The Comparative Effects of Myo-Inositol and Metformin Therapy on the Clinical and Biochemical Parameters of Women of Normal Weight Suffering from Polycystic Ovary Syndrome
Abstract
1. Introduction
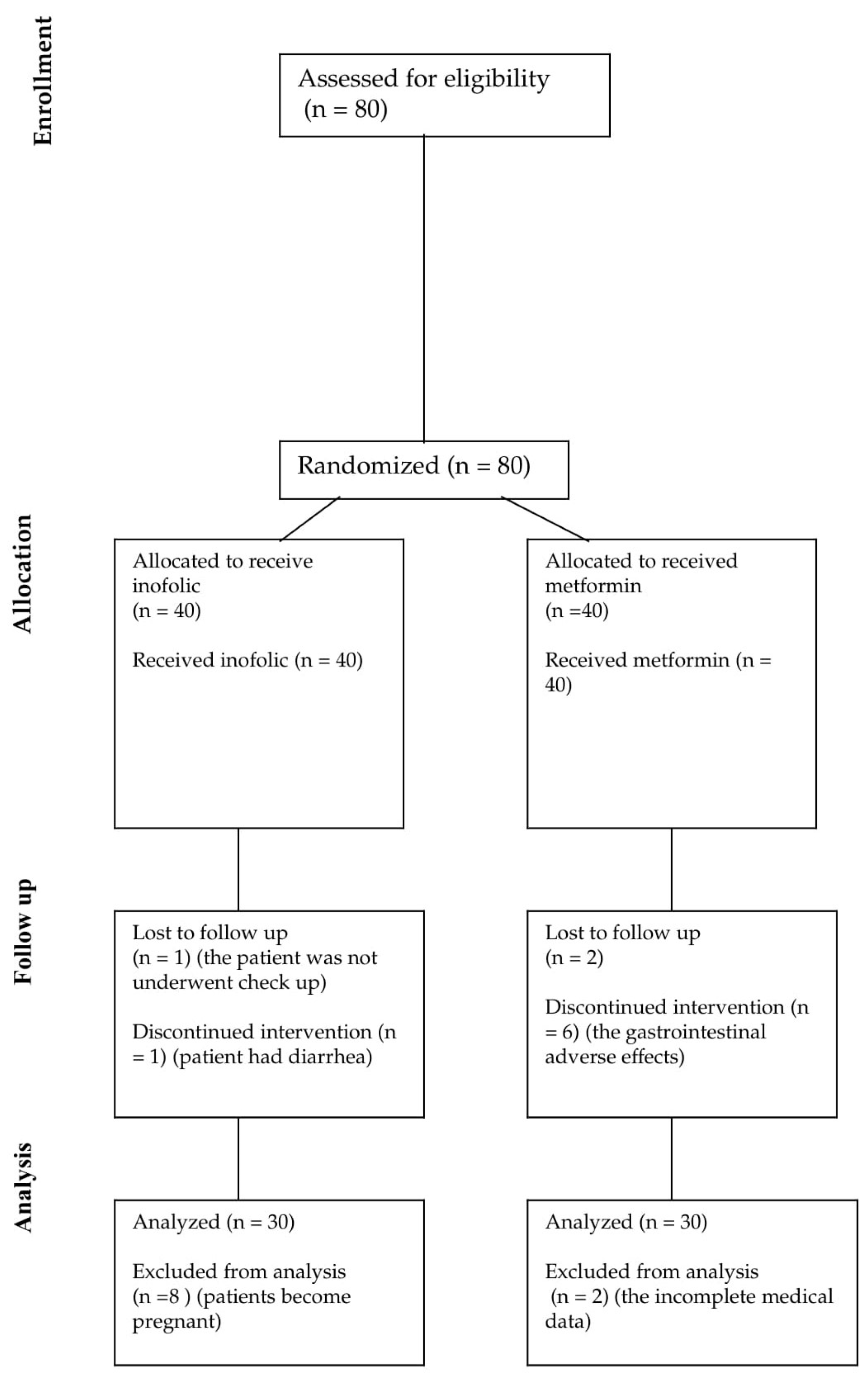
2. Materials and Methods
2.1. Patients
2.2. Study Protocol
2.2.1. Diagnostic Criteria and Normal Values
2.2.2. The Methods of Measuring Hormones Levels
2.3. Data Analysis Methods
3. Results
4. Discussion
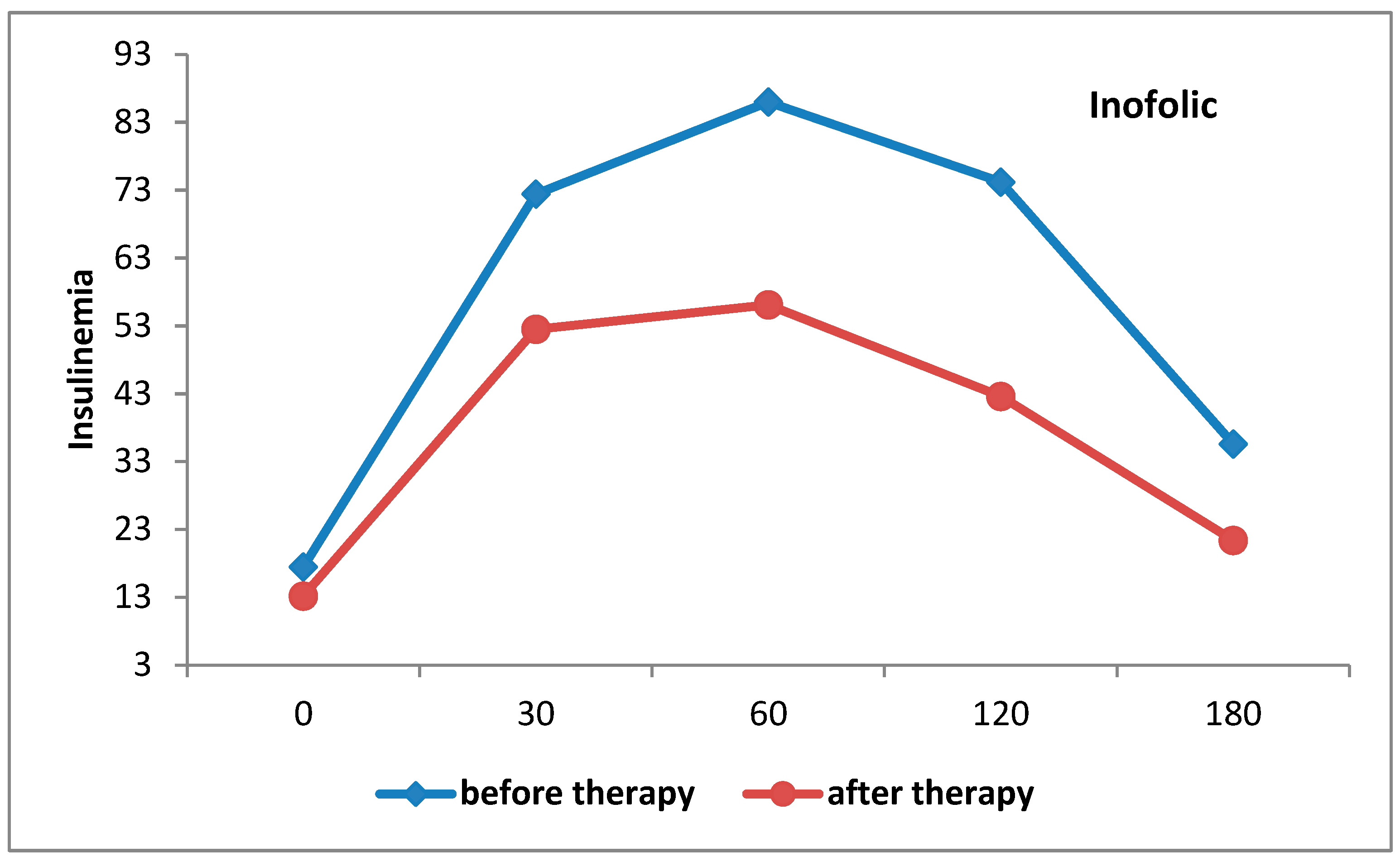
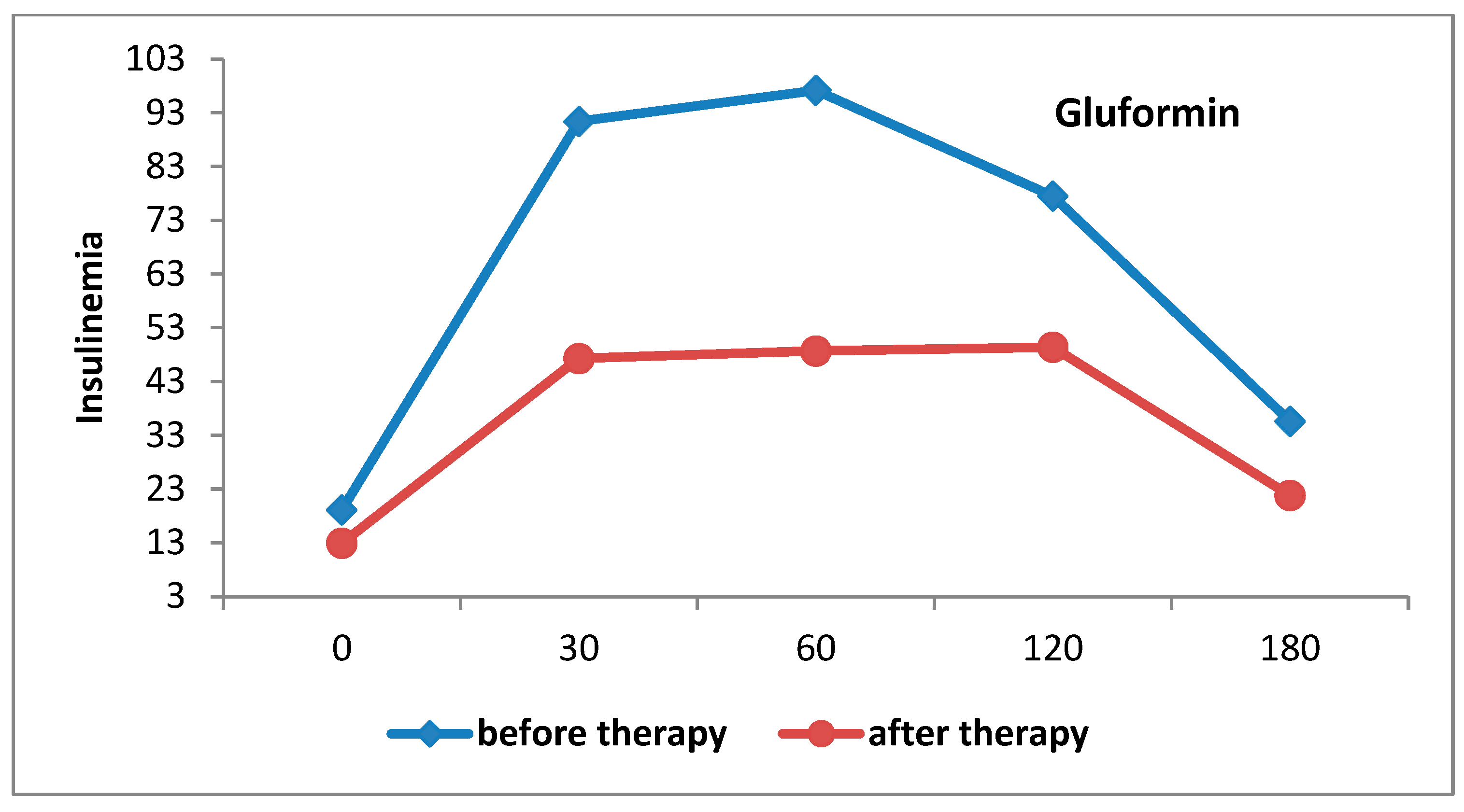
4.1. Metabolic Changes
4.2. Changes in the Androgens
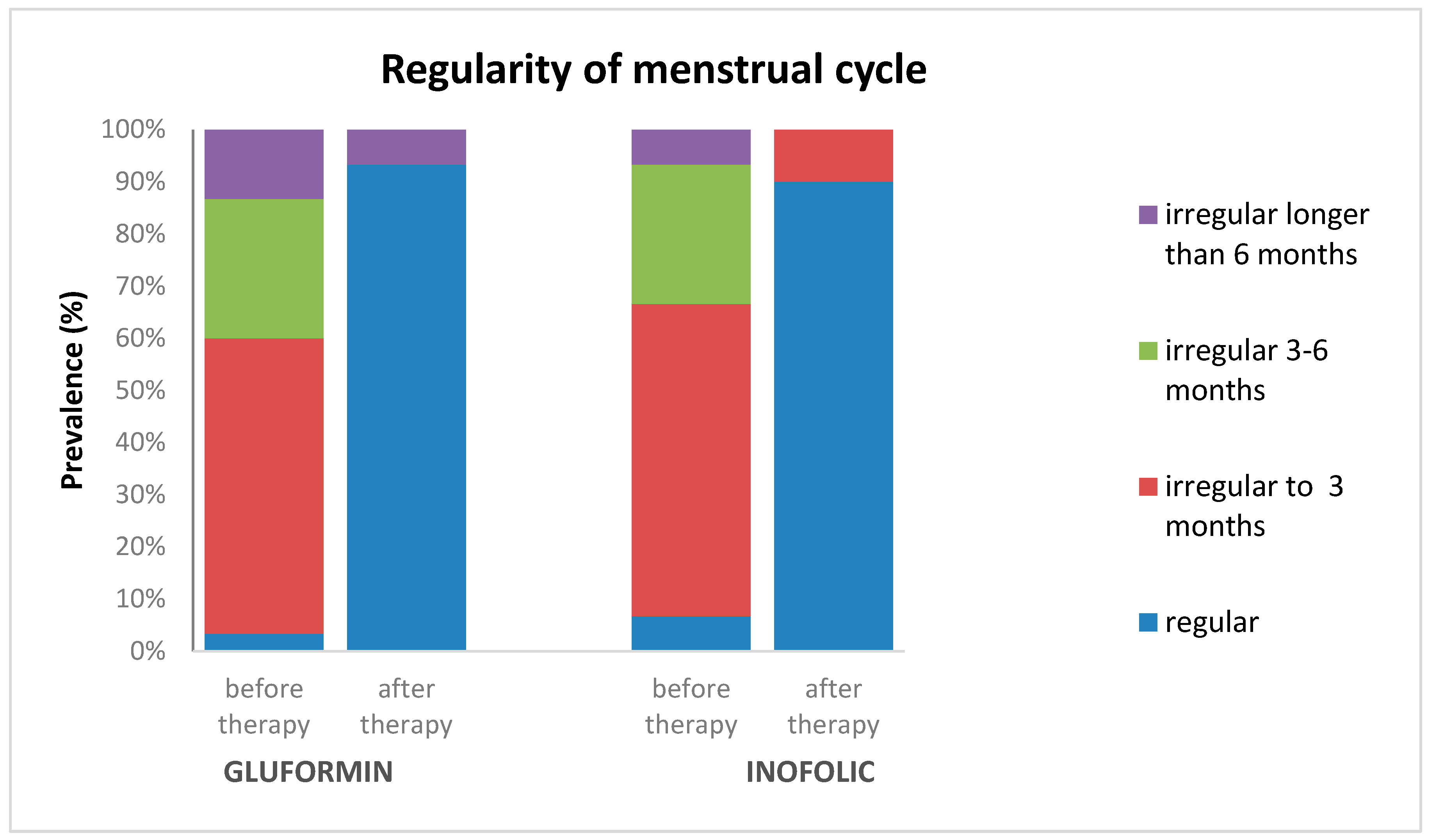
4.3. Menstrual Cycle Regularity
4.4. Adverse Effects, Non-Completion, and Pregnancy
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Teede, H.J.; Misso, M.L.; Costello, M.F.; Dokras, A.; Laven, J.; Moran, L.; Piltonen, T.; Norman, R.J.; on behalf of the International PCOS Network. Recommendations from the international evidence-based guideline for the assessment and management of polycystic ovary syndrome. Fertil. Steril. 2018, 110, 364–379. [Google Scholar] [CrossRef]
- Teede, H.J.; Tay, C.T.; Laven, J.J.; Dokras, A.; Moran, L.J.; Piltonen, T.T.; Costello, M.F.; Boivin, J.; Redman, L.M.; Boyle, J.; et al. Recommendations from the 2023 International Evidence-based Guideline for the Assessment and Management of Polycystic Ovary Syndrome. J. Clin. Endocrinol. Metab. 2023, 108, 2447–2469. [Google Scholar] [CrossRef]
- The Rotterdam ESHRE/ASRM-Sponsored PCOS Consensus Workshop Group. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome (PCOS). Hum. Reprod. 2004, 19, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Carmina, E.; Lobo, R.A. Comparing Lean and Obese PCOS in Different PCOS Phenotypes: Evidence That the Body Weight Is More Important than the Rotterdam Phenotype in Influencing the Metabolic Status. Diagnostics 2022, 12, 2313. [Google Scholar] [CrossRef]
- Armanini, D.; Boscaro, M.; Bordin, L.; Sabbadin, C. Controversies in the Pathogenesis, Diagnosis and Treatment of PCOS: Focus on Insulin Resistance, Inflammation, and Hyperandrogenism. Int. J. Mol. Sci. 2022, 23, 4110. [Google Scholar] [CrossRef]
- Facchinetti, F.; Orrù, B.; Grandi, G.; Unfer, V. Short-term effects of metformin and myo-inositol in women with polycystic ovarian syndrome (PCOS): A meta-analysis of randomized clinical trials. Gynecol. Endocrinol. 2019, 35, 198–206. [Google Scholar] [CrossRef]
- Sadeghi, H.M.; Adeli, I.; Calina, D.; Docea, A.O.; Mousavi, T.; Daniali, M.; Nikfar, S.; Tsatsakis, A.; Abdollahi, M. Polycystic Ovary Syndrome: A Comprehensive Review of Pathogenesis, Management, and Drug Repurposing. Int. J. Mol. Sci. 2022, 23, 583. [Google Scholar] [CrossRef] [PubMed]
- Stepto, N.K.; Cassar, S.; Joham, A.E.; Hutchison, S.K.; Harrison, C.L.; Goldstein, R.F.; Teede, H.J. Women with polycystic ovary syndrome have intrinsic insulin resistance on euglycaemic-hyperinsulaemic clamp. Hum. Reprod. 2013, 28, 777–784. [Google Scholar] [CrossRef]
- Toosy, S.; Sodi, R.; Pappachan, J.M. Lean polycystic ovary syndrome (PCOS): An evidence-based practical approach. J. Diabetes Metab. Disord. 2018, 17, 277–285. [Google Scholar] [CrossRef]
- Spritzer, P.M.; Marchesan, L.B.; Santos, B.R.; Fighera, T.M. Hirsutism, Normal Androgens and Diagnosis of PCOS. Diagnostics 2022, 12, 1922. [Google Scholar] [CrossRef]
- Bailey, C.J.; Turner, R.C. Metformin. N. Engl. J. Med. 1996, 334, 574–579. [Google Scholar] [CrossRef]
- Velazquez, E.; Mendoza, S.; Hamer, T.; Sosa, F.; Glueck, C. Metformin therapy in polycystic ovary syndrome reduces hyperinsulinemia, insulin resistance, hyperandrogenemia, and systolic blood pressure, while facilitating normal menses and pregnancy. Metabolism 1994, 43, 647–654. [Google Scholar] [CrossRef]
- Attia, G.R.; E Rainey, W.; Carr, B.R. Metformin directly inhibits androgen production in human thecal cells. Fertil. Steril. 2001, 76, 517–524. [Google Scholar] [CrossRef] [PubMed]
- Greff, D.; Juhász, A.E.; Váncsa, S.; Váradi, A.; Sipos, Z.; Szinte, J.; Park, S.; Hegyi, P.; Nyirády, P.; Ács, N.; et al. Inositol is an effective and safe treatment in polycystic ovary syndrome: A systematic review and meta-analysis of randomized controlled trials. Reprod. Biol. Endocrinol. 2023, 21, 10. [Google Scholar] [CrossRef]
- Kutenaei, M.A.; Teshnizi, S.H.; Ghaemmaghami, P.; Eini, F.; Roozbeh, N. The effects of myo-inositol vs. metformin on the ovarian function in the polycystic ovary syndrome: A systematic review and meta-analysis. Eur. Rev. Med. Pharmacol. Sci. 2021, 25, 3105–3115. [Google Scholar]
- Scherer. Ueber eine neue, aus dem Muskelfleische gewonnene Zuckerart. Justus Liebigs Ann. Chem. 1850, 73, 322–328. [Google Scholar] [CrossRef]
- Ijuin, T.; Takenawa, T. Regulation of Insulin Signaling and Glucose Transporter 4 (GLUT4) Exocytosis by Phosphatidylinositol 3,4,5-Trisphosphate (PIP3) Phosphatase, Skeletal Muscle, and Kidney Enriched Inositol Polyphosphate Phosphatase (SKIP). J. Biol. Chem. 2012, 287, 6991–6999. [Google Scholar] [CrossRef]
- Milewska, E.M.; Czyzyk, A.; Meczekalski, B.; Genazzani, A.D. Inositol and human reproduction. From cellular metabolism to clinical use. Gynecol. Endocrinol. 2016, 32, 690–695. [Google Scholar] [CrossRef]
- Christ, J.P.; Cedars, M.I. Current Guidelines for Diagnosing PCOS. Diagnostics 2023, 13, 1113. [Google Scholar] [CrossRef]
- Lizneva, D.; Suturina, L.; Walker, W.; Brakta, S.; Gavrilova-Jordan, L.; Azziz, R. Criteria, prevalence, and phenotypes of polycystic ovary syndrome. Fertil. Steril. 2016, 106, 6–15. [Google Scholar] [CrossRef]
- Matthews, D.R.; Hosker, J.P.; Rudenski, A.S.; Naylor, B.A.; Treacher, D.F.; Turner, R.C. Homeostasis model assessment: Insulin resistance and ?-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985, 28, 412–419. [Google Scholar] [CrossRef] [PubMed]
- Gardner, D.G.; Shoback, D. Normal Hormone Reference Ranges. Greenspan’s Basic & Clinical Endocrinology, 10th ed.; McGraw-Hill Education: New York, NY, USA, 2017. [Google Scholar]
- Purves, R.D. Optimum numerical integration methods for estimation of area-under-the-curve (AUC) and area-under-the-moment-curve (AUMC). J. Pharmacokinet. Biopharm. 1992, 20, 211–226. [Google Scholar] [CrossRef]
- Ravn, P.; Gram, F.; Andersen, M.S.; Glintborg, D. Myoinositol vs. Metformin in Women with Polycystic Ovary Syndrome: A Randomized Controlled Clinical Trial. Metabolites 2022, 12, 1183. [Google Scholar] [CrossRef] [PubMed]
- Coelho, M.; Oliveira, T.; Fernandes, R. State of the art paper Biochemistry of adipose tissue: An endocrine organ. Arch. Med. Sci. 2013, 2, 191–200. [Google Scholar] [CrossRef]
- De Leo, V.; Musacchio, M.C.; Cappelli, V.; Di Sabatino, A.; Tosti, C.; Piomboni, P. A Combined Treatment with Myo-Inositol and Monacolin K Improve the Androgen and Lipid Profiles of Insulin-Resistant PCOS Patients. J. Metab. Syndr. 2013, 2, 127. [Google Scholar] [CrossRef]
- Fruzzetti, F.; Perini, D.; Russo, M.; Bucci, F.; Gadducci, A. Comparison of two insulin sensitizers, metformin and myo-inositol, in women with polycystic ovary syndrome (PCOS). Gynecol. Endocrinol. 2017, 33, 39–42. [Google Scholar] [CrossRef]
- Shokrpour, M.; Foroozanfard, F.; Ebrahimi, F.A.; Vahedpoor, Z.; Aghadavod, E.; Ghaderi, A.; Asemi, Z. Comparison of myo-inositol and metformin on glycemic control, lipid profiles, and gene expression related to insulin and lipid metabolism in women with polycystic ovary syndrome: A randomized controlled clinical trial. Gynecol. Endocrinol. 2019, 35, 406–411. [Google Scholar] [CrossRef]
- Tagliaferri, V.; Romualdi, D.; Immediata, V.; De Cicco, S.; Di Florio, C.; Lanzone, A.; Guido, M. Metformin vs. myoinositol: Which is better in obese polycystic ovary syndrome patients? A randomized controlled crossover study. Clin. Endocrinol. 2017, 86, 725–730. [Google Scholar] [CrossRef]
- Jamilian, M.; Farhat, P.; Foroozanfard, F.; Ebrahimi, F.A.; Aghadavod, E.; Bahmani, F.; Badehnoosh, B.; Jamilian, H.; Asemi, Z. Expression of Concern: Comparison of myo-inositol and metformin on clinical, metabolic and genetic parameters in polycystic ovary syndrome: A randomized controlled clinical trial. Clin. Endocrinol. 2017, 87, 194–200. [Google Scholar] [CrossRef]
- Jamilian, H.; Jamilian, M.; Foroozanfard, F.; Ebrahimi, F.A.; Bahmani, F.; Asemi, Z. Comparison of myo-inositol and metformin on mental health parameters and biomarkers of oxidative stress in women with polycystic ovary syndrome: A randomized, double-blind, placebo-controlled trial. J. Psychosom. Obstet. Gynecol. 2018, 39, 307–314. [Google Scholar] [CrossRef]
- Fontes, A.F.S.; Reis, F.M.; Cândido, A.L.; Gomes, K.B.; Tosatti, J.A.G. Influence of metformin on hyperandrogenism in women with polycystic ovary syndrome: A systematic review and meta-analysis of randomized clinical trials. Eur. J. Clin. Pharmacol. 2023, 79, 445–460. [Google Scholar] [CrossRef] [PubMed]
- Rashid, R.; Mir, S.A.; Kareem, O.; Ali, T.; Ara, R.; Malik, A.; Amin, F.; Bader, G. Polycystic ovarian syndrome-current pharmacotherapy and clinical implications. Taiwan J. Obs. Gynecol. 2022, 61, 40–50. [Google Scholar] [CrossRef]
- Garzia, E.; Galiano, V.; Marfia, G.; Navone, S.; Grossi, E.; Marconi, A.M. Hyperandrogenism and menstrual imbalance are the best predictors of metformin response in PCOS patients. Reprod. Biol. Endocrinol. 2022, 20, 6. [Google Scholar] [CrossRef]
- Merviel, P.; James, P.; Bouée, S.; Le Guillou, M.; Rince, C.; Nachtergaele, C.; Kerlan, V. Impact of myo-inositol treatment in women with polycystic ovary syndrome in assisted reproductive technologies. Reprod. Health 2021, 18, 13. [Google Scholar] [CrossRef]
- Bonnet, F.; Scheen, A. Understanding and overcoming metformin gastrointestinal intolerance. Diabetes Obes. Metab. 2017, 19, 473–481. [Google Scholar] [CrossRef] [PubMed]
- Mumusoglu, S.; Yildiz, B.O. Polycystic ovary syndrome phenotypes and prevalence: Differential impact of diagnostic criteria and clinical versus unselected population. Curr. Opin. Endocr. Metab. Res. 2020, 12, 66–71. [Google Scholar] [CrossRef]
- Matossian, G.A.; Buklarewicz, I.M.; Moosa, V.A. The Effects of Metformin and Myo-Inositol on Hyperandrogenism, Insulin Sensitivity, and Fertility in Polycystic Ovary Syndrome: A Literature Review. Undergrad. Res. Nat. Clin. Sci. Technol. (URNCST) J. 2023, 7, 1–10. [Google Scholar] [CrossRef]
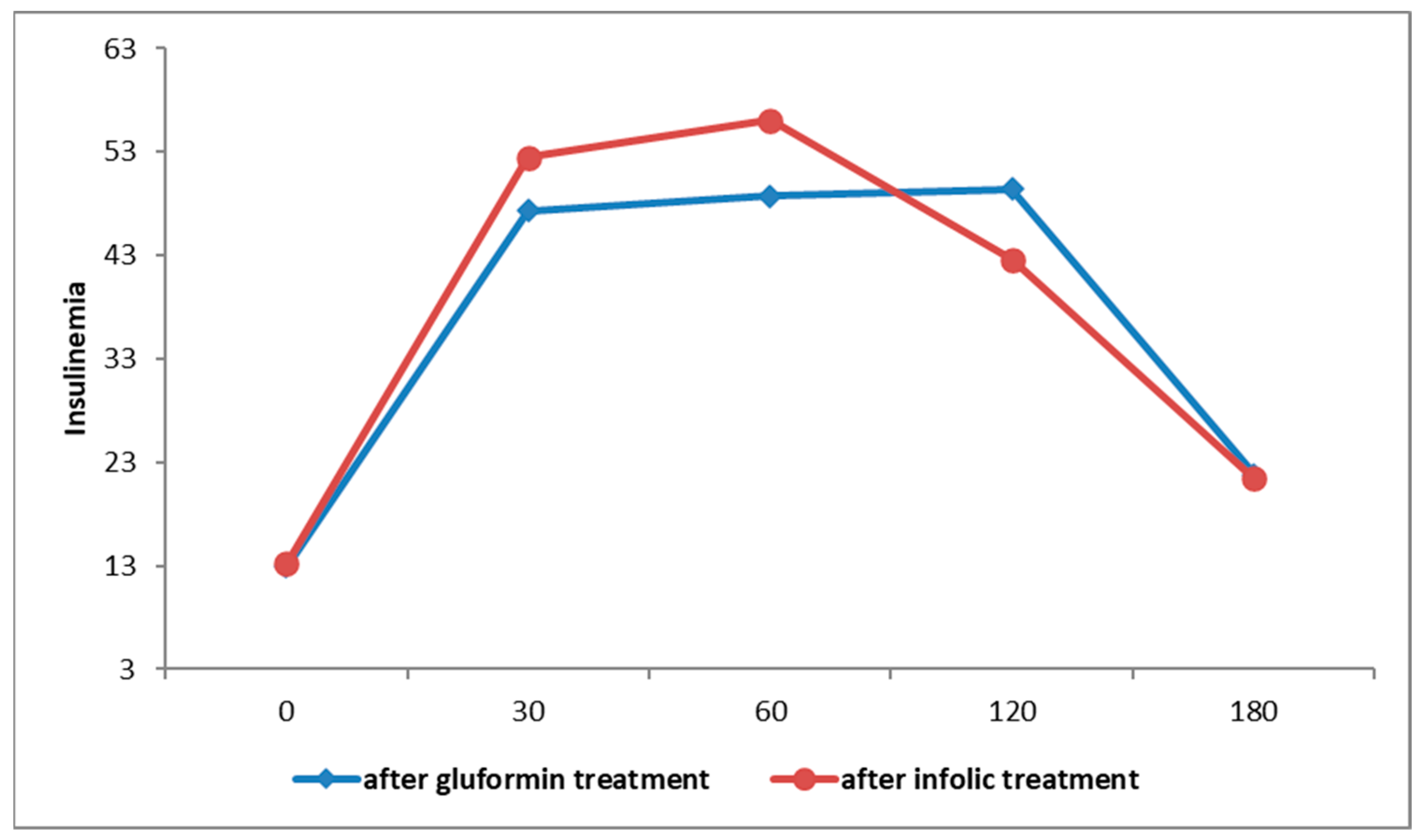
| Variable (Unit) | Metformin (n = 30) | Inofolic (n = 30) | Statistical Significance (p Value) |
|---|---|---|---|
| Age (years) | 28.0 ± 4.9 | 26.3 ± 4.3 | p = 0.147 |
| BMI (kg/m2) | 21.9 ± 2.9 | 21.6 ± 2.5 | p = 0.609 |
| Volume of right ovary (mm3) | 14.5 ± 4.8 | 12.7 ± 3.5 | p = 0.096 |
| Volume of left ovary (mm3) | 13.4 ± 3.7 | 12.0 ± 4.0 | p = 0.166 |
| AMH (ng/mL) | 9.1 ± 4.0 | 9.2 ± 3.1 | p = 0.945 |
| FSH (IU/L) | 4.8 ± 1.7 | 5.5 ± 1.9 | p = 0.072 |
| LH (IU/L) | 7.4 ± 4.5 | 9.0 ± 4.5 | p = 0.356 |
| LH/FSH ratio | 1.7 ± 1.2 | 1.8 ± 1.4 | p = 0.915 |
| Estradiol (pmol/L) | 151.6 ± 81.2 | 138.6 ± 60.2 | p = 0.709 |
| Progesteron (nmol/L) | 4.6 ± 2.2 | 3.8 ± 2.5 | p = 0.615 |
| Prolaktin (mIU/L) | 289.0 ± 161.3 | 306.9 ± 181.3 | p = 0.711 |
| TSH (mIU/L) | 2.2 ± 0.8 | 2.1 ± 0.7 | p = 0.960 |
| Testosteron (nmol/L) | 3.1 ± 1.1 | 2.9 ± 1.0 | p = 0.678 |
| Androstenedion (ng/mL) | 3.0 ± 1.3 | 2.9 ± 1.0 | p = 0.643 |
| DHEAs (μmol/L) | 9.3 ± 4.4 | 9.7 ± 4.9 | p = 0.114 |
| FAI | 8.0 ± 5.1 | 6.9 ± 4.0 | p = 0.673 |
| SHBG (nmol/L) | 52.9 ± 32.8 | 52.1 ± 30.8 | p = 0.742 |
| Fasting insulin (mIU/L) | 19.1 ± 8.3 | 17.5 ± 8.9 | p = 0.717 |
| Gluformin | Inofolic | |||||
|---|---|---|---|---|---|---|
| Varible | Before Therapy | After Therapy | Statistical Significance | Before Therapy | After Therapy | Statistical Significance |
| FSH (IU/L) | 4.8 ± 1.7 | 5.5 ± 1.7 | p = 0.018 | 5.5 ± 1.9 | 6.2 ± 1.9 | p = 0.018 |
| LH/FSH ratio | 1.7 ± 1.2 | 1.2 ± 0.8 | p = 0.058 | 1.8 ± 1.4 | 1.3 ± 1.1 | p = 0.058 |
| Testosteron (nmol/L) | 3.1 ± 1.1 | 2.0 ± 0.9 | p < 0.001 | 2.9 ± 1.0 | 2.0 ± 1.0 | p < 0.001 |
| Androstenedion, (ng/mL) | 3.0 ± 1.3 | 2.1 ± 0.6 | p < 0.001 | 2.9 ± 1.0 | 2.3 ± 0.7 | p < 0.001 |
| DHEAs (μmol/L) | 9.3 ± 4.4 | 7.1 ± 4.2 | p = 0.001 | 9.7 ± 4.9 | 8.6 ± 3.3 | p = 0.001 |
| FAI | 8.0 ± 5.1 | 3.6 ± 2.1 | p < 0.001 | 6.9 ± 4.0 | 3.6 ± 2.4 | p < 0.001 |
| SHBG (nmol/L) | 52.9 ± 32.8 | 69.3 ± 40.5 | p = 0.001 | 52.1 ± 30.8 | 73.6 ± 56.4 | p = 0.001 |
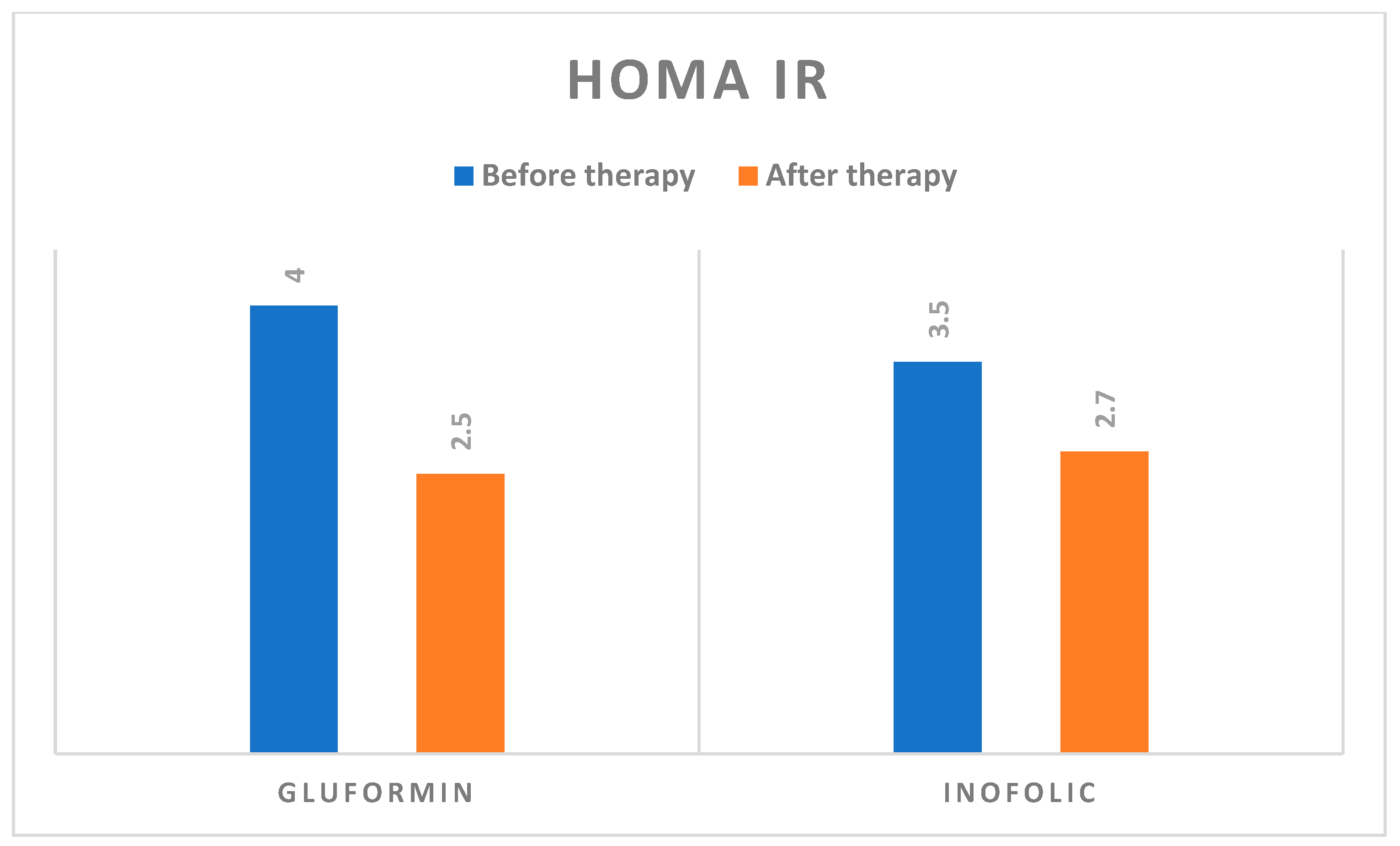
| Fasting insulin, (mIU/L) | 19.1 ± 8.3 | 12.9 ± 4.9 | p < 0.001 | 17.5 ± 8.9 | 13.2 ± 4.0 | p < 0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gudović, A.; Bukumirić, Z.; Milincic, M.; Pupovac, M.; Andjić, M.; Ivanovic, K.; Spremović-Rađenović, S. The Comparative Effects of Myo-Inositol and Metformin Therapy on the Clinical and Biochemical Parameters of Women of Normal Weight Suffering from Polycystic Ovary Syndrome. Biomedicines 2024, 12, 349. https://doi.org/10.3390/biomedicines12020349
Gudović A, Bukumirić Z, Milincic M, Pupovac M, Andjić M, Ivanovic K, Spremović-Rađenović S. The Comparative Effects of Myo-Inositol and Metformin Therapy on the Clinical and Biochemical Parameters of Women of Normal Weight Suffering from Polycystic Ovary Syndrome. Biomedicines. 2024; 12(2):349. https://doi.org/10.3390/biomedicines12020349
Chicago/Turabian StyleGudović, Aleksandra, Zoran Bukumirić, Milos Milincic, Miljan Pupovac, Mladen Andjić, Katarina Ivanovic, and Svetlana Spremović-Rađenović. 2024. "The Comparative Effects of Myo-Inositol and Metformin Therapy on the Clinical and Biochemical Parameters of Women of Normal Weight Suffering from Polycystic Ovary Syndrome" Biomedicines 12, no. 2: 349. https://doi.org/10.3390/biomedicines12020349
APA StyleGudović, A., Bukumirić, Z., Milincic, M., Pupovac, M., Andjić, M., Ivanovic, K., & Spremović-Rađenović, S. (2024). The Comparative Effects of Myo-Inositol and Metformin Therapy on the Clinical and Biochemical Parameters of Women of Normal Weight Suffering from Polycystic Ovary Syndrome. Biomedicines, 12(2), 349. https://doi.org/10.3390/biomedicines12020349






