Longitudinal 8-Epi-Prostaglandin F2-Alpha and Angiogenic Profile Mediator Evaluation during Pregnancy in Women with Suspected or Confirmed Pre-eclampsia
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Patient Selection
2.2. Diagnosis of PE
2.2.1. Diagnosis of Hypertension
2.2.2. Determination of Proteinuria
2.2.3. Obtaining Blood Samples for Angiogenic Profile and Oxidative Stress Mediator Assessment
2.3. Angiogenic and Oxidative Stress Mediator Assessment
2.4. Statistical Analysis
3. Results
3.1. Baseline Clinical Features, Profile Mediators, and Diagnosis at the Time of Inclusion
Baseline Angiogenic Profile and Oxidative Stress Mediators
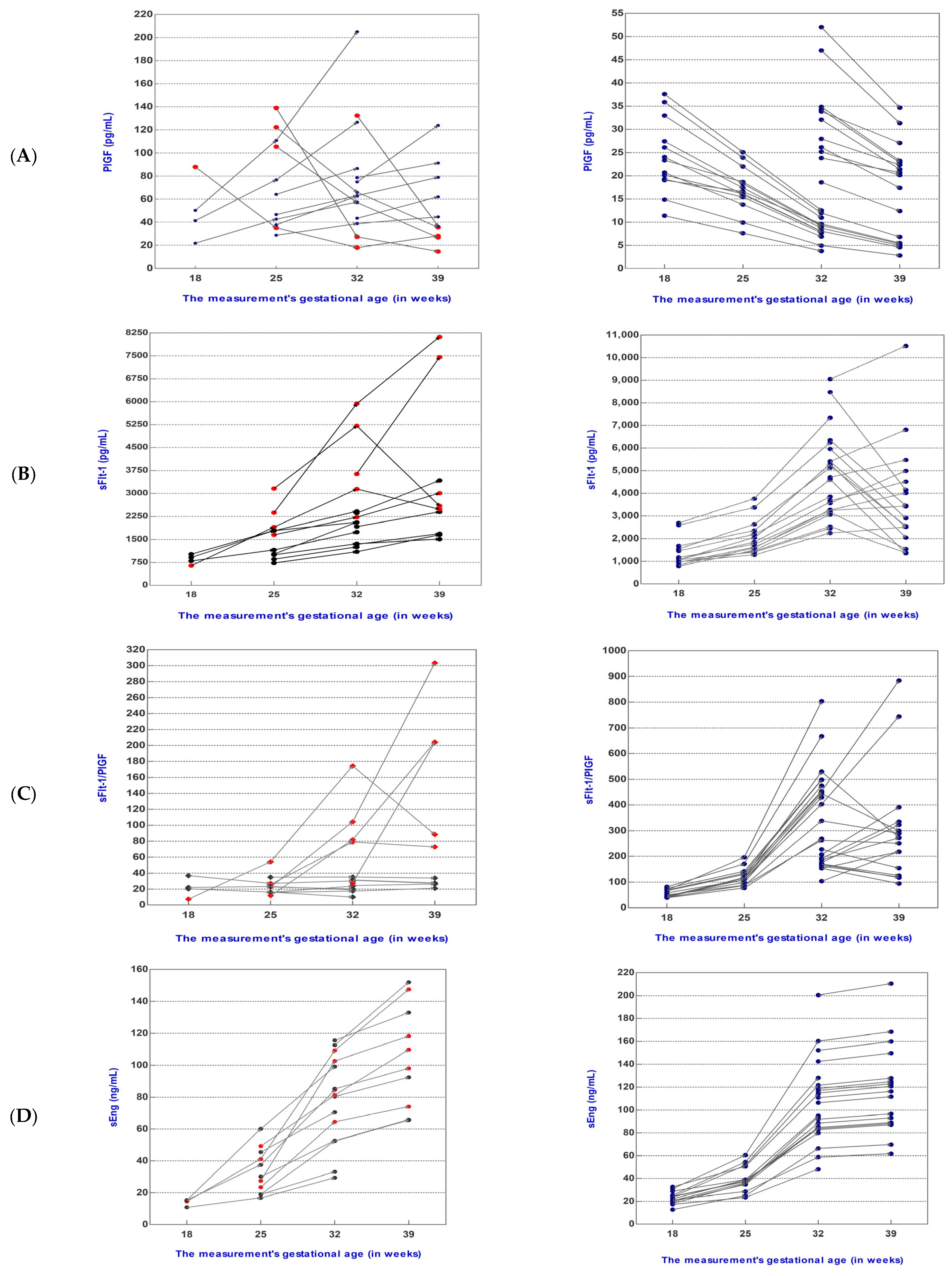
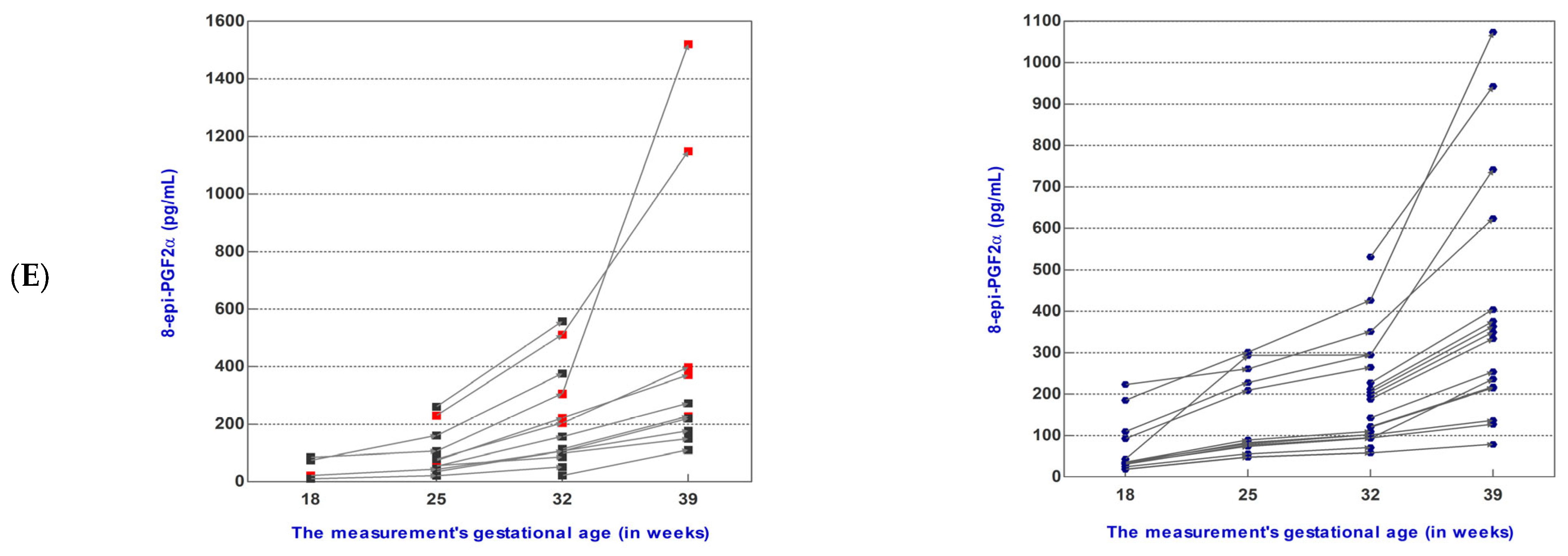
3.2. Angiogenic and Oxidative Stress Mediator Levels during Pregnancy Follow-Up
3.2.1. Dynamics of Mediator Levels during Pregnancy Follow-Up in the sFlt-1/PlGF Ratio ≤38 Group
3.2.2. Dynamics of Mediator Levels during Pregnancy Follow-Up in the sFlt-1/PlGF Ratio >38 Group
3.3. 8-epi-PGF2α Serum Levels Associated Positively with Angiogenic Mediator Levels at Baseline
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- ACOG. Practice Bulletin No. 202: Gestational Hypertension and Preeclampsia. Obstet. Gynecol. 2019, 133, 1. [Google Scholar] [CrossRef]
- Magee, L.A.; Brown, M.A.; Hall, D.R.; Gupte, S.; Hennessy, A.; Karumanchi, S.A.; Kenny, L.C.; McCarthy, F.; Myers, J.; Poon, L.C.; et al. The 2021 International Society for the Study of Hypertension in Pregnancy classification, diagnosis management recommendations for international practice. Pregnancy Hypertens. 2022, 27, 148–169. [Google Scholar] [CrossRef]
- Inversetti, A.; Pivato, C.A.; Cristodoro, M.; Latini, A.C.; Condorelli, G.; Di Simone, N.; Stefanini, G. Update on long-term cardiovascular risk after pre-eclampsia: A systematic review and meta-analysis. Eur. Heart J. Qual. Care Clin. Outcomes 2024, 10, 4–13. [Google Scholar] [CrossRef] [PubMed]
- Portelli, M.; Baron, B. Clinical Presentation of Preeclampsia and the Diagnostic Value of Proteins and Their Methylation Products as Biomarkers in Pregnant Women with Preeclampsia and Their Newborns. J. Pregnancy 2018, 2018, 2632637. [Google Scholar] [CrossRef] [PubMed]
- De Kat, A.C.; Hirst, J.; Woodward, M.; Kennedy, S.; Peters, S.A. Prediction models for preeclampsia: A systematic review. Pregnancy Hypertens. 2019, 16, 48–66. [Google Scholar] [CrossRef]
- Licini, C.; Avellini, C.; Picchiassi, E.; Mensà, E.; Fantone, S.; Ramini, D.; Tersigni, C.; Tossetta, G.; Castellucci, C.; Tarquini, F.; et al. Pre-eclampsia predictive ability of maternal miR-125b: A clinical and experimental study. Transl. Res. 2021, 228, 13–27. [Google Scholar] [CrossRef]
- Vatten, L.J.; Eskild, A.; Nilsen, T.I.; Jeansson, S.; Jenum, P.A.; Staff, A.C. Changes in circulating level of angiogenic factors from the first to second trimester as predictors of preeclampsia. Am. J. Obstet. Gynecol. 2007, 196, 239.e1–239.e2396. [Google Scholar] [CrossRef]
- Allen, R.E.; Rogozinska, E.; Cleverly, K.; Aquilina, J.; Thangaratinam, S. Abnormal blood biomarkers in early pregnancy are associated with preeclampsia: A meta-analysis. Eur. J. Obstet. Gynecol. Reprod. Biol. 2014, 182, 194–201. [Google Scholar] [CrossRef]
- Barton, J.R.; Woelkers, D.A.; Newman, R.B.; Combs, C.A.; How, H.Y.; Boggess, K.A.; Martin, J.N., Jr.; Kupfer, K.; Sibai, B.M.; PETRA (Preeclampsia Triage by Rapid Assay) Trial. Placental growth factor predicts time to delivery in women with signs or symptoms of early preterm preeclampsia: A prospective multicenter study. Am. J. Obstet. Gynecol. 2020, 222, 259.e1–259.e11. [Google Scholar] [CrossRef]
- Agrawal, S.; Shinar, S.; Cerdeira, A.S.; Redman, C.; Vatish, M. Predictive performance of PlGF (placental growth factor) for screening preeclampsia in asymptomatic women: A systematic review and meta-analysis. Hypertension 2019, 74, 1124–1135. [Google Scholar] [CrossRef]
- Alahakoon, T.I.; Zhang, W.; Trudinger, B.J.; Lee, V.W. Discordant clinical presentations of preeclampsia and intrauterine fetal growth restriction with similar pro- and anti-angiogenic profiles. J. Matern. Fetal Neonatal Med. 2014, 27, 1854–1859. [Google Scholar] [CrossRef]
- Herraiz, I.; Llurba, E.; Verlohren, S.; Galindo, A.; Spanish Group for the Study of Angiogenic Markers in Preeclampsia. Update on the Diagnosis and Prognosis of Preeclampsia with the Aid of the sFlt-1/PlGF Ratio in Singleton Pregnancies. Fetal Diagn. Ther. 2018, 43, 81–89. [Google Scholar] [CrossRef]
- The American College of Obstetricians and Gynecologists and the Committee on Obstetric Practice Society for Maternal–Fetal Medicine. ACOG Committee Opinion No. 743: Low-dose aspirin use during pregnancy. Obstet. Gynecol. 2018, 132, e44–e52. [Google Scholar] [CrossRef]
- National Institute of Health and Care Excellence (NICE). NICE Guidelines. PlGF-Based Testing to Help Diagnose Suspected Pre-Eclampsia (Triage PlGF Test, Elecsys Immunoassay sFlt-1/PlGF Ratio, DELFIA Xpress PlGF 1-2-3 Test, and BRAHMSsFlt-1Kryptor/BRAHMS PlGF Plus Kryptor PE Ratio). Available online: https://www.nice.org.uk/guidance/dg49 (accessed on 1 November 2023).
- Gratii, C. 2018 ESC Guidelines for the management of cardiovascular diseases during pregnancy. Eur. Heart J. 2018, 39, 3165–3241. [Google Scholar]
- German Society of Obstetrics and Gynecology (DGGG); Austrian Society of Obstetrics and Gynecology (OEGGG); Swiss Society for Obstetric and Gynecology (SGGG). Guidelines for Hypertensive Disorders in Pregnancy: Diagnosis and Therapy. Available online: https://register.awmf.org/assets/guidelines/015-018l_S2k_Diagnostik_Therapie_hypertensiver_Schwangerschaftserkrankungen_2019-07.pdf (accessed on 1 November 2023).
- Danish Society for Obstetrics and Gynecology: Danish 2018 National Clinical Guideline for Hypertensive Disorders in Pregnancy and Preeclampsia. Available online: https://static1.squarespace.com/static/5467abcce4b056d72594db79/t/5bac84e7652dea0a1b5fb489/1538032877105/180924+PE-guideline-final+sandbjerg.pdf (accessed on 1 November 2023).
- Spanish Society of Gynaecology and Obstetrics (SEGO). Practical assistance guide: Hypertensive disorders in pregnancy. Prog. Obstet. Ginecol. 2020, 63, 244–272. [Google Scholar]
- Venkatesha, S.; Toporsian, M.; Lam, C.; Hanai, J.; Mammoto, T.; Kim, Y.M.; Bdolah, Y.; Lim, K.H.; Yuan, H.T.; Libermann, T.A.; et al. Soluble endoglin contributes to the pathogenesis of preeclampsia. Nat. Med. 2003, 12, 642–649. [Google Scholar] [CrossRef]
- Levine, R.J.; Lam, C.; Qian, C.; Yu, K.F.; Maynard, S.E.; Sachs, B.P.; Sibai, B.M.; Epstein, F.H.; Romero, R.; Thadhani, R.; et al. Soluble endoglin and other circulating antiangiogenic factors in preeclampsia. N. Engl. J. Med. 2006, 355, 992–1005. [Google Scholar] [CrossRef] [PubMed]
- Rana, S.; Powe, C.E.; Salahuddin, S.; Verlohren, S.; Perschel, F.H.; Levine, R.J.; Lim, K.H.; Wenger, J.B.; Thadhani, R.; Karumanchi, S.A. Angiogenic factors and the risk of adverse outcomes in women with suspected preeclampsia. Circulation 2012, 125, 911–919. [Google Scholar] [CrossRef] [PubMed]
- Salahuddin, S.; Lee, Y.; Vadnais, M.; Sachs, B.P.; Karumanchi, S.A.; Lim, K.H. Diagnostic utility of soluble fms-like tyrosine kinase 1 and soluble endoglin in hypertensive diseases of pregnancy. Am. J. Obstet. Gynecol. 2007, 197, 28.e1–28.e286. [Google Scholar] [CrossRef] [PubMed]
- Nikuei, P.; Rajaei, M.; Malekzadeh, K.; Nejatizadeh, A.; Mohseni, F.; AtashAbParvar, A. Accuracy of Soluble Endoglin for Diagnosis of Preeclampsia and its Severity. Iran. Biomed. J. 2017, 21, 312–330. [Google Scholar] [CrossRef] [PubMed]
- Akbar, A.; Herdiyantini, M.; Aditiawarman, A. Comparison of serum soluble endoglin (sEng) level in early onset preeclampsia, late onset preeclampsia and normal pregnant woman. Maj. Obstet. Ginekol. 2018, 25, 10–15. [Google Scholar] [CrossRef]
- Krishnaveni, C.; Kiranmayee, P.; Raghuveer, C.V.; Sheela, S.R.; Kalyani, R.; Venkateshu, K.V. Maternal Serum Soluble Endoglin Levels as a biomarker in Preeclampsia: A Case Control Tertiary Care Hospital Based Study. Biomed. Pharmacol. J. 2022, 15, 1153–1160. [Google Scholar] [CrossRef]
- Schaarschmidt, W.; Rana, S.; Stepan, H. PP052. The course of sFlt-1 and PLGF reflects different progression patterns in early- versus late-onset preeclampsia and HELLP syndrome. Pregnancy Hypertens. 2012, 2, 269. [Google Scholar] [CrossRef]
- Baltajian, K.; Bajracharya, S.; Salahuddin, S.; Berg, A.H.; Geahchan, C.; Wenger, J.B.; Thadhani, R.; Karumanchi, S.A.; Rana, S. Sequential plasma angiogenic factors levels in women with suspected preeclampsia. Am. J. Obstet. Gynecol. 2016, 215, 89.e1–89.e10. [Google Scholar] [CrossRef] [PubMed]
- Verlohren, S.; Herraiz, I.; Lapaire, O.; Schlembach, D.; Zeisler, H.; Calda, P.; Sabria, J.; Markfeld-Erol, F.; Galindo, A.; Schoofs, K.; et al. New gestational phase-specific cutoff values for the use of the soluble fms-like tyrosine kinase-1/placental growth factor ratio as a diagnostic test for preeclampsia. Hypertension 2014, 63, 346–352. [Google Scholar] [CrossRef] [PubMed]
- Herraiz, I.; Simón, E.; Gómez-Arriaga, P.I.; Quezada, M.S.; García-Burguillo, A.; López-Jiménez, E.A.; Galindo, A. Clinical implementation of the sFlt-1/PlGF ratio to identify preeclampsia and fetal growth restriction: A prospective cohort study. Pregnancy Hypertens. 2018, 13, 279–285. [Google Scholar] [CrossRef]
- Zeisler, H.; Llurba, E.; Chantraine, F.; Vatish, M.; Staff, A.C.; Sennström, M.; Olovsson, M.; Brennecke, S.P.; Stepan, H.; Allegranza, D.; et al. Predictive Value of the sFlt-1:PlGF Ratio in Women with Suspected Preeclampsia. N. Engl. J. Med. 2016, 374, 13–22. [Google Scholar] [CrossRef]
- Bian, X.; Biswas, A.; Huang, X.; Lee, K.J.; Li, T.K.; Masuyama, H.; Ohkuchi, A.; Park, J.S.; Saito, S.; Tan, K.H.; et al. Short-Term Prediction of Adverse Outcomes Using the sFlt-1 (Soluble fms-Like Tyrosine Kinase 1)/PlGF (Placental Growth Factor) Ratio in Asian Women with Suspected Preeclampsia. Hypertension 2019, 74, 164–172. [Google Scholar] [CrossRef]
- Zeisler, H.; Llurba, E.; Chantraine, F.J.; Vatish, M.; Staff, A.C.; Sennström, M.; Olovsson, M.; Brennecke, S.P.; Stepan, H.; Allegranza, D.; et al. Soluble fms-like tyrosine kinase-1 to placental growth factor ratio: Ruling out pre-eclampsia for up to 4 weeks and value of retesting. Ultrasound Obstet. Gynecol. 2019, 53, 367–375. [Google Scholar] [CrossRef]
- Cerdeira, A.S.; O’Sullivan, J.; Ohuma, E.O.; Harrington, D.; Szafranski, P.; Black, R.; Mackillop, L.; Impey, L.; Greenwood, C.; James, T.; et al. Randomized Interventional Study on Prediction of Preeclampsia/Eclampsia in Women with Suspected Preeclampsia: INSPIRE. Hypertension 2019, 74, 983–990. [Google Scholar] [CrossRef]
- Cerdeira, A.S.; O’Sullivan, J.; Ohuma, E.O.; James, T.; Papageorghiou, A.T.; Knight, M.; Vatish, M. Performance of soluble fms-like tyrosine kinase-1-to-placental growth factor ratio of ≥85 for ruling in preeclampsia within 4 weeks. Am. J. Obstet. Gynecol. 2021, 224, 322–323. [Google Scholar] [CrossRef]
- Perales, A.; Delgado, J.L.; de la Calle, M.; García-Hernández, J.A.; Escudero, A.I.; Campillos, J.M.; Sarabia, M.D.; Laíz, B.; Duque, M.; Navarro, M.; et al. sFlt-1/PlGF for prediction of early-onset pre-eclampsia: STEPS (Study of Early Pre-eclampsia in Spain). Ultrasound Obstet. Gynecol. 2017, 50, 373–382. [Google Scholar] [CrossRef] [PubMed]
- Ishihara, O.; Hayashi, M.; Osawa, H.; Kobayashi, K.; Takeda, S.; Vessby, B.; Basu, S. Isoprostanes, prostaglandins and tocopherols in pre-eclampsia, normal pregnancy and non-pregnancy. Free. Radic. Res. 2004, 38, 913–918. [Google Scholar] [CrossRef] [PubMed]
- Boldeanu, L.; Văduva, C.-C.; Caragea, D.C.; Novac, M.B.; Manasia, M.; Siloși, I.; Manolea, M.M.; Boldeanu, M.V.; Dijmărescu, A.L. Association between Serum 8-Iso-Prostaglandin F2α as an Oxidative Stress Marker and Immunological Markers in a Cohort of Preeclampsia Patients. Life 2023, 13, 2242. [Google Scholar] [CrossRef]
- Turpin, C.A.; Sakyi, S.A.; Owiredu, W.K.; Ephraim, R.K.; Anto, E.O. Association between adverse pregnancy outcome and imbalance in angiogenic regulators and oxidative stress biomarkers in gestational hypertension and preeclampsia. BMC Pregnancy Childbirth 2015, 15, 189. [Google Scholar] [CrossRef] [PubMed]
- Sakyi, S.A.; Owiredu, W.K.; Anto, E.O.; Turpin, C.A.; Ephraim, R.K.; Linda, A.F. Individual and combined diagnostic accuracy of biochemical markers for detecting early onset preeclampsia. SOJ Gynecol. Obstet. Women’s Health 2016, 2, 9. [Google Scholar] [CrossRef]
- Anto, E.O.; Owiredu, W.K.B.A.; Sakyi, S.A.; Turpin, C.A.; Ephraim, R.K.D.; Fondjo, L.A.; Obirikorang, C.; Adua, E.; Acheampong, E. Adverse pregnancy outcomes and imbalance in angiogenic growth mediators and oxidative stress biomarkers is associated with advanced maternal age births: A prospective cohort study in Ghana. PLoS ONE 2018, 13, e0200581. [Google Scholar] [CrossRef]
- Anto, E.O.; Coall, D.A.; Addai-Mensah, O.; Wiafe, Y.A.; Owiredu, W.K.B.A.; Obirikorang, C.; Annani-Akollor, M.E.; Adua, E.; Tawiah, A.; Acheampong, E.; et al. Suboptimal Health Study Consortium (SHSC). Early gestational profiling of oxidative stress and angiogenic growth mediators as predictive, preventive and personalised (3P) medical approach to identify suboptimal health pregnant mothers likely to develop preeclampsia. EPMA J. 2021, 12, 517–534. [Google Scholar] [CrossRef] [PubMed]
- Anto, E.O.; Ofori Boadu, W.I.; Addai-Mensah, O.; Wiafe, Y.A.; Owiredu, W.K.; Obirikorang, C.; Annani-Akollor, M.E.; Adua, E.; Appiah, M.; Opoku, S.; et al. Association between micronutrients, oxidative stress biomarkers and angiogenic growth mediators in early and late-onset preeclamptic Ghanaian women. SAGE Open Med. 2023, 11, 20503121231175759. [Google Scholar] [CrossRef]
- Zeisler, H.; Llurba, E.; Chantraine, F.; Vatish, M.; Staff, A.C.; Sennström, M.; Olovsson, M.; Brennecke, S.P.; Stepan, H.; Allegranza, D.; et al. Soluble fms-Like Tyrosine Kinase-1-to-Placental Growth Factor Ratio and Time to Delivery in Women With Suspected Preeclampsia. Obstet. Gynecol. 2016, 128, 261–269. [Google Scholar] [CrossRef]
- Peguero, A.; Fernandez-Blanco, L.; Mazarico, E.; Benitez, L.; Gonzalez, A.; Youssef, L.; Crispi, F.; Hernandez, S.; Figueras, F. Added prognostic value of longitudinal changes of angiogenic factors in early-onset severe pre-eclampsia: A prospective cohort study. BJOG 2021, 128, 158–165. [Google Scholar] [CrossRef]
- Meler, E.; Scazzocchio, E.; Peguero, A.; Triunfo, S.; Gratacos, E.; Figueras, F. Role of maternal plasma levels of placental growth factor for the prediction of maternal complications in preeclampsia according to the gestational age at onset. Prenat. Diagn. 2014, 34, 706–710. [Google Scholar] [CrossRef] [PubMed]
- Saleh, L.; van den Meiracker, A.H.; Geensen, R.; Kaya, A.; Roeters van Lennep, J.E.; Duvekot, J.J.; Verdonk, K.; Steegers, E.A.P.; Russcher, H.; Danser, A.H.J. Soluble fms-like tyrosine kinase-1 and placental growth factor kinetics during and after pregnancy in women with suspected or confirmed pre-eclampsia. Ultrasound Obstet. Gynecol. 2018, 51, 751–757. [Google Scholar] [CrossRef] [PubMed]
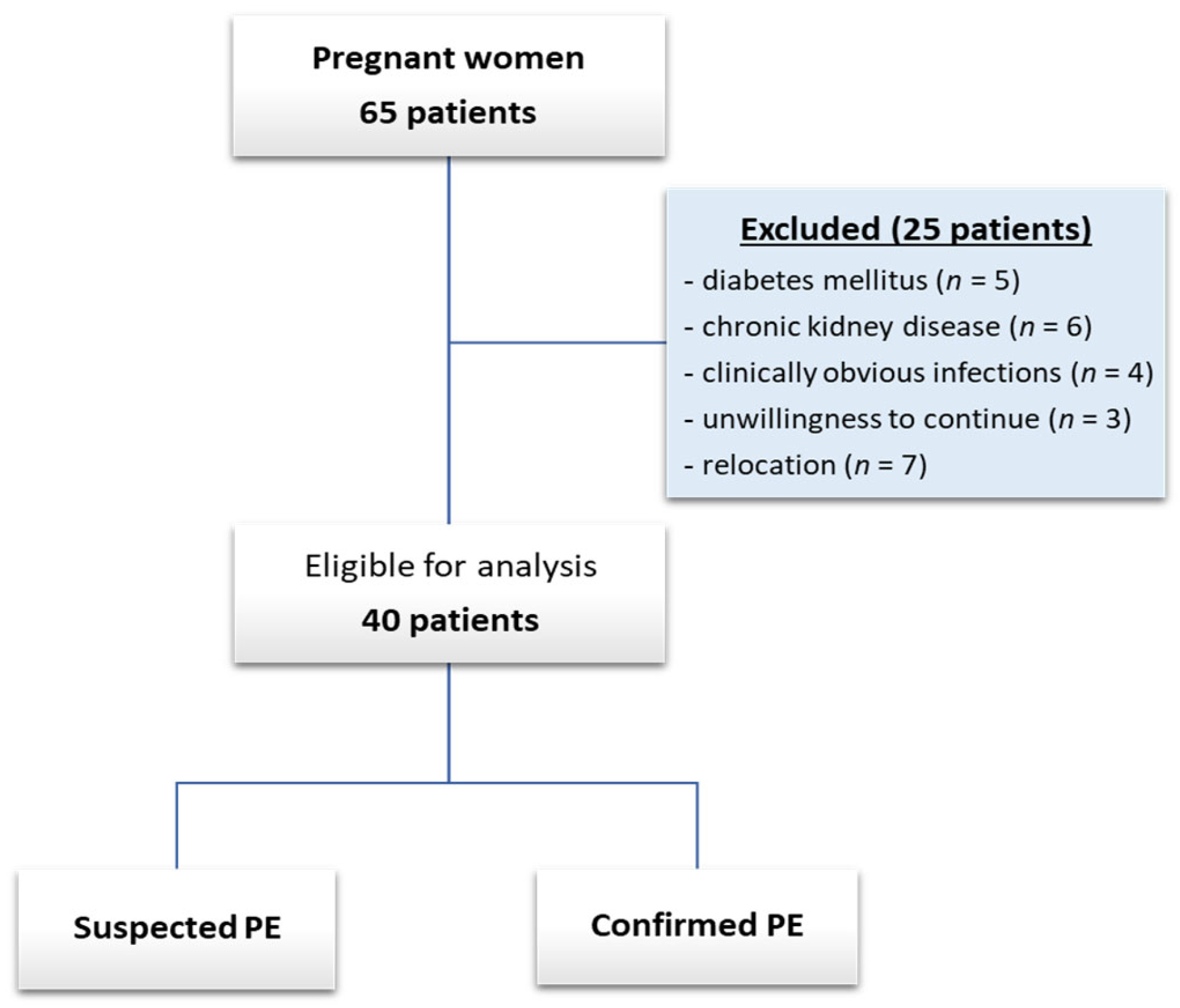
| Baseline Parameter | All Pregnant Women (n = 40) | sFlt-1/PlGF Ratio of ≤38 (n = 15) | sFlt-1/PlGF Ratio of >38 (n = 25) | p-Value |
|---|---|---|---|---|
| Clinical and paraclinical features | ||||
| Maternal age (years) [median (range)] | 29 (18–41) | 32 (20–41) | 28 (18–36) | 0.038 * |
| Gestational age (years) [median (range)] | 24 (18–28) | 23 (18–28) | 24 (18–28) | 0.287 ns |
| History of PE | 6 (24%) | - | 6 (24%) | - |
| Pre-existing hypertension | 7 (17.5%) | 3 (20%) | 4 (16%) | - |
| SBP [mmHg] (mean ± SD) | 143.00 ± 14.90 | 132.00 ± 15.80 | 149.00 ± 8.40 | 0.001 * |
| DBP [mmHg] (mean ± SD) | 89.70 ± 7.80 | 85.30 ± 6.10 | 92.40 ± 7.50 | 0.036 * |
| Platelets [109/L] (mean ± SD) | 248 (147–368) | 252 (165–322) | 212 (147–368) | 0.589 ns |
| AST [U/L] (mean ± SD) | 42.50 ± 20.50 | 28.10 ± 7.58 | 51.10 ± 21.10 | <0.0001 ** |
| ALT [U/L] (mean ± SD) | 46.90 ± 21.10 | 31.90 ± 11.60 | 55.90 ± 20.40 | 0.010 * |
| Creatinine [mg/dL] (mean ± SD) | 0.77 ± 0.13 | 0.68 ± 0.12 | 0.83 ± 0.11 | 0.008 * |
| Urea [mg/dL] (mean ± SD) | 30.80 ± 8.59 | 26.50 ± 7.41 | 33.30 ± 8.36 | 0.054 ns |
| Baseline angiogenic profile and oxidative stress mediators | ||||
| sFlt-1 [pg/mL] (mean ± SD) | 2586.00 ± 2182.00 | 1565.00 ± 934.20 | 3199.00 ± 2487.00 | 0.024 * |
| PlGF [pg/mL] (mean ± SD) | 44.50 ± 32.23 | 71.70 ± 38.32 | 28.17 ± 9.56 | <0.0001 ** |
| sFlt-1/PlGF [median (range)] | 44.24 (7.39–227.00) | 22.72 (7.39–36.77) | 76.00 (39.38–227.00) | <0.0001 ** |
| sEng [ng/mL] (mean ± SD) | 59.62 ± 51.40 | 44.24 ± 36.51 | 68.86 ± 57.26 | 0.131 ns |
| 8-epi-PGF2α [pg/mL] [median (range)] | 89.09 (10.98–531.20) | 74.67 (10.98–304.30) | 120.90 (19.06–531.20) | 0.039 * |
| PlGF/sFlt-1 [median (range)] | 0.023 (0.004–0.140) | 0.040 (0.030–0.140) | 0.013 (0.004–0.025) | <0.0001 ** |
| sEng/PlGF [median (range)] | 1.20 (0.16–8.41) | 0.41 (0.16–1.86) | 1.59 (0.46–8.41) | 0.0003 * |
| 8-epi-PGF2α/PlGF [median (range)] | 2.27 (0.26–15.70) | 1.45 (0.26–6.87) | 3.87 (0.51–15.70) | 0.003 * |
| Diagnosis at the time of inclusion | ||||
| Suspected PE [n (%)] | 25 (62.50%) | 10 (66.67%) | 15 (60%) | - |
| Gestational hypertension [n (%)] | 10 (25%) | - | 10 (40%) | - |
| PE/HELLP syndrome [n (%)] | 10 (25%) | 3 (20%) | 7 (28%) | - |
| Parameter | sFlt-1/PlGF Ratio of ≤38 Group | sFlt-1/PlGF Ratio of >38 Group | ||||||
|---|---|---|---|---|---|---|---|---|
| 18w | 25w | 32w | 39w | 18w | 25w | 32w | 39w | |
| sFlt-1 (mean) | 848.00 | 1587.00 | 2519.00 | 3434.00 | 1352.00 | 2058.00 | 4684.00 | 3771.00 |
| PlGF (mean) | 50.40 | 73.61 | 75.88 | 54.26 | 24.90 | 17.48 | 19.18 | 16.68 |
| sFlt-1/PlGF (median) | 21.22 | 22.72 | 30.23 | 53.34 | 48.75 | 115.70 | 268.80 | 271.80 |
| sEng (mean) | 13.96 | 33.59 | 78.24 | 105.60 | 23.05 | 39.56 | 108.30 | 117.50 |
| 8-epi-PGF2α (median) | 48.63 | 74.22 | 157.30 | 250.60 | 34.36 | 82.08 | 188.20 | 334.10 |
| PlGF/sFlt-1 (median) | 0.05 | 0.04 | 0.03 | 0.02 | 0.021 | 0.009 | 0.004 | 0.004 |
| sEng/PlGF (median) | 0.34 | 0.41 | 1.18 | 1.76 | 0.99 | 2.29 | 7.62 | 6.88 |
| 8-epi-PGF2α/PlGF (median) | 0.87 | 0.93 | 3.10 | 6.97 | 1.76 | 7.12 | 9.87 | 17.37 |
| Parameter | sFlt-1 | PlGF | sFlt-1/PlGF | sEng | 8-epi-PGF2α |
|---|---|---|---|---|---|
| sFlt-1 | rho = −0.754 p = 0.001 * | rho = 0.248 p = 0.373 | rho = 0.466 p = 0.056 ** | rho = 0.388 p = 0.053 ** | |
| PlGF | rho = −0.372 p = 0.172 | rho = 0.194 p = 0.488 | rho = 0.407 p = 0.132 | ||
| sFlt-1/PlGF | rho = 0.374 p = 0.170 | rho = 0.219 p = 0.046 * | |||
| sEng | rho = 0.330 p = 0.230 |
| Parameter | sFlt-1 | PlGF | sFlt-1/PlGF | sEng | 8-epi-PGF2α |
|---|---|---|---|---|---|
| sFlt-1 | rho = −0.650 p < 0.0001 * | rho = 0.856 p < 0.0001 * | rho = 0.669 p < 0.0001 * | rho = 0.724 p < 0.0001 * | |
| PlGF | rho = −0.248 p = 0.232 | rho = 0.285 p = 0.166 | rho = 0.471 p = 0.147 | ||
| sFlt-1/PlGF | rho = 0.306 p = 0.113 | rho = 0.529 p = 0.037 * | |||
| sEng | rho = 0.675 p < 0.0001 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dijmărescu, A.L.; Tănase, F.; Novac, M.B.; Siminel, M.A.; Rotaru, I.; Caragea, D.C.; Manolea, M.M.; Văduva, C.-C.; Boldeanu, M.V.; Boldeanu, L. Longitudinal 8-Epi-Prostaglandin F2-Alpha and Angiogenic Profile Mediator Evaluation during Pregnancy in Women with Suspected or Confirmed Pre-eclampsia. Biomedicines 2024, 12, 433. https://doi.org/10.3390/biomedicines12020433
Dijmărescu AL, Tănase F, Novac MB, Siminel MA, Rotaru I, Caragea DC, Manolea MM, Văduva C-C, Boldeanu MV, Boldeanu L. Longitudinal 8-Epi-Prostaglandin F2-Alpha and Angiogenic Profile Mediator Evaluation during Pregnancy in Women with Suspected or Confirmed Pre-eclampsia. Biomedicines. 2024; 12(2):433. https://doi.org/10.3390/biomedicines12020433
Chicago/Turabian StyleDijmărescu, Anda Lorena, Florentina Tănase, Marius Bogdan Novac, Mirela Anişoara Siminel, Ionela Rotaru, Daniel Cosmin Caragea, Maria Magdalena Manolea, Constantin-Cristian Văduva, Mihail Virgil Boldeanu, and Lidia Boldeanu. 2024. "Longitudinal 8-Epi-Prostaglandin F2-Alpha and Angiogenic Profile Mediator Evaluation during Pregnancy in Women with Suspected or Confirmed Pre-eclampsia" Biomedicines 12, no. 2: 433. https://doi.org/10.3390/biomedicines12020433
APA StyleDijmărescu, A. L., Tănase, F., Novac, M. B., Siminel, M. A., Rotaru, I., Caragea, D. C., Manolea, M. M., Văduva, C.-C., Boldeanu, M. V., & Boldeanu, L. (2024). Longitudinal 8-Epi-Prostaglandin F2-Alpha and Angiogenic Profile Mediator Evaluation during Pregnancy in Women with Suspected or Confirmed Pre-eclampsia. Biomedicines, 12(2), 433. https://doi.org/10.3390/biomedicines12020433







