The Implications of Cytochrome P450 2D6/CYP2D6 Polymorphism in the Therapeutic Response of Atypical Antipsychotics in Adolescents with Psychosis—A Prospective Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Inclusion and Exclusion Criteria
- An indication for treatment with one of the following atypical antipsychotics—risperidone, aripiprazole, or olanzapine;
- Aged between 13 and 18;
- A PANSS score ≥ 70.
2.2. Patient Medication
2.3. Clinical Assessments
2.4. Study Outcomes
Statistical Analysis
3. Results
- Group 1 (SNP genotype) included 3.6% (2/56) of the patients: slow metabolizers who have the AL-2 allele (CYP2D6*4) and do not have the functional AL-1 allele;
- Group 2 (CYP2D6*WT) included 69.6% (39/56) of the patients: extensive metabolizers carrying the functional AL-1 allele;
- Group 3 (WT/*4 genotype) included 26.8% (15/56) of the patients: intermediate metabolizers carrying a functional allele (WT) and a nonfunctional allele (*4).
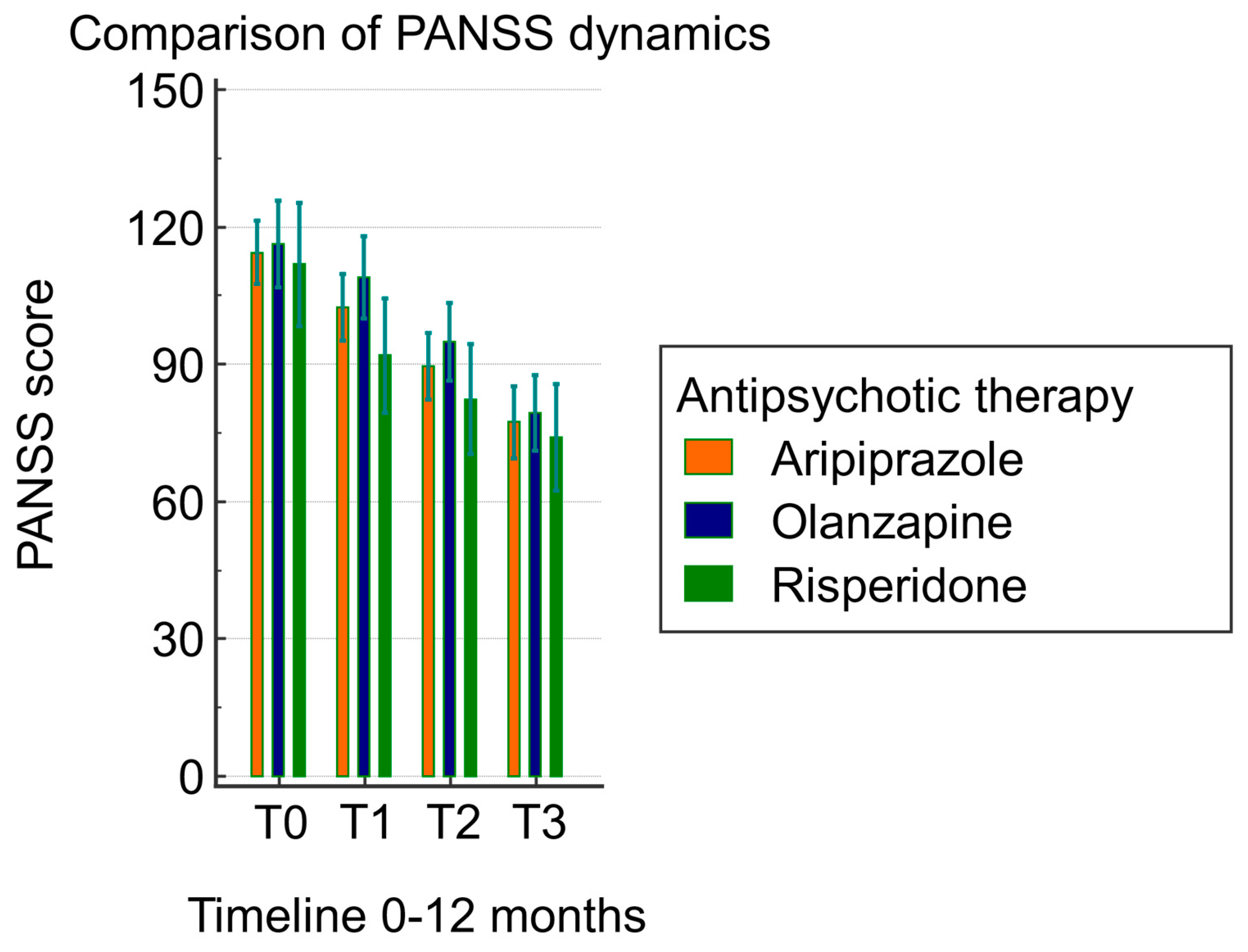
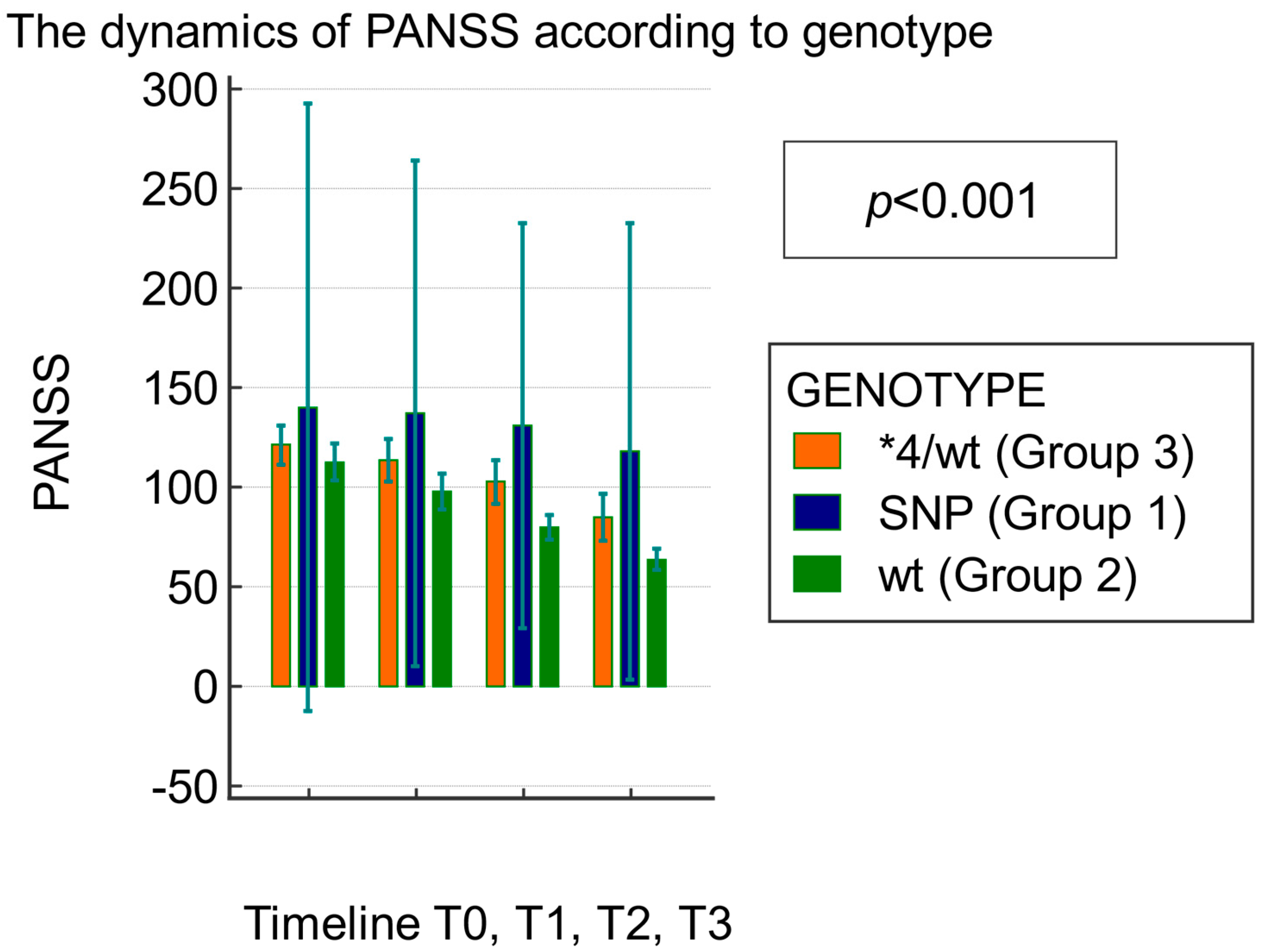
3.1. Clinical Efficacy of Atypical Antipsychotics Assessed Using the PANSS Score Based on the CYP2D6 Genotype
3.2. Adverse Reactions Recorded after Atypical Antipsychotic Medication
4. Discussions
- -
- Risperidone, CYP2D6 phenotype—poor, intermediate, and ultrarapid metabolizers—data are insufficient to calculate dose adjustment, but it is recommended to administer other antipsychotics (e.g., olanzapine, quetiapine, clozapine) or to pay close attention to adverse effects and adjust the dose depending on the patient’s clinical response;
- -
- Olanzapine, CYP2D6 phenotype—poor, intermediate, and ultrarapid metabolizers—there are no recommendations for adjusting the therapeutic dose;
- -
- Aripiprazole, CYP2D6 phenotype—intermediate and ultrarapid metabolizers. There are no recommendations for adjusting the therapeutic dose. Instead, for poor metabolizers, it is advised to reduce the maximum dose to 10mg/day (67% of the maximum recommended daily dose).
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jody, L.; Brown, D.A.B.; Laurence, M.; Angela, S. Psychosis in Children and Adolescents: Psych TLC Department of Psychiatry Division of Child & Adolescent Psychiatry University of Arkansas for Medical Sciences Psychiatric Research Institute. 2014. Available online: https://psychiatry.uams.edu/wp-content/uploads/2022/07/psychosis.pdf (accessed on 6 August 2023).
- McClellan, J. Psychosis in Children and Adolescents. J. Am. Acad. Child Adolesc. Psychiatry 2018, 57, 308–312. [Google Scholar] [CrossRef] [PubMed]
- Schulz, S.C.; Green, M.F.; Nelson, K.J. Schizophrenia and Psychotic Spectrum Disorders; Oxford University Press: Oxford, NY, USA, 2016; Volume XII, 382p. [Google Scholar]
- Altamura, C.A.; Fagiolini, A.; Galderisi, S.; Rocca, P.; Rossi, A. Schizophrenia today: Epidemiology, diagnosis, course and models of care. Off. J. Ital. Soc. Psychopathol. 2014, 20, 223–243. [Google Scholar]
- Courvoisie, H.; Labellarte, M.J.; Riddle, M.A. Psychosis in children: Diagnosis and treatment. Dialog. Clin. Neurosci. 2001, 3, 79–92. [Google Scholar] [CrossRef] [PubMed]
- Sikich, L. Diagnosis and Evaluation of Hallucinations and Other Psychotic Symptoms in Children and Adolescents. Child Adolesc. Psychiatr. Clin. North Am. 2013, 22, 655–673. [Google Scholar] [CrossRef] [PubMed]
- Dobrescu, I. Actualitati in Psihofarmacologia Copilului si Adolescentului; Almatea: Bucharest, Romania, 2004. [Google Scholar]
- Nussbaum, L.A.; Nussbaum, L.M. Management of Psychoses in Children and Adolescents; Editura Artpress: Timisoara, Romania, 2012. [Google Scholar]
- Dobrescu, I. Manual of Child and Adolescent Psychiatry; InfoMedica: Arad, Romania, 2016. [Google Scholar]
- Divac, N.; Prostran, M.; Jakovcevski, I.; Cerovac, N. Second-Generation Antipsychotics and Extrapyramidal Adverse Effects. BioMed Res. Int. 2014, 2014, 656370. [Google Scholar] [CrossRef] [PubMed]
- Davis, J.M.C.N.; Glick, I.D. A meta-analysis of the efficacy of second-generation antipsychotics. In Database of Abstracts of Re-views of Effects (DARE): Quality-Assessed Reviews; Centre for Reviews and Dissemination: York, UK, 2003. Available online: https://www.ncbi.nlm.nih.gov/books/NBK69643/ (accessed on 6 August 2023).
- Leo, R.J.; Regno, P.D. Atypical Antipsychotic Use in the Treatment of Psychosis in Primary Care. Prim Care Companion J. Clin. Psychiatry 2000, 2, 194–204. [Google Scholar] [CrossRef]
- Meltzer, H.Y.; McGurk, S.R. The Effects of Clozapine, Risperidone, and Olanzapine on Cognitive Function in Schizophrenia. Schizophr. Bull. 1999, 25, 233–255. [Google Scholar] [CrossRef]
- Kane, J.M. Antipsychotic medication in the treatment of schizophrenia. Isr. J. Psychiatry Relat. Sci. 1995, 32, 30–37. [Google Scholar]
- Fraguas, D.; Correll, C.U.; Merchán-Naranjo, J.; Rapado-Castro, M.; Parellada, M.; Moreno, C.; Arango, C. Efficacy and safety of sec-ond-generation antipsychotics in children and adolescents with psychotic and bipolar spectrum disorders: Comprehensive re-view of prospective head-to-head and placebo-controlled comparisons. Eur. Neuropsychopharmacol. 2011, 21, 621–645. [Google Scholar] [CrossRef]
- Lally, J.; MacCabe, J.H. Antipsychotic medication in schizophrenia: A review. Br. Med. Bull. 2015, 114, 169–179. [Google Scholar] [CrossRef]
- Leucht, S.; Cipriani, A.; Spineli, L.; Mavridis, D.; Orey, D.; Richter, F.; Samara, M.; Barbui, C.; Engel, R.R.; Geddes, J.R.; et al. Comparative efficacy and tolerability of 15 antipsychotic drugs in schizophrenia: A multiple-treatments meta-analysis. Lancet 2013, 382, 951–962. [Google Scholar] [CrossRef]
- Lambert, B.L.; Cunningham, F.E.; Miller, D.R.; Dalack, G.W.; Hur, K. Diabetes Risk Associated with Use of Olanzapine, Quetiapine, and Risperidone in Veterans Health Administration Patients with Schizophrenia. Am. J. Epidemiol. 2006, 164, 672–681. [Google Scholar] [CrossRef]
- Newcomer, J.W. Second-generation (atypical) antipsychotics and metabolic effects: A comprehensive literature review. CNS Drugs 2005, 19, 1–93. [Google Scholar] [CrossRef]
- Melkersson, K.; Dahl, M.L. Adverse metabolic effects associated with atypical antipsychotics: Literature review and clinical im-plications. Drugs 2004, 64, 701–723. [Google Scholar] [CrossRef]
- Nussbaum, L.A. Modern Treatment Approaches Based on Clinical Staging Model and Their Application in Early Psychosis. Available online: https://snpcar.ro/en/abordari-moderne-de-tratament-bazate-pe-modelul-stadializarii-clinice-si-aplicare-lor-in-psihoza-timpurie/ (accessed on 4 July 2023).
- Zanger, U.M.; Schwab, M. Cytochrome P450 enzymes in drug metabolism: Regulation of gene expression, enzyme activities, and impact of genetic variation. Pharmacol. Ther. 2013, 138, 103–141. [Google Scholar] [CrossRef]
- Bertilsson, L.; Dahl, M.; Dalén, P.; Al-Shurbaji, A. Molecular genetics of CYP2D6: Clinical relevance with focus on psychotropic drugs. Br. J. Clin. Pharmacol. 2002, 53, 111–122. [Google Scholar] [CrossRef] [PubMed]
- Cacabelos, R.; Hashimoto, R.; Takeda, M. Pharmacogenomics of antipsychotics efficacy for schizophrenia. Psychiatry Clin. Neurosci. 2011, 65, 3–19. [Google Scholar] [CrossRef] [PubMed]
- Nussbaum, L.A.; Dumitraşcu, V.; Tudor, A.; Grădinaru, R.; Andreescu, N.; Puiu, M. Molecular study of weight gain related to atypical antipsychotics: Clinical implications of the CYP2D6 genotype. Rom. J. Morphol. Embryol. Rev. Roum. Morphol. Embryol. 2014, 55, 877–884. [Google Scholar]
- Huang, M.L.; Van Peer, A.; Woestenborghs, R.; De Coster, R.; Heykants, J.; Jansen, A.A.; Zylicz, Z.; Visscher, H.W.; Jonkman, J.H.G. Pharmacokinetics of the novel antipsy-chotic agent risperidone and the prolactin response in healthy subjects. Clin. Pharmacol. Ther. 1993, 54, 257–268. [Google Scholar] [CrossRef] [PubMed]
- van der Weide, K.; van der Weide, J. The Influence of the CYP3A4*22 Polymorphism and CYP2D6 Polymorphisms on Serum Concentrations of Aripiprazole, Haloperidol, Pimozide, and Risperidone in Psychiatric Patients. J. Clin. Psychopharmacol. 2015, 35, 228–236. [Google Scholar] [CrossRef]
- Hendset, M.; Molden, E.; Knape, M.; Hermann, M. Serum Concentrations of Risperidone and Aripiprazole in Subgroups Encoding CYP2D6 Intermediate Metabolizer Phenotype. Ther. Drug Monit. 2014, 36, 80–85. [Google Scholar] [CrossRef] [PubMed]
- Callaghan, J.T.; Bergstrom, R.F.; Ptak, L.R.; Beasley, C.M. Olanzapine. Pharmacokinetic and pharmacodynamic profile. Clin. Pharmacokinet. 1999, 37, 177–193. [Google Scholar] [CrossRef] [PubMed]
- Opler, M.G.A.; Yavorsky, C.; Daniel, D.G. Positive and Negative Syndrome Scale (PANSS) Training: Challenges, Solutions, and Future Directions. Innov. Clin. Neurosci. 2017, 14, 77–81. [Google Scholar] [PubMed]
- Regier, D.A.; Kuhl, E.A.; Kupfer, D.J. The DSM-5: Classification and criteria changes. World Psychiatry 2013, 12, 92–98. [Google Scholar] [CrossRef]
- Kaufman, J.; Birmaher, B.; Brent, D.; Rao, U.; Flynn, C.; Moreci, P.; Williamson, D.; Ryan, N. Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime Version (K-SADS-PL): Initial Reliability and Validity Data. J. Am. Acad. Child Adolesc. Psychiatry 1997, 36, 980–988. [Google Scholar] [CrossRef]
- Lindström, E.; Lewander, T.; Malm, U.; Malt, U.F.; Lublin, H.; Ahlfors, U.G. Patient-rated versus clinician-rated side effects of drug treatment in schizophrenia. Clinical validation of a self-rating version of the UKU Side Effect Rating Scale (UKU-SERS-Pat). Nord. J Psychiatry 2001, 55, 5–69. [Google Scholar] [PubMed]
- Salvi, J. Calculated Decisions: Columbia-Suicide Severity Rating Scale (C-SSRS). Emerg. Med. Pract. 2019, 21, CD3–CD4. [Google Scholar]
- Barnes, T.R. A Rating Scale for Drug-Induced Akathisia. Br. J. Psychiatry 1989, 154, 672–676. [Google Scholar] [CrossRef]
- Grootenboer, E.M.; Giltay, E.J.; van der Lem, R.; van Veen, T.; van der Wee, N.J.; Zitman, F.G. Reliability and validity of the Global As-sessment of Functioning Scale in clinical outpatients with depressive disorders. J. Eval. Clin. Pract. 2012, 18, 502–507. [Google Scholar] [CrossRef]
- Liechti, S.; Capodilupo, G.; Opler, D.J.; Opler, M.; Yang, L.H. A Developmental History of the Positive and Negative Syndrome Scale (PANSS). Innov. Clin. Neurosci. 2017, 14, 12–17. [Google Scholar]
- Carbon, M.; Correll, C.U. Clinical predictors of therapeutic response to antipsychotics in schizophrenia. Dialog. Clin. Neurosci. 2014, 16, 505–524. [Google Scholar] [CrossRef] [PubMed]
- Wannasuphoprasit, Y.; Andersen, S.E.; Arranz, M.J.; Catalan, R.; Jurgens, G.; Kloosterboer, S.M.; Rasmussen, H.B.; Bhat, A.; Irizar, H.; Koller, D. CYP2D6 Genetic Variation and Antipsychotic-Induced Weight Gain: A Systematic Review and Meta-Analysis. Front. Psychol. 2021, 12, 768748. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, K.; Müller, D.J. Pharmacogenetics of Antipsychotic Drug Treatment: Update and Clinical Implications. Mol. Neuropsychiatry 2018, 5, 1–26. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Xiang, Q.; Zhao, X.; Ma, L.; Cui, Y. Association between aripiprazole pharmacokinetics and CYP2D6 phenotypes: A systematic review and meta-analysis. J. Clin. Pharm. Ther. 2019, 44, 163–173. [Google Scholar] [CrossRef] [PubMed]
- Xia, L.; Li, W.Z.; Liu, H.Z.; Hao, R.; Zhang, X.Y. Olanzapine Versus Risperidone in Children and Adolescents with Psychosis: A Meta-Analysis of Randomized Controlled Trials. J. Child Adolesc. Psychopharmacol. 2018, 28, 244–251. [Google Scholar] [CrossRef] [PubMed]
- Soria-Chacartegui, P.; Villapalos-García, G.; Zubiaur, P.; Abad-Santos, F.; Koller, D. Genetic Polymorphisms Associated with the Pharmacokinetics, Pharmacodynamics and Adverse Effects of Olanzapine, Aripiprazole and Risperidone. Front. Pharmacol. 2021, 12, 711940. [Google Scholar] [CrossRef] [PubMed]
- Kahn, S.E.; Hull, R.L.; Utzschneider, K.M. Mechanisms linking obesity to insulin resistance and type 2 diabetes. Nature 2006, 444, 840–846. [Google Scholar] [CrossRef]
- Holt, R.I.G. Association Between Antipsychotic Medication Use and Diabetes. Curr. Diabetes Rep. 2019, 19, 96. [Google Scholar] [CrossRef]
- American Diabetes Association; American Psychiatric Association; American Association of Clinical Endocrinologists; North American Association for the Study of Obesity. Consensus development conference on antipsychotic drugs and obesity and diabetes. Diabetes Care 2004, 27, 596–601. [Google Scholar] [CrossRef]
- Gill, S.S. Stable monotherapy with clozapine or Olanzapine increases the incidence of diabetes mellitus in people with schizo-phrenia. Evid. Based Ment. Health 2005, 8, 24. [Google Scholar] [CrossRef][Green Version]
- Austin-Zimmerman, I.; Wronska, M.; Wang, B.; Irizar, H.; Thygesen, J.H.; Bhat, A.; Denaxas, S.; Fatemifar, G.; Finan, C.; Harju-Seppänen, J.; et al. The Influence of CYP2D6 and CYP2C19 Genetic Variation on Diabetes Mellitus Risk in People Taking Antidepressants and Antipsychotics. Genes 2021, 12, 1758. [Google Scholar] [CrossRef]
- Calafato, M.S.; Austin-Zimmerman, I.; Thygesen, J.H.; Sairam, M.; Metastasio, A.; Marston, L.; Abad-Santos, F.; Bhat, A.; Harju-Seppänen, J.; Irizar, H.; et al. The effect of CYP2D6 variation on antipsychotic-induced hyperprolactinaemia: A systematic review and meta-analysis. Pharmacogenom. J. 2020, 20, 629–637. [Google Scholar] [CrossRef]
- Roke, Y.; van Harten, P.N.; Franke, B.; Galesloot, T.E.; Boot, A.M.; Buitelaar, J.K. The effect of the Taq1A variant in the dopamine D2 receptor gene and common CYP2D6 alleles on prolactin levels in risperidone-treated boys. Pharmacogenet. Genom. 2013, 23, 487–493. [Google Scholar] [CrossRef] [PubMed]
- Ozdemir, V.; Bertilsson, L.; Miura, J.; Carpenter, E.; Reist, C.; Harper, P.; Widén, J.; Svensson, J.-O.; Albers, L.J.; Kennedy, J.L.; et al. CYP2D6 genotype in relation to perphenazine con-centration and pituitary pharmacodynamic tissue sensitivity in Asians: CYP2D6-serotonin-dopamine crosstalk revisited. Pharmacogenet. Genom. 2007, 17, 339–347. [Google Scholar] [CrossRef]
- Puangpetch, A.; Vanwong, N.; Nuntamool, N.; Hongkaew, Y.; Chamnanphon, M.; Sukasem, C. CYP2D6 polymorphisms and their influence on risperidone treatment. Pharmacogenom. Pers. Med. 2016, 9, 131–147. [Google Scholar] [CrossRef]
- Bushe, C.; Shaw, M. Prevalence of hyperprolactinaemia in a naturalistic cohort of schizophrenia and bipolar outpatients during treatment with typical and atypical antipsychotics. J. Psychopharmacol. 2007, 21, 768–773. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Lakshmanan, D.A.M.; Khastgir, U.; Nair, R. Management of antipsychotic-induced hyperprolactinaemia. BJPsych Adv. 2017, 23, 278–286. [Google Scholar] [CrossRef]
- Ellingrod, V.L.; Miller, D.; Schultz, S.K.; Wehring, H.; Arndt, S. CYP2D6 polymorphisms and atypical antipsychotic weight gain. Psychiatr. Genet. 2002, 12, 55–58. [Google Scholar] [CrossRef]
- Lane, H.Y.; Liu, Y.C.; Huang, C.L.; Chang, Y.C.; Wu, P.L.; Lu, C.T.; Chang, W. Risperidone-related weight gain: Genetic and nongenetic predictors. J. Clin. Psychopharmacol. 2006, 26, 128–134. [Google Scholar] [CrossRef]
- Jallaq, S.A.; Verba, M.; Strawn, J.R.; Martin, L.J.; DelBello, M.P.; Ramsey, L.B. CYP2D6 Phenotype Influences Aripiprazole Tolerability in Pediatric Patients with Mood Disorders. J. Child Adolesc. Psychopharmacol. 2021, 31, 56–62. [Google Scholar] [CrossRef]
- Correia, C.T.; Almeida, J.P.; Santos, P.E.; Sequeira, A.F.; Marques, C.E.; Miguel, T.S.; Abreu, R.L.; Oliveira, G.G.; Vicente, A.M. Pharmacogenetics of risperidone therapy in autism: Association analysis of eight candidate genes with drug efficacy and adverse drug reactions. Pharmacogenomics J. 2010, 10, 418–430. [Google Scholar] [CrossRef]
- Arango, C.; Robles, O.; Parellada, M.; Fraguas, D.; Ruiz-Sancho, A.; Medina, O.; Zabala, A.; Bombín, I.; Moreno, D. Olanzapine compared to quetiapine in ado-lescents with a first psychotic episode. Eur. Child Adolesc. Psychiatry 2009, 18, 418–428. [Google Scholar] [CrossRef] [PubMed]
- Stroup, T.S.; Gray, N. Management of common adverse effects of antipsychotic medications. World Psychiatry 2018, 17, 341–356. [Google Scholar] [CrossRef] [PubMed]
- US Food and Drug Administration CfDEaR. Table of Pharmacogenomic Biomarkers in Drug Labeling. Available online: http://www.fda.gov/Drugs/ScienceResearch/ResearchAreas/Pharmacogenetics/ucm083378.htm (accessed on 3 January 2024).
- Kakihara, S.; Yoshimura, R.; Shinkai, K.; Matsumoto, C.; Goto, M.; Kaji, K.; Yamada, Y.; Ueda, N.; Ohmori, O.; Nakamura, J. Prediction of response to risperidone treatment with respect to plasma concencentrations of risperidone, catecholamine metabolites, and polymorphism of cytochrome P450 2D6. Int. Clin. Psychopharmacol. 2005, 20, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Rau, T.; Wohlleben, G.; Wuttke, H.; Thuerauf, N.; Lunkenheimer, J.; Lanczik, M.; Eschenhagen, T. Cyp2d6 genotype: Impact on adverse effects and nonresponse during treatment with antidepressants—A pilot study. Clin. Pharmacol. Ther. 2004, 75, 386–393. [Google Scholar] [CrossRef]
| Variable | Value | |
|---|---|---|
| Male % (n) | 51.7% (29/56) | |
| Age (years) | 15.14 ± 2 | |
| Weight (kg) | 69.1 ± 15.6 | |
| Fasting glycemia (mmol/L) | 4.5 ± 0.5 | |
| Insulin (µU/mL) | 14.2 ± 8.1 | |
| TGO (u/L) | 16.3 ± 4.4 | |
| TGP (u/L) | 13.1 ± 4.8 | |
| PANSS | 119.8 ± 32.4 | |
Genotype:
| 3.6% (2/56) 69.6% (39/56) 26.8% (15/56) | |
| Antipsychotic therapy | At baseline | After genotyping |
| 48.2% (27/56)
| 58.9% (33/56)
|
| Factors | Mean Difference | Std. Error | p a | 95% CI a | ||
|---|---|---|---|---|---|---|
| PANSS T0 | - | PANSS T1 | 11.815 | 1.235 | <0.0001 | 8.454 to 15.177 |
| - | PANSS T2 | 24.492 | 1.509 | <0.0001 | 20.383 to 28.602 | |
| - | PANSS T3 | 37.108 | 1.920 | <0.0001 | 31.879 to 42.337 | |
| PANSS T1 | - | PANSS T0 | −11.815 | 1.235 | <0.0001 | −15.177 to −8.454 |
| - | PANSS T2 | 12.677 | 0.972 | <0.0001 | 10.030 to 15.324 | |
| - | PANSS T3 | 25.292 | 1.571 | <0.0001 | 21.014 to 29.571 | |
| PANSS T2 | - | PANSS T0 | −24.492 | 1.509 | <0.0001 | −28.602 to −20.383 |
| - | PANSS T1 | −12.677 | 0.972 | <0.0001 | −15.324 to −10.030 | |
| - | PANSS T3 | 12.615 | 0.967 | <0.0001 | 9.981 to 15.249 | |
| PANSS T3 | - | PANSS T0 | −37.108 | 1.920 | <0.0001 | −42.337 to −31.879 |
| - | PANSS T1 | −25.292 | 1.571 | <0.0001 | −29.571 to −21.014 | |
| - | PANSS T2 | −12.615 | 0.967 | <0.0001 | −15.249 to −9.981 | |
| Test of Between-Subject Effects | ||||||
|---|---|---|---|---|---|---|
| Source of Variation | Sum of Squares | DF | Mean Square | F | p | |
| Groups (therapy) | 2792.316 | 2 | 1396.158 | 1.00 | 0.374 | |
| Test of within-subject effects | ||||||
| Factor | sphericity assumed | 40,892.965 | 3 | 13,630.988 | 220.08 | <0.001 |
| Greenhouse–Geisser | 40,892.965 | 1.7 | 24,045.001 | 220.08 | <0.001 | |
| Group × factor interaction | sphericity assumed | 794.166 | 6 | 132.361 | 2.14 | 0.051 |
| Greenhouse–Geisser | 794.166 | 3.4 | 233.484 | 2.14 | 0.092 | |
| Test of Between-Subject Effects | ||||||
|---|---|---|---|---|---|---|
| Source of Variation | Sum of Squares | DF | Mean Square | F | p | |
| Groups (genotype) | 18,576.847 | 2 | 9288.424 | 10.41 | <0.001 | |
| Test of within-subject effects | ||||||
| Factor | sphericity assumed | 10,157.967 | 3 | 3385.989 | 63.62 | <0.001 |
| Huynh–Feldt | 10,157.967 | 2.4 | 4083.290 | 63.62 | <0.001 | |
| Group × factor interaction | sphericity assumed | 1470.945 | 6 | 245.157 | 4.61 | <0.001 |
| Huynh–Feldt | 1470.945 | 4.9 | 295.645 | 4.61 | 0.001 | |
| Timeline | Genotype | N | Mean PANSS ± Standard Deviation | p |
|---|---|---|---|---|
| T0 | CYP2D6*WT (Group 2) | 39 | 116.70 ± 25.16 | 0.30 |
| WT/*4 (Group 3) | 15 | 125.30 ± 33.20 | ||
| T1 | CYP2D6*WT (Group 2) | 39 | 103.40 ± 23.60 | 0.02 |
| WT/*4 (Group 3) | 15 | 122.20 ± 32.62 | ||
| T2 | CYP2D6*WT (Group 2) | 39 | 90.80 ± 19.40 | 0.0009 |
| WT/*4 (Group 3) | 15 | 115.40 ± 30.37 | ||
| T3 | CYP2D6*WT (Group 2) | 39 | 75.60 ± 12.40 | <0.0001 |
| WT/*4 (Group 3) | 15 | 106.9 ± 26.84 |
| Systems/Apparatus Disorders | Side Effects Frequency | Antipsychotic Therapies after Genotyping | ||
|---|---|---|---|---|
| Overall % (n = 56) | Aripiprazole | Olanzapine | Risperidone | |
| Gastrointestinal disorders | ||||
| Nausea, vomiting, abdominal pain | 10.71% (6/56) | 1.78% (1/56) | 3.57% (2/56) | 5.35% (3/56) |
| Constipation, diarrhea | 19.64% (11/56) | 0% | 3.57% (2/56) | 16.07% (9/56) |
| Salivary hypersecretion | 1.78% (1/56) | 0% | 1.78% (1/56) | 0% |
| Nervous system disorders | ||||
| Headache | 17.85% (10/56) | 0% | 5.35% (3/56) | 12.5% (7/56) |
| Extrapyramidal symptoms | 17.85% (10/56) | 1.78% (1/56) | 0% | 16.07% (9/56) |
| Akathisia, tardive dyskinesia | 3.57% (2/56) | 0% | 0% | 3.57% (2/56) |
| Sleepiness | 16.07% (9/56) | 0% | 8.92% (5/56) | 7.14% (4/56) |
| Vertigo | 17.85% (10/56) | 0% | 7.14% (4/56) | 10.71% (6/56) |
| Adverse cognitive effects | 10.71% (6/56) | 0% | 3.57% (2/56) | 7.14% (4/56) |
| Psychiatric disorders | ||||
| Anxiety | 12.50% (7/56) | 1.78% (1/56) | 1.78% (1/56) | 8.92% (5/56) |
| Agitation | 14.28% (8/56) | 0% | 5.35% (3/56) | 8.92% (5/56) |
| Insomnia | 3.57% (2/56) | 1.78% (1/56) | 1.78% (1/56) | 0% |
| Aggressivity | 10.71% (6/56) | 0% | 5.35% (3/56) | 5.35% (3/56) |
| Suicidal ideation, suicidal attempt | 8.92% (5/56) | 0% | 5.35% (3/56) | 3.57% (2/56) |
| Metabolic disorders | ||||
| Hyperglycemia | 14.28% (8/56) | 0% | 1.78% (1/56) | 12.5% (7/56) |
| Hyponatremia | 1.78% (1/56) | 0% | 1.78% (1/56) | 0% |
| Increase in BMI (body mass index) | 32.1% (18/56) | 0% | 8.92%(5/56) | 23.21% (13/56) |
| Weight loss ≥ 7% | 3.57% (2/56) | 1.78% (1/56) | 1.78% (1/56) | 0% |
| Weight gain ≥ 7% | 10.71% (6/56) | 0% | 1.78% (1/56) | 8.92% (5/56) |
| Cardiovascular disorders | ||||
| Tachycardia/Bradycardia | 23.21% (13/56) | 0% | 5.35% (3/56) | 17.85% (10/56) |
| Arrhythmia/QT interval prolongation | 1.78% (1/56) | 0% | 1.78% (1/56) | 0% |
| Hypotension/Hypertension | 16.07% (9/56) | 0% | 5.35% (3/56) | 10.71% (6/56) |
| Endocrinological disorders | ||||
| Hyperprolactinemia | 25.00% (14/56) | 0% | 7.14% (4/56) | 17.85% (10/56) |
| Hyperthyroidism/Hypothyroidism | 3.57% (2/56) | 0% | 1.78% (1/56) | 1.78% (1/56) |
| Hematological disorders | ||||
| Anemia | 3.57% (2/56) | 1.78% (1/56) | 1.78% (1/56) | 0% |
| Leukopenia | 7.14% (4/56) | 0% | 1.78% (1/56) | 5.35% (3/56) |
| Neutropenia | 7.14% (4/56) | 0% | 1.78% (1/56) | 5.35% (3/56) |
| Thrombocytopenia | 10.71% (6/56) | 0% | 1.78% (1/56) | 5.35% (3/56) |
| Gynecological disorders | ||||
| Dysmenorrhea/Amenorrhea | 21.42% (12/56) | 0% | 7.14% (4/56) | 14.28% (8/56) |
| Galactorrhea/Gynecomastia | 14.28% (8/56) | 0% | 5.35% (3/56) | 12.5% (7/56) |
| Paraclinical analyses | ||||
| Glycosylated hemoglobin increase | 10.71% (6/56) | 0% | 1.78% (1/56) | 8.92% (5/56) |
| Increased creatine phosphokinase | 5.35% (3/56) | 0% | 0% | 5.35% (3/56) |
| Increased triglycerides | 8.92% (5/56) | 0% | 0% | 5.35% (3/56) |
| Increased bilirubin | 3.57% (2/56) | 0% | 1.78% (1/56) | 1.78% (1/56) |
| Increased uric acid | 3.57% (2/56) | 0% | 0% | 3.57% (2/56) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cojocaru, A.; Braha, A.; Jeleriu, R.; Andreescu, N.I.; Puiu, M.; Ageu, L.; Folescu, R.; Zamfir, C.L.; Nussbaum, L.A. The Implications of Cytochrome P450 2D6/CYP2D6 Polymorphism in the Therapeutic Response of Atypical Antipsychotics in Adolescents with Psychosis—A Prospective Study. Biomedicines 2024, 12, 494. https://doi.org/10.3390/biomedicines12030494
Cojocaru A, Braha A, Jeleriu R, Andreescu NI, Puiu M, Ageu L, Folescu R, Zamfir CL, Nussbaum LA. The Implications of Cytochrome P450 2D6/CYP2D6 Polymorphism in the Therapeutic Response of Atypical Antipsychotics in Adolescents with Psychosis—A Prospective Study. Biomedicines. 2024; 12(3):494. https://doi.org/10.3390/biomedicines12030494
Chicago/Turabian StyleCojocaru, Adriana, Adina Braha, Roxana Jeleriu, Nicoleta Ioana Andreescu, Maria Puiu, Luminita Ageu, Roxana Folescu, Carmen Lacramioara Zamfir, and Laura Alexandra Nussbaum. 2024. "The Implications of Cytochrome P450 2D6/CYP2D6 Polymorphism in the Therapeutic Response of Atypical Antipsychotics in Adolescents with Psychosis—A Prospective Study" Biomedicines 12, no. 3: 494. https://doi.org/10.3390/biomedicines12030494
APA StyleCojocaru, A., Braha, A., Jeleriu, R., Andreescu, N. I., Puiu, M., Ageu, L., Folescu, R., Zamfir, C. L., & Nussbaum, L. A. (2024). The Implications of Cytochrome P450 2D6/CYP2D6 Polymorphism in the Therapeutic Response of Atypical Antipsychotics in Adolescents with Psychosis—A Prospective Study. Biomedicines, 12(3), 494. https://doi.org/10.3390/biomedicines12030494







