Physical Health and Transition to Psychosis in People at Clinical High Risk
Abstract
1. Introduction
Aim of the Study
2. Materials and Methods
2.1. Design
2.2. Data Source
2.3. Study Population
2.4. Assessment Instruments
- The Fagerström Test for Nicotine Dependence (FTND) [58] is a standardised instrument consisting of six questions exploring daily cigarette consumption, compulsive use, and dependence. The score ranges from 0 to 10 (with higher scores indicating a most severe level of dependence on nicotine). More precisely, scores from 0 to 2 indicate a low level of dependence, from 3 to 4 low-moderate dependence, from 5 to 7 moderate dependence, and more than 8 a high level of dependence. For people that use other types of nicotine consumption other than cigarette smoking (e.g., e-cigarette, nicotine gum, or nicotine patches), we have investigated habits and reported information in adapted versions of FTND already used in previous literature (for instance, the equivalence of 10 vape nicotine puffs for a cigarette [59] or a re-worded test for gum users [60]).
- AUDIT (Alcohol Use Disorder Identification Test) [61] consists of 10 self-administered questions to investigate alcohol use disorder. When the AUDIT-C score, which includes core questions regarding alcohol units consumed and frequency of drinking, is equal to or above 5, it might indicate hazardous drinking. Regarding the AUDIT total score, a low level of risk is identified with an overall score between 0 and 7, and the range from 8 to 15 is the most appropriate for simple advice focused on the reduction of drinking. Higher scores (up to 19) suggest a need for brief counselling and continuous monitoring, while a complete diagnostic evaluation for alcoholic dependence is warranted for scores over 20.
- DINE (Dietary Instrument for Nutritional Education) [62] is a structured interview investigating dietary fibre and fat (unsaturated and saturated) intake. Scores for fibres and fats are rated into three different categories: low (under 30), medium (between 30 and 40), and high (more than 40). Scores for unsaturated fats are rated as low (less than 6), medium (6–9) and high (more than 9).
- IPAQ (International Physical Health Questionnaire) [63] rates the level of physical activity. This tool comprises three different categories of physical activity based on intensity (vigorous, moderate, and walking) and quantifies the amount of time spent sitting. Scores can also be expressed as a continuous variable with METs (estimating resting energy expenditure) [64].
2.5. Study Measures
- Sociodemographic parameters: age, sex, ethnicity.
- Physical health data:
- o
- Tobacco use: tobacco smoker status (yes/no), number of daily cigarettes, FTND score.
- o
- Alcohol use: alcohol drinker status (yes/no), AUDIT-C, and AUDIT total score.
- o
- Type of diet: DINE fibre score, DINE saturated fat score, DINE unsaturated fat score.
- o
- Physical activity: IPAQ vigorous, moderate, and walking activity (minutes per week), IPAQ time spent sitting (minutes per week), MET levels (continuous variable).
- o
- Physical parameters: BMI (body mass index), waist circumference in centimetres, heart rate in beats per minute (bpm), respiratory rate in acts per minute (apm), systolic and diastolic pressure in mmHg.
2.6. Statistical Analysis
3. Results
3.1. Sample Characteristics
3.2. Physical Health Data and Transition in the CHR-P Sample
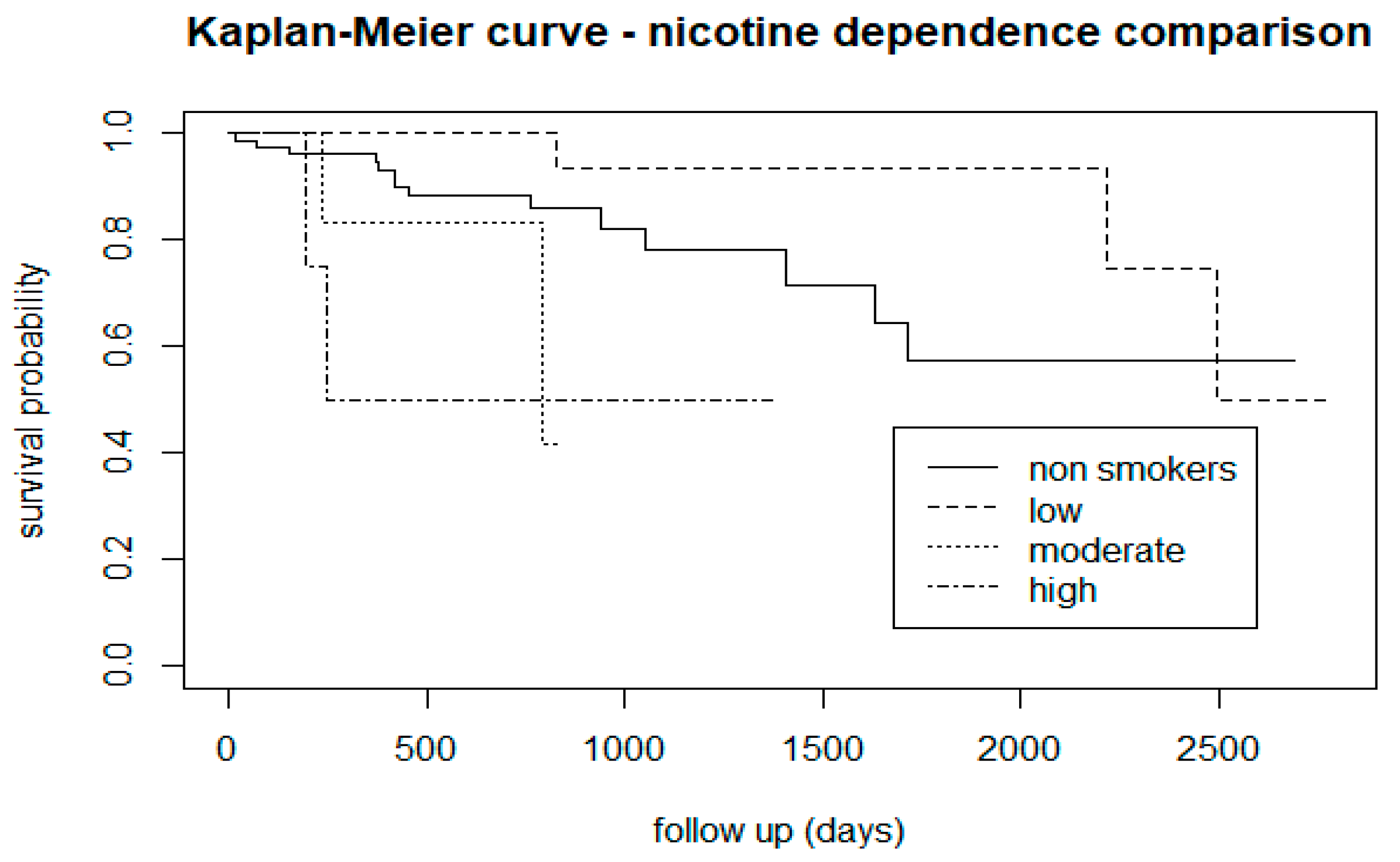
3.2.1. Tobacco Use
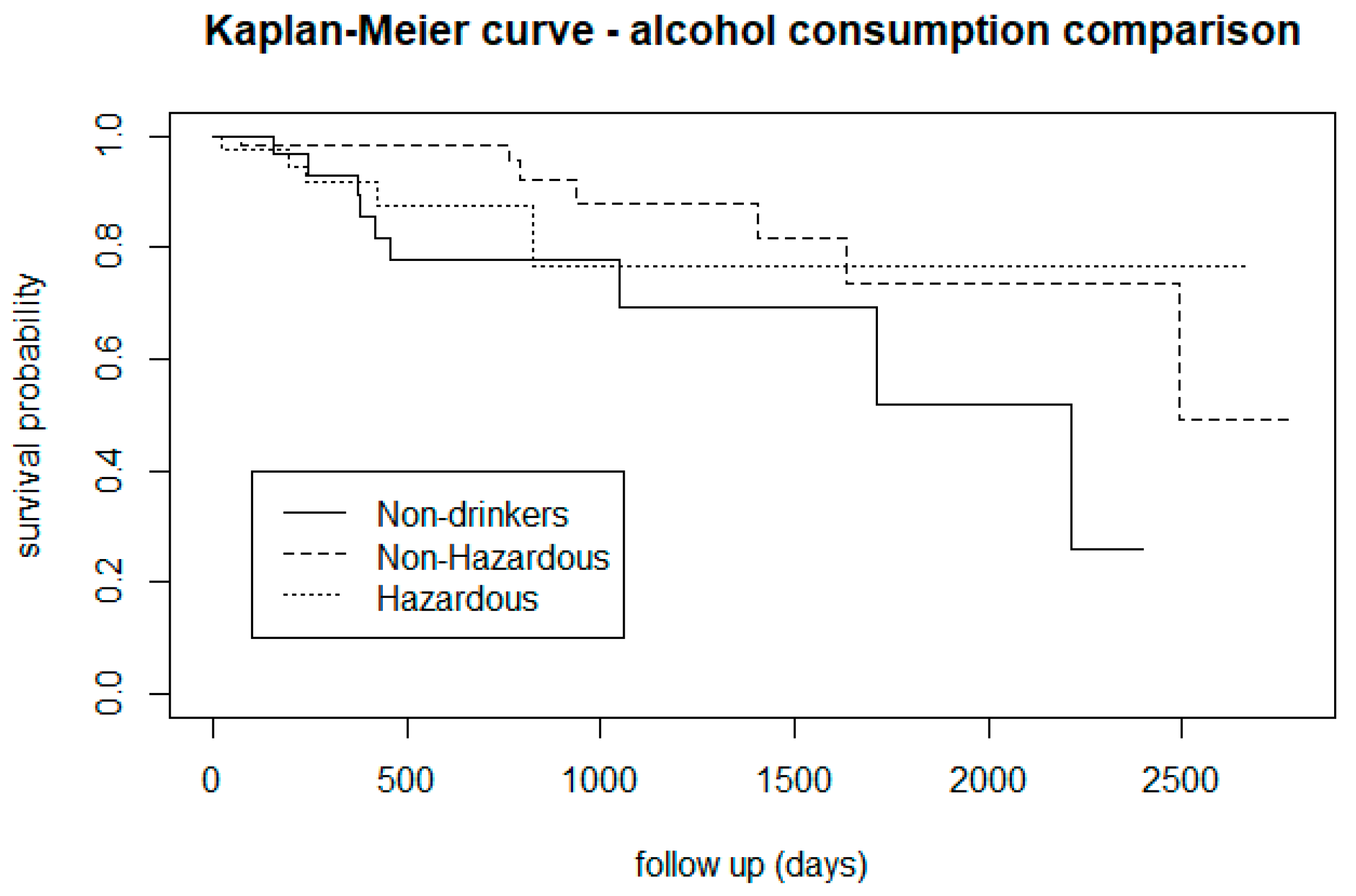
3.2.2. Alcohol Use
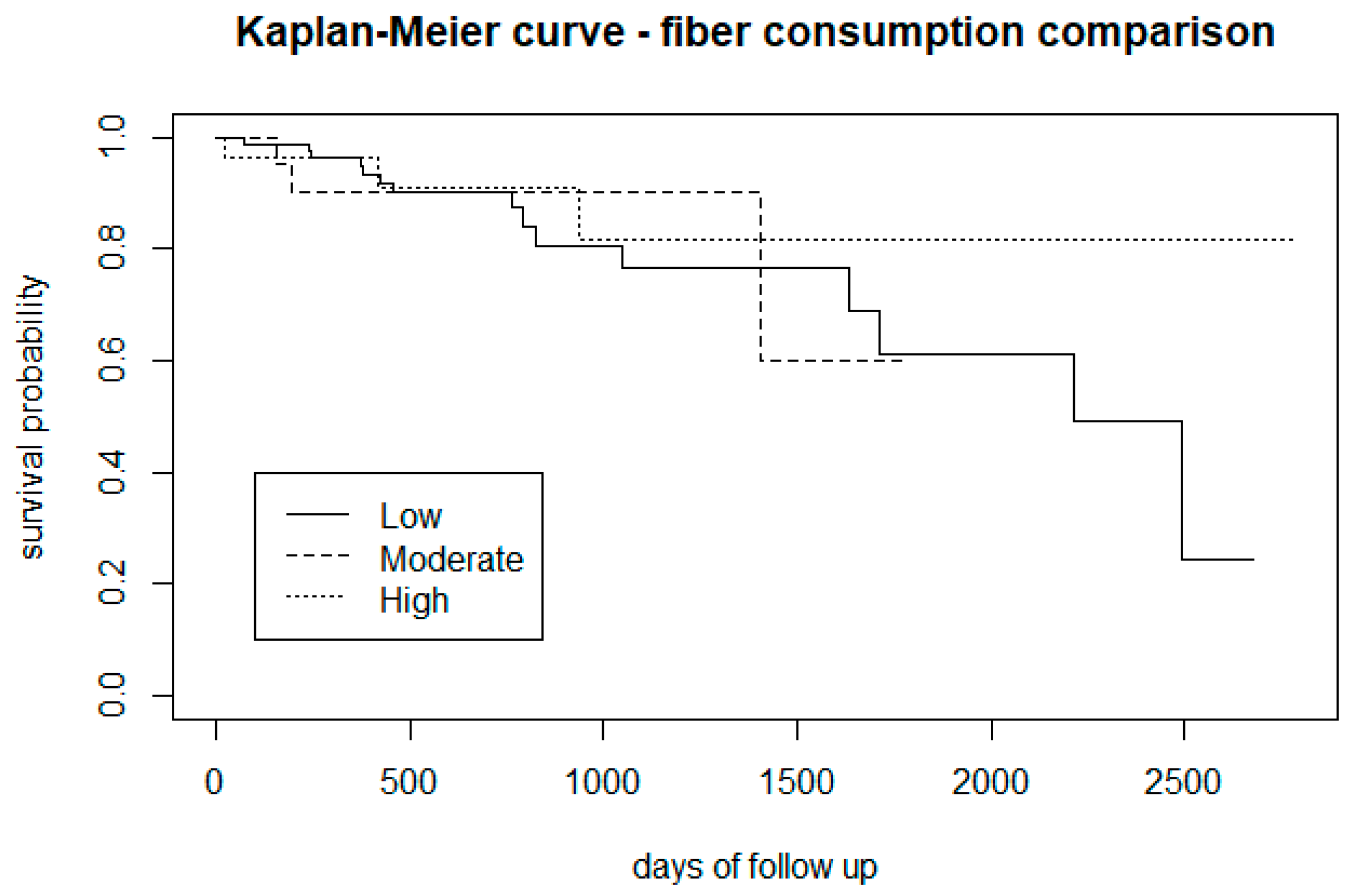
3.2.3. Type of Diet
3.2.4. Physical Activity
3.2.5. Physical Parameters
3.3. Log-Rank Tests
3.4. Cox Proportional Hazard Model
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fusar-Poli, P.; Borgwardt, S.; Bechdolf, A.; Addington, J.; Riecher-Rössler, A.; Schultze-Lutter, F.; Keshavan, M.; Wood, S.; Ruhrmann, S.; Seidman, L.J.; et al. The psychosis high-risk state: A comprehensive state-of-the-art review. JAMA Psychiatry 2013, 70, 107–120. [Google Scholar] [CrossRef] [PubMed]
- Yung, A.; McGorry, P. The prodromal phase of first-episode psychosis: Past and current conceptualizations. Schizophr. Bull. 1996, 22, 353–370. [Google Scholar] [CrossRef]
- Salazar de Pablo, G.; Radua, J.; Pereira, J.; Bonoldi, I.; Arienti, V.; Besana, F.; Soardo, L.; Cabras, A.; Fortea, L.; Catalan, A.; et al. Probability of Transition to Psychosis in Individuals at Clinical High Risk: An Updated Meta-analysis. JAMA Psychiatry 2021, 78, 970–978. [Google Scholar] [CrossRef]
- Yung, A.; Nelson, B.; Yuen, H.; Spiliotacopoulos, D.; Lin, A.; Simmons, M.; Bruxner, A.; Broussard, C.; Thompson, A.; McGorry, P. Long term outcome in an ultra high risk (“prodromal”) group. Schizophr. Bull. 2011, 37, 22–23. [Google Scholar] [CrossRef]
- Beck, K.; Studerus, E.; Andreou, C.; Egloff, L.; Leanza, L.; Simon, A.; Borgwardt, S.; Riecher-Rössler, A. Clinical and functional ultra-long-term outcome of patients with a clinical high risk (CHR) for psychosis. Eur. Psychiatry 2019, 62, 30–37. [Google Scholar] [CrossRef] [PubMed]
- Fusar-Poli, P.; Bechdolf, A.; Taylor, M.; Bonoldi, I.; Carpenter, W.; Yung, A.; McGuire, P. At risk for schizophrenic or affective psychoses? A meta-analysis of DSM/ICD diagnostic outcomes in individuals at high clinical risk. Schizophr. Bull. 2012, 39, 923–932. [Google Scholar] [CrossRef] [PubMed]
- Beck, K.; Andreou, C.; Studerus, E.; Heitz, U.; Ittig, S.; Leanza, L.; Riecher-Rössler, A. Clinical and functional long-term outcome of patients at clinical high risk (CHR) for psychosis without transition to psychosis: A systematic review. Schizophr. Res. 2019, 210, 39–47. [Google Scholar] [CrossRef]
- Yung, A.; Pan Yuen, H.; McGorry, P.; Phillips, L.; Kelly, D.; Dell’Olio, M.; Francey, S.; Cosgrave, E.; Killackey, E.; Stanford, C.; et al. Mapping the onset of psychosis: The Comprehensive Assessment of At-Risk Mental States. Aust. N. Z. J. Psychiatry 2005, 39, 964–971. [Google Scholar] [CrossRef]
- Rutigliano, G.; Valmaggia, L.; Landi, P.; Frascarelli, M.; Cappucciati, M.; Sear, V.; Rocchetti, M.; De Micheli, A.; Jones, C.; Palombini, E.; et al. Persistence or recurrence of non-psychotic comorbid mental disorders associated with 6-year poor functional outcomes in patients at ultra high risk for psychosis. J. Affect. Disord. 2016, 203, 101–110. [Google Scholar] [CrossRef]
- Haining, K.; Brunner, G.; Gajwani, R.; Gross, J.; Gumley, A.; Lawrie, S.; Schwannauer, M.; Schultze-Lutter, F.; Uhlhaas, P. The relationship between cognitive deficits and impaired short-term functional outcome in clinical high-risk for psychosis participants: A machine learning and modelling approach. Schizophr. Res. 2021, 231, 24–31. [Google Scholar] [CrossRef]
- Fusar-Poli, P.; Byrne, M.; Valmaggia, L.; Day, F.; Tabraham, P.; Johns, L.; McGuire, P. Social dysfunction predicts two years clinical outcome in people at ultra high risk for psychosis. J. Psychiatr. Res. 2010, 44, 294–301. [Google Scholar] [CrossRef]
- Velthorst, E.; Nieman, D.; Linszen, D.; Becker, H.; de Haan, L.; Dingemans, P.; Birchwood, M.; Patterson, P.; Salokangas, R.; Heinimaa, M.; et al. Disability in people clinically at high risk of psychosis. Br. J. Psychiatry 2010, 197, 278–284. [Google Scholar] [CrossRef]
- Fusar-Poli, P.; Tantardini, M.; De Simone, S.; Ramella-Cravaro, V.; Oliver, D.; Kingdon, J.; Kotlicka-Antczak, M.; Valmaggia, L.; Lee, J.; Millan, M.; et al. Deconstructing vulnerability for psychosis: Meta-analysis of environmental risk factors for psychosis in subjects at ultra high-risk. Eur. Psychiatry 2017, 40, 65–75. [Google Scholar] [CrossRef]
- Miller, T.; McGlashan, T.; Rosen, J.; Cadenhead, K.; Cannon, T.; Ventura, J.; McFarlane, W.; Perkins, D.; Pearlson, G.; Woods, S. Prodromal assessment with the structured interview for prodromal syndromes and the scale of prodromal symptoms: Predictive validity, interrater reliability, and training to reliability. Schizophr. Bull. 2003, 29, 703–715. [Google Scholar] [CrossRef] [PubMed]
- Hui, T.; Garvey, L.; Olasoji, M. Improving the physical health of young people with early psychosis with lifestyle interventions: Scoping review. Int. J. Ment. Health Nurs. 2021, 30, 1498–1524. [Google Scholar] [CrossRef]
- GBD 2016 Disease and Injury Incidence and Prevalence Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 328 diseased and injuries for 195 countries, 1990-2016 a systematic analysis for the Global Burden of Diseases Study 2016. Lancet 2017, 390, 1211–1259. [Google Scholar] [CrossRef]
- De Hert, M.; Cohen, D.; Bobes, J.; Cetkovich-Bakmas, M.; Leucht, S.; Ndetei, D.; Newcomer, J.; Uwakwe, R.; Asai, I.; Möller, H.; et al. Physical illness in patients with severe mental disorders. II. Barriers to care, monitoring and treatment guidelines, plus recommendations at the system and individual level. World Psychiatry 2011, 10, 138–151. [Google Scholar] [CrossRef] [PubMed]
- Wahlbeck, K.; Westman, J.; Nordentoft, M.; Gissier, M.; Laursen Munk, T. Outcomes of Nordic mental health systems: Life expectancy of patient with mental disorders. Br. J. Psychiatry 2011, 199, 453–458. [Google Scholar] [CrossRef] [PubMed]
- Saha, S.; Chant, D.; McGrath, J. A systematic review of mortality in schizophrenia: Is the differential mortality gap worsening over time? Arch. Gen. Psychiatry 2007, 64, 1123–1131. [Google Scholar] [CrossRef]
- Hjorthøj, C.; Stürup, A.; McGrath, J.; Nordentoft, M. Years of potential life lost and life expectancy in schizophrenia: A systematic review and meta-analysis. Lancet Psychiatry 2017, 4, 295–301. [Google Scholar] [CrossRef]
- Jayatilleke, N.; Hayes, R.; Dutta, R.; Shetty, H.; Hotopf, M.; Chang, C.; Stewart, R. Contributions of specific causes of death to lost life expectancy in severe mental illness. Eur. Psychiatry 2017, 43, 109–115. [Google Scholar] [CrossRef] [PubMed]
- Cordes, J.; Bechdolf, A.; Engelke, C.; Kahl, K.; Balijepalli, C.; Lösch, C.; Klosterkötter, J.; Wagner, M.; Maier, W.; Heinz, A.; et al. Prevalence of metabolic syndrome in female and male patients at risk of psychosis. Schizophr. Res. 2017, 181, 38–42. [Google Scholar] [CrossRef] [PubMed]
- De Micheli, A.; Provenzani, U.; Solmi, M.; Van Pabst, A.; Youssef, E.; McGuire, P.; Fusar-Poli, P. Prevalence of tobacco smoking in people at clinical high-risk for psychosis: Systematic review and meta-analysis. Schizophr. Res. 2023, 254, 109–115. [Google Scholar] [CrossRef] [PubMed]
- Provenzani, U.; De Micheli, A.; Damiani, S.; Oliver, D.; Brondino, N.; Fusar-Poli, P. Physical Health in Clinical High Risk for Psychosis Individuals: A Cross-Sectional Study. Brain Sci. 2023, 13, 128. [Google Scholar] [CrossRef] [PubMed]
- Carney, R.; Cotter, J.; Bradshaw, T.; Firth, J.; Yung, A. Cardiometabolic risk factors in young people at ultra-high risk for psychosis: A systematic review and meta-analysis. Schizophr. Res. 2016, 170, 290–300. [Google Scholar] [CrossRef]
- Newberry, R.; Dean, D.; Sayyah, M.; Mittal, V. What prevents youth at clinical high risk for psychosis from engaging in physical activity? An examination of the barriers to physical activity. Schizophr. Res. 2018, 201, 400–405. [Google Scholar] [CrossRef]
- Mittal, V.; Gupta, T.; Orr, J.; Pelletier-Baldelli, A.; Dean, D.; Lunsford-Avery, J.; Smith, A.; Robustelli, B.; Leopold, D.; Millman, Z.J. Physical activity level and medial temporal health in youth at ultra high-risk for psychosis. J. Abnorm. Psychol. 2013, 122, 1101–1110. [Google Scholar] [CrossRef]
- Colomer, L.; Anmella, G.; Vieta, E.; Grande, I. Physical health in affective disorders: A narrative review of the literature. Braz. J. Psychiatry 2021, 43, 621–630. [Google Scholar] [CrossRef] [PubMed]
- Tsiachristas, A.; Thomas, T.; Leal, J.; Lennox, B. Economic impact of early intervention in psychosis services: Results from a longitudinal retrospective controlled study in England. BMJ Open 2016, 6, e012611. [Google Scholar] [CrossRef] [PubMed]
- Smith, D.; Langan, J.; McLean, G.; Guthrie, B.; Mercer, S. Schizophrenia is associated with excess multiple physical-health comorbidities but low levels of recorded cardiovascular disease in primary care: Cross-sectional study. BMJ Open 2013, 3, e002808. [Google Scholar] [CrossRef]
- Damiani, S.; Rutigliano, G.; Fazia, T.; Merlino, S.; Berzuini, C.; Bernardinelli, L.; Politi, P.; Fusar-Poli, P. Developing and validating an individualized clinical prediction model to forecast psychotic recurrence in acute and transient psychotic disorders: Electronic health record cohort study. Schizophr. Bull. 2021, 47, 1695–1705. [Google Scholar] [CrossRef]
- Gage, S.; Jones, H.; Taylor, A.; Burgess, S.; Zammit, S.; Munafò, M. Investigating causality in associations between smoking initiation and schizophrenia using Mendelian randomization. Sci. Rep. 2017, 7, 40653. [Google Scholar] [CrossRef]
- Gurillo, P.; Jauhar, S.; Murray, R.; MacCabe, J. Does tobacco use cause psychosis? Systematic review and meta-analysis. Lancet Psychiatry 2015, 2, 718–725. [Google Scholar] [CrossRef]
- Cannon, T.; Cadenhead, K.; Cornblatt, B.; Woods, S.; Addington, J.; Walker, E.; Seidman, L.; Perkins, D.; Tsuang, M.; McGlashan, T.; et al. Prediction of psychosis in youth at high clinical risk: A multisite longitudinal study in North America. Arch. Gen. Psychiatry 2008, 65, 28–37. [Google Scholar] [CrossRef]
- Sormunen, E.; Saarinen, M.; Salokangas, R.; Telama, R.; Hutri-Kähönen, N.; Tammelin, T.; Viikari, J.; Raitakari, O.; Hietala, J. Effects of childhood and adolescence physical activity patterns on psychosis risk-a general population cohort study. NPJ Schizophr. 2017, 3, 5. [Google Scholar] [CrossRef]
- Pawełczyk, T.; Trafalska, E.; Kotlicka-Antczak, M.; Pawełczyk, A. The association between polyunsaturated fatty acid consumption and the transition to psychosis in ultra-high risk individuals. Prostaglandins Leukot. Essent. Fat. Acids 2016, 108, 30–37. [Google Scholar] [CrossRef]
- Buchy, L.; Perkins, D.; Woods, S.; Liu, L.; Addington, J. Impact of substance use on conversion to psychosis in youth at clinical high risk of psychosis. Schizophr. Res. 2014, 156, 277–280. [Google Scholar] [CrossRef]
- Amir, C.; Kapler, S.; Hoftman, G.; Kushan, L.; Zinberg, J.; Cadenhead, K.; Kennedy, L.; Cornblatt, B.; Keshavan, M.; Mathalon, D.; et al. Neurobehavioral risk factors influence prevalence and severity of hazardous substance use in youth at genetic and clinical high risk for psychosis. Front. Psychiatry 2023, 14, 1143315. [Google Scholar] [CrossRef]
- Abarca, M.; Pizarro, H.; Nuñez, R.; Arancibia, M. Physical exercise as an intervention in people at clinical high-risk for psychosis: A narrative review. Medvawe 2023, 23, e2724. [Google Scholar] [CrossRef]
- Carney, R.; Bradshaw, T.; Yung, A. Monitoring of physical health in services for young people at ultra-high risk of psychosis. Early Interv. Psychiatry 2018, 12, 153–159. [Google Scholar] [CrossRef]
- Thornicroft, G. Physical health disparities and mental illness: The scandal of premature mortality. Br. J. Psychiatry 2011, 199, 441–442. [Google Scholar] [CrossRef]
- Damme, K.; Gupta, T.; Ristanovic, I.; Kimhy, D.; Bryan, A.; Mittal, V. Exercise intervention in individuals at clinical high risk for psychosis: Benefits to fitness, symptoms, hippocampal volumes, and functional connectivity. Schizophr. Bull. 2022, 48, 1394–1405. [Google Scholar] [CrossRef]
- NHS UK: NHS Five Year Forward View (Page Last Reviewed: 8th March 2019). Available online: https://www.england.nhs.uk/five-year-forward-view/ (accessed on 20 July 2023).
- Lederman, O.; Rosenbaum, S.; Maloney, C.; Curtis, J.; Ward, P. Modifiable cardiometabolic risk factors in youth with at-risk mental states: A cross-sectional pilot study. Psychiatry Res. 2017, 257, 424–430. [Google Scholar] [CrossRef]
- Bhavsar, V.; Jauhar, S.; Murray, R.; Hotopf, M.; Hatch, S.; McNeill, A.; Boydell, J.; MacCabe, J. Tobacco smoking is associated with psychotic experiences in the general population of South London. Psychol. Med. 2018, 48, 123–131. [Google Scholar] [CrossRef]
- Benchimol, E.; Smeeth, L.; Guttmann, A.; Harron, K.; Moher, D.; Petersen, I.; Sørensen, H.T.; von Elm, E.; Langan, S. The REporting of studies Conducted using Observational Routinely-collected health Data (RECORD) statement. PLoS Med. 2015, 12, e1001885. [Google Scholar] [CrossRef]
- Perera, G.; Broadbent, M.; Callard, F.; Chang, C.; Downs, J.; Dutta, R.; Fernandes, A.; Hayes, R.; Henderson, M.; Jackson, R.; et al. Cohort profile of the South London and Maudsley NHS Foundation Trust Biomedical Research Centre (SLaM BRC) Case Register: Current status and recent enhancement of an Electronic Mental Health Record-derived data resource. BMJ Open 2016, 6, e008721. [Google Scholar] [CrossRef]
- Jongsma, H.; Gayer-Anderson, C.; Lasalvia, A.; Quattrone, D.; Mulè, A.; Szöke, A.; Selten, J.; Turner, C.; Arango, C.; Tarricone, I.; et al. Treated incidence of psychotic disorders in the multinational EU-GEI Study. JAMA Psychiatry 2018, 75, 36–46. [Google Scholar] [CrossRef]
- Stewart, R.; Soremekun, M.; Perera, G.; Broadbent, M.; Callard, F.; Denis, M.; Hotopf, M.; Thornicroft, G.; Lovestone, S. The South London and Maudsley NHS Foundation Trust Biomedical Research Centre (SLAM BRC) case register: Development and descriptive data. BMC Psychiatry 2009, 9, 51. [Google Scholar] [CrossRef]
- Fusar-Poli, P.; Spencer, T.; De Micheli, A.; Curzi, V.; Nandha, S.; McGuire, P. Outreach and support in South-London (OASIS) 2001–2020: Twenty years of early detection, prognosis and preventive care for young people at risk of psychosis. Eur. Neuropsychopharmacol. 2020, 39, 111–122. [Google Scholar] [CrossRef]
- Fusar-Poli, P.; Byrne, M.; Badger, S.; Valmaggia, L.; McGuire, P. Outreach and support in south London (OASIS), 2001–2011: Ten years of early diagnosis and treatment for young individuals at high clinical risk for psychosis. Eur. Psychiatry 2013, 28, 315–326. [Google Scholar] [CrossRef]
- Fusar-Poli, P.; Estradé, A.; Spencer, T.; Gupta, S.; Murguia-Asensio, S.; Eranti, S.; Wilding, K.; Andlauer, O.; Buhagiar, J.; Smith, M.; et al. Pan-London Network for Psychosis-Prevention (PNP). Front. Psychiatry 2019, 10, 707. [Google Scholar] [CrossRef] [PubMed]
- Fusar-Poli, P.; De Micheli, A.; Signorini, L.; Baldwin, H.; Salazar de Pablo, G.; McGuire, P. Real-world long-term outcomes in individuals at clinical risk for psychosis: The case for extending duration of care. EClinicalMedicine 2020, 28, 100578. [Google Scholar] [CrossRef]
- Fusar-Poli, P.; Schultze-Lutter, F. Predicting the onset of psychosis in patients at clinical high risk: Practical guide to probabilistic prognostic reasoning. Evid. Based Ment. Health 2016, 19, 10–15. [Google Scholar] [CrossRef]
- Fusar-Poli, P.; Palombini, E.; Davies, C.; Oliver, D.; Bonoldi, I.; Ramella-Cravaro, V.; McGuire, P. Why transition risk to psychosis is not declining at the OASIS ultra high risk service: The hidden role of stable pretest risk enrichment. Schizophr. Res. 2018, 192, 385–390. [Google Scholar] [CrossRef]
- Fusar-Poli, P.; Schultze-Lutter, F.; Cappucciati, M.; Rutigliano, G.; Bonoldi, I.; Stahl, D.; Borgwardt, S.; Riecher-Rössler, A.; Addington, J.; Perkins, D.; et al. The dark side of the moon: Meta-analytical impact of recruitment strategies on risk enrichment in the clinical high risk state for psychosis. Schizophr. Bull. 2016, 42, 732–743. [Google Scholar] [CrossRef]
- NICE. Surveillance Report 2017—Psychosis and Schizophrenia in Adults: Prevention and Management (2014) NICE Guideline CG178; National Institute for Health and Care Excellence (NICE): London, UK, 2017; Available online: https://www.nice.org.uk/guidance/cg178/evidence/full-guideline-490503565/ (accessed on 27 December 2023).
- Heatherton, T.; Kozlowski, L.; Frecker, R.; Fagerström, K. The Fagerström test for nicotine dependence: A revision of the Fagerström tolerance questionnaire. Br. J. Addict. 1991, 86, 1119–1127. [Google Scholar] [CrossRef]
- Etter, J.; Eissenberg, T. Dependence levels in users of electronic cigarettes, nicotine gums and tobacco cigarettes. Drug Alcohol Depend. 2015, 147, 68–75. [Google Scholar] [CrossRef]
- Etter, J. Dependence on the nicotine gum in former smokers. Addict. Behav. 2009, 34, 246–251. [Google Scholar] [CrossRef]
- Saunders, J.; Aasland, O.; Babor, T.; de la Fuente, J.; Grant, M. Development of the Alcohol Use Disorders Identification Test (AUDIT): WHO Collaborative project on early detection of persons with harmful alcohol consumption—II. Addiction 1993, 88, 791–804. [Google Scholar] [CrossRef] [PubMed]
- Little, P.; Barnett, J.; Margetts, B.; Kinmonth, A.; Gabbay, J.; Thompson, R.; Warm, D.; Warwick, H.; Wooton, S. The validity of dietary assessment in general practice. J. Epidemiol. Community Health 1999, 53, 165–172. [Google Scholar] [CrossRef]
- Hagströmer, M.; Oja, P.; Sjöström, M. The International Physical Activity Questionnaire (IPAQ): A study of concurrent and construct validity. Public Health Nutr. 2006, 9, 755–762. [Google Scholar] [CrossRef]
- Craig, C.; Marshall, A.; Sjöström, M.; Bauman, A.; Booth, M.; Ainsworth, B.; Pratt, M.; Ekelund, U.; Yngve, A.; Sallis, J.; et al. International physical activity questionnaire: 12-country reliability and validity. Med. Sci. Sports Exerc. 2003, 35, 1381–1395. [Google Scholar] [CrossRef]
- Kaplan, E.; Meier, P. Nonparametric Estimation from Incomplete Observations. J. Am. Stat. Assoc. 1958, 53, 457–481. [Google Scholar] [CrossRef]
- Mantel, N. Evaluation of survival data and two new rank order statistics arising in its consideration. Cancer Chemother. Rep. 1966, 50, 163–170. [Google Scholar]
- Cox, D. Regression models and life tables. J. R. Stat. Soc. Ser. B 1972, 34, 187–220. [Google Scholar] [CrossRef]
- Harrell, F. Regression modeling strategies. Bios 2017, 330, 14. Available online: https://www.google.com/url?sa=t&rct=j&q=&esrc=s&source=web&cd=&ved=2ahUKEwj1_5zsuq6DAxWg8rsIHbexCYYQFnoECBEQAQ&url=https%3A%2F%2Fwww.researchgate.net%2Fprofile%2FDavid-Booth-7%2Fpost%2FWhat_model_may_i_use_instead_of_multiple_regression_model%2Fattachment%2F6380dd2a97e2867d5070450f%2FAS%253A11431281100713611%25401669389609596%2Fdownload%2FcourseHarrell.pdf&usg=AOvVaw1bAPyF3Kf1cHhxGClsRJX6&opi=89978449/ (accessed on 27 December 2023).
- Benjamini, Y.; Hochberg, Y. Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. J. R. Stat. Soc. Ser. B 1995, 57, 289–300. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing. In R Foundation for Statistical Computing; R Core Team: Vienna, Austria, 2023; Available online: http://www.R-project.org/ (accessed on 29 August 2023).
- Parikh, V.; Kutlu, M.; Gould, T. nAChR dysfunction as a common substrate for schizophrenia and comorbid nicotine addiction: Current trends and perspectives. Schizophr. Res. 2016, 171, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Di Forti, M.; Quattrone, D.; Freeman, T.; Tripoli, G.; Gayer-Anderson, C.; Quigley, H.; Rodriguez, V.; Jongsma, H.; Ferraro, L.; La Cascia, C.; et al. The contribution of cannabis use to variation in the incidence of psychotic disorder across Europe (EU-GEI): A multicentre case-control study. Lancet Psychiatry 2019, 6, 427–436. [Google Scholar] [CrossRef] [PubMed]
- Marconi, A.; Di Forti, M.; Lewis, C.; Murray, R.; Vassos, E. Meta-analysis of the Association Between the Level of Cannabis Use and Risk of Psychosis. Schizophr. Bull. 2016, 42, 1262–1269. [Google Scholar] [CrossRef]
- MacCabe, J. It is time to start taking tobacco seriously as a risk factor for psychosis: Self-medication cannot explain the association. Acta Psychiatr. Scand. 2018, 138, 3–4. [Google Scholar] [CrossRef] [PubMed]
- van der Heijden, H.; Schirmbeck, F.; McGuire, P.; Valmaggia, L.; Kempton, M.; van der Gaag, M.; Nelson, B.; Riecher-Rössler, A.; Bressan, R.; Barrantes-Vidal, N.; et al. Association between tobacco use and symptomatology in individuals at ultra-high risk to develop a psychosis: A longitudinal study. Schizophr. Res. 2021, 236, 48–53. [Google Scholar] [CrossRef] [PubMed]
- Ward, H.; Lawson, M.; Addington, J.; Bearden, C.; Cadenhead, K.; Cannon, T.; Cornblatt, B.; Jeffries, C.; Mathalon, D.; McGlashan, T.; et al. Tobacco use and psychosis risk in persons at clinical high risk. Early Interv. Psychiatry 2019, 13, 1173–1181. [Google Scholar] [CrossRef]
- Buchy, L.; Cannon, T.; Anticevic, A.; Lyngberg, K.; Cadenhead, K.; Cornblatt, B.; McGlashan, T.; Perkins, D.; Seidman, L.; Tsuang, M.; et al. Evaluating the impact of cannabis use on thalamic connectivity in youth at clinical high risk of psychosis. BMC Psychiatry 2015, 15, 276. [Google Scholar] [CrossRef] [PubMed]
- Kristensen, K.; Cadenhead, K. Cannabis abuse and risk for psychosis in a prodromal sample. Psychiatry Res. 2007, 151, 151–154. [Google Scholar] [CrossRef] [PubMed]
- Buchy, L.; Cadenhead, K.; Cannon, T.; Cornblatt, B.; McGlashan, T.; Perkins, D.; Seidman, L.; Tsuang, M.; Walker, E.; Woods, S.; et al. Substance use in individuals at clinical high risk of psychosis. Psychol. Med. 2015, 45, 2275–2284. [Google Scholar] [CrossRef] [PubMed]
- Thornton, L.; Baker, A.; Johnson, M.; Kay-Lambkin, F.; Lewin, T. Reasons for substance use among people with psychotic disorders: Method triangulation approach. Psychol. Addict. Behav. 2012, 26, 279–288. [Google Scholar] [CrossRef]
- Auther, A.; Cadenhead, K.; Carrión, R.; Addington, J.; Bearden, C.; Cannon, T.; McGlashan, T.; Perkins, D.; Seidman, L.; Tsuang, M.; et al. Alcohol confounds relationship between cannabis misuse and psychosis conversion in a high-risk sample. Acta Psychiatr. Scand. 2015, 132, 60–68. [Google Scholar] [CrossRef]
- Teasdale, S.; Ward, P.; Samaras, K.; Firth, J.; Stubbs, B.; Tripodi, E.; Burrows, T. Dietary intake of people with severe mental illness: Systematic review and meta-analysis. Br. J. Psychiatry 2019, 214, 251–259. [Google Scholar] [CrossRef]
- Aucoin, M.; LaChance, L.; Cooley, K.; Kidd, S. Diet and Psychosis: A Scoping Review. Neuropsychobiology 2020, 79, 20–42. [Google Scholar] [CrossRef]
- Manzanares, N.; Monseny, R.; Ortega, L.; Montalvo, I.; Franch, J.; Gutiérrez-Zotes, A.; Reynolds, R.; Walker, B.; Vilella, E.; Labad, J. Unhealthy lifestyle in early psychoses: The role of life stress and the hypothalamic-pituitary-adrenal axis. Psychoneuroendocrinology 2014, 39, 1–10. [Google Scholar] [CrossRef]
- Labad, J.; Stojanovic-Pérez, A.; Montalvo, I.; Solé, M.; Cabezas, Á.; Ortega, L.; Moreno, I.; Vilella, E.; Martorell, L.; Reynolds, R.; et al. Stress biomarkers as predictors of transition to psychosis in at-risk mental states: Roles for cortisol, prolactin and albumin. J. Psychiatr. Res. 2015, 60, 163–169. [Google Scholar] [CrossRef]
- Cadenhead, K.; Minichino, A.; Kelsven, S.; Addington, J.; Bearden, C.; Cannon, T.; Cornblatt, B.; Mathalon, D.; McGlashan, T.; Perkins, D.; et al. Metabolic abnormalities and low dietary Omega 3 are associated with symptom severity and worse functioning prior to the onset of psychosis: Findings from the North American Prodrome Longitudinal Studies Consortium. Schizophr. Res. 2019, 204, 96–103. [Google Scholar] [CrossRef] [PubMed]
- Susai, S.; Sabherwal, S.; Mongan, D.; Föcking, M.; Cotter, D. Omega-3 fatty acid in ultra-high-risk psychosis: A systematic review based on functional outcome. Early Interv. Psychiatry 2022, 16, 3–16. [Google Scholar] [CrossRef] [PubMed]
- McGorry, P.; Nelson, B.; Markulev, C.; Yuen, H.; Schäfer, M.; Mossaheb, N.; Schlögelhofer, M.; Smesny, S.; Hickie, I.; Berger, G.; et al. Effect of ω-3 polyunsaturated fatty acids in young people at ultrahigh risk for psychotic disorders: The NEURAPRO randomized clinical trial. JAMA Psychiatry 2017, 74, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Amminger, G.; Mechelli, A.; Rice, S.; Kim, S.; Klier, C.; McNamara, R.; Berk, M.; McGorry, P.; Schäfer, M. Predictors of treatment response in young people at ultra-high risk for psychosis who received long-chain omega-3 fatty acids. Transl. Psychiatry 2015, 5, e495. [Google Scholar] [CrossRef]
- Amminger, G.; Schäfer, M.; Papageorgiou, K.; Klier, C.; Cotton, S.; Harrigan, S.; Mackinnon, A.; McGorry, P.; Berger, G. Long-chain omega-3 fatty acids for indicated prevention of psychotic disorders: A randomized, placebo-controlled trial. Arch. Gen. Psychiatry 2010, 67, 146–154. [Google Scholar] [CrossRef]
- Amminger, G.; Nelson, B.; Markulev, C.; Yuen, H.; Schäfer, M.; Berger, M.; Mossaheb, N.; Schlögelhofer, M.; Smesny, S.; Hickie, I.; et al. The NEURAPRO Biomarker Analysis: Long-Chain Omega-3 Fatty Acids Improve 6-Month and 12-Month Outcomes in Youths at Ultra-High Risk for Psychosis. Biol. Psychiatry 2020, 87, 243–252. [Google Scholar] [CrossRef]
- Teasdale, S.; Moerkl, S.; Moetteli, S.; Mueller-Stierlin, A. The Development of a Nutrition Screening Tool for Mental Health Settings Prone to Obesity and Cardiometabolic Complications: Study Protocol for the NutriMental Screener. Int. J. Environ. Res. Public Health 2021, 18, 11269. [Google Scholar] [CrossRef]
- Hancox, L.; Lee, P.; Armaghanian, N.; Hirani, V.; Wakefield, G. Nutrition risk screening methods for adults living with severe mental illness: A scoping review. Nutr. Diet. 2022, 79, 349–363. [Google Scholar] [CrossRef]
- Firth, J.; Stubbs, B.; Vancampfort, D.; Schuch, F.; Rosenbaum, S.; Ward, P.; Firth, J.A.; Sarris, J.; Yung, A. The validity and value of self-reported physical activity and accelerometry in people with schizophrenia: A population-scale study of the UK Biobank. Schizophr. Bull. 2018, 44, 1293–1300. [Google Scholar] [CrossRef]
- Vancampfort, D.; Firth, J.; Schuch, F.; Rosenbaum, S.; Mugisha, J.; Hallgren, M.; Probst, M.; Ward, P.; Gaughran, F.; De Hert, M.; et al. Sedentary behavior and physical activity levels in people with schizophrenia, bipolar disorder and major depressive disorder: A global systematic review and meta-analysis. World Psychiatry 2017, 16, 308–315. [Google Scholar] [CrossRef]
- Stubbs, B.; Firth, J.; Berry, A.; Schuch, F.; Rosenbaum, S.; Gaughran, F.; Veronesse, N.; Williams, J.; Craig, T.; Yung, A.; et al. How much physical activity do people with schizophrenia engage in? A systematic review, comparative meta-analysis and meta-regression. Schizophr. Res. 2016, 176, 431–440. [Google Scholar] [CrossRef]
- Correll, C.; Solmi, M.; Veronese, N.; Bortolato, B.; Rosson, S.; Santonastaso, P.; Thapa-Chhetri, N.; Fornaro, M.; Gallicchio, D.; Collantoni, E.; et al. Prevalence, incidence and mortality from cardiovascular disease in patients with pooled and specific severe mental illness: A large-scale meta-analysis of 3,211,768 patients and 113,383,368 controls. World Psychiatry 2017, 16, 163–180. [Google Scholar] [CrossRef]
- Vancampfort, D.; Correll, C.; Galling, B.; Probst, M.; De Hert, M.; Ward, P.; Rosenbaum, S.; Gaughran, F.; Lally, J.; Stubbs, B. Diabetes mellitus in people with schizophrenia, bipolar disorder and major depressive disorder: A systematic review and large scale meta-analysis. World Psychiatry 2016, 15, 166–174. [Google Scholar] [CrossRef]
- Vancampfort, D.; Stubbs, B.; Mitchell, A.; De Hert, M.; Wampers, M.; Ward, P.; Rosenbaum, S.; Correll, C. Risk of metabolic syndrome and its components in people with schizophrenia and related psychotic disorders, bipolar disorder and major depressive disorder: A systematic review and meta-analysis. World Psychiatry 2015, 14, 339–347. [Google Scholar] [CrossRef]
- NHS UK: Physical Activity Guidelines for Adults Aged 19 to 64 (Page Last Reviewed: 4th August 2021). Available online: https://www.nhs.uk/live-well/exercise/exercise-guidelines/physical-activity-guidelines-for-adults-aged-19-to-64/ (accessed on 20 July 2023).
- Brokmeier, L.; Firth, J.; Vancampfort, D.; Smith, L.; Deenik, J.; Rosenbaum, S.; Stubbs, B.; Schuch, F. Does physical activity reduce the risk of psychosis? A systematic review and meta-analysis of prospective studies. Psychiatry Res. 2020, 284, 112675. [Google Scholar] [CrossRef] [PubMed]
- Koivukangas, J.; Tammelin, T.; Kaakinen, M.; Mäki, P.; Moilanen, I.; Taanila, A.; Veijola, J. Physical activity and fitness in adolescents at risk for psychosis within the Northern Finland 1986 Birth Cohort. Schizophr. Res. 2010, 116, 152–158. [Google Scholar] [CrossRef] [PubMed]
- Keskinen, E.; Marttila, R.; Koivumaa-Honkanen, H.; Moilanen, K.; Keinänen-Kiukaanniemi, S.; Timonen, M.; Isohanni, M.; McGrath, J.; Miettunen, J.; Jääskeläinen, E. Search for protective factors for psychosis—A population-based sample with special interest in unaffected individuals with parental psychosis. Early Interv. Psychiatry 2018, 12, 869–878. [Google Scholar] [CrossRef]
- Firth, J.; Siddiqi, N.; Koyanagi, A.; Siskind, D.; Rosenbaum, S.; Galletly, C.; Allan, S.; Caneo, C.; Carney, R.; Carvalho, A.; et al. The Lancet Psychiatry Commission: A blueprint for protecting physical health in people with mental illness. Lancet Psychiatry 2019, 6, 675–712. [Google Scholar] [CrossRef] [PubMed]
- Damme, K.; Sloan, R.; Bartels, M.; Ozsan, A.; Ospina, L.; Kimhy, D.; Mittal, V. Psychosis risk individuals show poor fitness and discrepancies with objective and subjective measures. Sci. Rep. 2021, 11, 9851. [Google Scholar] [CrossRef]
- Firth, J.; Stubbs, B.; Vancampfort, D.; Schuch, F.; Lagopoulos, J.; Rosenbaum, S.; Ward, P. Effect of aerobic exercise on hippocampal volume in humans: A systematic review and meta-analysis. NeuroImage 2018, 166, 230–238. [Google Scholar] [CrossRef] [PubMed]
- Dean, D.; Bryan, A.; Newberry, R.; Gupta, T.; Carol, E.; Mittal, V. A supervised exercise intervention for youth at risk for psychosis: An open-label pilot study. J. Clin. Psychiatry 2017, 78, e1167–e1173. [Google Scholar] [CrossRef] [PubMed]
- Lederman, O.; Ward, P.; Rosenbaum, S.; Maloney, C.; Watkins, A.; Teasdale, S.; Morell, R.; Curtis, J. Stepping up early treatment for help-seeking youth with at-risk mental states: Feasibility and acceptability of a real-world exercise program. Early Interv. Psychiatry 2020, 14, 450–462. [Google Scholar] [CrossRef]
- Sormunen, E.; Saarinen, M.; Salokangas, R.; Hutri-Kähönen, N.; Viikari, J.; Raitakari, O.; Hietala, J. Body mass index trajectories in childhood and adolescence—Risk for non-affective psychosis. Schizophr. Res. 2019, 206, 313–317. [Google Scholar] [CrossRef]
- Abel, K.; Wicks, S.; Susser, E.; Dalman, C.; Pedersen, M.; Mortensen, P.; Webb, R. Birth weight, schizophrenia, and adult mental disorder: Is risk confined to the smallest babies? Arch. Gen. Psychiatry 2010, 67, 923–930. [Google Scholar] [CrossRef] [PubMed]
- Zammit, S.; Rasmussen, F.; Farahmand, B.; Gunnell, D.; Lewis, G.; Tynelius, P.; Brobert, G. Height and body mass index in young adulthood and risk of schizophrenia: A longitudinal study of 1 347 520 Swedish men. Acta Psychiatr. Scand. 2007, 116, 378–385. [Google Scholar] [CrossRef] [PubMed]
- Caravaggio, F.; Brucato, G.; Kegeles, L.; Lehembre-Shiah, E.; Arndt, L.; Colibazzi, T.; Girgis, R. Exploring the relationship between body mass index and positive symptom severity in persons at clinical high risk for psychosis. J. Nerv. Ment. Dis. 2017, 205, 893–895. [Google Scholar] [CrossRef]
- Lamichhane, S.; Dickens, A.; Sen, P.; Laurikainen, H.; Borgan, F.; Suvisaari, J.; Hyötyläinen, T.; Howes, O.; Hietala, J.; Orešič, M. Association between circulating lipids and future weight gain in individuals with an at-risk mental state and in first-episode psychosis. Schizophr. Bull. 2021, 47, 160–169. [Google Scholar] [CrossRef]
- Martorell, L.; Muntané, G.; Porta-López, S.; Moreno, I.; Ortega, L.; Montalvo, I.; Sanchez-Gistau, V.; Monseny, R.; Labad, J.; Vilella, E. Increased levels of serum leptin in the early stages of psychosis. J. Psychiatr. Res. 2019, 111, 24–29. [Google Scholar] [CrossRef]
- Alqarni, A.; Mitchell, T.; McGorry, P.; Nelson, B.; Markulev, C.; Yuen, H.; Schäfer, M.; Berger, M.; Mossaheb, N.; Schlögelhofer, M.; et al. Comparison of erythrocyte omega-3 index, fatty acids and molecular phospholipid species in people at ultra-high risk of developing psychosis and healthy people. Schizophr. Res. 2020, 226, 44–51. [Google Scholar] [CrossRef]
- Koivukangas, J.; Björnholm, L.; Tervonen, O.; Miettunen, J.; Nordström, T.; Kiviniemi, V.; Mäki, P.; Mukkala, S.; Moilanen, I.; Barnett, J.; et al. Body mass index and brain white matter structure in young adults at risk for psychosis—The Oulu Brain and Mind Study. Psychiatry Res. Neuroimaging 2016, 254, 169–176. [Google Scholar] [CrossRef]
- Pruessner, M.; Béchard-Evans, L.; Boekestyn, L.; Iyer, S.; Pruessner, J.; Malla, A. Attenuated cortisol response to acute psychosocial stress in individuals at ultra-high risk for psychosis. Schizophr. Res. 2013, 146, 79–86. [Google Scholar] [CrossRef]
- Veeneman, R.; Vermeulen, J.; Abdellaoui, A.; Sanderson, E.; Wootton, R.; Tadros, R.; Bezzina, C.; Denys, D.; Munafò, M.; Verweij, K.; et al. Exploring the relationship between schizophrenia and cardiovascular disease: A genetic correlation and multivariable Mendelian randomization study. Schizophr. Bull. 2022, 48, 463–473. [Google Scholar] [CrossRef]
- Latvala, A.; Kuja-Halkola, R.; Rück, C.; D’Onofrio, B.; Jernberg, T.; Almqvist, C.; Mataix-Cols, D.; Larsson, H.; Lichtenstein, P. Association of resting heart rate and blood pressure in late adolescence with subsequent mental disorders: A longitudinal population study of more than 1 million men in Sweden. JAMA Psychiatry 2016, 73, 1268–1275. [Google Scholar] [CrossRef]
- Thayer, J.; Hansen, A.; Saus-Rose, E.; Johnsen, B. Heart rate variability, prefrontal neural function, and cognitive performance: The neurovisceral integration perspective on self-regulation, adaptation, and health. Ann. Behav. Med. 2009, 37, 141–153. [Google Scholar] [CrossRef]
- Levy, M. Autonomic interactions in cardiac control. Ann. N. Y. Acad. Sci. 1990, 601, 209–221. [Google Scholar] [CrossRef]
- Montaquila, J.; Trachik, B.; Bedwell, J. Heart rate variability and vagal tone in schizophrenia: A review. J. Psychiatr. Res. 2015, 69, 57–66. [Google Scholar] [CrossRef]
- Chalmers, J.; Quintana, D.; Abbott, M.; Kemp, A. Anxiety disorders are associated with reduced heart rate variability: A meta-analysis. Front. Psychiatry 2014, 5, 80. [Google Scholar] [CrossRef]
- Clamor, A.; Sundag, J.; Lincoln, T. Specificity of resting-state heart rate variability in psychosis: A comparison with clinical high risk, anxiety, and healthy controls. Schizophr. Res. 2019, 206, 89–95. [Google Scholar] [CrossRef]
- Counotte, J.; Pot-Kolder, R.; van Roon, A.; Hoskam, O.; van der Gaag, M.; Veling, W. High psychosis liability is associated with altered autonomic balance during exposure to Virtual Reality social stressors. Schizophr. Res. 2017, 184, 14–20. [Google Scholar] [CrossRef]
- Kocsis, A.; Gajwani, R.; Gross, J.; Gumley, A.; Lawrie, S.; Schwannauer, M.; Schultze-Lutter, F.; Grent-’t-Jong, T.; Uhlhaas, P. Altered autonomic function in individuals at clinical high risk for psychosis. Front. Psychiatry 2020, 11, 580503. [Google Scholar] [CrossRef]
- Nordholm, D.; Jensen, M.; Kristiansen, J.; Glenthøj, L.; Kristensen, T.; Wenneberg, C.; Hjorthøj, C.; Garde, A.; Nordentoft, M. A longitudinal study on physiological stress in individuals at ultra high-risk of psychosis. Schizophr. Res. 2023, 254, 218–226. [Google Scholar] [CrossRef] [PubMed]
- Giannouli, V. Ethnicity, mortality, and severe mental illness. Lancet Psychiatry 2017, 4, 517. [Google Scholar] [CrossRef]
- Glick, I.; Stekoll, A.; Hays, S. The role of the family and improvement in treatment maintenance, adherence, and outcome for schizophrenia. J. Clin. Psychopharmacol. 2011, 31, 82–85. [Google Scholar] [CrossRef]
- McCullough, E.; Hoyt, W.; Larson, D.; Koenig, H.; Thoresen, C. Religious involvement and mortality: A meta-analytic review. Health Psychol. 2000, 19, 211–222. [Google Scholar] [CrossRef] [PubMed]





| Patient Characteristics Stratified by Psychosis Transition | ||
|---|---|---|
| Non-Transitioned | Transitioned | |
| n | 116 | 21 |
| Gender | ||
| Male | 68 (58.6) | 12 (57.1) |
| Female | 48 (41.4) | 9 (42.9) |
| Age Group | ||
| <20 | 34 (29.3) | 3 (14.3) |
| 20–25 | 38 (32.8) | 10 (47.6) |
| 26–30 | 24 (20.7) | 4 (19.0) |
| >30 | 20 (17.2) | 4 (19.0) |
| Ethnicity | ||
| White | 51 (44.0) | 2 (9.5) |
| Asian | 2 (1.7) | 2 (9.5) |
| Black African | 10 (8.6) | 5 (23.8) |
| Black Caribbean | 3 (2.6) | 1 (4.8) |
| Black British | 22 (19.0) | 7 (33.3) |
| Other | 28 (24.1) | 4 (19.0) |
| Smoker Status | ||
| Yes | 47 (40.5) | 8 (38.1) |
| No | 69 (59.5) | 13 (61.9) |
| Nicotine Dependence | ||
| Non-Smokers | 69 (59.5) | 14 (66.7) |
| Low | 40 (34.5) | 3 (14.3) |
| Moderate | 3 (2.6) | 2 (9.5) |
| High | 4 (3.4) | 2 (9.5) |
| Drinking Status | ||
| Yes | 93 (80.2) | 12 (57.1) |
| No | 23 (19.8) | 9 (42.9) |
| Alcohol Consumption | ||
| Non-Drinkers | 25 (21.6) | 9 (42.9) |
| Non-Hazardous | 57 (49.1) | 7 (33.3) |
| Hazardous | 34 (29.3) | 5 (23.8) |
| Fibre Consumption | ||
| Low | 72 (62.1) | 15 (71.4) |
| Moderate | 20 (17.2) | 3 (14.3) |
| High | 24 (20.7) | 3 (14.3) |
| Physical Activity | ||
| Active | 35 (30.2) | 3 (14.3) |
| Inactive | 81 (69.8) | 18 (85.7) |
| BMI Category | ||
| Underweight | 7 (6.3) | 1 (4.8) |
| Healthy Range | 67 (60.4) | 12 (57.1) |
| Overweight | 37 (33.3) | 8 (38.1) |
| Log-Rank Test Group Comparison | |||
|---|---|---|---|
| Feature | χ2 | Degrees of Freedom | p-Value |
| Nicotine dependence: low, moderate, high, and non-smokers | 12.8 | 3 | 0.005 |
| Nicotine dependence: non-smokers and low | 3.6 | 1 | 0.06 |
| Nicotine dependence: low and moderate or high | 14.1 | 1 | 0.002 |
| Nicotine dependence: non-smokers and moderate or high | 4.7 | 1 | 0.03 |
| Alcohol consumption: non-drinkers, non-hazardous, and hazardous | 6 | 2 | 0.05 |
| Fibre consumption: low, moderate, and high | 1.3 | 2 | 0.5 |
| Physical activity: active and non-active group | 0.8 | 1 | 0.4 |
| Body mass index (BMI) | 0.3 | 2 | 2 |
| Cox Regression Results | |||||
|---|---|---|---|---|---|
| Feature | Hazard Ratios | Lower 0.95 | Upper 0.95 | p-Value | Adjusted p-Value |
| FTND | 1.34 | 1.1 | 1.64 | 0.0034 | 0.01 |
| AUDIT | 1.04 | 0.95 | 1.143 | 0.34 | 0.45 |
| DINE (fibre score) | 0.99 | 0.96 | 1.01 | 0.7 | 0.7 |
| Physical Act (MET) | 0.99 | 0.96 | 1.01 | 0.22 | 0.44 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Micheli, A.; Provenzani, U.; Krakowski, K.; Oliver, D.; Damiani, S.; Brondino, N.; McGuire, P.; Fusar-Poli, P. Physical Health and Transition to Psychosis in People at Clinical High Risk. Biomedicines 2024, 12, 523. https://doi.org/10.3390/biomedicines12030523
De Micheli A, Provenzani U, Krakowski K, Oliver D, Damiani S, Brondino N, McGuire P, Fusar-Poli P. Physical Health and Transition to Psychosis in People at Clinical High Risk. Biomedicines. 2024; 12(3):523. https://doi.org/10.3390/biomedicines12030523
Chicago/Turabian StyleDe Micheli, Andrea, Umberto Provenzani, Kamil Krakowski, Dominic Oliver, Stefano Damiani, Natascia Brondino, Philip McGuire, and Paolo Fusar-Poli. 2024. "Physical Health and Transition to Psychosis in People at Clinical High Risk" Biomedicines 12, no. 3: 523. https://doi.org/10.3390/biomedicines12030523
APA StyleDe Micheli, A., Provenzani, U., Krakowski, K., Oliver, D., Damiani, S., Brondino, N., McGuire, P., & Fusar-Poli, P. (2024). Physical Health and Transition to Psychosis in People at Clinical High Risk. Biomedicines, 12(3), 523. https://doi.org/10.3390/biomedicines12030523






