Urinary Viral Spectrum in Patients with Interstitial Cystitis/Bladder Pain Syndrome and the Clinical Efficacy of Valacyclovir Treatment
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients and Valacyclovir Treatment Course
2.2. Power and Sample Size
2.3. Urinary Virus and Inflammatory Cytokine Investigation
2.4. Study Approval
2.5. Statistical Analysis
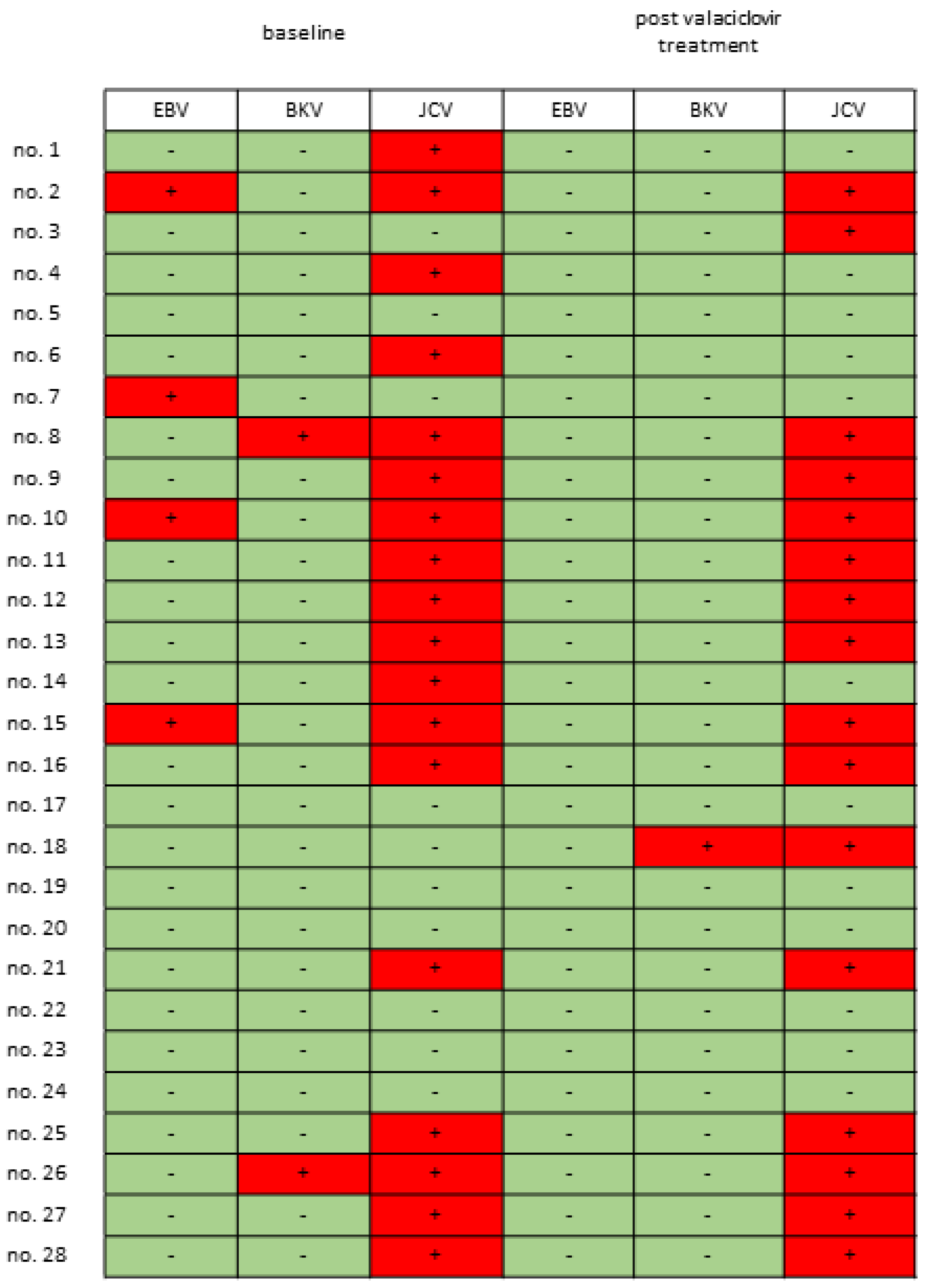
3. Results
3.1. Urinary Viral Profile of Patients with IC/BPS
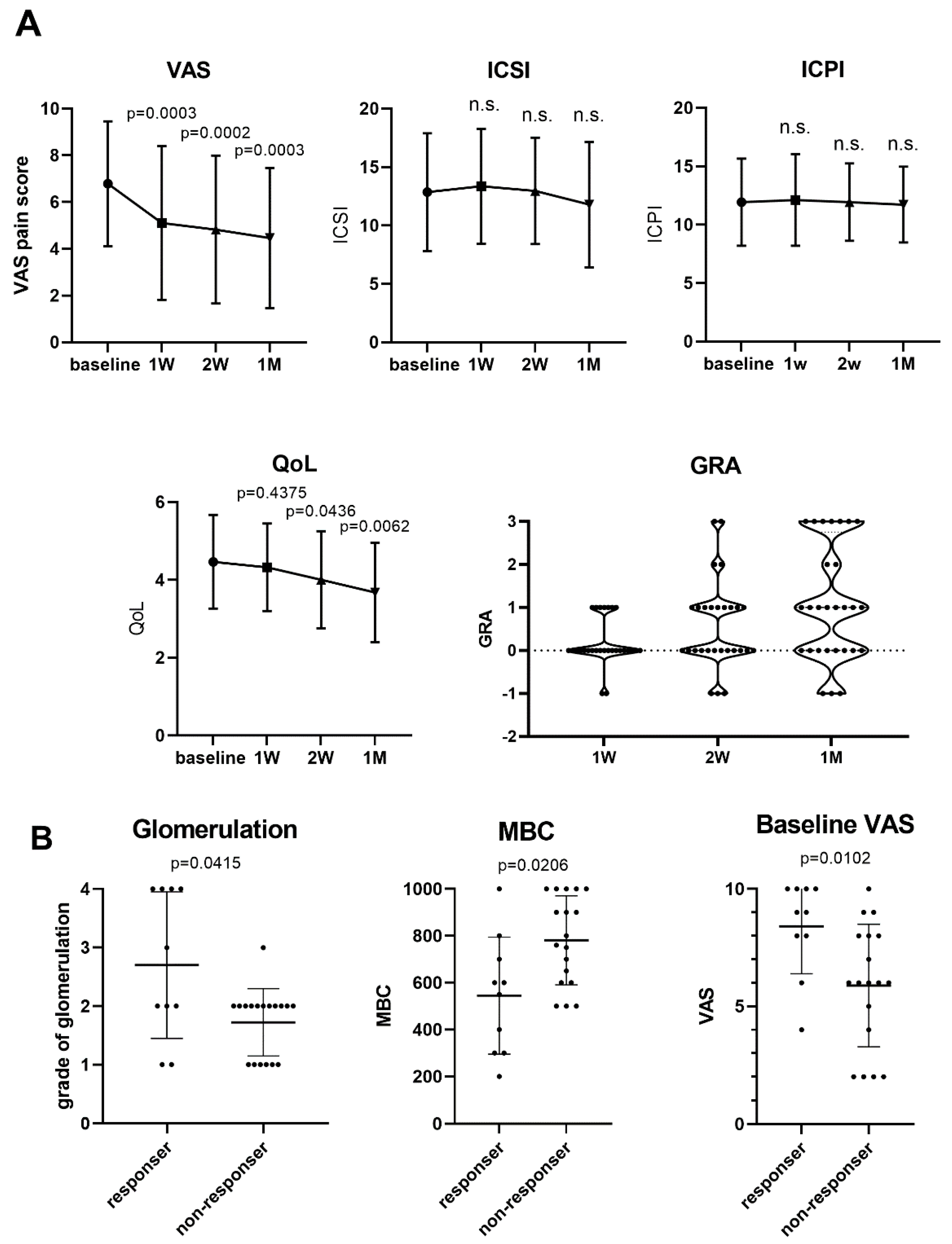
3.2. Valacyclovir for Pain Relief in Patients with IC/BPS
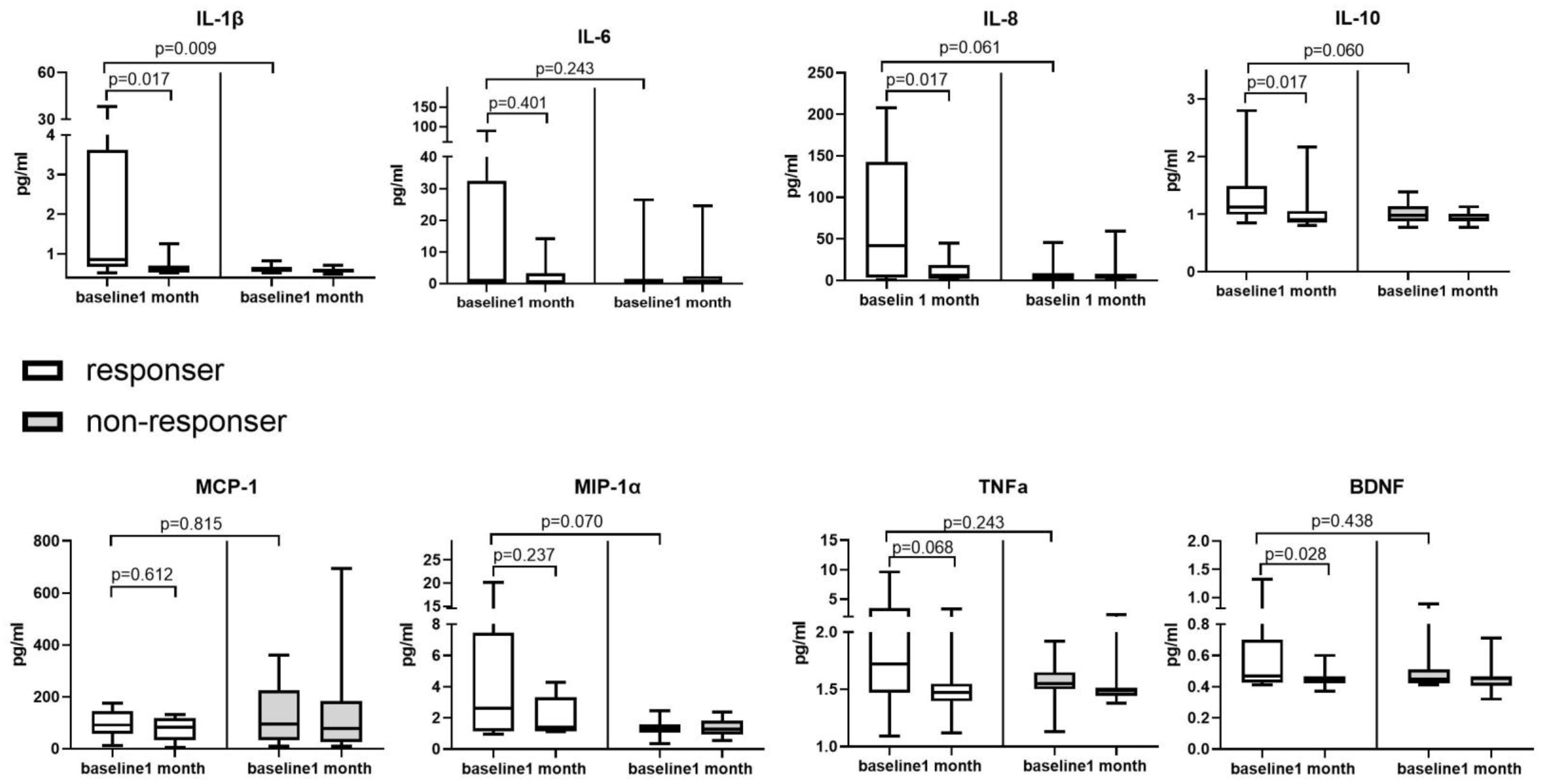
3.3. Changes in Urinary EBV and Inflammatory Cytokine Levels from before to after Valacyclovir Treatment
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Clemens, J.Q.; Erickson, D.R.; Varela, N.P.; Lai, H.H. Diagnosis and Treatment of Interstitial Cystitis/Bladder Pain Syndrome. J. Urol. 2022, 208, 34–42. [Google Scholar] [CrossRef] [PubMed]
- van de Merwe, J.P.; Nordling, J.; Bouchelouche, P.; Bouchelouche, K.; Cervigni, M.; Daha, L.K.; Elneil, S.; Fall, M.; Hohlbrugger, G.; Irwin, P.; et al. Diagnostic criteria, classification, and nomenclature for painful bladder syndrome/interstitial cystitis: An ESSIC proposal. Eur. Urol. 2008, 53, 60–67. [Google Scholar] [CrossRef] [PubMed]
- Anger, J.T.; Dallas, K.B.; Bresee, C.; De Hoedt, A.M.; Barbour, K.E.; Hoggatt, K.J.; Goodman, M.T.; Kim, J.; Freedland, S.J. National prevalence of IC/BPS in women and men utilizing veterans health administration data. Front. Pain Res. 2022, 3, 925834. [Google Scholar] [CrossRef] [PubMed]
- Regauer, S.; Gamper, M.; Fehr, M.K.; Viereck, V. Sensory Hyperinnervation Distinguishes Bladder Pain Syndrome/Interstitial Cystitis from Overactive Bladder Syndrome. J. Urol. 2017, 197, 159–166. [Google Scholar] [CrossRef] [PubMed]
- Kim, A.; Han, J.Y.; Ryu, C.M.; Yu, H.Y.; Lee, S.; Kim, Y.; Jeong, S.U.; Cho, Y.M.; Shin, D.M.; Choo, M.S. Histopathological characteristics of interstitial cystitis/bladder pain syndrome without Hunner lesion. Histopathology 2017, 71, 415–424. [Google Scholar] [CrossRef] [PubMed]
- Akiyama, Y.; Maeda, D.; Katoh, H.; Morikawa, T.; Niimi, A.; Nomiya, A.; Sato, Y.; Kawai, T.; Goto, A.; Fujimura, T.; et al. Molecular Taxonomy of Interstitial Cystitis/Bladder Pain Syndrome Based on Whole Transcriptome Profiling by Next-Generation RNA Sequencing of Bladder Mucosal Biopsies. J. Urol. 2019, 202, 290–300. [Google Scholar] [CrossRef]
- Logadottir, Y.; Delbro, D.; Lindholm, C.; Fall, M.; Peeker, R. Inflammation characteristics in bladder pain syndrome ESSIC type 3C/classic interstitial cystitis. Int. J. Urol. 2014, 21 (Suppl. S1), 75–78. [Google Scholar] [CrossRef] [PubMed]
- Jhang, J.F.; Hsu, Y.H.; Peng, C.W.; Jiang, Y.H.; Ho, H.C.; Kuo, H.C. Epstein-Barr Virus as a Potential Etiology of Persistent Bladder Inflammation in Human Interstitial Cystitis/Bladder Pain Syndrome. J. Urol. 2018, 200, 590–596. [Google Scholar] [CrossRef]
- Jhang, J.F.; Liu, C.D.; Hsu, Y.H.; Chen, C.C.; Chen, H.C.; Jiang, Y.H.; Wu, W.C.; Peng, C.W.; Kuo, H.C. EBV infection mediated BDNF expression is associated with bladder inflammation in interstitial cystitis/bladder pain syndrome with Hunner’s lesion. J. Pathol. 2022, 259, 276–290. [Google Scholar] [CrossRef]
- Winter, B.J.; O’Connell, H.E.; Bowden, S.; Carey, M.; Eisen, D.P. A Case Control Study Reveals that Polyomaviruria Is Significantly Associated with Interstitial Cystitis and Vesical Ulceration. PLoS ONE 2015, 10, e0137310. [Google Scholar] [CrossRef]
- Robles, M.T.S.; Cantalupo, P.G.; Duray, A.M.; Freeland, M.; Murkowski, M.; van Bokhoven, A.; Stephens-Shields, A.J.; Pipas, J.M.; Imperiale, M.J. Analysis of viruses present in urine from patients with interstitial cystitis. Virus Genes 2020, 56, 430–438. [Google Scholar] [CrossRef] [PubMed]
- Eisen, D.P.; Fraser, I.R.; Sung, L.M.; Finlay, M.; Bowden, S.; O’Connell, H. Decreased viral load and symptoms of polyomavirus-associated chronic interstitial cystitis after intravesical cidofovir treatment. Clin. Infect. Dis. 2009, 48, e86–e88. [Google Scholar] [CrossRef] [PubMed]
- Datta, A.K.; Colby, B.M.; Shaw, J.E.; Pagano, J.S. Acyclovir inhibition of Epstein-Barr virus replication. Proc. Natl. Acad. Sci. USA 1980, 77, 5163–5166. [Google Scholar] [CrossRef]
- Vere Hodge, R.A.; Field, H.J. Antiviral agents for herpes simplex virus. Adv. Pharmacol. 2013, 67, 1–38. [Google Scholar] [CrossRef]
- Yager, J.E.; Magaret, A.S.; Kuntz, S.R.; Selke, S.; Huang, M.L.; Corey, L.; Casper, C.; Wald, A. Valganciclovir for the Suppression of Epstein-Barr Virus Replication. J. Infect. Dis. 2017, 216, 198–202. [Google Scholar] [CrossRef] [PubMed]
- Giannantoni, A.; Gubbiotti, M.; Bini, V. Botulinum Neurotoxin A Intravesical Injections in Interstitial Cystitis/Bladder Painful Syndrome: A Systematic Review with Meta-Analysis. Toxins 2019, 11, 510. [Google Scholar] [CrossRef]
- Jiang, Y.H.; Jhang, J.F.; Hsu, Y.H.; Ho, H.C.; Wu, Y.H.; Kuo, H.C. Urine cytokines as biomarkers for diagnosing interstitial cystitis/bladder pain syndrome and mapping its clinical characteristics. Am. J. Physiol. Ren. Physiol. 2020, 318, F1391–F1399. [Google Scholar] [CrossRef]
- Hanash, K.A.; Pool, T.L. Interstitial and hemorrhagic cystitis: Viral, bacterial and fungal studies. J. Urol. 1970, 104, 705–706. [Google Scholar] [CrossRef]
- Fall, M.; Johansson, S.L.; Vahlne, A. A clinicopathological and virological study of interstitial cystitis. J. Urol. 1985, 133, 771–773. [Google Scholar] [CrossRef]
- Keay, S.; Schwalbe, R.S.; Trifillis, A.L.; Lovchik, J.C.; Jacobs, S.; Warren, J.W. A prospective study of microorganisms in urine and bladder biopsies from interstitial cystitis patients and controls. Urology 1995, 45, 223–229. [Google Scholar] [CrossRef]
- Hukkanen, V.; Haarala, M.; Nurmi, M.; Klemi, P.; Kiilholma, P. Viruses and interstitial cystitis: Adenovirus genomes cannot be demonstrated in urinary bladder biopsies. Urol. Res. 1996, 24, 235–238. [Google Scholar] [CrossRef]
- Landau, Z.; Gross, R.; Sanilevich, A.; Friedmann, A.; Mitrani-Rosenbaum, S. Presence of infective Epstein-Barr virus in the urine of patients with infectious mononucleosis. J. Med. Virol. 1994, 44, 229–233. [Google Scholar] [CrossRef]
- Walling, D.M.; Flaitz, C.M.; Nichols, C.M. Epstein-Barr virus replication in oral hairy leukoplakia: Response, persistence, and resistance to treatment with valacyclovir. J. Infect. Dis. 2003, 188, 883–890. [Google Scholar] [CrossRef] [PubMed]
- Pagano, J.S.; Whitehurst, C.B.; Andrei, G. Antiviral Drugs for EBV. Cancers 2018, 10, 197. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Xie, C.; Lung, H.L.; Lo, K.W.; Law, G.L.; Mak, N.K.; Wong, K.L. EBNA1-targeted inhibitors: Novel approaches for the treatment of Epstein-Barr virus-associated cancers. Theranostics 2018, 8, 5307–5319. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Xiao, S.; Li, J.; Long, X.; Zhang, Y.; Li, X. A Network Meta-Analysis of Randomized Clinical Trials to Assess the Efficacy and Safety of Antiviral Agents for Immunocompetent Patients with Herpes Zoster-Associated Pain. Pain Physician 2023, 26, 337–346. [Google Scholar] [PubMed]
- Andersson, J.; Skoldenberg, B.; Ernberg, I.; Britton, S.; Henle, W.; Andersson, U. Acyclovir treatment in primary Epstein-Barr virus infection. A double-blind placebo-controlled study. Scand. J. Infect. Dis. 1985, 47, 107. [Google Scholar]
- Pagano, J.S.; Sixbey, J.W.; Lin, J.-C. Acyclovir and Epstein—Barr virus infection. J. Antimicrob. Chemother. 1983, 12, 113–121. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, J.L.; Byron, K.S.; Brewster, F.E.; Sakamoto, K.; Shaw, J.E.; Pagano, J.S. Treatment of life-threatening Epstein-Barr virus infections with acyclovir. Am. J. Med. 1982, 73, 262–266. [Google Scholar] [CrossRef]
- Gershburg, E.; Pagano, J.S. Epstein–Barr virus infections: Prospects for treatment. J. Antimicrob. Chemother. 2005, 56, 277–281. [Google Scholar] [CrossRef]
- Tyring, S.K.; Douglas, J.J.M.; Corey, L.; Spruance, S.L.; Esmann, J. A Randomized, Placebo-Controlled Comparison of Oral Valacyclovir and Acyclovir in Immunocompetent Patients with Recurrent Genital Herpes Infections. Arch. Dermatol. 1998, 134, 185–191. [Google Scholar] [CrossRef] [PubMed]
- Hanno, P.M.; Erickson, D.; Moldwin, R.; Faraday, M.M. Diagnosis and Treatment of Interstitial Cystitis/Bladder Pain Syndrome: AUA Guideline Amendment. J. Urol. 2015, 193, 1545–1553. [Google Scholar] [CrossRef] [PubMed]
- Leone, P.A.; Trottier, S.; Miller, J.M. Valacyclovir for Episodic Treatment of Genital Herpes: A Shorter 3-Day Treatment Course Compared with 5-Day Treatment. Clin. Infect. Dis. 2002, 34, 958–962. [Google Scholar] [CrossRef]
- Tyring, S.K.; Baker, D.; Snowden, W. Valacyclovir for Herpes Simplex Virus Infection: Long-Term Safety and Sustained Efficacy after 20 Years’ Experience with Acyclovir. J. Infect. Dis. 2002, 186, S40–S46. [Google Scholar] [CrossRef] [PubMed]

| Baseline Characteristics | ||
|---|---|---|
| Patients with IC/BPS (n = 28) | Control Subjects (n = 30) | |
| Age | 55.3 ± 13.4 | 54.7 ± 5.70 |
| Sex | 24 female/4 male | All female |
| Hunner’s lesion | 4 HIC/24 NHIC | N/A |
| VAS | 6.79 ± 2.67 (7.5, [5.25–9.0]) | N/A |
| ICSI | 12.9 ± 5.04 (13, [11.25–16.5]) | N/A |
| ICPI | 11.9 ± 3.73 (12, [11–13.75]) | N/A |
| CBC (mL) | 240.2 ± 116.7 (239.5. [145.25–329.75]) | N/A |
| QoL | 4.46 ± 1.20 (4. [3–6]) | N/A |
| MBC (mL) | 664.6 ± 255.8 (700, [512.5–900]) | N/A |
| Grade of glomerulation hemorrhage | Gr. 1: 8; Gr. 2: 14; Gr. 3: 2; Gr. 4: 4 | N/A |
| EBV Positive n = 4 | EBV Negative n = 24 | p-Value | JCV Positive n = 18 | JCV Negative n = 10 | p-Value | Any Virus Positive n = 19 | Any Virus Negative n = 9 | p-Value | |
|---|---|---|---|---|---|---|---|---|---|
| ICSI | 19.5 | 12.5 | 0.128 | 13 | 13 | 0.384 | 13 | 12 | 0.758 |
| [9.25, 20.0] | [11.3, 15.0] | [9.0, 15.5] | [12.0, 20.0] | [10.3, 17.0] | [12.0, 14.8] | ||||
| ICPI | 16 | 12 | 0.074 | 12 | 12 | 0.640 | 12 | 12 | 0.434 |
| [11.5, 19.0] | [11.0, 13.0] | [10.8, 13.]) | [10.8, 16.0] | [11.3, 14.8] | [10.3, 12.8] | ||||
| VAS | 9.5 | 6 | 0.031 | 6 | 8 | 0.308 | 7 | 7.5 | 0.918 |
| [8.25, 10.0] | [4.25, 8.75] | [3.50, 9.0] | [5.75, 9.25] | [4.5, 9.75] | [5.25, 8.75] | ||||
| CBC (mL) | 124.5 | 188.5 | 0.131 | 162 | 231 | 0.314 | 153 | 298 | 0.042 |
| [68.5, 167] | [110, 307) | [103, 247] | [91.0, 340] | [91.8, 241] | [181, 341] | ||||
| MBC (mL) | 400 | 700 | 0.007 | 575 | 900 | 0.005 | 575 | 900 | 0.002 |
| [225, 500] | [563, 790) | [500, 713] | [675, 1000] | [500, 738] | [713, 1000] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kuo, H.-C.; Peng, C.-W.; Jiang, Y.-H.; Jhang, J.-F. Urinary Viral Spectrum in Patients with Interstitial Cystitis/Bladder Pain Syndrome and the Clinical Efficacy of Valacyclovir Treatment. Biomedicines 2024, 12, 522. https://doi.org/10.3390/biomedicines12030522
Kuo H-C, Peng C-W, Jiang Y-H, Jhang J-F. Urinary Viral Spectrum in Patients with Interstitial Cystitis/Bladder Pain Syndrome and the Clinical Efficacy of Valacyclovir Treatment. Biomedicines. 2024; 12(3):522. https://doi.org/10.3390/biomedicines12030522
Chicago/Turabian StyleKuo, Hann-Chorng, Chih-Wen Peng, Yuan-Hong Jiang, and Jia-Fong Jhang. 2024. "Urinary Viral Spectrum in Patients with Interstitial Cystitis/Bladder Pain Syndrome and the Clinical Efficacy of Valacyclovir Treatment" Biomedicines 12, no. 3: 522. https://doi.org/10.3390/biomedicines12030522
APA StyleKuo, H.-C., Peng, C.-W., Jiang, Y.-H., & Jhang, J.-F. (2024). Urinary Viral Spectrum in Patients with Interstitial Cystitis/Bladder Pain Syndrome and the Clinical Efficacy of Valacyclovir Treatment. Biomedicines, 12(3), 522. https://doi.org/10.3390/biomedicines12030522








