Fulminant Leptospirosis Presenting with Rapidly Developing Acute Renal Failure and Multiorgan Failure
Abstract
1. Introduction
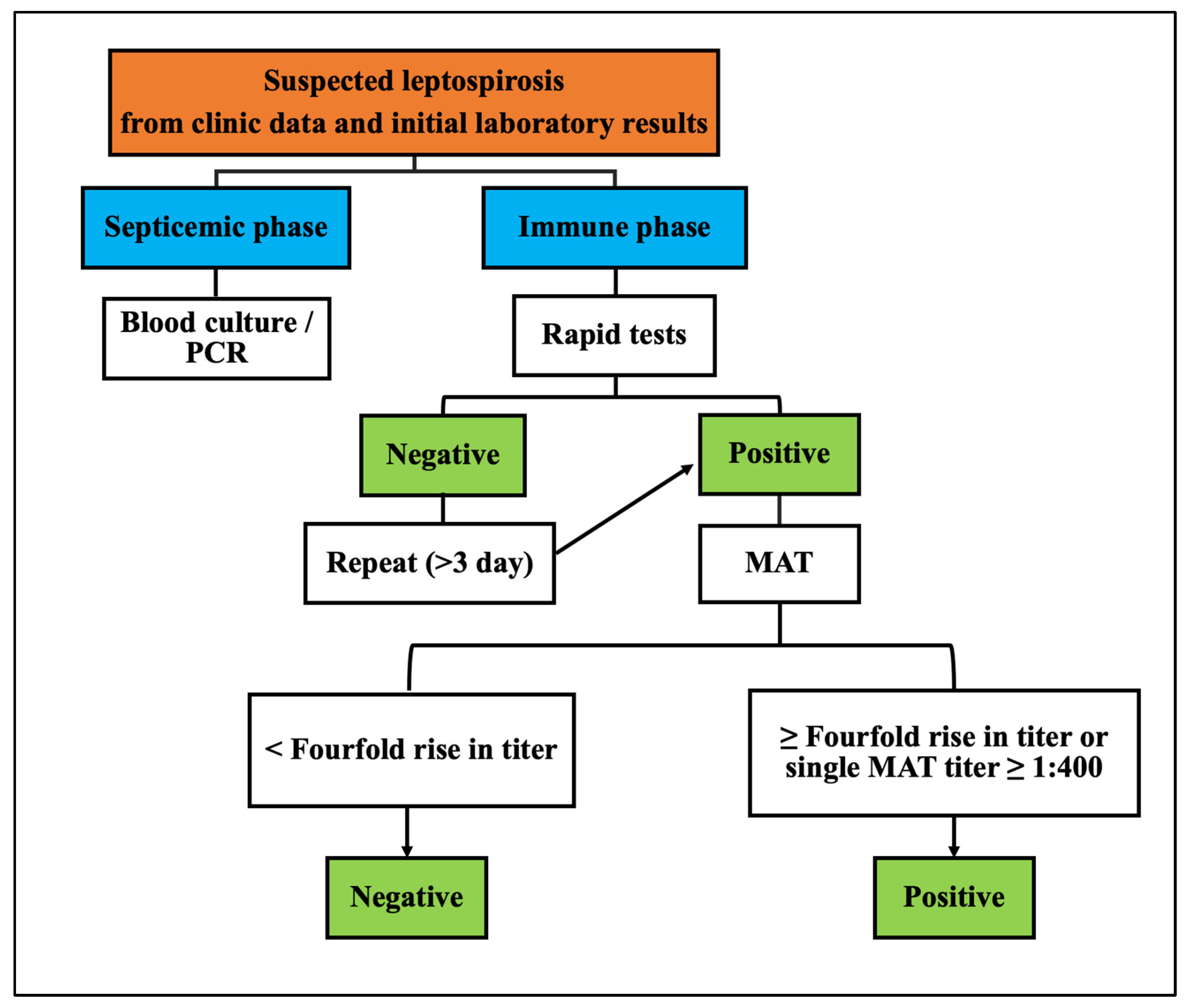
2. Materials and Methods
3. Results
3.1. Clinical Presentation and Initial Treatment Response of Leptospirosis
3.2. Intensive Care Unit Admission and Evolving Treatment Strategies of Leptospirosis
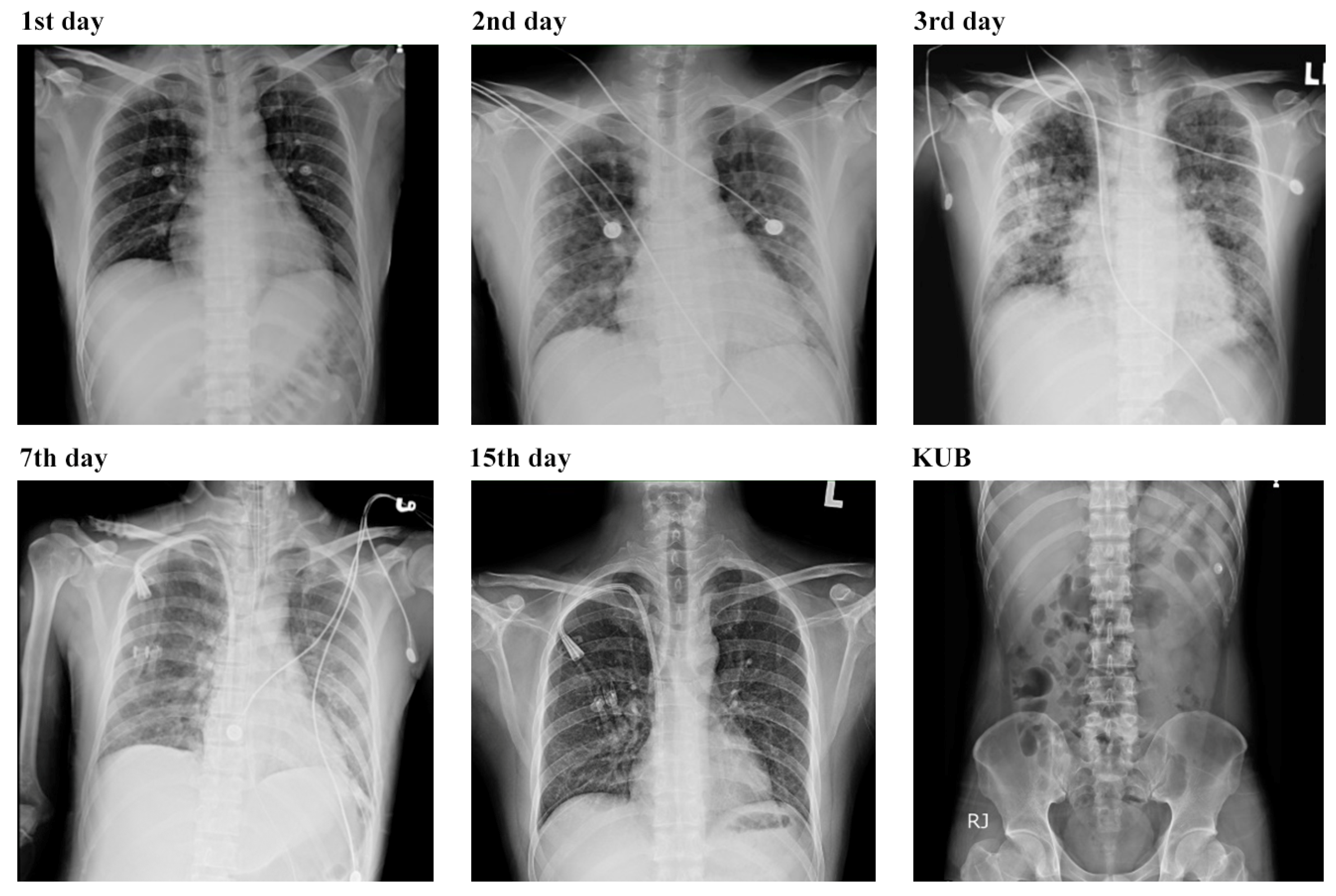
3.3. Respiratory Complications and Multifaceted Treatment Outcome of Leptospirosis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Adler, B.; de la Peña Moctezuma, A. Leptospira and leptospirosis. Vet. Microbiol. 2010, 140, 287–296. [Google Scholar] [CrossRef] [PubMed]
- Forbes, A.; Zochowski, W.; Dubrey, S.; Sivaprakasam, V. Leptospirosis and Weil’s disease in the UK. QJM 2012, 105, 1151–1162. [Google Scholar] [CrossRef]
- Haake, D.A.; Levett, P.N. Leptospirosis in humans. Curr. Top. Microbiol. Immunol. 2015, 387, 65–97. [Google Scholar]
- Lopes, A.A.; Costa, E.; Costa, Y.A.; Sacramento, E.; Oliveira Junior, A.R.R.d.; Lopes, M.B.; Lopes, G.B. Comparative study of the in-hospital case-fatality rate of leptospirosis between pediatric and adult patients of different age groups. Rev. Inst. Med. Trop. 2004, 46, 19–24. [Google Scholar] [CrossRef]
- Gulati, S.; Maheshwari, A. Dengue fever-like illnesses: How different are they from each other? Scand. J. Infect. Dis. 2012, 44, 522–530. [Google Scholar] [CrossRef] [PubMed]
- Ko, C.H.; Lan, Y.W.; Chen, Y.C.; Cheng, T.T.; Yu, S.F.; Cidem, A.; Liu, Y.H.; Kuo, C.W.; Yen, C.C.; Chen, W.; et al. Effects of mean artery pressure and blood pH on survival rate of patients with acute kidney injury combined with acute hypoxic respiratory failure: A retrospective study. Medicina 2021, 57, 1243. [Google Scholar] [CrossRef] [PubMed]
- Dolhnikoff, M.; Mauad, T.; Bethlem, E.P.; Carvalho, C.R.R. Pathology and pathophysiology of pulmonary manifestations in leptospirosis. Braz. J. Infect. Dis. 2007, 11, 142–148. [Google Scholar] [CrossRef]
- Goris, M.G.; Hartskeerl, R.A. Leptospirosis serodiagnosis by the microscopic agglutination test. Curr. Protoc. Microbiol. 2014, 32, Unit 12E.15. [Google Scholar] [CrossRef]
- Gravekamp, C.; Korver, H.; Montgomery, J.; Everard, C.O.; Carrington, D.; Ellis, W.; Terpstra, W.J. Leptospires isolated from toads and frogs on the Island of Barbados. Zentralbl. Bakteriol. 1991, 275, 403–411. [Google Scholar] [CrossRef]
- Michel, V.; Branger, C.; Andre-Fontaine, G. Epidemiology of leptospirosis. Rev. Cubana. Med. Trop. 2002, 54, 7–10. [Google Scholar]
- World Health Organization. Human Leptospirosis: Guidance for Diagnosis, Surveillance and Control. 2003. Available online: https://apps.who.int/iris/handle/10665/42667 (accessed on 1 August 2023).
- Nguyen, L.; Chimunda, T. Neuro-leptospirosis–A batty diagnostic enigma. IDCases 2023, 32, e01731. [Google Scholar] [CrossRef]
- Levett, P. Leptospirosis. Clin. Microbiol. Rev. 2001, 14, 296–326. [Google Scholar] [CrossRef] [PubMed]
- Norris, M.; Smythe, L.; Symonds, M.; Dohnt, M.; Scott, J. Review of leptospirosis notifications in Queensland 1997, 1985 to 1996. Commun. Dis. Intell. 1997, 21, 17–20. [Google Scholar]
- Shetty, A.; Kundu, S.; Vernel-Pauillac, F.; Ratet, G.; Werts, C.; Gomes-Solecki, M. Transient presence of live Leptospira interrogans in murine testes. Microbiol. Spectr. 2022, 10, e0277521. [Google Scholar] [CrossRef]
- Ghazaei, C. Pathogenic Leptospira: Advances in understanding the molecular pathogenesis and virulence. Open Vet. J. 2018, 8, 13–24. [Google Scholar] [CrossRef] [PubMed]
- Kassegne, K.; Hu, W.; Ojcius, D.M.; Sun, D.; Ge, Y.; Zhao, J.; Yang, X.F.; Li, L.; Yan, J. Identification of collagenase as a critical virulence factor for invasiveness and transmission of pathogenic Leptospira species. J. Infect. Dis. 2014, 209, 1105–1115. [Google Scholar] [CrossRef]
- King, A.M.; Pretre, G.; Bartpho, T.; Sermswan, R.W.; Toma, C.; Suzuki, T.; Eshghi, A.; Picardeau, M.; Adler, B.; Murray, G.L. High-temperature protein G is an essential virulence factor of Leptospira interrogans. Infect. Immun. 2014, 82, 1123–1131. [Google Scholar] [CrossRef]
- Wang, N.; Han, Y.-H.; Sung, J.-Y.; Lee, W.-S.; Ou, T.-Y. Atypical leptospirosis: An overlooked cause of aseptic meningitis. BMC Res. Notes 2016, 9, 154. [Google Scholar] [CrossRef]
- Levett, P.N.; Haake, D.A. Leptospira species (leptospirosis). In Principles and Practice of Infectious Diseases, Churchill Livingtsone; Elsevier: Philadelphia, PA, USA, 2010; pp. 3059–3065. [Google Scholar]
- Maroun, E.; Kushawaha, A.; El-Charabaty, E.; Mobarakai, N.; El-Sayegh, S. Fulminant Leptospirosis (Weil’s disease) in an urban setting as an overlooked cause of multiorgan failure: A case report. J. Med. Case Rep. 2011, 5, 7. [Google Scholar] [CrossRef]
- Faine, S.; Adler, B.; Bolin, C.; Perlat, P. Leptospira and Leptospirosis, 2nd ed.; MediSci: Melbourne, Australia, 1999. [Google Scholar]
- Guerreiro, H.; Croda, J.; Flannery, B.; Mazel, M.; Matsunaga, J.; Galvão Reis, M.; Levett, P.N.; Ko, A.I.; Haake, D.A. Leptospiral proteins recognized during the humoral immune response to leptospirosis in humans. Infect. Immun. 1999, 69, 4958–4968. [Google Scholar] [CrossRef]
- Salkade, H.P.; Divate, S.; Deshpande, J.R.; Kawishwar, V.; Chaturvedi, R.; Kandalkar, B.M.; Vaideeswar, P. A study of autopsy findings in 62 cases of leptospirosis in a metropolitan city in India. J. Postgrad. Med. 2005, 51, 169–173. [Google Scholar] [PubMed]
- Martínez Garcia, M.A.; de Diego Damia, A.; Menendez Villanueva, R.; Lopez Hontagas, J. Pulmonary involvement in leptospirosis. Eur. J. Clin. Microbiol. Infect. Dis. 2000, 19, 471–474. [Google Scholar] [CrossRef]
- Griffith, M.E.; Hospenthal, D.R.; Murray, C.K. Antimicrobial therapy of leptospirosis. Curr. Opin. Infect. Dis. 2006, 19, 533–537. [Google Scholar] [CrossRef] [PubMed]
- Marotto, P.C.; Nascimento, C.M.; Eluf-Neto, J.; Marotto, M.S.; Andrade, L.; Sztajnbok, J.; Seguro, A.C. Acute lung injury in leptospirosis: Clinical and laboratory features, outcome, and factors associated with mortality. Clin. Infect. Dis. 1999, 29, 1561–1563. [Google Scholar] [CrossRef]
- Chawla, V.; Trivedi, T.; Yeolekar, M. Epidemic of leptospirosis: An ICU experience. J. Assoc. Physicians India 2004, 52, 619–622. [Google Scholar]
- Thalji, M.; Qunibi, H.; Muhtasib, L.; Hroob, H.; Al-Zughayyar, A.; Salhab, R.; Abu Asbeh, Y. Case report: Leptospirosis with multi-organ failure complicated by massive upper gastrointestinal bleeding in a non-epidemic setting with successful management. Front. Surg. 2023, 10, 1131659. [Google Scholar] [CrossRef]
- Zahornacky, O.; Porubčin, Š.; Rovňáková, A.; Fedačko, J.; Jarčuška, P. Uncovering a Rarely Diagnosed Disease: Severe Leptospirosis with Multiorgan Failure in Slovakia. Microbiol. Res. 2023, 14, 1524–1533. [Google Scholar] [CrossRef]
- Hung, C.C.; Chang, C.T.; Tian, Y.C.; Wu, M.S.; Yu, C.C.; Pan, M.J.; Vandewalle, A.; Yang, C.W. Leptospiral membrane proteins stimulate pro-inflammatory chemokines secretion by renal tubule epithelial cells through toll-like receptor 2 and p38 mitogen activated protein kinase. Nephrol. Dial. Transplant. 2006, 21, 898–910. [Google Scholar] [CrossRef]
- Reis, E.A.; Hagan, J.E.; Ribeiro, G.S.; Teixeira-Carvalho, A.; Martins-Filho, O.A.; Montgomery, R.R.; Shaw, A.C.; Ko, A.I.; Reis, M.G. Cytokine response signatures in disease progression and development of severe clinical outcomes for leptospirosis. PLoS Negl. Trop. Dis. 2013, 7, e2457. [Google Scholar] [CrossRef]
- Carvalho, C.R.; Bethlem, E.P. Pulmonary complications of leptospirosis. Clin. Chest Med. 2002, 23, 469–478. [Google Scholar] [CrossRef]
- Sitprija, V. Renal dysfunction in leptospirosis: A view from the tropics. Nat. Clin. Pract. Nephrol. 2006, 2, 658–659. [Google Scholar] [CrossRef]
- Yang, C.W. Leptospirosis renal disease: Understanding the initiation by Toll-like receptors. Kidney Int. 2007, 72, 918–925. [Google Scholar] [CrossRef]
- Lin, C.L.; Wu, M.S.; Yang, C.W.; Huang, C.C. Leptospirosis associated with hypokalaemia and thick ascending limb dysfunction. Nephrol. Dial. Transplant. 1999, 14, 193–195. [Google Scholar] [CrossRef]
- Yang, C.W.; Wu, M.S.; Pan, M.J.; Hong, J.J.; Yu, C.C.; Vandewalle, A.; Huang, C.C. Leptospira outer membrane protein activates NF-κB and downstream genes expressed in medullary thick ascending limb cells. J. Am. Soc. Nephrol. 2000, 11, 2017–2026. [Google Scholar] [CrossRef] [PubMed]
- Cetin, B.D.; Harmankaya, O.; Hasman, H.; Gunduz, A.; Oktar, M.; Seber, E. Acute renal failure: A common manifestation of leptospirosis. Ren. Fail. 2004, 26, 655–661. [Google Scholar] [CrossRef]
- Magaldi, A.J.; Yasuda, P.N.; Kudo, L.H.; Seguro, A.C.; Rocha, A.S. Renal involvement in leptospirosis: A pathophysiologic study. Nephron 1992, 62, 332–339. [Google Scholar] [CrossRef]
- Fajgenbaum, D.C.; June, C.H. Cytokine storm. N. Engl. J. Med. 2020, 383, 2255–2273. [Google Scholar] [CrossRef]
- Kundu, S.; Shetty, A.; Gomes-Solecki, M. Necroptosis contributes to persistent inflammation during acute leptospirosis. Front. Immunol. 2022, 13, 810834. [Google Scholar] [CrossRef]
- Shetty, A.; Kundu, S.; Gomes-Solecki, M. Inflammatory signatures of pathogenic and non-pathogenic leptospira infection in susceptible C3H-HeJ mice. Front. Cell. Infect. Microbiol. 2021, 11, 677999. [Google Scholar] [CrossRef]
- Ittyachen, A.; Lakshmanakumar, V.; Eapen, C.; Joseph, M. Methylprednisolone as adjuvant in treatment of acute respiratory distress syndrome owing to leptospirosis-a pilot study. Indian J. Crit. Care Med. 2005, 9, 133–136. [Google Scholar] [CrossRef]
- Ittyachen, A.M. COVID-19 and leptospirosis: Cytokine storm and the use of steroids. Trop. Doct. 2021, 51, 128–130. [Google Scholar] [CrossRef]
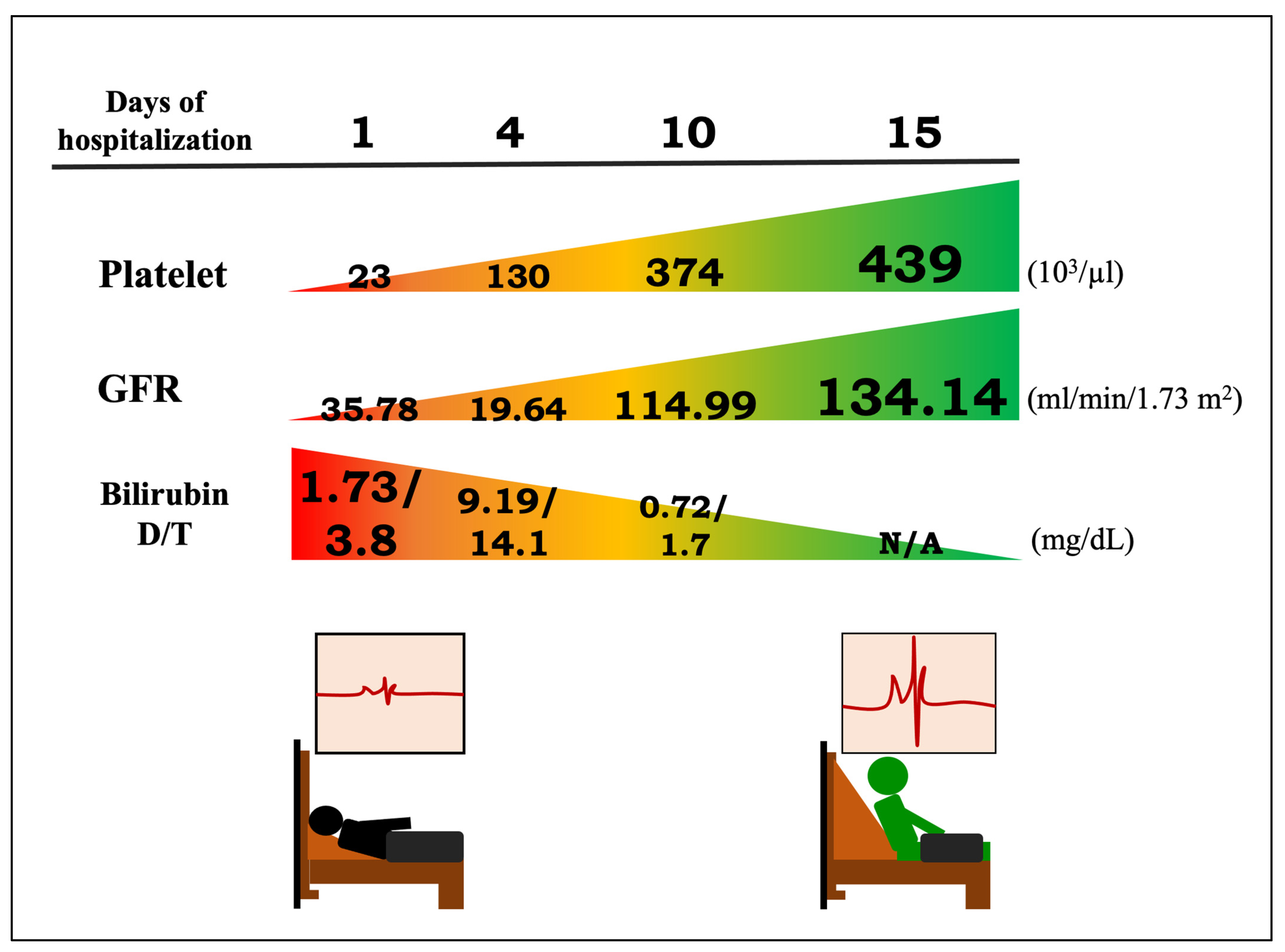


| Days of Hospitalization | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 15 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Temperature (°C) | 39 | 38.4 | 37.5 | 37.5 | 38.7 | 37.6 | 38 | 37.6 | 39 | 37.5 | 37.5 | 37 |
| Blood pressure (mmHg) | 87/51 | 80/44 | 88/52 | 100/64 | 112/58 | 89/65 | 106/96 | 106/83 | 107/74 | 115/68 | 113/73 | 113/78 |
| Dialysis | ▲ | ▲ | ▲ | |||||||||
| Endo. Tube | ★ | ★ | ★ | ★ | ★ | ★ | ★ | |||||
| WBC (103/μL) (3.9~10.6) | 13,760 | 13,310 | 17,160 | 16,100 | 26,010 | 35,700 | 24,770 | 17,560 | 11,050 | |||
| Neutrophils (%) (40–75) | 80 | 91 | 84 | 78 | 70 | 73 | 71 | 69 | 70 | |||
| Lymphocytes (%) (20–45) | 11 | 5 | 10 | 5 | 8 | 9 | 12 | 25 | 24 | |||
| Monocyte (%) (2–10) | 1 | 3 | 3 | 2 | 4 | 8 | 3 | 5 | 4 | |||
| Eosinophil (%) (0.2–6) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |||
| Band (5) (0–5) | 0 | 0 | 3 | 0 | 5 | 5 | 1 | 0 | 0 | |||
| Hb (106/μL) (13.5–17.5) | 10.8 | 5 | 6.1 | 7.5 | 7.6 | 9.3 | 9.3 | 10 | 10.5 | |||
| Platelet (103/μL) (150–400) | 23 | 20 | 45 | 130 | 196 | 179 | 219 | 374 | 439 | |||
| ESR (mm) (0–10) | 115 | 66 | ||||||||||
| Blood Gas Analysis | Nasal Cannula 3 L | BiPAP 15 L/min | Ventilator O2 70% | Ventilator O2 30% | ||||||||
| PH (7.35–7.45) | 7.394 | 7.44 | 7.41 | 7.49 | 7.49 | |||||||
| PCO2 (mmHg) (35–45) | 32.2 | 35 | 43 | 43 | 38 | |||||||
| PO2 (mmHg) (80–100) | 66.9 | 58 | 51 | 158 | 109 | |||||||
| HCO3 (mmol/L) | 19.2 | 23.8 | 27.6 | 32.6 | 29 | |||||||
| O2 SAT(%) (95–100) | 92.6 | NA | 86 | 100 | 99 | |||||||
| BUN (mg/dL) (7~25) | 25 | 63 | 55 | 59 | 31 | |||||||
| Cr (mg/dL) (0.7–1.3) | 2.2 | 3.9 | 5.5 | 3.7 | 1.3 | 0.8 | 0.7 | |||||
| GFR (ml/min/1.73 m2) (>90) | 35.78 | 18.48 | 12.43 | 19.64 | 65.66 | 114.99 | 134.14 | |||||
| Na (mmol/L) (136–146) | 131 | 132 | 132 | 155 | 159 | 138 | ||||||
| K (mmol/L) (3.5–5.1) | 3.5 | 3.8 | 3 | 2.6 | 2.7 | 3.4 | 4.4 | |||||
| Ca (mg/dL) (8.4–10.2) | 8.2 | |||||||||||
| AST (mmol/L) (13–39) | 375 | 402 | 88 | 5.7 | 56 | 53 | ||||||
| ALT (U/L) (7–25) | 39 | 54 | 66 | 48 | 36 | 38 | 46 | |||||
| LDH (U/L) (140–270) | 314 | 476 | ||||||||||
| CK (U/L) (30–223) | 8047 | 14,947 | ||||||||||
| Bilirubin D/T (mg/dL) (0.03–0.18, 0.3–1.0) | 1.73/3.8 | 2.66/4.7 | 9.19/14.1 | 2.98/4.8 | 1.13/2.3 | 0.92/2.0 | 0.72/1.7 | |||||
| Glucose (mg/dL) (70–100) | 109 | 107 | 112 | |||||||||
| CRP (mg/dL) (<0.3) | 21.94 | 9.15 | 5.53 | |||||||||
| Albumin (g/dL) (3.5–5.7) | 2.8 | 3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.-H.; Chen, Y.-H.; Chen, C.-M. Fulminant Leptospirosis Presenting with Rapidly Developing Acute Renal Failure and Multiorgan Failure. Biomedicines 2024, 12, 435. https://doi.org/10.3390/biomedicines12020435
Liu Y-H, Chen Y-H, Chen C-M. Fulminant Leptospirosis Presenting with Rapidly Developing Acute Renal Failure and Multiorgan Failure. Biomedicines. 2024; 12(2):435. https://doi.org/10.3390/biomedicines12020435
Chicago/Turabian StyleLiu, Yu-Hsien, Yu-Hsuan Chen, and Chuan-Mu Chen. 2024. "Fulminant Leptospirosis Presenting with Rapidly Developing Acute Renal Failure and Multiorgan Failure" Biomedicines 12, no. 2: 435. https://doi.org/10.3390/biomedicines12020435
APA StyleLiu, Y.-H., Chen, Y.-H., & Chen, C.-M. (2024). Fulminant Leptospirosis Presenting with Rapidly Developing Acute Renal Failure and Multiorgan Failure. Biomedicines, 12(2), 435. https://doi.org/10.3390/biomedicines12020435






