Ribonucleic Acid Sequencing Reveals the Upregulation and Resolution of Inflammation and Extracellular Matrix Remodeling in Lidocaine-Treated Human Acute Monocytic Leukemia Cell Line THP-1
Abstract
1. Introduction
2. Materials and Methods
2.1. THP-1 Cell Culture
2.2. Cell Viability
2.3. RNA Sequencing
2.4. Bioinformatic Analysis
2.5. Reverse Transcription Polymerase Chain Reaction
2.6. Statistical Analysis
3. Results
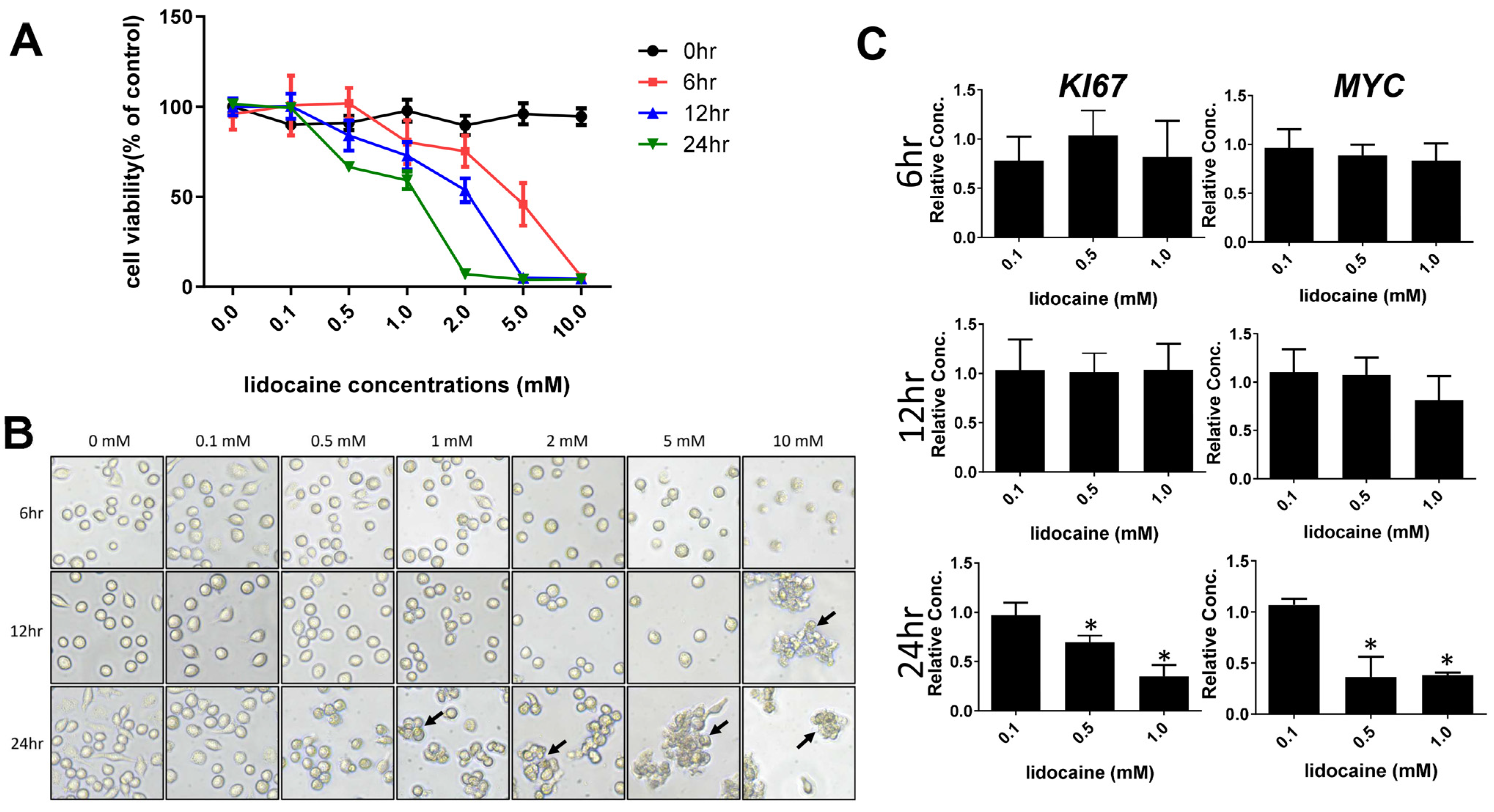
3.1. High Concentrations of Lidocaine Affect Macrophage Viability
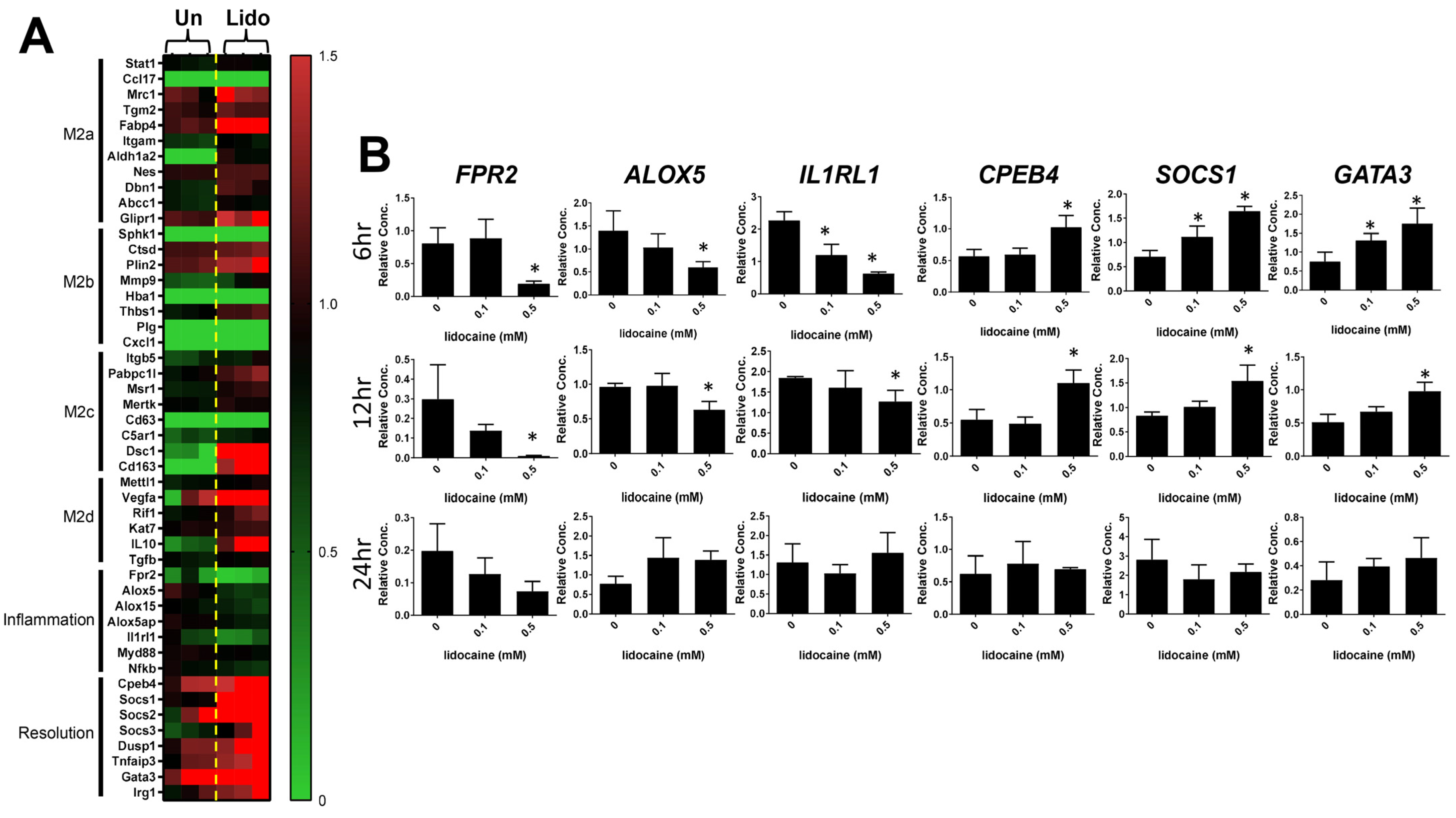
3.2. Transcriptome Analysis of Lidocaine-Treated Macrophages Revealed Significant Upregulation of Tissue Remodeling Cassettes in Macrophages
3.3. Lidocaine Increases the Resolution of Inflammation-Associated Gene Expression in Macrophages
4. Discussion
5. Conclusions
6. Limitations
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gordh, T.; Gordh, T.E.; Lindqvist, K.; Warner, D.S. Lidocaine: The Origin of a Modern Local Anesthetic. J. Am. Soc. Anesthesiol. 2010, 113, 1433–1437. [Google Scholar] [CrossRef]
- Raftery, P. The Gold Standard in Local Anaesthesia. Dent. Nurs. 2016, 12, 448–449. [Google Scholar] [CrossRef]
- Fozzard, H.; Lee, P.; Lipkind, G. Mechanism of Local Anesthetic Drug Action on Voltage-Gated Sodium Channels. Curr. Pharm. Des. 2005, 11, 2671–2686. [Google Scholar] [CrossRef]
- Sekhar, G.R.; Nagaraju, T.; Nandagopal, V.; Sudheer, R. Is Palatal Injection Mandatory Prior to Extraction of Permanent Maxillary Tooth: A Preliminary Study. Indian J. Dent. Res. 2011, 22, 100. [Google Scholar]
- Larjava, H. Oral Wound Healing: Cell Biology and Clinical Management; John Wiley & Sons: Hoboken, NJ, USA, 2012; ISBN 0-8138-0481-7. [Google Scholar]
- Discepoli, N.; Vignoletti, F.; Laino, L.; De Sanctis, M.; Muñoz, F.; Sanz, M. Early Healing of the Alveolar Process after Tooth Extraction: An Experimental Study in the Beagle Dog. J. Clin. Periodontol. 2013, 40, 638–644. [Google Scholar] [CrossRef] [PubMed]
- Goldberg, M. Pulp Healing and Regeneration: More Questions than Answers. Adv. Dent. Res. 2011, 23, 270–274. [Google Scholar] [CrossRef]
- DeCroos, F.; Liao, J.; Ramey, N.; Li, I. Management of Odontogenic Orbital Cellulitis. J. Med. Life 2011, 4, 314. [Google Scholar] [PubMed]
- Koh, T.J.; DiPietro, L.A. Inflammation and Wound Healing: The Role of the Macrophage. Expert Rev. Mol. Med. 2011, 13, e23. [Google Scholar] [CrossRef]
- Ferrante, C.J.; Leibovich, S.J. Regulation of Macrophage Polarization and Wound Healing. Adv. Wound Care 2012, 1, 10–16. [Google Scholar] [CrossRef]
- Kloc, M.; Ghobrial, R.M.; Wosik, J.; Lewicka, A.; Lewicki, S.; Kubiak, J.Z. Macrophage Functions in Wound Healing. J. Tissue Eng. Regen. Med. 2019, 13, 99–109. [Google Scholar] [CrossRef] [PubMed]
- Murray, P.J. Macrophage Polarization. Annu. Rev. Physiol. 2017, 79, 541–566. [Google Scholar] [CrossRef]
- Bigger, J., Jr.; Heissenbuttel, R. The Use of Procaine Amide and Lidocaine in the Treatment of Cardiac Arrhythmias. Prog. Cardiovasc. Dis. 1969, 11, 515–534. [Google Scholar] [CrossRef] [PubMed]
- Ali, Z.A.; El-Mallakh, R.S. Nebulized Lidocaine in COVID-19, an Hypothesis. Med. Hypotheses 2020, 144, 109947. [Google Scholar] [CrossRef]
- Caracas, H.C.P.M.; Maciel, J.V.B.; de Souza, M.M.G.; Maia, L.C. The Use of Lidocaine as an Anti-Inflammatory Substance: A Systematic Review. J. Dent. 2009, 37, 93–97. [Google Scholar] [CrossRef] [PubMed]
- Sakaguchi, M.; Kuroda, Y.; Hirose, M. The Antiproliferative Effect of Lidocaine on Human Tongue Cancer Cells with Inhibition of the Activity of Epidermal Growth Factor Receptor. Anesth. Analg. 2006, 102, 1103–1107. [Google Scholar] [CrossRef] [PubMed]
- Chamaraux-Tran, T.-N.; Mathelin, C.; Aprahamian, M.; Joshi, G.P.; Tomasetto, C.; Diemunsch, P.; Akladios, C. Antitumor Effects of Lidocaine on Human Breast Cancer Cells: An in Vitro and in Vivo Experimental Trial. Anticancer Res. 2018, 38, 95–105. [Google Scholar] [PubMed]
- Lin, S.; Jin, P.; Shao, C.; Lu, W.; Xiang, Q.; Jiang, Z.; Zhang, Y.; Bian, J. Lidocaine Attenuates Lipopolysaccharide-Induced Inflammatory Responses and Protects against Endotoxemia in Mice by Suppressing HIF1α-Induced Glycolysis. Int. Immunopharmacol. 2020, 80, 106150. [Google Scholar] [CrossRef]
- Robinson, M.D.; McCarthy, D.J.; Smyth, G.K. EdgeR: A Bioconductor Package for Differential Expression Analysis of Digital Gene Expression Data. Bioinformatics 2010, 26, 139–140. [Google Scholar] [CrossRef]
- Love, M.I.; Huber, W.; Anders, S. Moderated Estimation of Fold Change and Dispersion for RNA-Seq Data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef]
- Sherman, B.T.; Hao, M.; Qiu, J.; Jiao, X.; Baseler, M.W.; Lane, H.C.; Imamichi, T.; Chang, W. DAVID: A Web Server for Functional Enrichment Analysis and Functional Annotation of Gene Lists (2021 Update). Nucleic Acids Res. 2022, 50, W216–W221. [Google Scholar] [CrossRef]
- Huang, D.W.; Sherman, B.T.; Lempicki, R.A. Systematic and Integrative Analysis of Large Gene Lists Using DAVID Bioinformatics Resources. Nat. Protoc. 2009, 4, 44–57. [Google Scholar] [CrossRef] [PubMed]
- Gray, A.; Marrero-Berrios, I.; Weinberg, J.; Manchikalapati, D.; SchianodiCola, J.; Schloss, R.S.; Yarmush, J. The Effect of Local Anesthetic on Pro-Inflammatory Macrophage Modulation by Mesenchymal Stromal Cells. Int. Immunopharmacol. 2016, 33, 48–54. [Google Scholar] [CrossRef] [PubMed]
- Chang, Z.; Wang, Y.; Liu, C.; Smith, W.; Kong, L. Natural Products for Regulating Macrophages M2 Polarization. Curr. Stem Cell Res. Ther. 2020, 15, 559–569. [Google Scholar] [CrossRef] [PubMed]
- Corminboeuf, O.; Leroy, X. FPR2/ALXR Agonists and the Resolution of Inflammation. J. Med. Chem. 2015, 58, 537–559. [Google Scholar] [CrossRef] [PubMed]
- Kain, V.; Jadapalli, J.K.; Tourki, B.; Halade, G.V. Inhibition of FPR2 Impaired Leukocytes Recruitment and Elicited Non-Resolving Inflammation in Acute Heart Failure. Pharmacol. Res. 2019, 146, 104295. [Google Scholar] [CrossRef] [PubMed]
- Petri, M.H.; Thul, S.; Andonova, T.; Lindquist-Liljeqvist, M.; Jin, H.; Skenteris, N.-T.; Arnardottir, H.; Maegdefessel, L.; Caidahl, K.; Perretti, M. Resolution of Inflammation through the Lipoxin and ALX/FPR2 Receptor Pathway Protects against Abdominal Aortic Aneurysms. JACC Basic Transl. Sci. 2018, 3, 719–727. [Google Scholar] [CrossRef] [PubMed]
- Kain, V.; Ingle, K.A.; Kabarowski, J.; Barnes, S.; Limdi, N.A.; Prabhu, S.D.; Halade, G.V. Genetic Deletion of 12/15 Lipoxygenase Promotes Effective Resolution of Inflammation Following Myocardial Infarction. J. Mol. Cell. Cardiol. 2018, 118, 70–80. [Google Scholar] [CrossRef]
- Halade, G.V.; Kain, V.; Hossain, S.; Parcha, V.; Limdi, N.A.; Arora, P. Arachidonate 5-Lipoxygenase Is Essential for Biosynthesis of Specialized pro-Resolving Mediators and Cardiac Repair in Heart Failure. Am. J. Physiol.-Heart Circ. Physiol. 2022, 323, H721–H737. [Google Scholar] [CrossRef]
- Malamed, S.F. Handbook of Local Anesthesia; Elsevier: Rio de Janeiro, Brazil, 2004; ISBN 0-323-02449-1. [Google Scholar]
- Weinberg, L.; Peake, B.; Tan, C.; Nikfarjam, M. Pharmacokinetics and Pharmacodynamics of Lignocaine: A Review. World J. Anesthesiol. 2015, 4, 17–29. [Google Scholar] [CrossRef]
- Oliveira, A.C.; Rodríguez, I.Á.; Garzón, I.; Martín-Piedra, M.Á.; Alfonso-Rodríguez, C.A.; García, J.M.; Sánchez-Quevedo, M.D.C.; Alaminos, M. An Early and Late Cytotoxicity Evaluation of Lidocaine on Human Oral Mucosa Fibroblasts. Exp. Biol. Med. 2014, 239, 71–82. [Google Scholar] [CrossRef]
- Woo, M.S.; Park, J.; Ok, S.-H.; Park, M.; Sohn, J.-T.; Cho, M.S.; Shin, I.-W.; Kim, Y.A. The Proper Concentrations of Dextrose and Lidocaine in Regenerative Injection Therapy: In Vitro Study. Korean J. Pain 2021, 34, 19–26. [Google Scholar] [CrossRef]
- Xu, K.; Wang, J.; Yang, L.; Wan, L.; Wang, Y. Effect of Lidocaine on the Safety of Postoperative Skin Reconstruction after Malignant Melanoma Resection. Exp. Ther. Med. 2019, 18, 949–954. [Google Scholar] [CrossRef] [PubMed]
- Hwang, J.S.; Ma, D.J.; Choi, J.; Shin, Y.J. COL8A2 Regulates the Fate of Corneal Endothelial Cells. Investig. Ophthalmol. Vis. Sci. 2020, 61, 26. [Google Scholar] [CrossRef]
- Karlsson, T.; Lagerholm, B.C.; Vikström, E.; Loitto, V.M.; Magnusson, K.-E. Water Fluxes through Aquaporin-9 Prime Epithelial Cells for Rapid Wound Healing. Biochem. Biophys. Res. Commun. 2013, 430, 993–998. [Google Scholar] [CrossRef]
- Camilleri, S.; McDonald, F. Runx2 and Dental Development. Eur. J. Oral Sci. 2006, 114, 361–373. [Google Scholar] [CrossRef] [PubMed]
- Cascone, S.; Lamberti, G. Hydrogel-Based Commercial Products for Biomedical Applications: A Review. Int. J. Pharm. 2020, 573, 118803. [Google Scholar] [CrossRef]
- Jablonski, K.A.; Amici, S.A.; Webb, L.M.; de Dios Ruiz-Rosado, J.; Popovich, P.G.; Partida-Sanchez, S.; Guerau-de-Arellano, M. Novel Markers to Delineate Murine M1 and M2 Macrophages. PLoS ONE 2015, 10, e0145342. [Google Scholar] [CrossRef]
- Porcheray, F.; Viaud, S.; Rimaniol, A.; Leone, C.; Samah, B.; Dereuddre-Bosquet, N.; Dormont, D.; Gras, G. Macrophage Activation Switching: An Asset for the Resolution of Inflammation. Clin. Exp. Immunol. 2005, 142, 481–489. [Google Scholar] [CrossRef]
- Watanabe, S.; Alexander, M.; Misharin, A.V.; Budinger, G.S. The Role of Macrophages in the Resolution of Inflammation. J. Clin. Investig. 2019, 129, 2619–2628. [Google Scholar] [CrossRef]

| (A) The top 20 enriched GO terms of upregulated genes | |||
| Category | Term | Count | p Value |
| BP | signal transduction | 64 | 1.9 × 10−5 |
| BP | cell adhesion | 50 | 4.3 × 10−12 |
| BP | cell differentiation | 40 | 4.4 × 10−5 |
| BP | proteolysis | 26 | 5.2 × 10−4 |
| BP | nervous system development | 23 | 4.7 × 10−3 |
| BP | protein phosphorylation | 23 | 5.9 × 10−2 |
| BP | spermatogenesis | 22 | 2.1 × 10−2 |
| CC | plasma membrane | 224 | 4.7 × 10−11 |
| CC | integral component of membrane | 210 | 2.2 × 10−7 |
| CC | extracellular region | 90 | 3.4 × 10−4 |
| CC | integral component of plasma membrane | 89 | 6.9 × 10−12 |
| CC | extracellular exosome | 78 | 4.0 × 10−2 |
| CC | extracellular space | 71 | 3.2 × 10−2 |
| CC | cell surface | 37 | 1.4 × 10−4 |
| CC | apical plasma membrane | 34 | 3.7 × 10−8 |
| CC | synapse | 34 | 3.3 × 10−5 |
| CC | neuron projection | 29 | 2.7 × 10−6 |
| CC | external side of plasma membrane | 29 | 2.7 × 10−4 |
| CC | dendrite | 24 | 5.1 × 10−3 |
| CC | glutamatergic synapse | 22 | 7.5 × 10−3 |
| MF | identical protein binding | 61 | 7.5 × 10−2 |
| MF | calcium ion binding | 47 | 1.9 × 10−6 |
| MF | protein homodimerization activity | 38 | 1.0 × 10−3 |
| MF | sequence-specific double-stranded DNA binding | 30 | 2.2 × 10−3 |
| MF | transcription factor activity, sequence-specific DNA binding | 29 | 4.6 × 10−3 |
| MF | protein serine/threonine/tyrosine kinase activity | 24 | 5.5 × 10−3 |
| MF | receptor binding | 22 | 9.0 × 10−3 |
| (B) The top 20 enriched GO terms of downregulated genes | |||
| Category | Term | Count | p Value |
| BP | signal transduction | 83 | 2.6 × 10−4 |
| BP | cell adhesion | 57 | 6.1 × 10−9 |
| BP | nervous system development | 50 | 2.5 × 10−10 |
| BP | cell differentiation | 44 | 1.1 × 10−2 |
| BP | chemical synaptic transmission | 36 | 1.6 × 10−9 |
| BP | positive regulation of gene expression | 35 | 9.0 × 10−3 |
| BP | cell–cell signaling | 34 | 1.4 × 10−9 |
| BP | positive regulation of cell proliferation | 33 | 4.6 × 10−2 |
| BP | proteolysis | 30 | 1.0 × 10−2 |
| BP | inflammatory response | 30 | 1.2 × 10−2 |
| BP | intracellular signal transduction | 29 | 4.2 × 10−2 |
| BP | brain development | 27 | 7.2 × 10−5 |
| BP | axon guidance | 26 | 7.5 × 10−7 |
| BP | positive regulation of cell migration | 26 | 1.9 × 10−4 |
| BP | cell surface receptor signaling pathway | 26 | 3.8 × 10−3 |
| BP | homophilic cell adhesion via plasma membrane adhesion molecules | 24 | 1.9 × 10−6 |
| BP | positive regulation of protein phosphorylation | 22 | 3.9 × 10−4 |
| BP | extracellular matrix organization | 20 | 4.7 × 10−4 |
| BP | visual perception | 20 | 2.2 × 10−3 |
| CC | plasma membrane | 346 | 3.9 × 10−19 |
| CC | integral component of membrane | 309 | 2.5 × 10−9 |
| CC | extracellular region | 157 | 5.5 × 10−11 |
| CC | extracellular space | 138 | 6.0 × 10−9 |
| CC | integral component of plasma membrane | 136 | 1.0 × 10−18 |
| CC | Golgi apparatus | 63 | 3.2 × 10−2 |
| CC | synapse | 58 | 2.6 × 10−10 |
| CC | cell surface | 53 | 1.4 × 10−5 |
| CC | dendrite | 52 | 2.0 × 10−10 |
| CC | glutamatergic synapse | 49 | 2.9 × 10−10 |
| CC | neuronal cell body | 46 | 2.2 × 10−10 |
| CC | neuron projection | 43 | 7.5 × 10−9 |
| CC | axon | 41 | 3.6 × 10−8 |
| CC | perinuclear region of cytoplasm | 40 | 9.8 × 10−2 |
| CC | Golgi membrane | 38 | 6.2 × 10−2 |
| CC | endoplasmic reticulum lumen | 36 | 1.4 × 10−7 |
| CC | apical plasma membrane | 33 | 4.1 × 10−4 |
| CC | extracellular matrix | 30 | 2.1 × 10−6 |
| CC | external side of plasma membrane | 26 | 3.7 × 10−2 |
| CC | perikaryon | 26 | 2.3 × 10−8 |
| MF | calcium ion binding | 72 | 2.1 × 10−9 |
| MF | receptor binding | 36 | 1.9 × 10−4 |
| MF | sequence-specific double-stranded DNA binding | 36 | 3.0 × 10−2 |
| MF | macromolecular complex binding | 28 | 5.9 × 10−3 |
| MF | growth factor activity | 22 | 1.9 × 10−5 |
| MF | cytokine activity | 22 | 1.5 × 10−4 |
| MF | integrin binding | 21 | 2.8 × 10−5 |
| MF | transmembrane signaling receptor activity | 21 | 3.1 × 10−4 |
| MF | signaling receptor activity | 21 | 2.9 × 10−3 |
| (A) The KEGG pathway enrichment analysis of upregulated genes | ||
| Term | Count | p Value |
| Pathways in cancer | 30 | 9.4 × 10−4 |
| Neuroactive ligand–receptor interaction | 25 | 2.1 × 10−4 |
| Calcium signaling pathway | 22 | 1.7 × 10−5 |
| PI3K-Akt signaling pathway | 19 | 1.7 × 10−2 |
| Cytokine–cytokine receptor interaction | 16 | 3.0 × 10−2 |
| MAPK signaling pathway | 15 | 6.4 × 10−2 |
| cGMP-PKG signaling pathway | 14 | 1.3 × 10−3 |
| Hematopoietic cell lineage | 13 | 3.2 × 10−5 |
| Platelet activation | 13 | 2.9 × 10−4 |
| Oxytocin signaling pathway | 13 | 2.0 × 10−3 |
| (B) The KEGG pathway enrichment analysis of downregulated genes | ||
| Term | Count | p Value |
| Neuroactive ligand–receptor interaction | 37 | 2.3 × 10−6 |
| Pathways in cancer | 32 | 4.6 × 10−2 |
| Cytokine–cytokine receptor interaction | 24 | 4.2 × 10−3 |
| Calcium signaling pathway | 21 | 5.9 × 10−3 |
| MAPK signaling pathway | 21 | 3.4 × 10−2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Feng, S.-W.; Lin, W.-C.; Lee, I.-T.; Luo, S.-D.; Wang, C.-S. Ribonucleic Acid Sequencing Reveals the Upregulation and Resolution of Inflammation and Extracellular Matrix Remodeling in Lidocaine-Treated Human Acute Monocytic Leukemia Cell Line THP-1. Biomedicines 2024, 12, 509. https://doi.org/10.3390/biomedicines12030509
Feng S-W, Lin W-C, Lee I-T, Luo S-D, Wang C-S. Ribonucleic Acid Sequencing Reveals the Upregulation and Resolution of Inflammation and Extracellular Matrix Remodeling in Lidocaine-Treated Human Acute Monocytic Leukemia Cell Line THP-1. Biomedicines. 2024; 12(3):509. https://doi.org/10.3390/biomedicines12030509
Chicago/Turabian StyleFeng, Sheng-Wei, Wei-Chun Lin, I-Ta Lee, Sheng-Dean Luo, and Ching-Shuen Wang. 2024. "Ribonucleic Acid Sequencing Reveals the Upregulation and Resolution of Inflammation and Extracellular Matrix Remodeling in Lidocaine-Treated Human Acute Monocytic Leukemia Cell Line THP-1" Biomedicines 12, no. 3: 509. https://doi.org/10.3390/biomedicines12030509
APA StyleFeng, S.-W., Lin, W.-C., Lee, I.-T., Luo, S.-D., & Wang, C.-S. (2024). Ribonucleic Acid Sequencing Reveals the Upregulation and Resolution of Inflammation and Extracellular Matrix Remodeling in Lidocaine-Treated Human Acute Monocytic Leukemia Cell Line THP-1. Biomedicines, 12(3), 509. https://doi.org/10.3390/biomedicines12030509








