Therapeutic Potential of Cannabinoid Profiles Identified in Cannabis L. Crops in Peru
Abstract
1. Introduction
1.1. Regulation and Cannabis Cultivars in Peru
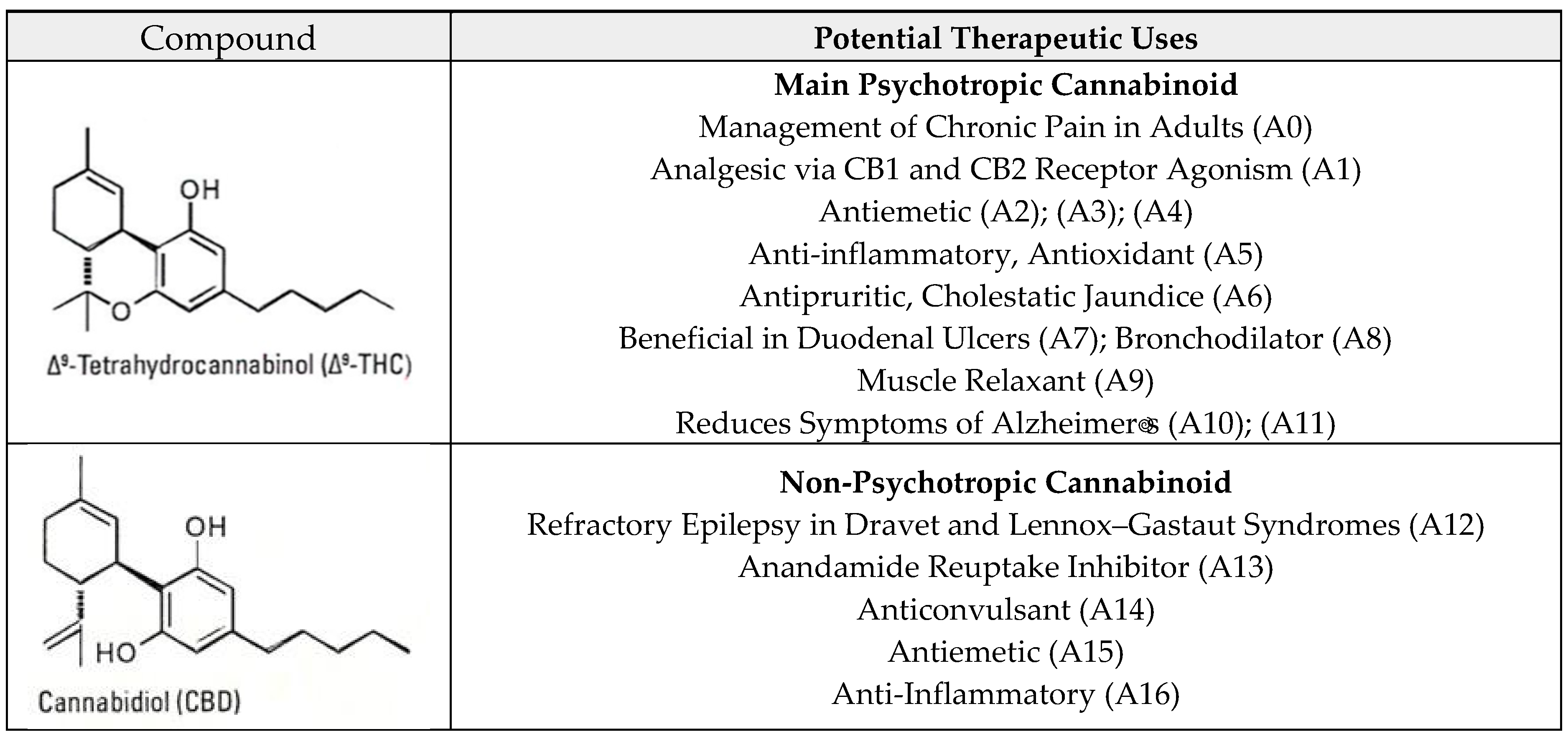
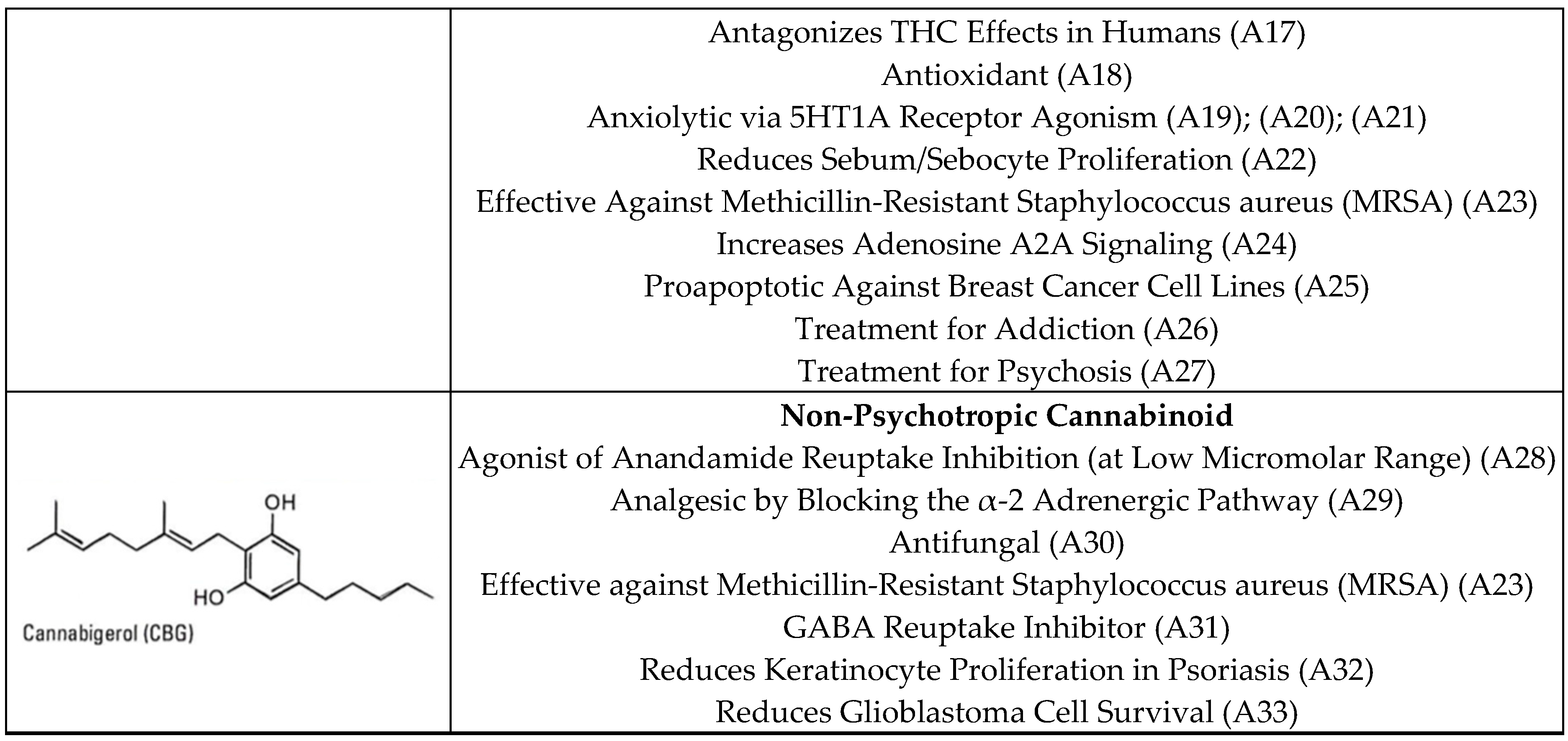
1.2. Phytocannabinoids
1.3. Cannabis Chemical Profiles or “Chemotypes”
2. Materials and Methods
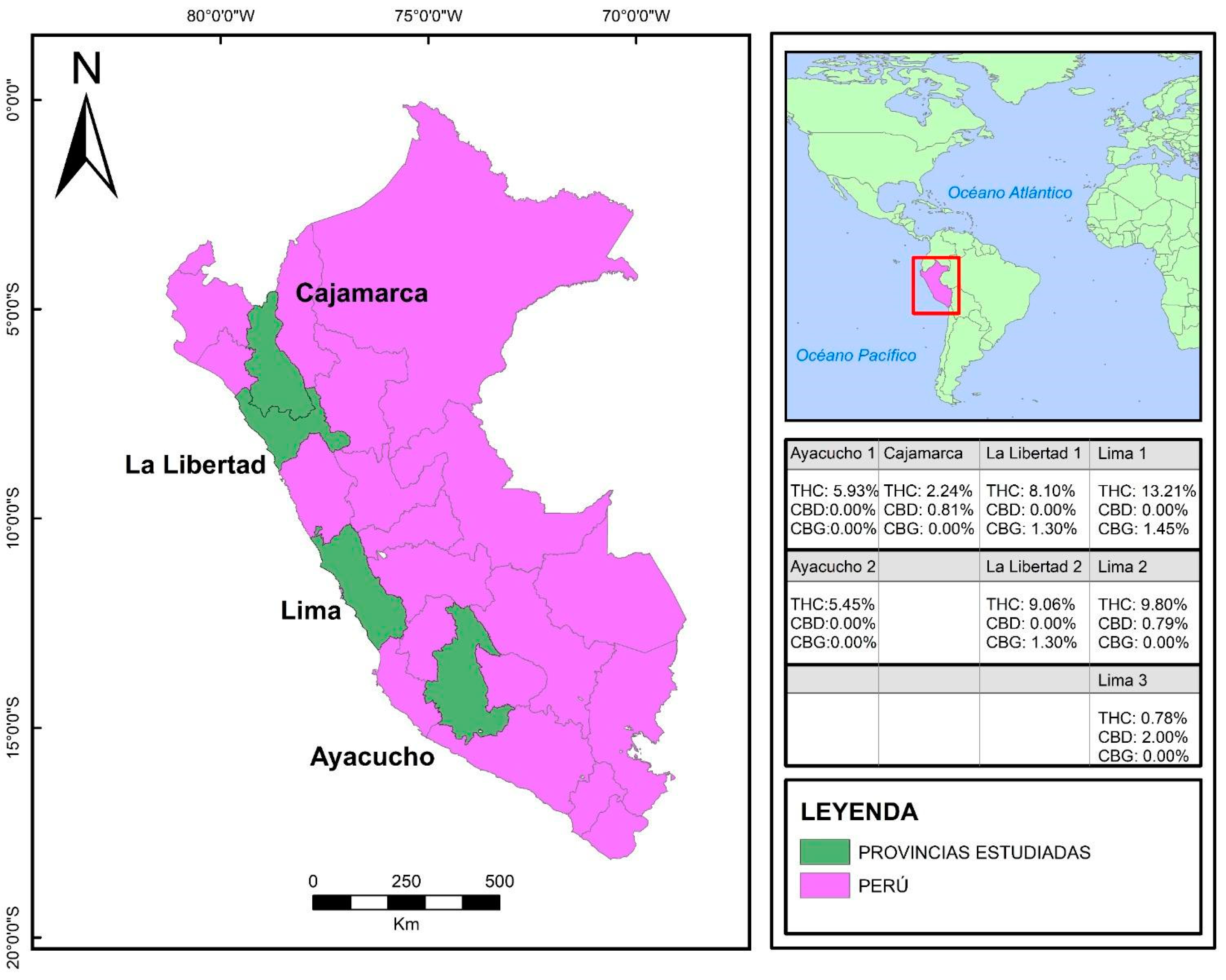
2.1. Study Design and Settings
2.2. Crop and Cannabinoid Identification
2.2.1. Crops and Inclusion Criteria
2.2.2. Botanical Characterization and Cannabinoid Quantification
2.2.3. Potential Therapeutic Profiles
2.3. Data Analysis
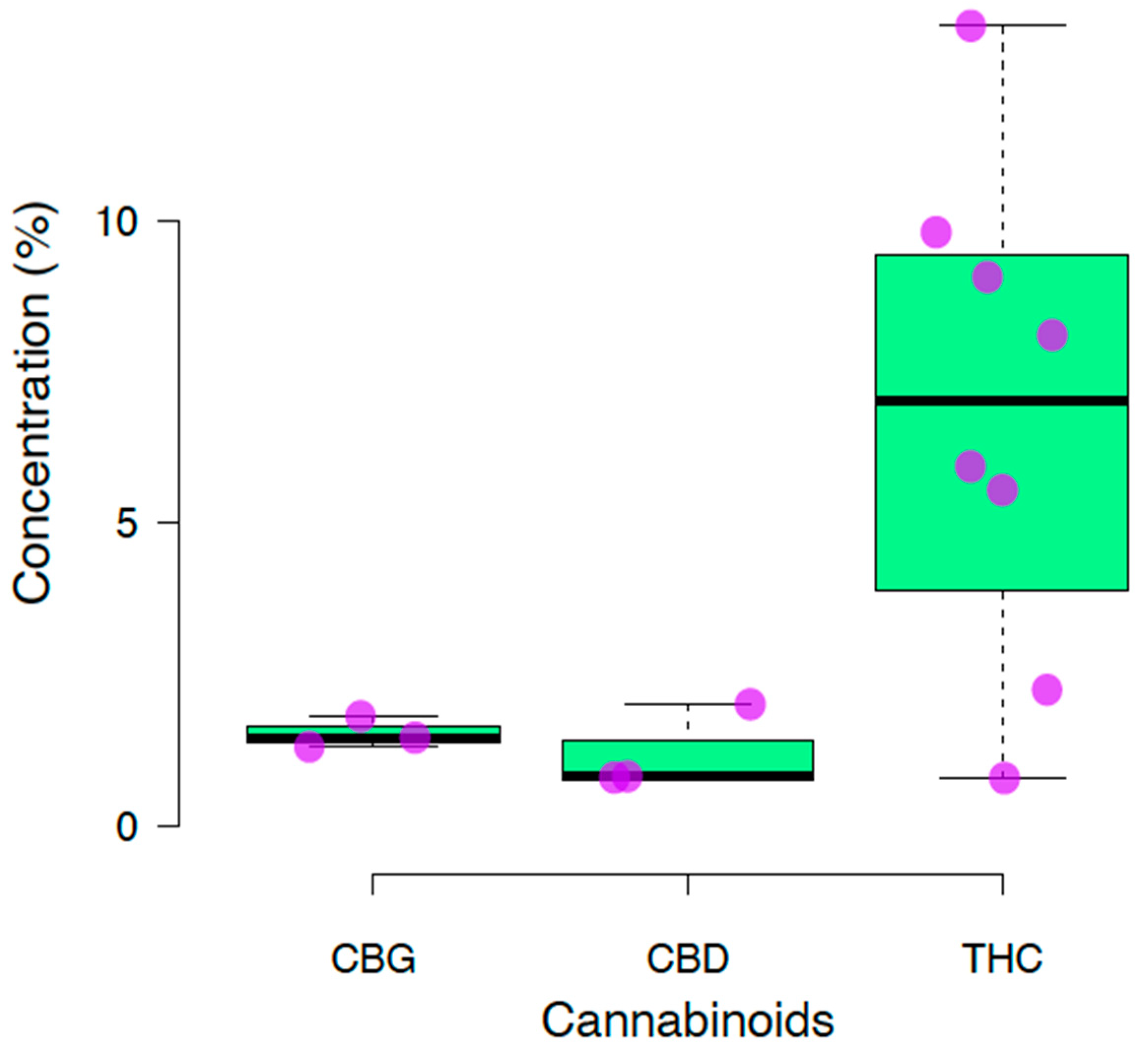
3. Results
4. Discussion
4.1. Strengths
4.2. Main Findings
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A


References
- Pain, S. A potted history. Nature 2015, 525, S10–S11. [Google Scholar] [CrossRef] [PubMed]
- Long, T.; Wagner, M.; Demske, D.; Leipe, C.; Tarasov, P.E. Cannabis in Eurasia: Origin of human use and Bronze Age trans-continental connections. Veg. Hist. Archaeobot. 2017, 26, 245–258. [Google Scholar] [CrossRef]
- Maccarrone, M. Endocannabinoid Signaling: Methods and Protocols, 2nd ed.; Humana Press: London, UK, 2022. [Google Scholar]
- Whiting, P.F.; Wolff, R.F.; Deshpande, S.; Di Nisio, M.; Duffy, S.; Hernandez, A.V.; Keurentjes, J.C.; Lang, S.; Misso, K.; Ryder, S.; et al. Cannabinoids for Medical Use: A Systematic Review and Meta-analysis. JAMA 2015, 313, 2456–2473. [Google Scholar] [CrossRef] [PubMed]
- Connor, J.P.; Stjepanović, D.; Le Foll, B.; Hoch, E.; Budney, A.J.; Hall, W.D. Cannabis use and cannabis use disorder. Nat. Rev. Dis. Primers 2021, 7, 16. [Google Scholar] [CrossRef] [PubMed]
- Levine, J. Understanding Medical Cannabis: Critical Issues and Perspectives for Human Service Professionals; Routledge: London, UK, 2020. [Google Scholar]
- Di Marzo, V.; Wang, J. The Endocannabinoidome: The World of Endocannabinoids and Related Mediators; Academic Press: London, UK, 2021. [Google Scholar]
- Devinsky, O.; Cross, J.H.; Laux, L.; Marsh, E.; Miller, I.; Nabbout, R.; Scheffer, I.E.; Thiele, E.A.; Wright, S. Trial of cannabidiol for drug-resistant seizures in the Dravet syndrome. N. Engl. J. Med. 2017, 376, 2011–2020. [Google Scholar] [CrossRef] [PubMed]
- Bonnett, M. Historical Overview on the Evolution and Legislation of Cannabis in Latin America. Meritas. Available online: https://www.meritas.org/insight/article/Historical-Overview-on-the-evolution-and-legislation-of-cannabis-in-latin-america (accessed on 16 August 2023).
- Ministério da Saúde. Resolução da Diretoria Colegiada—RDC Nº 327. Available online: https://www.in.gov.br/en/web/dou/-/resolucao-da-diretoria-colegiada-rdc-n-327-de-9-de-dezembro-de-2019-232669072 (accessed on 16 August 2023).
- Ministerio de Salud. LEY Nº 27.350 Investigacion Medica y Cientifica de Uso Medicinal de la Planta de Cannabis y Sus Derivados. Available online: https://www.argentina.gob.ar/normativa/nacional/ley-27350-273801/normas-modifican (accessed on 16 August 2023).
- La República. Reglamento del Minsa No Beneficia a los Usuarios del Cannabis Medicinal. Available online: https://larepublica.pe/sociedad/2023/03/10/reglamento-del-minsa-no-beneficia-a-los-usuarios-del-cannabis-medicinal-489440 (accessed on 16 August 2023).
- Ministerio del Interior. LEY No 22095 Ley de Represión de Tráfico Ilícito de Drogas. Available online: https://www.gob.pe/institucion/corahperu/normas-legales/850797-22095 (accessed on 16 August 2023).
- Ministerio de Salud. DS 023—2001SA Reglamento de Estupefacientes y Psicotrópicos. Available online: https://www.gob.pe/institucion/minsa/normas-legales/255646-023-2001-sa (accessed on 16 August 2023).
- Comisión Nacional para el Desarrollo y Vida sin Droga. Politica Nacional Contra las Drogas al 2030; DEVIDA: Lima, Peru, 2022. [Google Scholar]
- ElSohly, M.A.; Slade, D. Chemical constituents of marijuana: The complex mixture of natural cannabinoids. Life Sci. 2005, 78, 539–548. [Google Scholar] [CrossRef] [PubMed]
- Cicaloni, V.; Salvini, L.; Vitalini, S.; Garzoli, S. Chemical Profiling and Characterization of Different Cultivars of Cannabis sativa L. Inflorescences by SPME-GC-MS and UPLC-MS. Separations 2022, 9, 90. [Google Scholar] [CrossRef]
- Murovec, J.; Eržen, J.J.; Flajšman, M.; Vodnik, D. Analysis of Morphological Traits, Cannabinoid Profiles, THCAS Gene Sequences, and Photosynthesis in Wide and Narrow Leaflet High-Cannabidiol Breeding Populations of Medical Cannabis. Front. Plant Sci. 2022, 13, 786161. [Google Scholar] [CrossRef] [PubMed]
- Tipparat, P.; Natakankitkul, S.; Chamnivikaipong, P.; Chutiwat, S. Characteristics of cannabinoids composition of Cannabis plants grown in Northern Thailand and its forensic application. Forensic Sci. Int. 2012, 215, 164–170. [Google Scholar] [CrossRef] [PubMed]
- San Nicolas, M.; Villate, A.; Gallastegi, M.; Aizpurua-Olaizola, O.; Olivares, M.; Etxebarria, N.; Usobiaga, A. Chapter Three—Analysis of cannabinoids in plants, marijuana products and biological tissues. Compreh. Anal. Chem. 2020, 90, 65–102. [Google Scholar] [CrossRef]
- National Academies of Sciences, Engineering, and Medicine. Health and Medicine Division; Board on Population Health and Public Health Practice; Committee on the Health Effects of Marijuana: An Evidence Review and Research Agenda. In The Health Effects of Cannabis and Cannabinoids: The Current State of Evidence and Recommendations for Research; National Academies Press: Washington, DC, USA, 2017. [Google Scholar]
- Gaoni, Y.; Mechoulam, R. Isolation, Structure, and Partial Synthesis of an Active Constituent of Hashish. J. Am. Chem. Soc. 1964, 86, 1646–1647. [Google Scholar] [CrossRef]
- Food and Drug Aadministration. MARINOL (Dronabinol) Capsules, for Oral Use. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/018651s029lbl.pdf (accessed on 16 August 2023).
- Meccariello, R.; Chianese, R. Cannabinoids in Health and Disease; InTech: London, UK, 2016. [Google Scholar]
- Fućak, T.; Kreft, S.; Svedružić, Ž.M.; Tavčar, E. Mechanism and kinetics of CBDA decarboxylation into CBD in hemp. J. Plant Biochem. Biotechnol. 2023, 32, 608–621. [Google Scholar] [CrossRef]
- Ministerio de Salud. Resolución 673/2022. Available online: https://www.boletinoficial.gob.ar/detalleAviso/primera/259987/20220329 (accessed on 16 August 2023).
- Cascio, M.G.; Gauson, L.A.; Stevenson, L.A.; Ross, R.A.; Pertwee, R.G. Evidence that the plant cannabinoid cannabigerol is a highly potent α 2-adrenoceptor agonist and moderately potent 5HT 1A receptor antagonist. Br. J. Pharmacol. 2010, 159, 129–141. [Google Scholar] [CrossRef] [PubMed]
- Food and Drug Adminsitration. EPIDIOLEX® USPI. Highlights of Prescribing Information: EPIDIOLEX® (Cannabidiol) Oral Solution. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/210365s005s006s007lbl.pdf (accessed on 16 August 2023).
- Ministerio de Salud. Resolución Directoral N.° 140-2012-DIGEMID-DG-MINSA Listado de Referencias Bibliográficas que Sustentan la Seguridad de Uso y Uso Tradicional de Recursos Naturales o Sus Asociaciones del Ministerio de Salud del Perú. Available online: https://www.gob.pe/institucion/minsa/normas-legales/241178-140-2012-digemid-dg-minsa (accessed on 16 August 2023).
- Von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gøtzsche, P.C.; Vandenbroucke, J.P. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: Guidelines for reporting observational studies. J. Clin. Epidemiol. 2008, 61, 344–349. [Google Scholar] [CrossRef] [PubMed]
- Lazarjani, M.P.; Young, O.; Kebede, L.; Seyfoddin, A. Processing and extraction methods of medicinal cannabis: A narrative review. J. Cannabis Res. 2021, 3, 32. [Google Scholar] [CrossRef] [PubMed]
- Chasiotis, V.; Tsakirakis, A.; Termentzi, A.; Machera, K.; Filios, A. Drying and quality characteristics of Cannabis sativa L. inflorescences under constant and time-varying convective drying temperature schemes. Therm. Sci. Eng. Prog. 2022, 28, 101076. [Google Scholar] [CrossRef]
- United Nations Office on Drugs and Crime. World Drug Report 2023; UNDOC: Geneva, Switzerland, 2023. [Google Scholar]
- Fernández, S.; Castro, R.; López-Radcenco, A.; Rodriguez, P.; Carrera, I.; García-Carnelli, C.; Moyna, G. Beyond cannabinoids: Application of NMR-based metabolomics for the assessment of Cannabis sativa L. crop health. Front. Plant Sci. 2023, 14, 1025932. [Google Scholar] [CrossRef] [PubMed]
- Editorial. El 70% de Pacientes de Cannabis no Compra su Medicina en Farmacias y Boticas Debido a Trabas. La República. Available online: https://larepublica.pe/sociedad/2022/05/26/cannabis-el-70-de-pacientes-no-compra-su-medicina-en-farmacias-o-boticas-autorizadas-debido-a-trabas-marihuana-minsa-atmp (accessed on 16 August 2023).
- Kane, M. Peru Destroys Massive Marijuana Crop, Pointing to Possible Rise in Cultivation. InSight Crime. Available online: https://insightcrime.org/news/brief/peru-destroys-massive-marijuana-crop-pointing-to-possible-rise-in-cultivation/ (accessed on 16 August 2023).
- Wong-Salgado, P.; Moya-Salazar, M.M.; Goicochea-Palomino, E.; Moya-Salazar, J. The current context of a cannabis regulatory change: Analysis of a Peruvian course. Rev. Cub. Med. Mil. 2023, 52, e02302434. Available online: https://revmedmilitar.sld.cu/index.php/mil/article/view/2434 (accessed on 10 July 2023).

| Region | M.A.S.L. | Climate | Average Humidity (%) | Average Temperature °C/F |
|---|---|---|---|---|
| Lima | 200 | Arid subtropical | 80 | 17.4 (63.3) |
| Ayacucho | 2760 | Inter-Andean valley | 56 | 17.5 (63.5) |
| Trujillo | 34 | Arid subtropical | 89 | 19.7 (67.46) |
| Cajamarca | 2750 | Inter-Andean valley | 72 | 13 (55.4) |
| Sample | Region of Origin | Geographical Region | Cannabinoid Profile | Potential Therapeutic Effect |
|---|---|---|---|---|
| F1 | Cajamarca | Highlands | THC: 2.24% CBD: 0.81% CBG: ND | Potency of effect: Low. Medical areas of potential: Chronic pain management, antiemetic use, anti-inflammatory use, antipruritic use, duodenal ulcer treatment, bronchodilators, muscle relaxants, and treating Alzheimer’s disease symptoms due to the presence of THC. Refractory epilepsy treatment, anxiolytic use, sebum reduction, proapoptotic use in breast cancer, and are effective on MRSA and in addiction and psychosis treatments due to the presence of CBD. Psychotropic effect: Very small. |
| F2 | Lima | Coast | THC: 13.21% CBD: ND CBG: 1.45% | Potency of effect: Very high. Medical areas of potential: Chronic pain management, antiemetic use, anti-inflammatory use, antipruritic use, duodenal ulcer treatment, bronchodilators, muscle relaxants, and treating Alzheimer’s disease symptoms due to the presence of THC in high amounts; effective on MRSA, in controlling psoriasis, and in glioblastoma treatment due to the presence of CBG. Psychotropic effect: Large. |
| F3 | Lima | Coast | THC: 9.80% CBD: 0.79% CBG: ND | Potency of effect: High. Medical areas of potential: Chronic pain management, antiemetic use, anti-inflammatory use, antipruritic use, duodenal ulcer treatment, bronchodilators, muscle relaxants, and treating Alzheimer’s disease symptoms due to the presence of THC. Refractory epilepsy treatment, anxiolytic use, sebum reduction, proapoptotic in breast cancer, and are effective on MRSA and in addiction and psychosis treatments due to the presence of CBD. Psychotropic effect: Moderate. |
| F4 | Lima | Coast | THC: 0.78% CBD: 2.0% CBG: ND | Potency of effect: Moderate. Medical areas of potential: Chronic pain management, antiemetic use, anti-inflammatory use, antipruritic use, duodenal ulcer treatment, bronchodilators, muscle relaxants, and treating Alzheimer’s disease symptoms due to the presence of THC. Refractory epilepsy treatment, anxiolytic use, sebum reduction, proapoptotic in breast cancer, and are effective on MRSA and in addiction, and psychosis treatments due to the presence of CBD. Psychotropic effect: Null. |
| F5 | La Libertad | Highlands | THC: 8.1% CBD: ND CBG: 1.30% | Potency of effect: High. Medical areas of potential: Chronic pain management, antiemetic use, anti-inflammatory use, antipruritic use, duodenal ulcer treatment, bronchodilators, muscle relaxants, and treating Alzheimer’s disease symptoms due to the presence of THC in high amounts; effective on MRSA, in controlling psoriasis, and in glioblastoma treatments due to the presence of CBG. Psychotropic effect: Large. |
| F6 | La Libertad | Highlands | THC: 9.06% CBD: ND CBG: 1.83% | Potency of effect: High. Medical areas of potential: Chronic pain management, antiemetic use, anti-inflammatory use, antipruritic use, duodenal ulcer treatment, bronchodilators, muscle relaxants, and treating Alzheimer’s disease symptoms due to the presence of THC in high amounts; effective on MRSA, in controlling psoriasis, and in glioblastoma treatments due to the presence of CBG. Psychotropic effect: Large. |
| F7 | Ayacucho | Highlands | THC: 5.93% CBD: ND CBG: ND | Potency of effect: Moderate. Medical areas of potential: Chronic pain management, antiemetic use, anti-inflammatory use, antipruritic use, duodenal ulcer treatment, bronchodilators, muscle relaxants, and treating Alzheimer’s disease symptoms due to the presence of THC; small psychotropic effect. Psychotropic effect: Moderate. |
| F8 | Ayacucho | Highlands | THC: 5.45% CBD: ND CBG: ND | Potency of effect: Moderate. Medical areas of potential: Chronic pain management, antiemetic use, anti-inflammatory use, antipruritic use, duodenal ulcer treatment, bronchodilators, muscle relaxants, and treating Alzheimer’s disease symptoms due to the presence of THC; small psychotropic effect. Psychotropic effect: Moderate. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wong-Salgado, P.; Soares, F.; Moya-Salazar, J.; Ramírez-Méndez, J.F.; Moya-Salazar, M.M.; Apesteguía, A.; Castro, A. Therapeutic Potential of Cannabinoid Profiles Identified in Cannabis L. Crops in Peru. Biomedicines 2024, 12, 306. https://doi.org/10.3390/biomedicines12020306
Wong-Salgado P, Soares F, Moya-Salazar J, Ramírez-Méndez JF, Moya-Salazar MM, Apesteguía A, Castro A. Therapeutic Potential of Cannabinoid Profiles Identified in Cannabis L. Crops in Peru. Biomedicines. 2024; 12(2):306. https://doi.org/10.3390/biomedicines12020306
Chicago/Turabian StyleWong-Salgado, Pedro, Fabiano Soares, Jeel Moya-Salazar, José F. Ramírez-Méndez, Marcia M. Moya-Salazar, Alfonso Apesteguía, and Americo Castro. 2024. "Therapeutic Potential of Cannabinoid Profiles Identified in Cannabis L. Crops in Peru" Biomedicines 12, no. 2: 306. https://doi.org/10.3390/biomedicines12020306
APA StyleWong-Salgado, P., Soares, F., Moya-Salazar, J., Ramírez-Méndez, J. F., Moya-Salazar, M. M., Apesteguía, A., & Castro, A. (2024). Therapeutic Potential of Cannabinoid Profiles Identified in Cannabis L. Crops in Peru. Biomedicines, 12(2), 306. https://doi.org/10.3390/biomedicines12020306




