Correlation Between Clinical Indicators and Liver Pathology in Children with Chronic Hepatitis B
Abstract
1. Introduction
2. Methods and Methods
2.1. Patients
2.2. Ethical Clearance and Informed Consent for Participation
2.3. Demographic and Clinical Characteristics
2.4. Histological Examination
2.5. Statistical Analysis
3. Results
3.1. Propensity Score Matching
3.2. Basic Demographic Information and Characteristics
3.3. Grading of Liver Inflammation and Staging of Fibrosis
3.4. Power Analysis Results
3.5. Correlation Analysis of Inflammation and Fibrosis with Clinical Indicators
3.6. RCS Plots for Predictors of Inflammation and Fibrosis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Polaris Observatory Collaborators. Global prevalence, cascade of care, and prophylaxis coverage of hepatitis B in 2022: A modelling study. Lancet Gastroenterol. Hepatol. 2023, 8, 879–907. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Wu, Y.; Zhang, C.; Ji, D.; Wang, F.S. The burden of cirrhosis and other chronic liver diseases due to hepatitis B in children and adolescents: Results from global burden of disease study 2019. Front. Public Health 2023, 11, 1315392. [Google Scholar] [CrossRef]
- Hou, J.; Cui, F.; Ding, Y.; Dou, X.; Duan, Z.; Han, G.; Jia, J.; Mao, Q.; Li, J.; Li, Z.; et al. Management Algorithm for Interrupting Mother-to-Child Transmission of Hepatitis B Virus. Clin. Gastroenterol. Hepatol. 2019, 17, 1929–1936. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Shen, M.; Ong, J.J.; Cui, F.; Hu, W.; Chan, P.; Zou, Z.; Su, S.; Liu, H.; Zhang, L.; et al. Blueprint to hepatitis B elimination in China: A modelling analysis of clinical strategies. JHEP Rep. 2023, 5, 100833. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Zhuang, T.; Xia, R.; Zou, Z.; Zhang, L.; Shen, M.; Zhuang, G. Modelling the prevalence of hepatitis B towards eliminating it as a major public health threat in China. BMC Public Health 2022, 22, 1179. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.F.; Dai, C.Y.; Huang, C.F.; Tsai, P.C.; Yeh, M.L.; Hsu, P.Y.; Huang, S.F.; Bair, M.J.; Hou, N.J.; Huang, C.I.; et al. First-in-Asian double-blind randomized trial to assess the efficacy and safety of insulin sensitizer in nonalcoholic steatohepatitis patients. Hepatol. Int. 2021, 15, 1136–1147. [Google Scholar] [CrossRef]
- Wirth, S.; Zhang, H.; Hardikar, W.; Schwarz, K.B.; Sokal, E.; Yang, W.; Fan, H.; Morozov, V.; Mao, Q.; Deng, H.; et al. Efficacy and Safety of Peginterferon Alfa-2a (40KD) in Children With Chronic Hepatitis B: The PEG-B-ACTIVE Study. Hepatology 2018, 68, 1681–1694. [Google Scholar] [CrossRef] [PubMed]
- Dezsőfi, A.; Baumann, U.; Dhawan, A.; Durmaz, O.; Fischler, B.; Hadzic, N.; Hierro, L.; Lacaille, F.; McLin, V.A.; Nobili, V.; et al. Liver biopsy in children: Position paper of the ESPGHAN Hepatology Committee. J. Pediatr. Gastroenterol. Nutr. 2015, 60, 408–420. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Wu, X.; Ye, Y.; Ye, L.; Han, S.; Wang, X.; Yu, H. Liver histology of treatment-naive children with chronic hepatitis B virus infection in Shanghai China. Int. J. Infect. Dis. 2022, 123, 112–118. [Google Scholar]
- Liang, C.; Chang, Y.; Peng, X.; He, Y.; Chen, M.; Peng, M.; Hu, P.; Ren, H.; Xu, H. Analysis of liver pathology characteristics and exploration of non-invasive indicators of liver fibrosis in children with chronic hepatitis B. Chin. J. Hepatol. 2021, 29, 551–557. [Google Scholar]
- Forna, L.; Bozomitu, L.; Lupu, A.; Lupu, V.V.; Cojocariu, C.; Anton, C.; Girleanu, I.; Singeap, A.M.; Muzica, C.M.; Trifan, A. Insights into the Natural and Treatment Courses of Hepatitis B in Children: A Retrospective Study. Biomedicines 2024, 12, 1585. [Google Scholar] [CrossRef]
- Luo, H.; Peng, S.; Ouyang, W.; Tan, Y.; Jiang, T.; Tang, L.; Li, S.; Qiu, J.; Zhou, C. Assessment of liver fibrosis by transient elastography and multi-parameters model in young children with chronic hepatitis B virus infection. BMC Infect. Dis. 2022, 22, 160. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.L.; Chen, X.Q.; Lv, Z.L.; Tang, Q.; Shan, Q.W. A simple noninvasive model to predict significant fibrosis in children with chronic hepatitis B. Medicine 2021, 100, e26462. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Baez, N.; Murray, K.F.; Kleiner, D.E.; Ling, S.C.; Rosenthal, P.; Carlin, K.; Cooper, K.; Schwarz, K.B.; Schwarzenberg, S.J.; Teckman, J.H.; et al. Hepatic Histology in Treatment-naive Children With Chronic Hepatitis B Infection Living in the United States and Canada. J. Pediatr. Gastroenterol. Nutr. 2020, 71, 99–105. [Google Scholar] [CrossRef] [PubMed]
- Mozer-Lisewska, I.; Mania, A.; Słuzewski, W.; Kemnitz, P.; Prusinowska, J.; Kowala-Piaskowska, A. Factors influencing clinical course and histological findings in children with chronic hepatitis B. Eur. J. Gastroenterol. Hepatol. 2009, 21, 1400–1406. [Google Scholar] [CrossRef]
- Mozer-Lisewska, I.; Słuzewski, W.; Mania, A.; Walewska-Zielecka, B.; Bujnowska, A.; Kowala-Piaskowska, A.; Figlerowicz, M. Histopathological evaluation of liver biopsy specimens in children with chronic hepatitis B. Hepatol. Res. 2006, 34, 9–14. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Wang, Y.; Liu, S.; Zhai, X.; Zhou, G.; Lu, F.; Zhao, J. Nonalcoholic fatty liver disease is associated with lower hepatitis B viral load and antiviral response in pediatric population. J. Gastroenterol. 2019, 54, 1096–1105. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Zhu, S.; Xu, X. Expert consensus on the prevention and treatment of chronic hepatitis B in children. Infect. Dis. Immun. 2024, 3, 106–120. [Google Scholar]
- World Medical Association. Declaration of Helsinki: Ethical principles for medical research involving human subjects. J. Int. Bioethique 2004, 15, 124–129. [Google Scholar]
- Goodman, Z.D. Grading and staging systems for inflammation and fibrosis in chronic liver diseases. J. Hepatol. 2007, 47, 598–607. [Google Scholar] [CrossRef]
- Torbenson, M.S.; Arnold, C.A.; Graham, R.P.; Jain, D.; Kakar, S.; Lam-Himlin, D.M.; Naini, B.V.; Wu, T.T.; Yeh, M. Identification of key challenges in liver pathology: Data from a multicenter study of extramural consults. Hum. Pathol. 2019, 87, 75–82. [Google Scholar] [CrossRef]
- Zhou, J.; Zhang, Q.; Wang, W.; Sun, L.; Li, R.; Zhao, S.; Wang, D. The benefits of R anastomotic technique for Billroth-II reconstruction with Braun anastomosis during totally laparoscopic distal gastrectomy: A propensity score matching analysis. Int. J. Surg. 2024, 110, 23–31. [Google Scholar] [CrossRef]
- van Putten, M.J.A.M.; Ruijter, B.J.; Horn, J.; van Rootselaar, A.F.; Tromp, S.C.; van Kranen-Mastenbroek, V.; Gaspard, N.; Hofmeijer, J.; TELSTAR Investigators. Quantitative Characterization of Rhythmic and Periodic EEG Patterns in Patients in a Coma After Cardiac Arrest and Association With Outcome. Neurology 2024, 103, e209608. [Google Scholar] [CrossRef] [PubMed]
- Indolfi, G.; Easterbrook, P.; Dusheiko, G.; Siberry, G.; Chang, M.H.; Thorne, C.; Bulterys, M.; Chan, P.L.; El-Sayed, M.H.; Giaquinto, C.; et al. Hepatitis B virus infection in children and adolescents. Lancet Gastroenterol. Hepatol. 2019, 4, 466–476. [Google Scholar] [CrossRef]
- Mason, W.S.; Gill, U.S.; Litwin, S.; Zhou, Y.; Peri, S.; Pop, O.; Hong, M.L.; Naik, S.; Quaglia, A.; Bertoletti, A.; et al. HBV DNA Integration and Clonal Hepatocyte Expansion in Chronic Hepatitis B Patients Considered Immune Tolerant. Gastroenterology 2016, 151, 986–998. [Google Scholar] [CrossRef]
- Wu, J.F.; Song, S.H.; Lee, C.S.; Chen, H.L.; Ni, Y.H.; Hsu, H.Y.; Wu, T.C.; Chang, M.H. Clinical Predictors of Liver Fibrosis in Patients With Chronic Hepatitis B Virus Infection From Children to Adults. J. Infect. Dis. 2018, 217, 1408–1416. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhu, L.; Zhang, Z.; Zhang, S.; Pan, Y.; Li, Y.; Cao, F.; Jiang, C.; Fan, T.; Xiong, Y.; et al. Lower HBV DNA level is associated with more severe liver fibrosis in HBeAg-positive chronic hepatitis B with normal alanine transaminase. Virol. J. 2024, 21, 127. [Google Scholar] [CrossRef]
- Xu, J.; Zhang, Y.; Zhu, L.; Tang, S.; Xu, H.; Zhang, D.; Chen, H.; Zhou, J. Non-Invasive Monitoring of the Impact of Low-Level Viremia on Liver Fibrosis in Treated Chronic Hepatitis B Patients. Infect. Drug Resist. 2024, 17, 2751–2758. [Google Scholar] [CrossRef] [PubMed]
- Tang, S.; Qin, R.; Zhang, D.; He, X.; Yu, C.; Chen, D.; Li, X.; Liu, S. Liver injury and prolonged hospitalization as indicators of severity in patients with adenovirus infections. BMC Infect. Dis. 2024, 24, 430. [Google Scholar] [CrossRef]
- Takano, T.; Tajiri, H.; Hosono, S.; Inui, A.; Murakami, J.; Ushijima, K.; Miyoshi, Y.; Etani, Y.; Abukawa, D.; Suzuki, M.; et al. Natural history of chronic hepatitis B virus infection in children in Japan: A comparison of mother-to-child transmission with horizontal transmission. J. Gastroenterol. 2017, 52, 1041–1050. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Y.W.; Di, F.L.; Liu, C.; Zhang, X.C.; Bi, J.F.; Li, Y.L.; Wu, S.Q.; Dong, H.; Liu, L.M.; He, J.; et al. Hepatitis B virus basal core promoter/precore mutants and association with liver cirrhosis in children with chronic hepatitis B virus infection. Clin. Microbiol. Infect. 2016, 22, 371–379. [Google Scholar] [CrossRef] [PubMed]
- Zeng, S.; Liu, Z.; Ke, B.; Zhang, Y.; Wang, Q.; Tan, S. The non-invasive serum biomarkers contributes to indicate liver fibrosis staging and evaluate the progress of chronic hepatitis B. BMC Infect. Dis. 2024, 24, 638. [Google Scholar] [CrossRef]
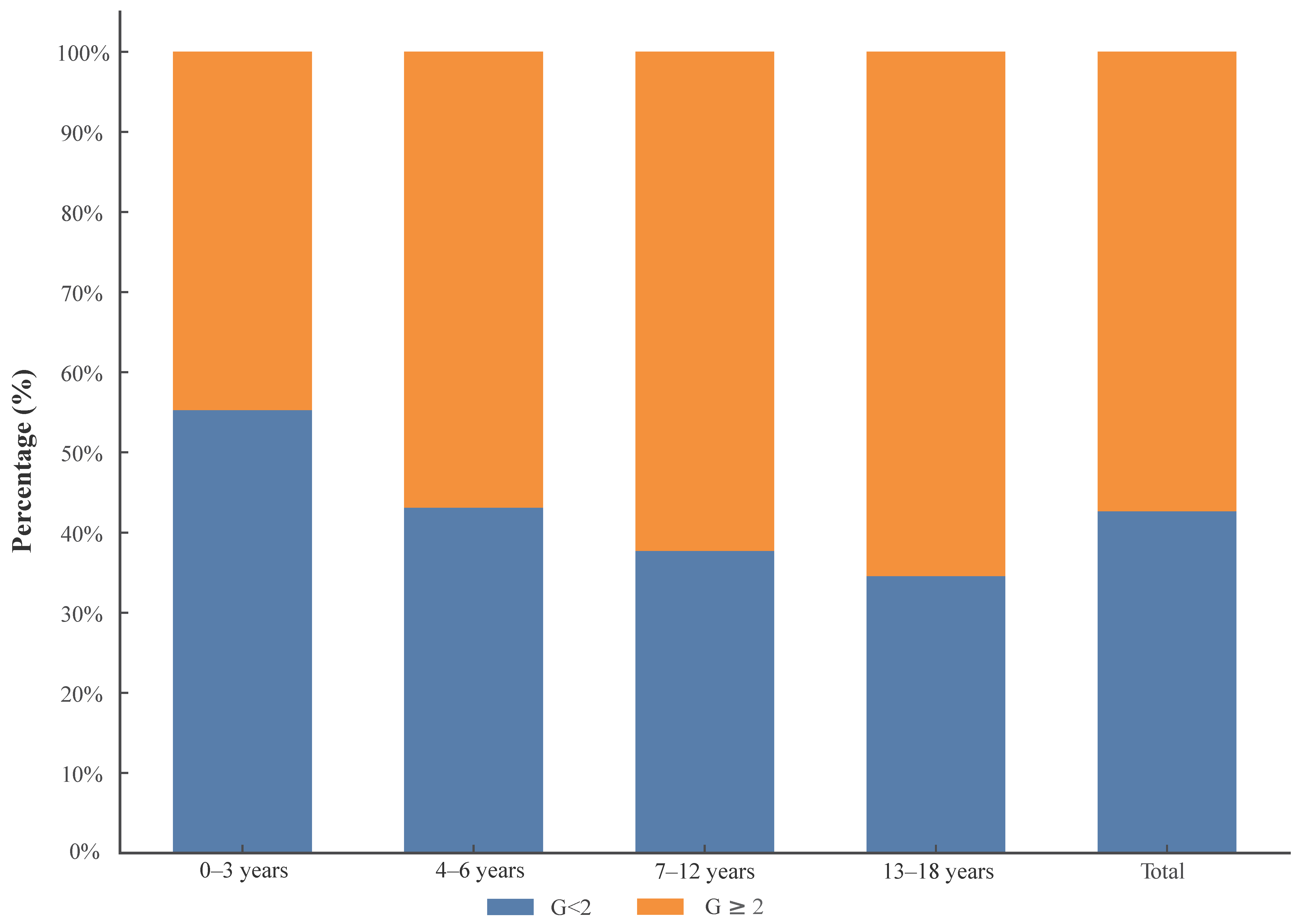



| Variable | S0–1 Mean (SD) Pre-Match | S2–4 Mean (SD) Pre-Match | G0–1 Mean (SD) Post-Match | G2–4 Mean (SD) Post-Match | Fibrosis SMD Pre-Match | Fibrosis SMD Post-Match | G0–1 Mean (SD) Pre-Match | G2–4 Mean (SD) Pre-Match | G0–1 Mean (SD) Post-Match | G2–4 Mean (SD) Post-Match | Inflammation SMD Pre-Match | Inflammation SMD Post-Match |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Age (years) | 6.5 (3.1) | 7.2 (3.0) | 6.8 (3.1) | 6.9 (3.0) | 5:31:12 | 1:12:00 | 6.5 (3.1) | 7.2 (3.0) | 6.8 (3.1) | 6.9 (3.0) | 5:31:12 | 1:12:00 |
| Sex (% male) | 66.3 (0.47) | 68.2 (0.47) | 67.1 (0.47) | 67.8 (0.47) | 0:57:36 | 0:28:48 | 66.3 (0.47) | 68.2 (0.47) | 67.1 (0.47) | 67.8 (0.47) | 0:57:36 | 0:28:48 |
| Genotype B (%) | 34.2 (0.48) | 35.6 (0.48) | 34.9 (0.48) | 35.2 (0.48) | 0:43:12 | 0:14:24 | 34.2 (0.48) | 35.6 (0.48) | 34.9 (0.48) | 35.2 (0.48) | 0:43:12 | 0:14:24 |
| HBeAg (% positive) | 55.0 (0.50) | 53.0 (0.50) | 54.0 (0.50) | 54.5 (0.50) | 0:57:36 | 0:14:24 | 55.0 (0.50) | 53.0 (0.50) | 54.0 (0.50) | 54.5 (0.50) | 0:57:36 | 0:14:24 |
| HBV-DNA (lgIU/mL) | 4.7 (1.2) | 5.0 (1.2) | 4.8 (1.2) | 4.9 (1.2) | 6:00:00 | 1:55:12 | 4.7 (1.2) | 5.0 (1.2) | 4.8 (1.2) | 4.9 (1.2) | 6:00:00 | 1:55:12 |
| PLT (109/L) | 231.2 (54.6) | 224.7 (56.3) | 229.5 (55.2) | 227.4 (55.9) | 2:52:48 | 0:57:36 | 231.2 (54.6) | 224.7 (56.3) | 229.5 (55.2) | 227.4 (55.9) | 2:52:48 | 0:57:36 |
| HB (g/L) | 130.0 (15.0) | 128.5 (14.5) | 129.8 (14.8) | 129.1 (14.6) | 2:24:00 | 0:28:48 | 130.0 (15.0) | 128.5 (14.5) | 129.8 (14.8) | 129.1 (14.6) | 2:24:00 | 0:28:48 |
| RBC (109/L) | 4.5 (0.6) | 4.4 (0.5) | 4.5 (0.6) | 4.5 (0.5) | 4:48:00 | 1:12:00 | 4.5 (0.6) | 4.4 (0.5) | 4.5 (0.6) | 4.5 (0.5) | 4:48:00 | 1:12:00 |
| WBC (1012/L) | 5.5 (1.2) | 5.4 (1.1) | 5.5 (1.2) | 5.5 (1.1) | 3:36:00 | 0:43:12 | 5.5 (1.2) | 5.4 (1.1) | 5.5 (1.2) | 5.5 (1.1) | 3:36:00 | 0:43:12 |
| AFP (ng/mL) | 10.0 (5.0) | 10.5 (4.8) | 10.2 (4.9) | 10.3 (4.7) | 1:55:12 | 0:28:48 | 10.0 (5.0) | 10.5 (4.8) | 10.2 (4.9) | 10.3 (4.7) | 1:55:12 | 0:28:48 |
| TBiL (μmol/L) | 20.0 (5.0) | 21.5 (5.2) | 20.8 (5.1) | 21.0 (5.0) | 4:19:12 | 0:57:36 | 20.0 (5.0) | 21.5 (5.2) | 20.8 (5.1) | 21.0 (5.0) | 4:19:12 | 0:57:36 |
| DBiL (μmol/L) | 5.0 (1.5) | 5.2 (1.6) | 5.1 (1.5) | 5.1 (1.6) | 2:24:00 | 0:43:12 | 5.0 (1.5) | 5.2 (1.6) | 5.1 (1.5) | 5.1 (1.6) | 2:24:00 | 0:43:12 |
| AST (U/L) | 45.0 (10.0) | 46.0 (9.5) | 45.5 (9.8) | 45.8 (9.6) | 2:24:00 | 0:28:48 | 45.0 (10.0) | 46.0 (9.5) | 45.5 (9.8) | 45.8 (9.6) | 2:24:00 | 0:28:48 |
| ALT (U/L) | 50.0 (15.0) | 51.5 (14.5) | 50.8 (14.8) | 51.0 (14.6) | 3:36:00 | 0:43:12 | 50.0 (15.0) | 51.5 (14.5) | 50.8 (14.8) | 51.0 (14.6) | 3:36:00 | 0:43:12 |
| ALP (U/L) | 80.0 (20.0) | 82.5 (19.5) | 81.8 (19.8) | 82.0 (19.6) | 3:07:12 | 0:57:36 | 80.0 (20.0) | 82.5 (19.5) | 81.8 (19.8) | 82.0 (19.6) | 3:07:12 | 0:57:36 |
| ALB (g/L) | 40.0 (5.0) | 39.5 (4.8) | 39.8 (4.9) | 39.7 (4.7) | 1:55:12 | 0:28:48 | 40.0 (5.0) | 39.5 (4.8) | 39.8 (4.9) | 39.7 (4.7) | 1:55:12 | 0:28:48 |
| GGT (U/L) | 25.0 (10.0) | 26.5 (9.5) | 25.8 (9.8) | 26.0 (9.6) | 4:19:12 | 0:43:12 | 25.0 (10.0) | 26.5 (9.5) | 25.8 (9.8) | 26.0 (9.6) | 4:19:12 | 0:43:12 |
| Variables | Range * |
|---|---|
| Age (years) | 7.0 (3.0–12.0) |
| Sex | |
| Male | 1086 (66.7%) |
| Female | 543 (33.3%) |
| Genotype | |
| B | 604 (37.1%) |
| C | 1025 (62.9%) |
| HBeAg | |
| Positive | 1566 (96.1%) |
| Negative | 118 (3.9%) |
| HBV-DNA (lgIU/mL) | 6.88 (5.96–7.55) |
| PLT (109/L) | 247 (200–296) |
| HB (g/L) | 129 (123–138) |
| RBC (1012/L) | 4.60 (4.35–4.87) |
| WBC (×109/L) | 6.70 (5.37–8.50) |
| AFP (ng/mL) | 6 (4–11) |
| TBiL (μmol/L) | 6.7 (5.0–9.6) |
| DBiL (μmol/L) | 2.00 (1.30–3.03) |
| AST (U/L) | 72 (49–118) |
| ALT (U/L) | 89.0 (53.4–148.0) |
| ALP (U/L) | 277.1 (231.1–322.2) |
| ALB (g/L) | 42.0 (39.0–44.0) |
| GGT (U/L) | 19 (13–42) |
| Inflammation Grading | 0–3 Years | 4–6 Years | 7–12 Years | 13–18 Years | Total |
|---|---|---|---|---|---|
| G0–1 | 236 | 144 | 177 | 138 | 695 |
| G2–4 | 191 | 190 | 292 | 261 | 934 |
| Fibrosis Staging | |||||
| S0 | 42 | 19 | 25 | 28 | 114 |
| S1 | 233 | 160 | 206 | 175 | 774 |
| S2 | 107 | 80 | 166 | 113 | 466 |
| S3 | 27 | 62 | 41 | 54 | 184 |
| S4 | 18 | 13 | 31 | 29 | 91 |
| Total | 427 | 334 | 469 | 399 | 1629 |
| Variables | Univariable | p | Multivariable | p |
|---|---|---|---|---|
| OR (95% CI) | OR (95% CI) | |||
| Age (years) | ||||
| 0–3 years | Reference | |||
| 4–6 years | 1.82 (1.20–2.76) | 0.005 | 2.21 (1.34–3.63) | 0.002 |
| 7–12 years | 2.21 (1.46–3.35) | <0.001 | 1.82 (1.05–3.15) | 0.033 |
| 13–18 years | 1.67 (1.07–2.60) | 0.025 | 1.55 (0.84–2.83) | 0.158 |
| Sex | ||||
| Female | Reference | |||
| Male | 1.04 (0.75–1.43) | 0.831 | ||
| Genotype | ||||
| B | Reference | |||
| C | 1.87 (1.33–2.63) | <0.001 | 1.85 (1.25–2.76) | 0.002 |
| HBeAg | ||||
| Negative | Reference | |||
| Positive | 0.47 (0.14–1.56) | 0.219 | ||
| HBV-DNA (lgIU/mL) | 0.86 (0.77–0.96) | 0.008 | 0.88 (0.78–1.01) | 0.064 |
| PLT (109/L) | 1.00 (0.99–1.00) | <0.001 | 1.00 (0.99–1.00) | 0.007 |
| Hb (g/L) | 1.01 (1.00–1.02) | 0.232 | ||
| RBC (109/L) | 0.60 (0.41–0.87) | 0.007 | 0.61 (0.38–0.97) | 0.037 |
| WBC (1012/L) | 0.95 (0.90–1.01) | 0.102 | 1.07 (0.98–1.16) | 0.142 |
| AFP (ng/mL) | 1.01 (1.00–1.02) | 0.052 | 1.00 (1.00–1.00) | 0.57 |
| TBiL (μmol/L) | 1.11 (1.06–1.15) | <0.001 | 1.06 (0.98–1.15) | 0.133 |
| DBiL (μmol/L) | 1.23 (1.12–1.35) | <0.001 | 0.94 (0.80–1.11) | 0.48 |
| AST (U/L) | 1.01 (1.01–1.01) | <0.001 | 1.01 (1.00–1.01) | 0.013 |
| ALT (U/L) | 1.01 (1.01–1.01) | <0.001 | 1.00 (1.00–1.01) | 0.023 |
| ALP (U/L) | 1.00 (1.00–1.00) | 0.203 | ||
| ALB (g/L) | 1.01 (0.99–1.03) | 0.195 | 1.01 (0.99–1.02) | 0.567 |
| GGT (U/L) | 1.04 (1.03–1.05) | <0.001 | 1.02 (1.01–1.03) | <0.001 |
| Variables | Univariable | p | Multivariable | p |
|---|---|---|---|---|
| OR (95% CI) | OR (95% CI) | |||
| Age (years) | ||||
| 0–3 years | Reference | |||
| 4–6 years | 1.65 (1.08–2.52) | 0.021 | 1.82 (1.12–2.96) | 0.015 |
| 7–12 years | 2.62 (1.72–3.99) | <0.001 | 2.22 (1.31–3.77) | 0.003 |
| 13–18 years | 1.46 (0.92–2.29) | 0.105 | 1.03 (0.56–1.88) | 0.934 |
| Sex | ||||
| Female | Reference | |||
| Male | 0.93 (0.68–1.29) | 0.674 | ||
| Genotype | ||||
| B | Reference | |||
| C | 2.26 (1.56–3.25) | <0.001 | 1.80 (1.20–2.69) | 0.004 |
| HBeAg | ||||
| Negative | Reference | |||
| Positive | 0.91 (0.30–2.74) | 0.868 | ||
| HBV-DNA (lgIU/mL) | 0.86 (0.77–0.95) | 0.005 | 0.91 (0.80–1.03) | 0.121 |
| PLT (109/L) | 0.99 (0.99–1.00) | 0.001 | 1.00 (0.99–1.00) | 0.001 |
| Hb (g/L) | 1.01 (1.00–1.02) | 0.057 | 1.02 (1.00–1.04) | 0.114 |
| RBC (109/L) | 0.46 (0.31–0.69) | <0.001 | 0.37 (0.20–0.68) | 0.001 |
| WBC (1012/L) | 0.94 (0.89–1.00) | 0.057 | 1.10 (1.01–1.20) | 0.025 |
| AFP (ng/mL) | 1.00 (1.00–1.00) | 0.365 | ||
| TBiL (μmol/L) | 1.10 (1.05–1.14) | <0.001 | 1.05 (0.97–1.13) | 0.235 |
| DBiL (μmol/L) | 1.18 (1.09–1.29) | <0.001 | 0.97 (0.83–1.14) | 0.716 |
| AST (U/L) | 1.00 (1.00–1.00) | <0.001 | 1.00 (1.00–1.00) | 0.561 |
| ALT (U/L) | 1.00 (1.00–1.00) | <0.001 | 1.00 (1.00–1.00) | 0.528 |
| ALP (U/L) | 1.00 (1.00–1.00) | 0.443 | ||
| ALB (g/L) | 1.01 (1.00–1.03) | 0.142 | 1.01 (0.99–1.04) | |
| GGT (U/L) | 1.02 (1.02–1.03) | <0.001 | 1.01 (1.01–1.02) | 0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, C.; Lu, Y.; Wang, Z.; Jiang, Q.; Dong, Y.; Cao, L.; Yan, J.; Xu, Z.; Wang, F.; Gao, Y.; et al. Correlation Between Clinical Indicators and Liver Pathology in Children with Chronic Hepatitis B. Biomedicines 2024, 12, 2903. https://doi.org/10.3390/biomedicines12122903
Huang C, Lu Y, Wang Z, Jiang Q, Dong Y, Cao L, Yan J, Xu Z, Wang F, Gao Y, et al. Correlation Between Clinical Indicators and Liver Pathology in Children with Chronic Hepatitis B. Biomedicines. 2024; 12(12):2903. https://doi.org/10.3390/biomedicines12122903
Chicago/Turabian StyleHuang, Chenyang, Ying Lu, Ziwei Wang, Qiyu Jiang, Yi Dong, Lili Cao, Jianguo Yan, Zhiqiang Xu, Fuchuan Wang, Yinjie Gao, and et al. 2024. "Correlation Between Clinical Indicators and Liver Pathology in Children with Chronic Hepatitis B" Biomedicines 12, no. 12: 2903. https://doi.org/10.3390/biomedicines12122903
APA StyleHuang, C., Lu, Y., Wang, Z., Jiang, Q., Dong, Y., Cao, L., Yan, J., Xu, Z., Wang, F., Gao, Y., Fu, J., Zhang, M., & Wang, F.-S. (2024). Correlation Between Clinical Indicators and Liver Pathology in Children with Chronic Hepatitis B. Biomedicines, 12(12), 2903. https://doi.org/10.3390/biomedicines12122903






