagenomic Next-Generation Sequencing. J. Clin. Microbiol. 2016, 54, 2348–2353. [Google Scholar] [CrossRef] [PubMed]
- Wilson, M.R.; Zimmermann, L.L.; Crawford, E.D.; Sample, H.A.; Soni, P.R.; Baker, A.N.; Khan, L.M.; DeRisi, J.L. Acute West Nile Virus Meningoencephalitis Diagnosed Via Metagenomic Deep Sequencing of Cerebrospinal Fluid in a Renal Transplant Patient. Am. J. Transplant. Off. J. Am. Soc. Transplant. Am. Soc. Transpl. Surg. 2017, 17, 803–808. [Google Scholar] [CrossRef]
- Altona Diagnostics. RealStar®® Rift Valley Fever Virus RT-PCR Kit 1.0. Available online: https://www.altona-diagnostics.com/files/public/Content%20Homepage/-%2002%20RealStar/MAN%20-%20CE%20-%20EN/RealStar%20RVFV%20RT-PCR%20Kit%201.0_WEB_CE_EN-S02.pdf (accessed on 15 January 2024).
- Petrova, V.; Kristiansen, P.; Norheim, G.; Yimer, S.A. Rift Valley Fever: Diagnostic Challenges and Investment Needs for Vaccine Development. BMJ Glob. Health 2020, 5, e002694. [Google Scholar] [CrossRef]
- WOAH (World Organisation for Animal Health). Report of the Meeting of the OIE Biological Standards Commission; World Organisation for Animal Health: Paris, France, 2013; Available online: https://www.woah.org/fileadmin/Home/eng/Internationa_Standard_Setting/docs/pdf/BSC/A_BSC_Feb2013.pdf (accessed on 17 January 2024).
- Hoste, A.C.R.; Ruiz, T.; Fernández-Pacheco, P.; Jiménez-Clavero, M.Á.; Djadjovski, I.; Moreno, S.; Brun, A.; Edwards, T.A.; Barr, J.N.; Rueda, P.; et al. Development of a Multiplex Assay for Antibody Detection in Serum against Pathogens Affecting Ruminants. Transbound. Emerg. Dis. 2021, 68, 1229–1239. [Google Scholar] [CrossRef]
- Surtees, R.; Stern, D.; Ahrens, K.; Kromarek, N.; Lander, A.; Kreher, P.; Weiss, S.; Hewson, R.; Punch, E.K.; Barr, J.N.; et al. Development of a Multiplex Microsphere Immunoassay for the Detection of Antibodies against Highly Pathogenic Viruses in Human and Animal Serum Samples. PLoS Negl. Trop. Dis. 2020, 14, e0008699. [Google Scholar] [CrossRef]
- Ragan, I.K.; Schuck, K.N.; Upreti, D.; Odendaal, L.; Richt, J.A.; Trujillo, J.D.; Wilson, W.C.; Davis, A.S. Rift Valley Fever Viral RNA Detection by In Situ Hybridization in Formalin-Fixed, Paraffin-Embedded Tissues. Vector Borne Zoonotic Dis. 2019, 19, 553–556. [Google Scholar] [CrossRef] [PubMed]
- Tesh, R.B.; Peters, C.J.; Meegan, J.M. Studies on the Antigenic Relationship among Phleboviruses. Am. J. Trop. Med. Hyg. 1982, 31, 149–155. [Google Scholar] [CrossRef] [PubMed]
- Xu, F.; Liu, D.; Nunes, M.R.T.; DA Rosa, A.P.A.T.; Tesh, R.B.; Xiao, S.-Y. Antigenic and Genetic Relationships among Rift Valley Fever Virus and Other Selected Members of the Genus Phlebovirus (Bunyaviridae). Am. J. Trop. Med. Hyg. 2007, 76, 1194–1200. [Google Scholar] [CrossRef]
- World Organisation for Animal Health (OIE). Manual of Diagnostic Tests and Vaccines for Terrestrial Animals, Twelfth Edition 2023. Available online: https://www.woah.org/fileadmin/Home/eng/Health_standards/tahm/A_summry.htm (accessed on 20 December 2023).
- Smith, M.R.; Schirtzinger, E.E.; Wilson, W.C.; Davis, A.S. Rift Valley Fever Virus: Propagation, Quantification, and Storage. Curr. Protoc. Microbiol. 2019, 55, e92. [Google Scholar] [CrossRef]
- Anderson, G.W.; Saluzzo, J.F.; Ksiazek, T.G.; Smith, J.F.; Ennis, W.; Thureen, D.; Peters, C.J.; Digoutte, J.P. Comparison of in Vitro and in Vivo Systems for Propagation of Rift Valley Fever Virus from Clinical Specimens. Res. Virol. 1989, 140, 129–138. [Google Scholar] [CrossRef]
- Odendaal, L.; Clift, S.J.; Fosgate, G.T.; Davis, A.S. Lesions and Cellular Tropism of Natural Rift Valley Fever Virus Infection in Adult Sheep. Vet. Pathol. 2019, 56, 61–77. [Google Scholar] [CrossRef]
- Digoutte, J.P.; Jouan, A.; Le Guenno, B.; Riou, O.; Philippe, B.; Meegan, J.; Ksiazek, T.G.; Peters, C.J. Isolation of the Rift Valley Fever Virus by Inoculation into Aedes Pseudoscutellaris Cells: Comparison with Other Diagnostic Methods. Res. Virol. 1989, 140, 31–41. [Google Scholar] [CrossRef] [PubMed]
- World Organisation for Animal Health (OIE). Rift Valley Fever (Infection with Rift Valley Fever Virus). Available online: https://www.woah.org/app/uploads/2021/03/3-01-18-rvf-1.pdf (accessed on 15 January 2024).
- Euroimmun. IFA per Infettivologia. Available online: https://www.euroimmun.it/prodotti/infettivologia/ifa-2/ (accessed on 1 November 2023).
- Paweska, J.T.; Smith, S.J.; Wright, I.M.; Williams, R.; Cohen, A.S.; Van Dijk, A.A.; Grobbelaar, A.A.; Croft, J.E.; Swanepoel, R.; Gerdes, G.H. Indirect Enzyme-Linked Immunosorbent Assay for the Detection of Antibody against Rift Valley Fever Virus in Domestic and Wild Ruminant Sera. Onderstepoort J. Vet. Res. 2003, 70, 49–64. [Google Scholar] [PubMed]
- Jansen van Vuren, P.; Potgieter, A.C.; Paweska, J.T.; van Dijk, A.A. Preparation and Evaluation of a Recombinant Rift Valley Fever Virus N Protein for the Detection of IgG and IgM Antibodies in Humans and Animals by Indirect ELISA. J. Virol. Methods 2007, 140, 106–114. [Google Scholar] [CrossRef] [PubMed]
- Fafetine, J.M.; Tijhaar, E.; Paweska, J.T.; Neves, L.C.B.G.; Hendriks, J.; Swanepoel, R.; Coetzer, J.a.W.; Egberink, H.F.; Rutten, V.P.M.G. Cloning and Expression of Rift Valley Fever Virus Nucleocapsid (N) Protein and Evaluation of a N-Protein Based Indirect ELISA for the Detection of Specific IgG and IgM Antibodies in Domestic Ruminants. Vet. Microbiol. 2007, 121, 29–38. [Google Scholar] [CrossRef]
- Cêtre-Sossah, C.; Billecocq, A.; Lancelot, R.; Defernez, C.; Favre, J.; Bouloy, M.; Martinez, D.; Albina, E. Evaluation of a Commercial Competitive ELISA for the Detection of Antibodies to Rift Valley Fever Virus in Sera of Domestic Ruminants in France. Prev. Vet. Med. 2009, 90, 146–149. [Google Scholar] [CrossRef]
- van Vuren, P.J.; Paweska, J.T. Comparison of Enzyme-Linked Immunosorbent Assay-Based Techniques for the Detection of Antibody to Rift Valley Fever Virus in Thermochemically Inactivated Sheep Sera. Vector Borne Zoonotic Dis. 2010, 10, 697–699. [Google Scholar] [CrossRef]
- Fafetine, J.M.; Jansen van Vuren, P.; Paweska, J.T. Comparison of a Recombinant Nucleocapsid IgG Indirect ELISA with an IgG Sandwich ELISA for the Detection of Antibodies to Rift Valley Fever Virus in Small Ruminants. Vector Borne Zoonotic Dis. 2012, 12, 1062–1064. [Google Scholar] [CrossRef]
- Kortekaas, J.; Kant, J.; Vloet, R.; Cêtre-Sossah, C.; Marianneau, P.; Lacote, S.; Banyard, A.C.; Jeffries, C.; Eiden, M.; Groschup, M.; et al. European Ring Trial to Evaluate ELISAs for the Diagnosis of Infection with Rift Valley Fever Virus. J. Virol. Methods 2013, 187, 177–181. [Google Scholar] [CrossRef]
- Paweska, J.T.; Burt, F.J.; Swanepoel, R. Validation of IgG-Sandwich and IgM-Capture ELISA for the Detection of Antibody to Rift Valley Fever Virus in Humans. J. Virol. Methods 2005, 124, 173–181. [Google Scholar] [CrossRef]
- Faburay, B.; Wilson, W.C.; Secka, A.; Drolet, B.; McVey, D.S.; Richt, J.A. Evaluation of an Indirect Enzyme-Linked Immunosorbent Assay Based on Recombinant Baculovirus-Expressed Rift Valley Fever Virus Nucleoprotein as the Diagnostic Antigen. J. Clin. Microbiol. 2019, 57, e01058-19. [Google Scholar] [CrossRef]
- UNEP. Law and Environment Assistance Platform. Commission Implementing Regulation (EU) 2018/1882 on the Application of Certain Disease Prevention and Control Rules to Categories of Listed Diseases and Establishing a List of Species and Groups of Species Posing a Considerable Risk for the Spread of Those Listed Diseases. Available online: https://leap.unep.org/en/countries/eu/national-legislation/commission-implementing-regulation-eu-20181882-application (accessed on 14 August 2023).
- EFSA Panel on Animal Health and Welfare (EFSA AHAW Panel); Nielsen, S.S.; Alvarez, J.; Bicout, D.J.; Calistri, P.; Depner, K.; Drewe, J.A.; Garin-Bastuji, B.; Gonzales Rojas, J.L.; Gortázar Schmidt, C.; et al. Rift Valley Fever—Assessment of Effectiveness of Surveillance and Control Measures in the EU. EFSA J. Eur. Food Saf. Auth. 2020, 18, e06292. [Google Scholar] [CrossRef]
- Kasari, T.R.; Carr, D.A.; Lynn, T.V.; Weaver, J.T. Evaluation of Pathways for Release of Rift Valley Fever Virus into Domestic Ruminant Livestock, Ruminant Wildlife, and Human Populations in the Continental United States. J. Am. Vet. Med. Assoc. 2008, 232, 514–529. [Google Scholar] [CrossRef] [PubMed]
- Rolin, A.I.; Berrang-Ford, L.; Kulkarni, M.A. The Risk of Rift Valley Fever Virus Introduction and Establishment in the United States and European Union. Emerg. Microbes Infect. 2013, 2, e81. [Google Scholar] [CrossRef]
- Oyas, H.; Holmstrom, L.; Kemunto, N.P.; Muturi, M.; Mwatondo, A.; Osoro, E.; Bitek, A.; Bett, B.; Githinji, J.W.; Thumbi, S.M.; et al. Enhanced Surveillance for Rift Valley Fever in Livestock during El Niño Rains and Threat of RVF Outbreak, Kenya, 2015–2016. PLoS Negl. Trop. Dis. 2018, 12, e0006353. [Google Scholar] [CrossRef]
- Kim, H.-J.; Lyoo, H.-R.; Park, J.-Y.; Choi, J.-S.; Lee, J.-Y.; Jeoung, H.-Y.; Cho, Y.-S.; Cho, I.-S.; Yoo, H.S. Surveillance of Rift Valley Fever Virus in Mosquito Vectors of the Republic of Korea. Vector Borne Zoonotic Dis. 2016, 16, 131–135. [Google Scholar] [CrossRef]
- Anyamba, A.; Linthicum, K.J.; Small, J.; Britch, S.C.; Pak, E.; de La Rocque, S.; Formenty, P.; Hightower, A.W.; Breiman, R.F.; Chretien, J.-P.; et al. Prediction, Assessment of the Rift Valley Fever Activity in East and Southern Africa 2006–2008 and Possible Vector Control Strategies. Am. J. Trop. Med. Hyg. 2010, 83, 43–51. [Google Scholar] [CrossRef]
- Gregor, K.M.; Michaely, L.M.; Gutjahr, B.; Rissmann, M.; Keller, M.; Dornbusch, S.; Naccache, F.; Schön, K.; Jansen, S.; Heitmann, A.; et al. Rift Valley Fever Virus Detection in Susceptible Hosts with Special Emphasis in Insects. Sci. Rep. 2021, 11, 9822. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lapa, D.; Pauciullo, S.; Ricci, I.; Garbuglia, A.R.; Maggi, F.; Scicluna, M.T.; Tofani, S. Rift Valley Fever Virus: An Overview of the Current Status of Diagnostics. Biomedicines 2024, 12, 540. https://doi.org/10.3390/biomedicines12030540
Lapa D, Pauciullo S, Ricci I, Garbuglia AR, Maggi F, Scicluna MT, Tofani S. Rift Valley Fever Virus: An Overview of the Current Status of Diagnostics. Biomedicines. 2024; 12(3):540. https://doi.org/10.3390/biomedicines12030540
Chicago/Turabian StyleLapa, Daniele, Silvia Pauciullo, Ida Ricci, Anna Rosa Garbuglia, Fabrizio Maggi, Maria Teresa Scicluna, and Silvia Tofani. 2024. "Rift Valley Fever Virus: An Overview of the Current Status of Diagnostics" Biomedicines 12, no. 3: 540. https://doi.org/10.3390/biomedicines12030540
APA StyleLapa, D., Pauciullo, S., Ricci, I., Garbuglia, A. R., Maggi, F., Scicluna, M. T., & Tofani, S. (2024). Rift Valley Fever Virus: An Overview of the Current Status of Diagnostics. Biomedicines, 12(3), 540. https://doi.org/10.3390/biomedicines12030540






