Abstract
Nucleotide-binding oligomerization domain-like (NOD) receptors rely on the interface between immunity and metabolism. Dietary factors constitute critical players in the activation of innate immunity and modulation of the gut microbiota. The latter have been involved in worsening or improving the control and promotion of diseases such as obesity, type 2 diabetes, metabolic syndrome, diseases known as non-communicable metabolic diseases (NCDs), and the risk of developing cancer. Intracellular NODs play key coordinated actions with innate immune ‘Toll-like’ receptors leading to a diverse array of gene expressions that initiate inflammatory and immune responses. There has been an improvement in the understanding of the molecular and genetic implications of these receptors in, among others, such aspects as resting energy expenditure, insulin resistance, and cell proliferation. Genetic factors and polymorphisms of the receptors are determinants of the risk and severity of NCDs and cancer, and it is conceivable that dietary factors may have significant differential consequences depending on them. Host factors are difficult to influence, while environmental factors are predominant and approachable with a preventive and/or therapeutic intention in obesity, T2D, and cancer. However, beyond the recognition of the activation of NODs by peptidoglycan as its prototypical agonist, the underlying molecular response(s) and its consequences on these diseases remain ill-defined. Metabolic (re)programming is a hallmark of NCDs and cancer in which nutritional strategies might play a key role in preventing the unprecedented expansion of these diseases. A better understanding of the participation and effects of immunonutritional dietary ingredients can boost integrative knowledge fostering interdisciplinary science between nutritional precision and personalized medicine against cancer. This review summarizes the current evidence concerning the relationship(s) and consequences of NODs on immune and metabolic health.
1. Introduction
Today, more than 1600 million people (aged 15 years and more) worldwide are overweight or obese and, according to the World Health Organization (WHO), this number will increase to 2300 million in 2050. Worldwide, hyperglycemia kills some 3.4 million people a year. In the EU, approximately 60% of adults and 20% of children of school age are overweight or obese (WHO, (https://www.who.int/, accessed on 1 November 2023). In the USA, the situation is very similar where being overweight and obese occurs in the population in an approximate proportion of 3/4 of adults (>20 years) and 1/5 of children and adolescents (2–19 years) [1]. The WHO forecasts that deaths from type 2 diabetes (T2D) will double between 2005 and 2030. In these contexts, the metabolic (re)programming of certain subphenotypes of immune system components, which are key to inducing obesity (i.e., innate and macrophage lymphoid cells), is a distinctive stamp in alterations to the homeostasis of nutrients in which nutritional strategies can play a key role. Data from epidemiological studies reveal that obesity and T2D significantly contribute to increasing the risk for a number of cancer types [2]. Overall, a reduction can be seen when considering dietary risks as the origin of carcinogenic processes [3]. However, it is worth noting the increasing trend in the incidence of cancer if we consider factors such as weight gain and metabolic pathologies such as diabetes [4]. Cancer represents a scourge of today’s societies [1]; there were an estimated 18.1 million cancer cases around the world in 2020. Of these, 9.3 million cases were in men and 8.8 million in women.
The role of food ingredients, beyond their nutritional value, has been a subject of an open debate over the past few decades. In this sense, the European Food Safety Authority (EFSA) has issued specific guidelines referring to health claims pertaining to various foods/food constituents [5] as well as to intestinal immunity and defense against pathogens [6]. These guidelines stress the need for establishing a cause–effect relationship and definition of specific functions of the immune system to be improved by foods/food constituents. Nowadays, most nutritional interventions possess a clear observational perspective rather than a hypothesis-driven point of view. Despite significant advances in the understanding of metabolic diseases such as obesity, type 2 diabetes, metabolic syndrome, and cancer, dietary recommendations have remained general and, at this point, apply to all patients regardless of the disease features. In this sense, “Precision nutrition” has emerged as a discipline with the potential to provide significant contributions to successfully implement personalized medicine [7,8]. Understanding how a host’s intrinsic features determine the metabolic variability between individuals is used to tailor specific nutritional strategies to manage diseases.
Previous research has largely focused on total calorie intake and an adequate nutrient intake so as to develop proper immune system response(s) [9]. NCDs share common features, such as liver dysfunction and tissue inflammation, where the monocyte/macrophage population has multifaceted effects [10,11]. Recent findings also provided new insights into how innate and adaptive lymphocytes operate sequentially and in distinct ways during normal development to establish steady-state commensalism and lipid homeostasis [12]. Studies on the intricate relationship between the maturation of the intestinal immune system and the induction of obesity revealed that innate lymphoid cells (group 2)—ILC2s—are determinants in the induction of diet-induced obesity [13]. Later research demonstrated that liver macrophages are determinants in the diet-regulated control of hepatic fat accumulation [14]. In this sense, recent data evidence that dietary nutrients able to promote beneficial immune response(s)—immunonutritional agonists—can be even more important determinants of intestinal, liver, metabolic, and cardiovascular health [15]. These effects appear to be derived from a selective functional differentiation of the monocyte/macrophage population towards an M1-like phenotype [15]. In addition, recent research also associated the expansion of ILC2 and ILC3 populations with a reduction in the accumulation of fats into the liver [16].
Collectively, a critical overview of the scientific literature indicates that NCDs and cancer require effective nutritional intervention strategies. The latter is preferably based on immunonutritional agonists enabling the oriented expansion and activity of innate immune populations. The modulation of immune responses via innate immune pattern recognition receptors by immunonutritional agonists are strategies that could have determinant influences on promoting the metabolic and immune health of the gut–liver axis. In particular, nucleotide-binding oligomerization domain-like (NOD) receptors rely on the interface between immunity and metabolism. Thus, the integration of signaling molecule regulators of these receptors constitutes a promising strategy for approaching, with a preventive and/or therapeutic intention, immunometabolic diseases such as obesity, T2D and cancer. A better understanding of these processes can help to integrate the potential use of NODs in targeted precision nutrition strategies as coadjuvant to the classical pharmacological treatments (Figure 1).
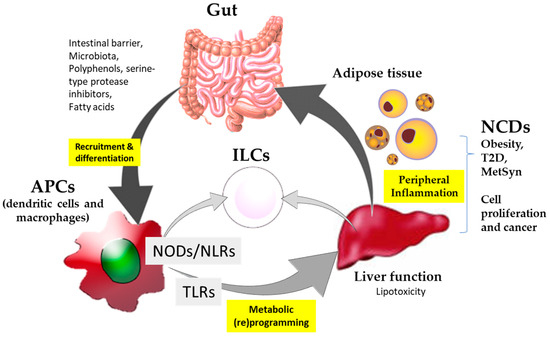
Figure 1.
Schematic summary of the participation of immunonutritional agonists in interaction with the nucleotide-binding oligomerization domain-like (NODs) and ‘Toll-like’ (TLRs) receptors and their implication for non-communicable diseases (NCDs) and cancer.
This review summarizes the current evidence concerning the relationship(s) and consequences of NODs on immune and metabolic health. An extensive search was carried out to identify as many studies as possible relevant to NOD receptors. Particular efforts were devoted to compiling human intervention studies as preclinical studies are enormously informative, but the effects may not be recapitulated in human trials. All relevant studies are classified according to the reported biomarkers associated with NCDs and cancer, and to affect the metabolic and innate immune function.
2. Current Challenges in the Nutritional Approach to NODs in NCDs and Cancer
Over the last decade, there has been a significant implementation of nutritional approaches for NCD and cancer patients [7,17,18]. Only a few of the main drivers responsible for consistent clinical outcomes in diabetes (i.e., type 1, 2, or prediabetes) have been identified as nutrient-targeted processes [18]. Nutritional obesity management approaches the modulation of inflammation to improve insulin sensitivity, and consequently fat accumulation [17]. In 2022, a panel of experts on the nutritional approach for cancer patients in Spain agreed upon the extended incidence of malnutrition in cancer patients [19]. In general terms, nutritional approaches rely on the energetic and biological value of nutrients. The study of interindividual dissimilarities based on exposome heterogeneity (i.e., genetic/epigenetic, lifestyle, microbiome and behavioral/psychological features) significantly contributed to improving disease management [7]. Eminently, these studies attempted to condition the intensity and severity of potential interactions to augment the magnitude of the effects attributable to nutrient utilization. There exists a link between immunocompetent cells (i.e., the monocyte/macrophage population and ILCs) and functions and the development of NCDs and cancer [12,13,14]. However, addressing these processes to modulate the oriented activation and function of these immune effectors by foods/food constituents remains poorly exploited.
Preclinical studies have reported opposing results concerning the implication and role of NOD1 in the promotion and severity of obesity [20,21]. These effects vary from the protective role of NOD1 deficiency against obesity-induced inflammation and insulin resistance [20], to a deleterious effect accelerating diet-induced obesity and liver steatosis in HFD-fed mice [21]. NCDs are characterized by low-grade inflammation. In this context, peripheral monocytes and macrophages derived from these appear as central players in the progression of liver dysfunction, worsening or improving the disease. This inducible population of innate immune effectors acquires a specific phenotype due to the integration of signals (i.e., immune and metabolic) originating (i.e., tissular and systemic) within the gut–liver axis. In NCDs, the role played by M1-like macrophages under chronic/sterile inflammation allows us to consider a situation where NOD1 is subjected to inhibitory regulation. This assumption is based on reduced macrophage apoptosis derived from NOD1 deficiency [22], and the contrasting apoptosis-mediated clearance of macrophages from resolving inflammation [23].
Prior research has shown the key role of intestinal innate immunity in determining lipid homeostasis and gut microbiota composition [12,15]. These studies revealed deep links between the immune system and body organ metabolism. While ILC3s have been shown to exhibit a clear role in the establishment of a tolerable commensal state and influence lipid homeostasis [12], ILC2s appear as essential determinants of fat absorption [13]. In addition, taking advantage of preclinical models—deficient in adaptive immune effectors, Rag2−/− mice and ILCs, Rag2−/−IL2−/− mice—it has been shown that the promotion of an M1-like phenotype (CD68+F4/80+) of the monocyte/macrophage population was associated with the expansion of ILC2/3 (i.e., CD117+KLRG+ group ILC2s and CD56+CD117+Nkp46+ group ILC3s) precursors that benefit the control of HFD-induced obesity [16]. The latter appeared as a consequence of the adaptations of lipid homeostasis, which could not be associated with changes in the gut microbiota. Altogether, these results are concordant with the previously reported role as responsible for NOD2 sensing to selectively activate inflammatory cytokine production from ILC2/3s [24].
Overall, there are substantial knowledge gaps in relation to the integration of NODs into nutrition strategies, eco-nutritional conditions, immunity, and the specificity of the effects (Figure 1): (i) understanding food-derived ingredients’ influence on NODs at the systems’ biology level allowing for the comparison of different sources, (ii) producing new knowledge on stimulatory and inhibitory effects, as well as (iii) integrating information as complementary to the classical pharmacological approach. Despite the well-known role of NOD receptor proteins in the interplay between the microbiota and gastrointestinal immune adaptations [25], their regulation by foods/food constituents in NCDs is less explored. Most available information identifies the influence of phytochemicals and extracts of aromatic and medicinal plants [25,26] as signaling molecule modulators. These compounds may display the potential to access the cellular plasmatic membrane, thus impairing TLR4 relocation and distribution to lipid rafts [27]. However, the impossibility to control the effective concentration of these compounds to modulate signaling in an oriented fashion by directly interacting with the receptors limits their use with a clinical preventive and/or therapeutic intention.
3. NOD/NLR Signaling: Implications for NCDs and Cancer
Elegant reviews have compiled information concerning the structure and gene encoding for NODs [28,29]. NOD1 and NOD2 are located in the cytoplasm and are composed of an LRR ligand-binding domain, an oligomerization domain with NACHT homology, and a caspase recruitment domain (CARD) that transmits the signal. Despite these significant advances and improvements in the understanding of the genetic basis for these receptors, their importance in NCDs and cancer could be underscored by the fact that single-nucleotide polymorphisms (SNPs) occurring in different subpopulations of patients have been barely elucidated [30,31]
NOD1/2 and NOD-like receptors (NLRs) belong to the superclass of pattern recognition receptors (i.e., PAMPs, DAMPs), among which are also the innate immune ‘Toll-like’ receptors (TLRs). Pattern recognition receptors play key roles in determining the function and activity of innate immune effectors. Both NODs/NLRs and TLRs are well-recognized mediators of the immune and metabolic imbalances occurring in NCDs and cancer [32,33,34]. It is well known that microbial peptidoglycans—γ-D-glutamyl-meso-diaminopimelic acid and muramyl dipeptide—act as the prototypical agonist of NODs, and despite the known and described functions as microbial sensors, they also affect the development of extraintestinal diseases and cancer [35]. For example, NOD1/2 activation and deficiency as well as the intake of a diet rich in lipids and certain signals of cellular damage are closely related to cell proliferation and the response(s) to chemotherapy in hepatocellular carcinoma (HCC) [32]. The balance between the anti/pro-tumor consequences from NOD signaling appears to depend on several factors (i.e., the type and stage of the tumor, the microenvironment, and the interactions between the cells of the immune system) [36]. The convergence between NODs/NLRs and TLRs may constitute the way to integrate the different stimuli determining the underlying signaling and the receptors’ contribution to disease development.
Recent work identified the participation of Rho GTPases as part of the molecular signaling associated with NODs [37]. In humans and mice, the widespread expression of Rho kinases, which represent target molecules in regulating metabolic function and energy storage, is known [38]. A clear example of the importance of NCDs is that molecules such as Rac1 play important positive roles in insulin-stimulated glucose uptake. The latter effect constitutes a possible link between obesity and T2D. Rac1 also represents a regulator of cell migration and a potential target for cancer therapy and contributes to the maximal activation of STAT3 in IFN-γ stimulation. Rho kinases are also relevant players in cancer and neurodegeneration [39]. Not surprisingly, Rho kinases, also exert various activities to effectively modulate the immune system and have been identified as potential therapeutic targets in cancer immunotherapy [40]. Protein kinase inhibitors can be found in several foods where bioactive compounds displaying inhibitory activity occur naturally [39]. Unfortunately, the inhibitory role appears unspecific and reversible, as in the case of polyphenols. In addition, some of these activities have their origin in both bacterial production and their metabolic capacities [41]. Notwithstanding, recent work has shown the potential of serine-type protease inhibitors to up-regulate the Rho GDP-dissociation inhibitor in macrophages via interaction with TLR4 (MyD88-independent) signaling [42]. This effect was accompanied by the downregulatory effect of several glycolytic mediators, thus allowing us to hypothesize a negative regulatory effect on NOD signaling. Collectively, these observations suggest that imbalances of cellular metabolic homeostasis are sensed by NOD1/2, thereby contributing to the early glycolytic reprogramming of human monocyte-derived macrophages [33]. The implication of NOD2 in the production of type I interferons, associated with the interconnected signaling with TLRs, could be responsible, at least in part, for boosting the loss of regulatory capacity on the proinflammatory processes in NCDs.
A direct consequence of the metabolic imbalances occurring during NCDs and cancer is increased stress on the endoplasmic reticulum [43]. This organelle plays a critical role in the development of NCDs and cancer since the maintenance of its homeostasis is essential in the regulation of TLR4 expression. In this context, the capacity of NOD1/2 for sensing ER stress may also be of relevance for these pathologies in which the receptors could significantly influence the organelle’s function. ER stress has been identified as a central feature of the peripheral insulin resistance occurring in T2D [44]. In obesity, increased and sustained concentrations of fatty acids in the peripheral bloodstream contribute to establishing lipotoxic stimuli and a chronic inflammatory state impairing ER stress [45]. A clear consequence of these homeostatic alterations is the activation of the unfolded protein response (UPR), commonly upregulated in cancer [46].
A critical revision of the scientific literature, concerning the influence of food ingredients on NOD/NLR expression, shows their potential to partially, either with a down-/up-regulatory effect, affect NODs/NLRs [42,47]. In addition, both Gram (+) and (−) bacteria release extracellular vesicles, but knowledge of the genus Lactobacillus and Bifidobacterium remains poor and comprehensive and integrated knowledge is needed to understand their capacity to activate/control NOD signaling. Similarly, compelling revisions can be found regarding the potential of naturally occurring bioactive compounds (i.e., mostly polyphenols and associated molecules) displaying inhibitory activity in regard to protein kinases sensed by NODs [48]. However, a common gap in knowledge is that the exact mechanism remains to be elucidated. These aspects limit the attribution of added value to food ingredients as modulators of the NOD/NLR axis with a preventive or therapeutic intention.
4. Immunonutritional Interventions on NOD Signaling
The innate immune system is deeply and complexly linked not only to immune, but also to metabolic homeostasis, and thus associated with different diseases [49]. Despite preclinical mouse models having provided a way to study human innate immune pathways, many cases lacking translation to humans can be found, which is most likely attributable to developmental and evolutionary aspects [50]. The divergence of NODs between humans and mice appears to not be so extensive in the inflammasome-forming NLRP and IPAF subfamilies [51]. Otherwise, different genomic content in the TLRs as well as regulated response(s) in mouse and human macrophages has been reported. Thus, immune and metabolic processes in NCDs [52,53,54,55] and cancer [56] can be exacerbated during disease development. These differences can have significant implications for devising the potential of immunonutritional strategies to influence NODs.
The scientific literature reveals scarce information from patients concerning the involvement of specific NODs in NCDs [57,58]. NOD1/2 were shown to be up-regulated (mRNA) in monocytes from patients with T2D. This result allows us to hypothesize that nutrient utilization by monocytes is going to be affected in such a way that insulin resistance is increased thereby signaling events that promote an inflammatory phenotype [59]. Synergistic effects between NOD-like receptors (NLRs) and TLRs in human B lymphocytes [60] have been reported. Thus, immunometabolic adaptations in the myeloid population promote a cascade of events involving adaptive T-cells [61] leading to increased, ultimately aberrant and uncontrolled, inflammatory processes. In obese individuals, it was shown that NLRP3 activation could be attenuated by acutely raising the plasmatic concentration of β-hydroxybutyrate with the ingestion of exogenous ketones [58]. This effect was reflected in the decreased production of plasmatic IL-1β. Together with IL-1α, these cytokines significantly contribute to the promotion of insulin resistance impairing the function of adipocytes in promoting inflammation. In addition to low-grade inflammation, NCDs [62] share other features such as imbalances in the composition of the gut microbiota, which have also been associated with cancer [63]. In this sense, patients suffering from intestinal metaplasia display a modified cluster of microbes after H. pylori eradication [64]. Despite the abundance of pathogenic bacteria and the presence of oral microbes enabling the production of peptidoglycan(s), decreased NOD-like signaling was found. This effect appears to contrast with that expected from the endotoxin-like properties of the peptidoglycan(s) from pathogens. The microbial cluster of these patients was composed of, among others, members of the Prevotella, Rothia, and Granulicatella genera, which could represent a good source of butyrate-producing bacteria [65,66]. It is worth noting the butyrate-induced effects limiting natural killer (NK) cell function [67], which could allow hypothesizing this effect to explain, at least in part, the reported data on patients suffering intestinal metaplasia. It is known that NK cells play a dual role, and in cases of immune evasion even help promote metastases [68]. This hypothesis could also be supported by the anti-inflammatory effects derived from probiotic administration. Despite the widespread use of probiotics, most of which include the Bifidobacterium and Lactobacillus genera producing peptidoglycans, there is scarce information from controlled trials concerning their direct association either with the expression or modulation of NODs and NOD-like receptors (NLRs). In this regard, we can hypothesize that the production of peptidoglycan hydrolases by these genera could present a different activity than those produced by Gram (−) bacteria. Thus, despite the generation of ligands for NOD2 by Gram (+) bacteria [69], the signaling of this receptor results in effects that may not synergize with TLRs, which would partly explain the absence of inflammatory effects (Figure 2). Otherwise, a significant bulk of scientific reports exists confirming that cytokine production and innate immune as well as adaptive cells are modulated by lactobacilli and bifidobacteria resulting in improved regulatory immune responses [70].
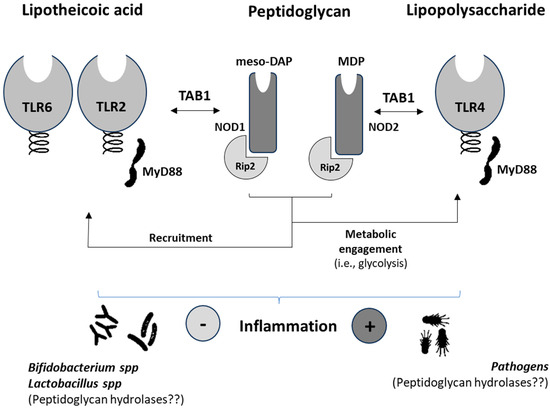
Figure 2.
Schematic diagram summarizing the synergies between nucleotide-binding oligomerization domain-containing proteins (NOD) and Toll-like receptors in human immune and metabolic systems and their main agonists. meso-DAP, γ-D-glutamyl-meso-diaminopimelic acid; MDP, muramyl dipeptide; TAB1, mitogen-activated protein kinase kinase 7-interacting protein-1; Rip2, receptor-interacting protein 2.
Currently, most of the prominent information concerning the key role of NODs and NLRs in immunity and metabolism has come from preclinical models [71,72]. In addition to identifying the different signaling pathways and interaction with PAMPs and DAMPs, studies have demonstrated the enormous involvement of these receptors in different immunonutritional-based processes and diseases, including cancer [73,74,75]. These studies constitute good examples of the many immune and metabolic diseases associated with NODs and NLRs. Prior research defined the protective role of NOD1/2 deficiency from HFD-induced inflammation, lipid accumulation, and peripheral insulin intolerance [20]. Further studies also defined an active role for NOD1 in the inflammatory environment associated with both experimental and human diabetic cardiac disease [76]. In addition, recent data point out the protective function of NOD1 reducing low-grade inflammation and thereby obesity development [21]. Alterations in glucose metabolism have been previously identified as a major driver of B cell lymphopoiesis and function [77]. Recently, a key—microbial ligand-independent—role has been demonstrated for NOD1in regulating proliferative response(s) in adaptive immunity [75]. Notably, the latter response(s) were associated with NOD1-mediated contrasting effects preventing colitis, while impairing T cell maturation and activity. Thus, important consequences during anti-tumoral immunity development can be expected. A clear example is the recently described microbiota-dependent activation of CD4+ T cells inducing the CTLA-4 blockade via Fcγ receptors [78]. However, the Bifidobacterium and Lactobacillus genera prevent immunological alterations, generally causing severe inflammation, associated with the activation of CTLA4 [79,80]. Supplementation with probiotics can help to integrate the diverse biological response(s) derived from NOD/NLR activation, PAMPs, and DAMPs, the lack of activation by their peptidoglycan(s), but control of the proinflammatory milieu and TLR signaling (Figure 2). This speculation could be supported by in vivo studies using a probiotic mixture (i.e., Bifidobacterium strains and Lacticaseibacillus rhamnosus) as a supplement for preterm infants, where it was proven effective in reducing the level of calprotectin as well as IFN-γ and IL-22 [81], the latter mediating the synergic and inhibitory effects, respectively, of NOD activity and expression. The administration of prebiotic inulin, which increased the abundances of Akkermansia and Bifidobacterium that was reflected in a decrease in the ratio Firmicutes/Bacteroidetes, was shown to cause the down-regulation of NLRP3 [82]. Also, some other symbionts such as Bacterioides fragilis, Enterococcus fecalis, and Lactobacillus plantarum have been identified as microbes that do not induce the NLRC4-dependent release of IL-1β [83].
Obesity and T2D result in enhanced oxidative damage to circulating lipoproteins in the plasma, playing a key role in the pathogenesis of the diseases. It is not surprising that molecular components of LDL such as phospholipids as well as parts of the cell membrane become oxidized (oxPLs), thus exerting critical functions as immunomodulatory (DAMPs) signals [84]. These oxPLs can interact with CD14 and be recognized by CD36 accessing the cytosol to activate caspases 1 and 11, the NLRP3 regulators [85]. In addition, in vitro studies have also shown the role of oxPLs in inducing the chemotaxis and intracellular calcium influx in natural killer (NKs) cells [86] and the release of IL-6 in human monocytes [87]. It is worth noting here that NOD-like receptors may directly mediate signaling at the endoplasmic reticulum affecting calcium influx [34]. These signals end up in the development of an inflammatory milieu and could drive the hyperactivation of innate lymphoid cells (ILCs) group 1, which includes the conventional and unconventional subsets of NK cells. Notably, ILCs aggravate adipose tissue fibrosis and the development of diabetes in obesity [88].
From an immunonutritional perspective, n-3 PUFAs represent important food ingredients with the potential to improve, at least in part, NCD-associated immune and metabolic imbalances alleviating obesity, T2D, and metabolic syndrome, and they could also slow down tumor growth and increase the efficacy of chemotherapy. In particular, n-3 PUFAs have been shown to be effective in inhibiting NF-kappaB and IL-8 expression induced by NOD receptors in HCT116 [89]. So far, the results derived from the administration of n-3 PUFAs in humans on the expansion and activation of the immunological effectors of the innate branch—NKs and macrophages—are not conclusive. The scarce literature on the matter reveals the variability of biological responses and their meaning [90,91,92,93]. The consumption of n-3 PUFAs had a significant influence on the functional polarization of the macrophage population in adipose tissue, promoting a drift toward its M2 subphenotype while reducing insulin resistance [93]. Recent research suggests that the body’s composition in PUFAs appears to be associated with TLR4 signaling [16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86,87,88,89,90,91,92,93,94]. The latter is attributed to a protein fraction (~30 kDa MWCO) isolated from C. quinoa with advantageous effects, either in healthy or cancer-developing mice—improving mechanisms to control inflammatory processes and liver macrophage and ILC expansion in animals under HFD. These results allow us to hypothesize that the derivation of signaling in TLRs can condition and modify the severity and orientation of NOD control over metabolic and immunological responses.
5. Conclusions and Future Perspectives
While significant progress has been made to better understand the role of NODs/NLRs in human diseases, immunity, and inflammation, the relation to NCDs and the interaction with immunonutritional compounds remain poorly defined from a biomedical point of view. A critical review of the literature clearly shows the scarcity of existing information, and the non-specific effects of food/food-derived ingredients in interactions with NOD/NLR receptors. Lacking ‘defined’ and ‘oriented mechanism(s)’ limits the use of food with a preventive and/or therapeutic intention further than considering their nutritional profile. It remains to be understood how these receptors differentiate between peptidoglycan from pathogenic bacteria and beneficial probiotics. In addition, how NODs/NLRs integrate the stimuli and TLR-derived signaling enabled by immunonutritional compounds needs to be elucidated. Altogether, only in conjunction with the dynamics of immune and metabolic alterations occurring at NCDs can the role, worsening or improving disease, be elucidated. A better understanding of their molecular implication and influence on innate immune effector cells—myeloid monocytes/macrophages and the expansion of ILCs—can significantly contribute to control and/or intervention in NCDs and cancer (Figure 1). The use of biocompatible vesicles could help in improving the bioavailability of biofunctional food compounds to influence the activity of NODs/NLRs.
NCDs and cancer could benefit from early and preventive immunonutritional strategies, which result in less aggressive to physiological processes. Although NODs/NLRs have been considered in various clinical interventions, improved research into immunonutritional trials is needed along with large-scale studies. The integration of this knowledge and the definition of the lack of understanding will significantly contribute to paving the way to transdisciplinary interventions, enabling nutrition precision interventions with a preventive or therapeutic intention in regard to immune and metabolic imbalances leading to NCDs and cancer. Understanding the role of NODs/NLRs may represent an important molecular checkpoint for devising effective strategies for the innate immune control of these diseases.
Author Contributions
Conceptualization, J.M.L.L.; writing—original draft preparation, C.J.A., M.D., A.G.T. and J.M.L.L.; writing—review and editing, C.J.A., M.D. and A.G.T.; supervision, J.M.L.L. and L.B.; funding acquisition, J.M.L.L. All authors have read and agreed to the published version of the manuscript.
Funding
This work was financially supported by grants Food4ImNut (PID2019-107650RB-C22), PID2020-113238RB-I00 from MICIU/AEI 13039/501100011033, and Centro de Investigación Biomédica en Red en Enfermedades Cardiovasculares (CB16/11/00222) from the Instituto de Salud Carlos III (co-financed by the European Development Regional Fund “A Way to Achieve Europe”, by the “European Union” and by the “European Union NextGeneration EU/PRTR”); Comunidad de Madrid Programa Biociencias (S2022-BMD-7223).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Wang, M.; Sperrin, M.; Rutter, M.K.; Renehan, A.G. Cancer is becoming the leading cause of death in diabetes. Lancet 2023, 401, 1849. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Wei, B.; Zhai, Z.; Zheng, Y.; Wang, S.; Xiang, D.; Hu, J.; Ye, X.; Yang, S.; Wu, Y.; et al. Dietary Risk-Related Colorectal Cancer Burden: Estimates From 1990 to 2019. Front. Nutr. 2021, 8, 690663. [Google Scholar] [CrossRef] [PubMed]
- Murray, C.J.L.; Aravkin, A.Y.; Zheng, P.; Abbafati, C.; Abbas, K.M.; Abbasi-Kangevari, M.; Abd-Allah, F.; Abdelalim, A.; Abdollahi, M.; Abdollahpour, I.; et al. Global Burden of 87 Risk Factors in 204 Countries and Territories, 1990–2019: A Systematic Analysis for the Global Burden of Disease Study 2019. Lancet 2020, 396, 1223–1249. [Google Scholar] [CrossRef] [PubMed]
- Panel, E.; Nda, A. Scientific Opinion on the Substantiation of Health Claims Related to Various Foods/Food Constituents and “Immune Function/Immune System” (ID 573, 586, 1374, 1566, 1628, 1778, 1793, 1817, 1829, 1939, 2155, 2485, 2486, 2859, 3521, 3774, 3896), Contribution. EFSA J. 2011, 9, 2061. [Google Scholar] [CrossRef][Green Version]
- EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA). Guidance on the Scientific Requirements for Health Claims Related to the Immune System, the Gastrointestinal Tract and Defence against Pathogenic Microorganisms. EFSA J. 2016, 14, 4369. [Google Scholar] [CrossRef]
- González-Muniesa, P.; Martínez, J.A. Precision Nutrition and Metabolic Syndrome Management. Nutrients 2019, 11, 2411. [Google Scholar] [CrossRef]
- Lee, B.Y.; Ordovás, J.M.; Parks, E.J.; Anderson, C.A.M.; Barabási, A.-L.; Clinton, S.K.; de la Haye, K.; Duffy, V.B.; Franks, P.W.; Ginexi, E.M.; et al. Research Gaps and Opportunities in Precision Nutrition: An NIH Workshop Report. Am. J. Clin. Nutr. 2022, 116, 1877–1900. [Google Scholar] [CrossRef]
- Meydani, S.N.; Erickson, K.L. Nutrients as Regulators of Immune Function: Introduction1. FASEB J. 2001, 15, 2555. [Google Scholar] [CrossRef]
- Cipolletta, D. Adipose Tissue-Resident Regulatory T Cells: Phenotypic Specialization, Functions and Therapeutic Potential. Immunology 2014, 142, 517–525. [Google Scholar] [CrossRef]
- Barreby, E.; Chen, P.; Aouadi, M. Macrophage Functional Diversity in NAFLD—More than Inflammation. Nat. Rev. Endocrinol. 2022, 18, 461–472. [Google Scholar] [CrossRef]
- Mao, K.; Baptista, A.P.; Tamoutounour, S.; Zhuang, L.; Bouladoux, N.; Martins, A.J.; Huang, Y.; Gerner, M.Y.; Belkaid, Y.; Germain, R.N. Innate and Adaptive Lymphocytes Sequentially Shape the Gut Microbiota and Lipid Metabolism. Nature 2018, 554, 255–259. [Google Scholar] [CrossRef]
- Sasaki, T.; Moro, K.; Kubota, T.; Kubota, N.; Kato, T.; Ohno, H.; Nakae, S.; Saito, H.; Koyasu, S. Innate Lymphoid Cells in the Induction of Obesity. Cell Rep. 2019, 28, 202–217.e7. [Google Scholar] [CrossRef] [PubMed]
- Cox, N.; Crozet, L.; Holtman, I.R.; Loyher, P.-L.; Lazarov, T.; White, J.B.; Mass, E.; Stanley, E.R.; Elemento, O.; Glass, C.K.; et al. Diet-Regulated Production of PDGFcc by Macrophages Controls Energy Storage. Science 2021, 373, eabe9383. [Google Scholar] [CrossRef]
- Bouzas Muñoz, A.; Giménez-Bastida, J.A.; García-Tejedor, A.; Haros, C.M.; Gómez de Cedrón, M.; Ramírez de Molina, A.; Laparra, J.M. Intestinal Intervention Strategy Targeting Myeloid Cells to Improve Hepatic Immunity during Hepatocarcinoma Development. Biomedicines 2021, 9, 1633. [Google Scholar] [CrossRef]
- García-Tejedor, A.; Haros, C.M.; Laparra, J.M. Chenopodium Quinoa’s Ingredients Improve Control of the Hepatic Lipid Disturbances Derived from a High-Fat Diet. Foods 2023, 12, 3321. [Google Scholar] [CrossRef] [PubMed]
- Botchlett, R.; Woo, S.-L.; Liu, M.; Pei, Y.; Guo, X.; Li, H.; Wu, C. Nutritional Approaches for Managing Obesity-Associated Metabolic Diseases. J. Endocrinol. 2017, 233, JOE-16. [Google Scholar] [CrossRef]
- Wylie-Rosett, J.; Hu, F.B. Nutritional Strategies for Prevention and Management of Diabetes: Consensus and Uncertainties. Diabetes Care 2019, 42, 727–730. [Google Scholar] [CrossRef]
- Suárez-Llanos, J.P.; Vera-García, R.; Contreras-Martinez, J. The Determination of a Consensus Nutritional Approach for Cancer Patients in Spain Using the Delphi Methodology. Nutrients 2022, 14, 1404. [Google Scholar] [CrossRef] [PubMed]
- Schertzer, J.D.; Tamrakar, A.K.; Magalhães, J.G.; Pereira, S.; Bilan, P.J.; Fullerton, M.D.; Liu, Z.; Steinberg, G.R.; Giacca, A.; Philpott, D.J.; et al. NOD1 Activators Link Innate Immunity to Insulin Resistance. Diabetes 2011, 60, 2206–2215. [Google Scholar] [CrossRef]
- González-Ramos, S.; Fernández-García, V.; Recalde, M.; Rodríguez, C.; Martínez-González, J.; Andrés, V.; Martín-Sanz, P.; Boscá, L. Deletion or Inhibition of NOD1 Favors Plaque Stability and Attenuates Atherothrombosis in Advanced Atherogenesis. Cells 2020, 9, 2067. [Google Scholar] [CrossRef]
- González-Ramos, S.; Paz-García, M.; Fernández-García, V.; Portune, K.J.; Acosta-Medina, E.F.; Sanz, Y.; Castrillo, A.; Martín-Sanz, P.; Obregon, M.J.; Boscá, L. NOD1 Deficiency Promotes an Imbalance of Thyroid Hormones and Microbiota Homeostasis in Mice Fed High Fat Diet. Sci. Rep. 2020, 10, 12317. [Google Scholar] [CrossRef]
- Gautier, E.L.; Ivanov, S.; Lesnik, P.; Randolph, G.J. Local Apoptosis Mediates Clearance of Macrophages from Resolving Inflammation in Mice. Blood 2013, 122, 2714–2722. [Google Scholar] [CrossRef]
- Zhou, L.; Chu, C.; Teng, F.; Bessman, N.J.; Goc, J.; Santosa, E.K.; Putzel, G.G.; Kabata, H.; Kelsen, J.R.; Baldassano, R.N.; et al. Innate Lymphoid Cells Support Regulatory T Cells in the Intestine through Interleukin-2. Nature 2019, 568, 405–409. [Google Scholar] [CrossRef]
- Fernández-García, V.; González-Ramos, S.; Martín-Sanz, P.; Portillo, F.G.; Laparra, J.M.; Boscá, L. NOD1 in the Interplay between Microbiota and Gastrointestinal Immune Adaptations. Pharmacol. Res. 2021, 171, 105775. [Google Scholar] [CrossRef]
- Dou, X.; Yan, D.; Liu, S.; Gao, L.; Shan, A. Thymol Alleviates LPS-Induced Liver Inflammation and Apoptosis by Inhibiting NLRP3 Inflammasome Activation and the AMPK-mTOR-Autophagy Pathway. Nutrients 2022, 14, 2809. [Google Scholar] [CrossRef]
- Choudhari, A.S.; Mandave, P.C.; Deshpande, M.; Ranjekar, P.; Prakash, O. Phytochemicals in Cancer Treatment: From Preclinical Studies to Clinical Practice. Front. Pharmacol. 2020, 10, 1614, Erratum in Front. Pharmacol.2020, 11, 175.. [Google Scholar] [CrossRef]
- Płóciennikowska, A.; Hromada-Judycka, A.; Borzęcka, K.; Kwiatkowska, K. Co-operation of TLR4 and raft proteins in LPS-induced pro-inflammatory signaling. Cell Mol. Life Sci. 2015, 72, 557–581. [Google Scholar] [CrossRef]
- Chamaillard, M.; Girardin, S.E.; Viala, J.; Philpott, D.J. Nods, Nalps and Naip: Intracellular Regulators of Bacterial-Induced Inflammation. Cell. Microbiol. 2003, 5, 581–592. [Google Scholar] [CrossRef]
- Ting, J.P.-Y.; Davis, B.K. CATERPILLER: A Novel Gene Family Important in Immunity, Cell Death, and Diseases. Annu. Rev. Immunol. 2004, 23, 387–414. [Google Scholar] [CrossRef]
- Zhong, Y.; Kinio, A.; Saleh, M. Functions of NOD-Like Receptors in Human Diseases. Front Immunol. 2013, 4, 333. [Google Scholar] [CrossRef]
- Zangara, M.T.; Johnston, I.; Johnson, E.E.; McDonald, C. Mediadores del metabolismo: Un papel no convencional para NOD1 y NOD2. Int. J. Mol. Sci. 2021, 22, 1156. [Google Scholar] [CrossRef]
- Dapito, D.H.; Mencin, A.; Gwak, G.-Y.; Pradere, J.-P.; Jang, M.-K.; Mederacke, I.; Caviglia, J.M.; Khiabanian, H.; Adeyemi, A.; Bataller, R.; et al. Promotion of Hepatocellular Carcinoma by the Intestinal Microbiota and TLR4. Cancer Cell 2012, 21, 504–516. [Google Scholar] [CrossRef]
- Murugina, N.E.; Budikhina, A.S.; Dagil, Y.A.; Maximchik, P.V.; Balyasova, L.S.; Murugin, V.V.; Melnikov, M.V.; Sharova, V.S.; Nikolaeva, A.M.; Chkadua, G.Z.; et al. Glycolytic Reprogramming of Macrophages Activated by NOD1 and TLR4 Agonists: No Association with Proinflammatory Cytokine Production in Normoxia. J. Biol. Chem. 2020, 295, 3099–3114. [Google Scholar] [CrossRef]
- Philpott, D.J.; Sorbara, M.T.; Robertson, S.J.; Croitoru, K.; Girardin, S.E. NOD Proteins: Regulators of Inflammation in Health and Disease. Nat. Rev. Immunol. 2014, 14, 9–23. [Google Scholar] [CrossRef]
- Ma, X.; Qiu, Y.; Zhu, L.; Zhao, Y.; Lin, Y.; Ma, D.; Qin, Z.; Sun, C.; Shen, X.; Li, T.; et al. NOD1 Inhibits Proliferation and Enhances Response to Chemotherapy via Suppressing SRC-MAPK Pathway in Hepatocellular Carcinoma. J. Mol. Med. 2020, 98, 221–232. [Google Scholar] [CrossRef]
- Rakoff-Nahoum, S.; Medzhitov, R. Toll-like Receptors and Cancer. Nat. Rev. Cancer 2009, 9, 57–63. [Google Scholar] [CrossRef]
- Keestra-Gounder, A.M.; Tsolis, R.M. NOD1 and NOD2: Beyond Peptidoglycan Sensing. Trends Immunol. 2017, 38, 758–767. [Google Scholar] [CrossRef]
- Wei, L.; Shi, J. Insight Into Rho Kinase Isoforms in Obesity and Energy Homeostasis. Front. Endocrinol. 2022, 13, 886534. [Google Scholar] [CrossRef] [PubMed]
- Mertsch, S.; Krämer, O.H. The Interplay between Histone Deacetylases and Rho Kinases Is Important for Cancer and Neurodegeneration. Cytokine Growth Factor Rev. 2017, 37, 29–45. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.I.; Cassella, C.R.; Byrne, K.T. Tumor Burden and Immunotherapy: Impact on Immune Infiltration and Therapeutic Outcomes. Front. Immunol. 2021, 11, 629722. [Google Scholar] [CrossRef]
- Proteau, P.J.; Gerwick, W.H.; Garcia-Pichel, F.; Castenholz, R. The Structure of Scytonemin, an Ultraviolet Sunscreen Pigment from the Sheaths of Cyanobacteria. Experientia 1993, 49, 825–829. [Google Scholar] [CrossRef] [PubMed]
- Srdić, M.; Ovčina, I.; Fotschki, B.; Haros, C.M.; Laparra, J.M. C. quinoa and S. hispanica L. Seeds Provide Immunonutritional Agonists to Selectively Polarize Macrophages. Cells 2020, 9, 593. [Google Scholar] [CrossRef] [PubMed]
- Pagliassotti, M.J.; Kim, P.Y.; Estrada, A.L.; Stewart, C.M.; Gentile, C.L. Endoplasmic Reticulum Stress in Obesity and Obesity-Related Disorders: An Expanded View. Metabolism 2016, 65, 1238–1246. [Google Scholar] [CrossRef]
- Özcan, U.; Cao, Q.; Yilmaz, E.; Lee, A.-H.; Iwakoshi, N.N.; Özdelen, E.; Tuncman, G.; Görgün, C.; Glimcher, L.H.; Hotamisligil, G.S. Endoplasmic Reticulum Stress Links Obesity, Insulin Action, and Type 2 Diabetes. Science 2004, 306, 457–461. [Google Scholar] [CrossRef]
- Cunha, D.A.; Hekerman, P.; Ladrière, L.; Bazarra-Castro, A.; Ortis, F.; Wakeham, M.C.; Moore, F.; Rasschaert, J.; Cardozo, A.K.; Bellomo, E.; et al. Initiation and Execution of Lipotoxic ER Stress in Pancreatic β-Cells. J. Cell Sci. 2008, 121, 2308–2318. [Google Scholar] [CrossRef]
- Ojha, R.; Amaravadi, R.K. Targeting the Unfolded Protein Response in Cancer. Pharmacol. Res. 2017, 120, 258–266. [Google Scholar] [CrossRef] [PubMed]
- Kurata, A.; Kiyohara, S.; Imai, T.; Yamasaki-Yashiki, S.; Zaima, N.; Moriyama, T.; Kishimoto, N.; Uegaki, K. Characterization of Extracellular Vesicles from Lactiplantibacillus Plantarum. Sci. Rep. 2022, 12, 13330. [Google Scholar] [CrossRef]
- Baier, A.; Szyszka, R. Compounds from Natural Sources as Protein Kinase Inhibitors. Biomolecules 2020, 10, 1546. [Google Scholar] [CrossRef]
- Rosenstiel, P.; Till, A.; Schreiber, S. NOD-like Receptors and Human Diseases. Microbes Infect. 2007, 9, 648–657. [Google Scholar] [CrossRef]
- Ariffin, J.K.; Sweet, M.J. Differences in the Repertoire, Regulation and Function of Toll-like Receptors and Inflammasome-Forming Nod-like Receptors between Human and Mouse. Curr. Opin. Microbiol. 2013, 16, 303–310. [Google Scholar] [CrossRef]
- Schroder, K.; Tschopp, J. The Inflammasomes. Cell 2010, 140, 821–832. [Google Scholar] [CrossRef]
- Nosalski, R.; Guzik, T.J. Perivascular Adipose Tissue Inflammation in Vascular Disease. Br. J. Pharmacol. 2017, 174, 3496–3513. [Google Scholar] [CrossRef]
- Hägglöf, T.; Vanz, C.; Kumagai, A.; Dudley, E.; Ortega, V.; Siller, M.; Parthasarathy, R.; Keegan, J.; Koenigs, A.; Shute, T.; et al. T-Bet+ B Cells Accumulate in Adipose Tissue and Exacerbate Metabolic Disorder during Obesity. Cell Metab. 2022, 34, 1121–1136.e6. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Wang, Y.; He, Y.; Gao, Y.; Wan, R.; Cai, M.; Li, W.; Chen, R.; Walker, E.; Zhai, X.; et al. Non-Obese Type 2 Diabetes Patients Present Intestinal B Cell Dysregulations Associated with Hyperactive Intestinal Tfh Cells. Mol. Immunol. 2018, 97, 27–32. [Google Scholar] [CrossRef]
- Clement, C.C.; Osan, J.; Buque, A.; Nanaware, P.P.; Chang, Y.-C.; Perino, G.; Shetty, M.; Yamazaki, T.; Tsai, W.L.; Urbanska, A.M.; et al. PDIA3 Epitope-Driven Immune Autoreactivity Contributes to Hepatic Damage in Type 2 Diabetes. Sci. Immunol. 2022, 7, eabl3795. [Google Scholar] [CrossRef] [PubMed]
- Loscocco, G.G.; Piccini, M.; Vergoni, F.; Vannucchi, A.M.; Bosi, A. A Case of Disseminated Blastic Plasmocytoid Dendritic Cell Neoplasm. Am. J. Hematol. 2018, 93, 1433–1434. [Google Scholar] [CrossRef]
- Shiny, A.; Regin, B.; Balachandar, V.; Gokulakrishnan, K.; Mohan, V.; Babu, S.; Balasubramanyam, M. Convergence of Innate Immunity and Insulin Resistance as Evidenced by Increased Nucleotide Oligomerization Domain (NOD) Expression and Signaling in Monocytes from Patients with Type 2 Diabetes. Cytokine 2013, 64, 564–570. [Google Scholar] [CrossRef]
- Neudorf, H.; Myette-Côté, É.; Little, J.P. The Impact of Acute Ingestion of a Ketone Monoester Drink on LPS-Stimulated NLRP3 Activation in Humans with Obesity. Nutrients 2020, 12, 854. [Google Scholar] [CrossRef]
- Makhijani, P.; Basso, P.J.; Chan, Y.T.; Chen, N.; Baechle, J.; Khan, S.; Furman, D.; Tsai, S.; Winer, D.A. Regulation of the Immune System by the Insulin Receptor in Health and Disease. Front. Endocrinol. 2023, 14, 1128622. [Google Scholar] [CrossRef]
- Petterson, T.; Jendholm, J.; Månsson, A.; Bjartell, A.; Riesbeck, K.; Cardell, L.-O. Effects of NOD-like Receptors in Human B Lymphocytes and Crosstalk between NOD1/NOD2 and Toll-like Receptors. J. Leukoc. Biol. 2011, 89, 177–187. [Google Scholar] [CrossRef] [PubMed]
- DeFuria, J.; Belkina, A.C.; Jagannathan-Bogdan, M.; Snyder-Cappione, J.; Carr, J.D.; Nersesova, Y.R.; Markham, D.; Strissel, K.J.; Watkins, A.A.; Zhu, M.; et al. B Cells Promote Inflammation in Obesity and Type 2 Diabetes through Regulation of T-Cell Function and an Inflammatory Cytokine Profile. Proc. Natl. Acad. Sci. USA 2013, 110, 5133–5138. [Google Scholar] [CrossRef]
- Fernández-Musoles, R.; García-Tejedor, A.; Laparra, J.M. Immunonutritional Contribution of Gut Microbiota to Fatty Liver Disease. Nutr. Hosp. 2020, 37, 193–206. [Google Scholar] [CrossRef]
- Park, E.M.; Chelvanambi, M.; Bhutiani, N.; Kroemer, G.; Zitvogel, L.; Wargo, J.A. Targeting the Gut and Tumor Microbiota in Cancer. Nat. Med. 2022, 28, 690–703. [Google Scholar] [CrossRef]
- Sung, J.; Coker, O.; Chu, E.; Szeto, C.H.; Luk, S.; Lau, H.; Yu, J. Gastric Microbes Associated with Gastric Inflammation, Atrophy and Intestinal Metaplasia 1 Year after Helicobacter Pylori Eradication. Gut 2020, 69, 1572–1580. [Google Scholar] [CrossRef]
- Jiang, S.; Xie, S.; Lv, D.; Zhang, Y.; Deng, J.; Zeng, L.; Chen, Y. A Reduction in the Butyrate Producing Species Roseburia Spp. and Faecalibacterium Prausnitzii Is Associated with Chronic Kidney Disease Progression. Antonie Van Leeuwenhoek 2016, 109, 1389–1396. [Google Scholar] [CrossRef]
- Ren, W.; Yan, H.; Yu, B.; Walsh, M.C.; Yu, J.; Zheng, P.; Huang, Z.; Luo, J.; Mao, X.; He, J.; et al. Prevotella-Rich Enterotype May Benefit Gut Health in Finishing Pigs Fed Diet with a High Amylose-to-Amylopectin Ratio. Anim. Nutr. 2021, 7, 400–411. [Google Scholar] [CrossRef]
- Zaiatz-Bittencourt, V.; Jones, F.; Tosetto, M.; Scaife, C.; Cagney, G.; Jones, E.; Doherty, G.A.; Ryan, E.J. Butyrate Limits Human Natural Killer Cell Effector Function. Sci. Rep. 2023, 13, 2715. [Google Scholar] [CrossRef]
- Poggi, A.; Benelli, R.; Venè, R.; Costa, D.; Ferrari, N.; Tosetti, F.; Zocchi, M.R. Human Gut-Associated Natural Killer Cells in Health and Disease. Front. Immunol. 2019, 10, 961. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Wang, L.; Jiang, J.; Xu, Q.; Zeng, N.; Lu, B.; Yuan, P.; Sun, K.; Zhou, H.; He, X. A Probiotic Bi-Functional Peptidoglycan Hydrolase Sheds NOD2 Ligands to Regulate Gut Homeostasis in Female Mice. Nat. Commun. 2023, 14, 3338. [Google Scholar] [CrossRef] [PubMed]
- Vlasova, A.N.; Kandasamy, S.; Chattha, K.S.; Rajashekara, G.; Saif, L.J. Comparison of Probiotic Lactobacilli and Bifidobacteria Effects, Immune Responses and Rotavirus Vaccines and Infection in Different Host Species. Vet. Immunol. Immunopathol. 2016, 172, 72–84. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Shaw, M.H.; Kim, Y.-G.; Nuñez, G. NOD-Like Receptors: Role in Innate Immunity and Inflammatory Disease. Annu. Rev. Pathol. Mech. Dis. 2009, 4, 365–398. [Google Scholar] [CrossRef] [PubMed]
- Platnich, J.M.; Muruve, D.A. NOD-like Receptors and Inflammasomes: A Review of Their Canonical and Non-Canonical Signaling Pathways. Arch. Biochem. Biophys. 2019, 670, 4–14. [Google Scholar] [CrossRef] [PubMed]
- Velloso, F.J.; Trombetta-Lima, M.; Anschau, V.; Sogayar, M.C.; Correa, R.G. NOD-like Receptors: Major Players (and Targets) in the Interface between Innate Immunity and Cancer. Biosci. Rep. 2019, 39, BSR20181709. [Google Scholar] [CrossRef] [PubMed]
- Fernández-García, V.; González-Ramos, S.; Martín-Sanz, P.; Laparra, J.M.; Boscá, L. NOD1-Targeted Immunonutrition Approaches: On the Way from Disease to Health. Biomedicines 2021, 9, 519. [Google Scholar] [CrossRef]
- Iwamura, C.; Ohnuki, H.; Flomerfelt, F.A.; Zheng, L.; Carletti, A.; Wakashin, H.; Mikami, Y.; Brooks, S.R.; Kanno, Y.; Gress, R.E.; et al. Microbial Ligand-Independent Regulation of Lymphopoiesis by NOD1. Nat. Immunol. 2023, 24, 2080–2090. [Google Scholar] [CrossRef]
- Prieto, P.; Vallejo-Cremades, M.T.; Benito, G.; González-Peramato, P.; Francés, D.; Agra, N.; Terrón, V.; Gónzalez-Ramos, S.; Delgado, C.; Ruiz-Gayo, M.; et al. NOD1 Receptor Is Up-Regulated in Diabetic Human and Murine Myocardium. Clin. Sci. 2014, 127, 665–677. [Google Scholar] [CrossRef]
- Tan, J.; Ni, D.; Wali, J.A.; Cox, D.A.; Pinget, G.V.; Taitz, J.; Daïen, C.I.; Senior, A.; Read, M.N.; Simpson, S.J.; et al. Dietary Carbohydrate, Particularly Glucose, Drives B Cell Lymphopoiesis and Function. iScience 2021, 24, 102835. [Google Scholar] [CrossRef]
- Lo, B.C.; Kryczek, I.; Yu, J.; Vatan, L.; Caruso, R.; Matsumoto, M.; Sato, Y.; Shaw, M.H.; Inohara, N.; Xie, Y.; et al. Microbiota-dependent activation of CD4+ T cells induces CTLA-4 blockade-associated colitis via Fcγ receptors. Science 2024, 383, 62–70. [Google Scholar] [CrossRef]
- Wang, F.; Yin, Q.; Chen, L.; Davis, M.M. Bifidobacterium can mitigate intestinal immunopathology in the context of CTLA-4 blockade. Proc. Natl. Acad. Sci. USA 2018, 115, 157–161. [Google Scholar] [CrossRef]
- Zhuo, Q.; Yu, B.; Zhou, J.; Zhang, J.; Zhang, R.; Xie, J.; Wang, Q.; Zhao, S. Lysates of Lactobacillus acidophilus combined with CTLA-4-blocking antibodies enhance antitumor immunity in a mouse colon cancer model. Sci. Rep. 2019, 9, 20128. [Google Scholar] [CrossRef] [PubMed]
- Samara, J.; Moossavi, S.; Alshaikh, B.; Ortega, V.A.; Pettersen, V.K.; Ferdous, T.; Hoops, S.L.; Soraisham, A.; Vayalumkal, J.; Dersch-Mills, D.; et al. Supplementation with a Probiotic Mixture Accelerates Gut Microbiome Maturation and Reduces Intestinal Inflammation in Extremely Preterm Infants. Cell Host Microbe 2022, 30, 696–711.e5. [Google Scholar] [CrossRef]
- Bao, T.; He, F.; Zhang, X.; Zhu, L.; Wang, Z.; Lu, H.; Wang, T.; Li, Y.; Yang, S.; Wang, H. Inulin Exerts Beneficial Effects on Non-Alcoholic Fatty Liver Disease via Modulating Gut Microbiome and Suppressing the Lipopolysaccharide-Toll-Like Receptor 4-Mψ-Nuclear Factor-ΚB-Nod-Like Receptor Protein 3 Pathway viaGut-Liver Axis in Mice. Front. Pharmacol. 2020, 11, 558525. [Google Scholar] [CrossRef]
- Franchi, L.; Kamada, N.; Nakamura, Y.; Burberry, A.; Kuffa, P.; Suzuki, S.; Shaw, M.; Kim, Y.-G.; Nunez, G. NLRC4-Driven Production of IL-1 Beta Discriminates between Pathogenic and Commensal Bacteria and Promotes Host Intestinal Defense. Nat. Immunol. 2012, 13, 449–456. [Google Scholar] [CrossRef]
- Zhivaki, D.; Kagan, J.C. Innate Immune Detection of Lipid Oxidation as a Threat Assessment Strategy. Nat. Rev. Immunol. 2022, 22, 322–330. [Google Scholar] [CrossRef]
- Zanoni, I.; Tan, Y.; Di Gioia, M.; Broggi, A.; Ruan, J.; Shi, J.; Donado, C.A.; Shao, F.; Wu, H.; Springstead, J.R.; et al. An Endogenous Caspase-11 Ligand Elicits Interleukin-1 Release from Living Dendritic Cells. Science 2016, 352, 1232–1236. [Google Scholar] [CrossRef] [PubMed]
- Rolin, J.; Al-Jaderi, Z.; Maghazachi, A.A. Oxidized Lipids and Lysophosphatidylcholine Induce the Chemotaxis and Intracellular Calcium Influx in Natural Killer Cells. Immunobiology 2013, 218, 875–883. [Google Scholar] [CrossRef]
- Rolin, J.; Vego, H.; Maghazachi, A.A. Oxidized Lipids and Lysophosphatidylcholine Induce the Chemotaxis, Up-Regulate the Expression of CCR9 and CXCR4 and Abrogate the Release of IL-6 in Human Monocytes. Toxins 2014, 6, 2840–2856. [Google Scholar] [CrossRef]
- Wang, H.; Shen, L.; Sun, X.; Liu, F.; Feng, W.; Jiang, C.; Chu, X.; Ye, X.; Jiang, C.; Wang, Y.; et al. Adipose Group 1 Innate Lymphoid Cells Promote Adipose Tissue Fibrosis and Diabetes in Obesity. Nat. Commun. 2019, 10, 3254. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Kwon, M.-J.; Huang, S.; Lee, J.Y.; Fukase, K.; Inohara, N.; Hwang, D.H. Differential Modulation of Nods Signaling Pathways by Fatty Acids in Human Colonic Epithelial HCT116 Cells. J. Biol. Chem. 2007, 282, 11618–11628. [Google Scholar] [CrossRef]
- Thies, F.; Nebe-von-Caron, G.; Powell, J.R.; Yaqoob, P.; Newsholme, E.A.; Calder, P.C. Dietary Supplementation with Eicosapentaenoic Acid, but Not with Other Long-Chain N−3 or N−6 Polyunsaturated Fatty Acids, Decreases Natural Killer Cell Activity in Healthy Subjects Aged >55 Y123. Am. J. Clin. Nutr. 2001, 73, 539–548. [Google Scholar] [CrossRef] [PubMed]
- Mukaro, V.R.; Costabile, M.; Murphy, K.J.; Hii, C.S.; Howe, P.R.; Ferrante, A. Leukocyte Numbers and Function in Subjects Eating N-3 Enriched Foods: Selective Depression of Natural Killer Cell Levels. Arthritis Res. Ther. 2008, 10, R57. [Google Scholar] [CrossRef] [PubMed]
- Miles, E.A.; Banerjee, T.; Wells, S.J.; Calder, P.C. Limited Effect of Eicosapentaenoic Acid on T-Lymphocyte and Natural Killer Cell Numbers and Functions in Healthy Young Males. Nutrition 2006, 22, 512–519. [Google Scholar] [CrossRef] [PubMed]
- Poledne, R.; Malinska, H.; Kubatova, H.; Fronek, J.; Thieme, F.; Kauerova, S.; Kralova Lesna, I. Polarization of Macrophages in Human Adipose Tissue Is Related to the Fatty Acid Spectrum in Membrane Phospholipids. Nutrients 2020, 12, 8. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).