The Role and Mechanism of Deubiquitinase USP7 in Tumor-Associated Inflammation
Abstract
1. Introduction
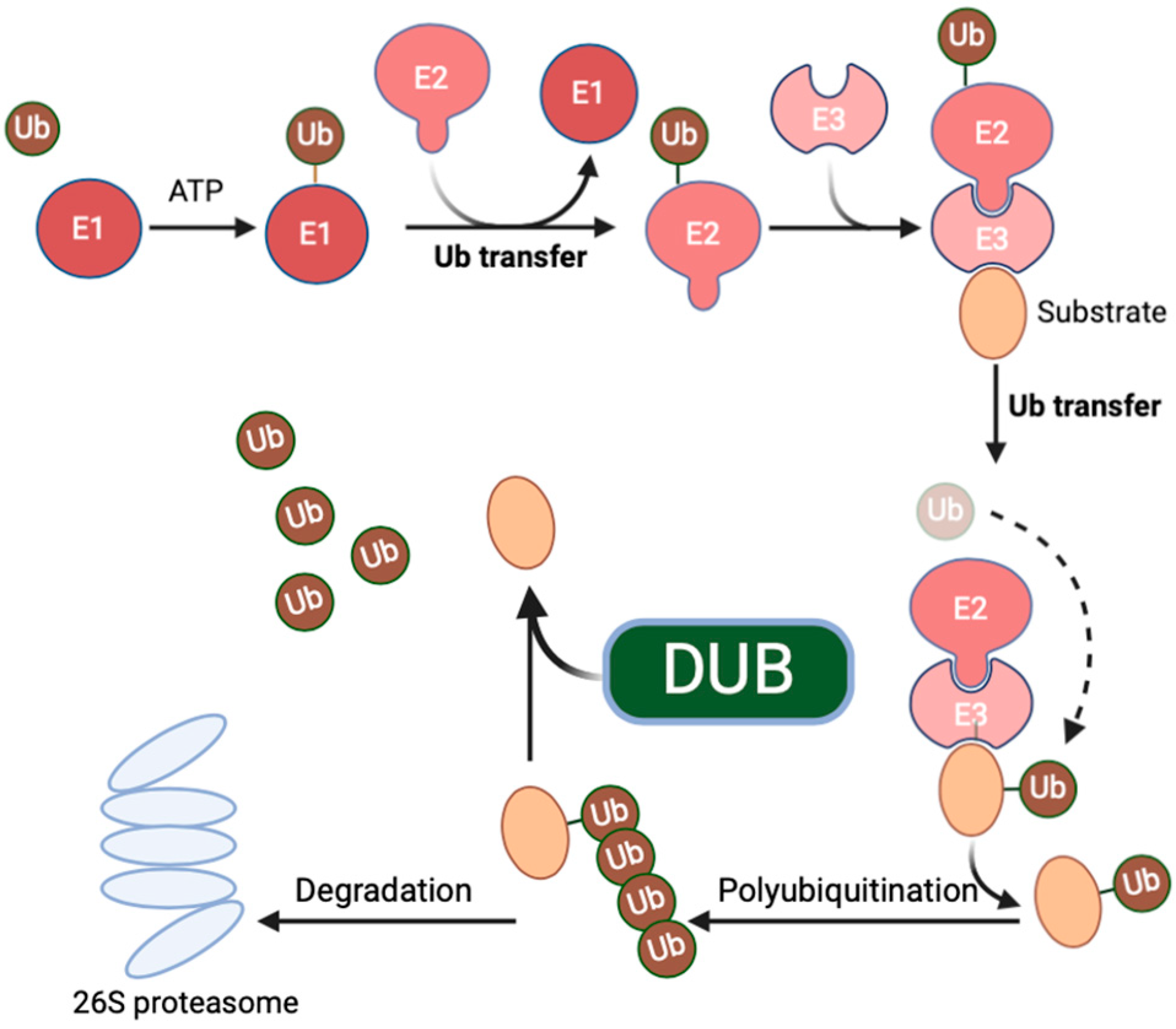
2. The Structure, Function, and Expression of USP7
2.1. Structure and Function of USP7
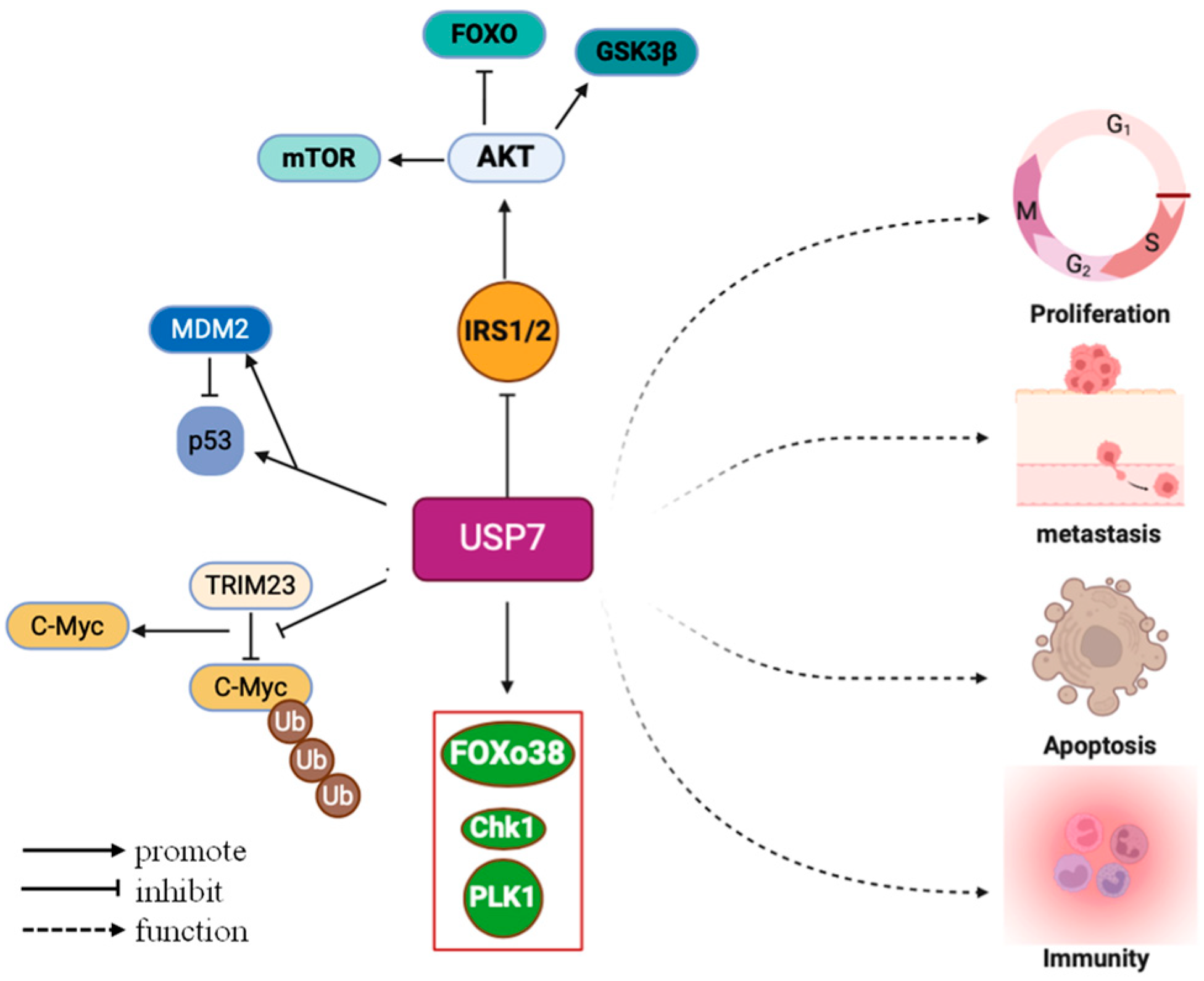
2.2. USP7 Expression and Cancer Initiation, Progression, and Drug Resistance
- P53 signaling pathway: USP7 stabilizes MDM2 and MDMX through ubiquitination; these are the main negative regulatory factors of p53. In this way, USP7 inhibits p53 degradation and thereby suppresses p53-mediated cell cycle arrest and apoptosis, thereby promoting tumor cell survival and proliferation [3]. In addition, USP7 was found to stabilize LSD1, further inhibit the p53 signaling pathway, and promote tumorigenesis and metastasis of glioblastoma [34].
- The PI3K/Akt/FOXO and AMPK signaling pathways: Knocking down or knocking out USP7 can increase the expression of AMPK beta, caspase 7, and PPP2R3A while reducing the expression of ATP6V0 and PEX11B, thereby inhibiting the proliferation of melanoma cells [35].
- Wnt/β-catenin signaling pathway: USP7 can directly bind to β-catenin, activating the Wnt/β-catenin signaling pathway and inducing epithelial–mesenchymal transition (EMT), a crucial process in tumor cell invasion and metastasis [36].
- PI3K/AKT signaling pathway: Ubiquitin-specific protease 7 promotes osteosarcoma cell metastasis by inducing epithelial–mesenchymal transition [37].
- NF-κ B/PD-L1 signaling pathway: USP7 promotes cervical cancer initiation and progression by upregulating EZH2 expression, downregulating TIMP2 expression, and consequently activating the NF-κB/PD-L1 signaling pathway [14].
- Insulin/IGF signaling pathway: USP7 is an IRS-1/2 deubiquitinase that establishes a negative feedback loop in insulin/IGF signaling [38].
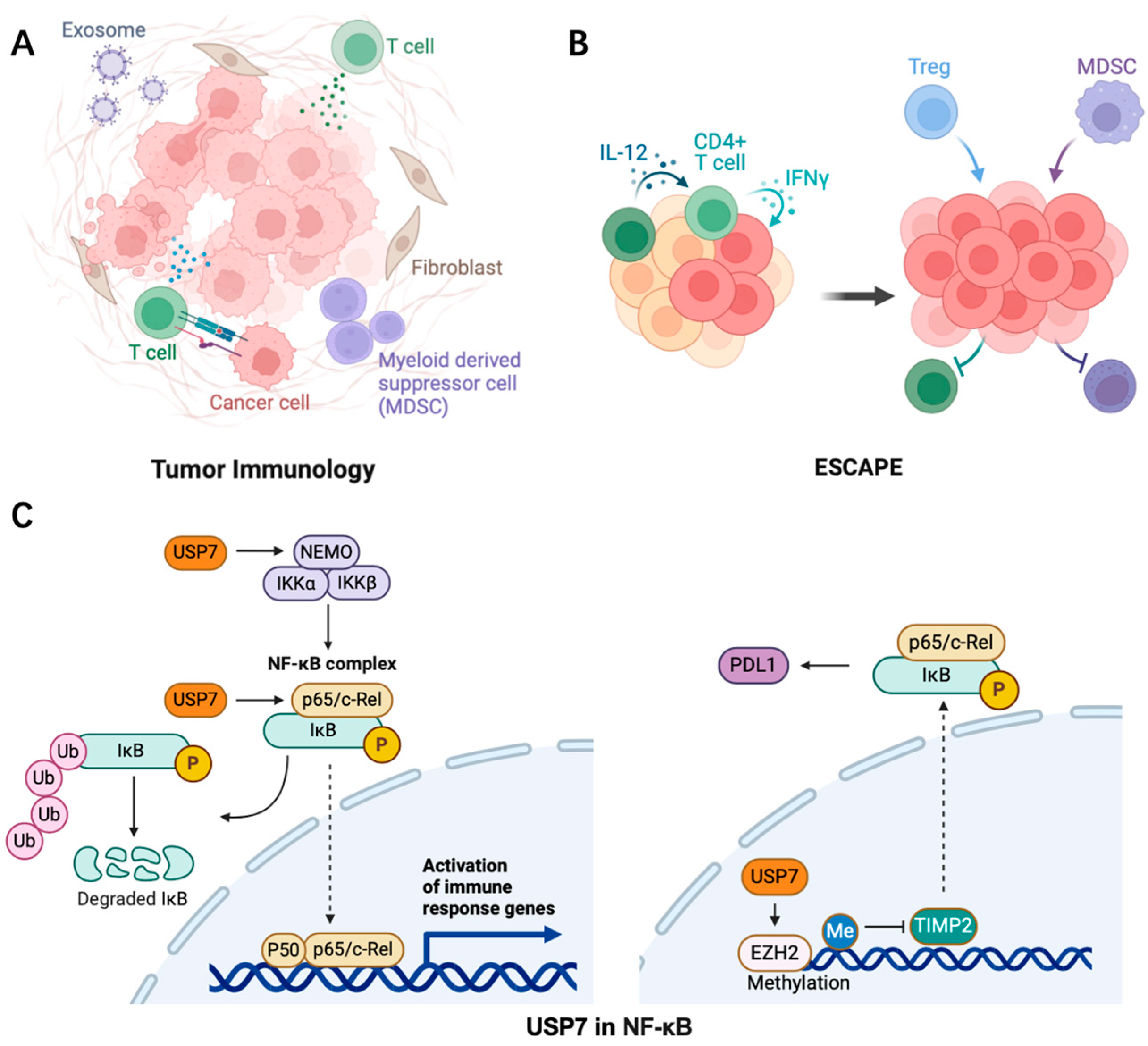
3. The Role of USP7 in Tumor Inflammation
3.1. Initiation of Inflammatory Tumor Microenvironment
3.2. USP7 Regulates NF-κB Signaling
4. Discussion
Author Contributions
Funding
Conflicts of Interest
References
- Snyder, N.A.; Silva, G.M. Deubiquitinating enzymes (DUBs): Regulation, homeostasis, and oxidative stress response. J. Biol. Chem. 2021, 297, 101077. [Google Scholar] [CrossRef] [PubMed]
- Pozhidaeva, A.; Bezsonova, I. USP7: Structure, substrate specificity, and inhibition. DNA Repair 2019, 76, 30–39. [Google Scholar] [CrossRef] [PubMed]
- Qi, S.M.; Cheng, G.; Cheng, X.D.; Xu, Z.; Xu, B.; Zhang, W.D.; Qin, J.J. Targeting USP7-Mediated Deubiquitination of MDM2/MDMX-p53 Pathway for Cancer Therapy: Are We There Yet? Front. Cell Dev. Biol. 2020, 8, 233. [Google Scholar] [CrossRef] [PubMed]
- Dai, X.; Lu, L.; Deng, S.; Meng, J.; Wan, C.; Huang, J.; Sun, Y.; Hu, Y.; Wu, B.; Wu, G.; et al. USP7 targeting modulates anti-tumor immune response by reprogramming Tumor-associated Macrophages in Lung Cancer. Theranostics 2020, 10, 9332–9347. [Google Scholar] [CrossRef]
- Ying, H.; Zhang, B.; Cao, G.; Wang, Y.; Zhang, X. Role for ubiquitin-specific protease 7 (USP7) in the treatment and the immune response to hepatocellular carcinoma: Potential mechanisms. Transl. Cancer Res. 2023, 12, 3016–3033. [Google Scholar] [CrossRef]
- Zhang, N.; Wang, F.; Zhang, G.; Zhang, Q.; Liu, Y.; Wang, Q.; Elsharkawy, M.S.; Zheng, M.; Wen, J.; Zhao, G.; et al. USP7 Promotes deubiquitination and stabilization of MyD88 to enhance immune responses. Front. Immunol. 2022, 13, 900243. [Google Scholar] [CrossRef]
- Dong, X.; Yang, C.; Luo, Y.; Dong, W.; Xu, X.; Wu, Y.; Wang, J. USP7 Attenuates Endoplasmic Reticulum Stress and NF-κB Signaling to Modulate Chondrocyte Proliferation, Apoptosis, and Inflammatory Response under Inflammation. Oxidative Med. Cell. Longev. 2022, 2022, 1835900. [Google Scholar] [CrossRef]
- Cockram, P.E.; Kist, M.; Prakash, S.; Chen, S.H.; Wertz, I.E.; Vucic, D. Ubiquitination in the regulation of inflammatory cell death and cancer. Cell Death Differ. 2021, 28, 591–605. [Google Scholar] [CrossRef]
- Al-Eidan, A.; Wang, Y.; Skipp, P.; Ewing, R.M. The USP7 protein interaction network and its roles in tumorigenesis. Genes Dis. 2022, 9, 41–50. [Google Scholar] [CrossRef]
- Wang, C.; Zhu, Y.; Zhu, X.; Chen, R.; Zhang, X.; Lian, N. USP7 regulates HMOX-1 via deubiquitination to suppress ferroptosis and ameliorate spinal cord injury in rats. Neurochem. Int. 2023, 168, 105554. [Google Scholar] [CrossRef]
- Park, H.B.; Baek, K.H. Current and future directions of USP7 interactome in cancer study. Biochim. Biophys. Acta Rev. Cancer 2023, 1878, 188992. [Google Scholar] [CrossRef] [PubMed]
- Hu, M.; Gu, L.; Li, M.; Jeffrey, P.D.; Gu, W.; Shi, Y. Structural basis of competitive recognition of p53 and MDM2 by HAUSP/USP7: Implications for the regulation of the p53-MDM2 pathway. PLoS Biol. 2006, 4, e27. [Google Scholar] [CrossRef] [PubMed]
- Pozhidaeva, A.K.; Mohni, K.N.; Dhe-Paganon, S.; Arrowsmith, C.H.; Weller, S.K.; Korzhnev, D.M.; Bezsonova, I. Structural Characterization of Interaction between Human Ubiquitin-specific Protease 7 and Immediate-Early Protein ICP0 of Herpes Simplex Virus-1. J. Biol. Chem. 2015, 290, 22907–22918. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Geng, F.; Liang, S.M.; Qin, X. USP7 inhibits TIMP2 by up-regulating the expression of EZH2 to activate the NF-κB/PD-L1 axis to promote the development of cervical cancer. Cell. Signal. 2022, 96, 110351. [Google Scholar] [CrossRef]
- Yi, J.; Li, H.; Chu, B.; Kon, N.; Hu, X.; Hu, J.; Xiong, Y.; Kaniskan, H.U.; Jin, J.; Gu, W. Inhibition of USP7 induces p53-independent tumor growth suppression in triple-negative breast cancers by destabilizing FOXM1. Cell Death Differ. 2023, 30, 1799–1810. [Google Scholar] [CrossRef]
- Korenev, G.; Yakukhnov, S.; Druk, A.; Golovina, A.; Chasov, V.; Mirgayazova, R.; Ivanov, R.; Bulatov, E. USP7 Inhibitors in Cancer Immunotherapy: Current Status and Perspective. Cancers 2022, 14, 5539. [Google Scholar] [CrossRef]
- Do, H.A.; Baek, K.H. Protein phosphatase 2A regulated by USP7 is polyubiquitinated and polyneddylated. Oncol. Rep. 2022, 48, 124. [Google Scholar] [CrossRef]
- Liu, J.; Zhou, T.; Dong, X.; Guo, Q.; Zheng, L.; Wang, X.; Zhang, N.; Li, D.; Ren, L.; Yi, F.; et al. De-ubiquitination of SAMHD1 by USP7 promotes DNA damage repair to overcome oncogenic stress and affect chemotherapy sensitivity. Oncogene 2023, 42, 1843–1856. [Google Scholar] [CrossRef]
- Oliveira, R.I.; Guedes, R.A.; Salvador, J.A.R. Highlights in USP7 inhibitors for cancer treatment. Front. Chem. 2022, 10, 1005727. [Google Scholar] [CrossRef]
- Bojagora, A.; Saridakis, V. USP7 manipulation by viral proteins. Virus Res. 2020, 286, 198076. [Google Scholar] [CrossRef]
- Qian, J.; Pentz, K.; Zhu, Q.; Wang, Q.; He, J.; Srivastava, A.K.; Wani, A.A. USP7 modulates UV-induced PCNA monoubiquitination by regulating DNA polymerase eta stability. Oncogene 2015, 34, 4791–4796. [Google Scholar] [CrossRef] [PubMed]
- Kashiwaba, S.; Kanao, R.; Masuda, Y.; Kusumoto-Matsuo, R.; Hanaoka, F.; Masutani, C. USP7 Is a Suppressor of PCNA Ubiquitination and Oxidative-Stress-Induced Mutagenesis in Human Cells. Cell Rep. 2015, 13, 2072–2080. [Google Scholar] [CrossRef] [PubMed]
- Georges, A.; Coyaud, E.; Marcon, E.; Greenblatt, J.; Raught, B.; Frappier, L. USP7 Regulates Cytokinesis through FBXO38 and KIF20B. Sci. Rep. 2019, 9, 2724. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.W.; Feng, N.; Liu, Y.C.; Guo, Q.; Wang, J.K.; Bai, Y.Z.; Ye, X.M.; Yang, Z.; Yang, H.; Liu, Y.; et al. Neuroinflammation inhibition by small-molecule targeting USP7 noncatalytic domain for neurodegenerative disease therapy. Sci. Adv. 2022, 8, eabo0789. [Google Scholar] [CrossRef]
- Kumagai, J.; Kiuchi, M.; Kokubo, K.; Yagyu, H.; Nemoto, M.; Tsuji, K.; Nagahata, K.; Sasaki, A.; Hishiya, T.; Onoue, M.; et al. The USP7-STAT3-granzyme-Par-1 axis regulates allergic inflammation by promoting differentiation of IL-5-producing Th2 cells. Proc. Natl. Acad. Sci. USA 2023, 120, e2302903120. [Google Scholar] [CrossRef]
- Guo, N.J.; Wang, B.; Zhang, Y.; Kang, H.Q.; Nie, H.Q.; Feng, M.K.; Zhang, X.Y.; Zhao, L.J.; Wang, N.; Liu, H.M.; et al. USP7 as an emerging therapeutic target: A key regulator of protein homeostasis. Int. J. Biol. Macromol. 2024, 263, 130309. [Google Scholar] [CrossRef]
- Nicklas, S.; Hillje, A.L.; Okawa, S.; Rudolph, I.M.; Collmann, F.M.; van Wuellen, T.; Del Sol, A.; Schwamborn, J.C. A Complex of the Ubiquitin Ligase TRIM32 and the Deubiquitinase USP7 Balances the Level of C-Myc Ubiquitination and Thereby Determines Neural Stem Cell Fate Specification. Cell Death Differ. 2019, 26, 728–740. [Google Scholar] [CrossRef]
- Chang, Y.C.; Lin, K.; Baxley, R.M.; Durrett, W.; Wang, L.; Stojkova, O.; Billmann, M.; Ward, H.; Myers, C.L.; Bielinsky, A.K. RNF4 and USP7 cooperate in ubiquitin-regulated steps of DNA replication. Open Biol. 2023, 13, 230068. [Google Scholar] [CrossRef]
- Zhang, C.; Lu, J.; Zhang, Q.W.; Zhao, W.; Guo, J.H.; Liu, S.L.; Wu, Y.L.; Jiang, B.; Gao, F.H. USP7 promotes cell proliferation through the stabilization of Ki-67 protein in non-small cell lung cancer cells. Int. J. Biochem. Cell Biol. 2016, 79, 209–221. [Google Scholar] [CrossRef]
- Zeng, Q.; Li, Z.; Zhao, X.; Guo, L.; Yu, C.; Qin, J.; Zhang, S.; Zhang, Y.; Yang, X. Ubiquitin-specific protease 7 promotes osteosarcoma cell metastasis by inducing epithelial-mesenchymal transition. Oncol. Rep. 2019, 41, 543–551. [Google Scholar] [CrossRef]
- Yang, X.; Jin, J.; Yang, J.; Zhou, L.; Mi, S.; Qi, G. Expression of Ubiquitin-specific protease 7 in oral squamous cell carcinoma promotes tumor cell proliferation and invasion. Genet. Mol. Biol. 2021, 44, e20210058. [Google Scholar] [CrossRef] [PubMed]
- Cai, J.B.; Shi, G.M.; Dong, Z.R.; Ke, A.W.; Ma, H.H.; Gao, Q.; Shen, Z.Z.; Huang, X.Y.; Chen, H.; Yu, D.D.; et al. Ubiquitin-Specific Protease 7 Accelerates P14(ARF) Degradation by Deubiquitinating Thyroid Hormone Receptor-Interacting Protein 12 and Promotes Hepatocellular Carcinoma Progression. Hepatology 2015, 61, 1603–1614. [Google Scholar] [CrossRef] [PubMed]
- Xiang, M.; Liang, L.; Kuang, X.; Xie, Z.; Liu, J.; Zhao, S.; Su, J.; Chen, X.; Liu, H. Pharmacological inhibition of USP7 suppresses growth and metastasis of melanoma cells in vitro and in vivo. J. Cell Mol. Med. 2021, 25, 9228–9240. [Google Scholar] [CrossRef] [PubMed]
- Yi, L.; Cui, Y.; Xu, Q.; Jiang, Y. Stabilization of LSD1 by Deubiquitinating Enzyme USP7 Promotes Glioblastoma Cell Tumorigenesis and Metastasis through Suppression of the P53 Signaling Pathway. Oncol. Rep. 2016, 36, 2935–2945. [Google Scholar] [CrossRef]
- Gao, L.; Zhu, D.; Wang, Q.; Bao, Z.; Yin, S.; Qiang, H.; Wieland, H.; Zhang, J.; Teichmann, A.; Jia, J. Proteome Analysis of USP7 Substrates Revealed Its Role in Melanoma Through PI3K/Akt/FOXO and AMPK Pathways. Front. Oncol. 2021, 11, 650165. [Google Scholar] [CrossRef]
- Ye, M.; He, J.; Zhang, J.; Liu, B.; Liu, X.; Xie, L.; Wei, M.; Dong, R.; Li, K.; Ma, D.; et al. USP7 promotes hepatoblastoma progression through activation of PI3K/AKT signaling pathway. Cancer Biomark. 2021, 31, 107–117. [Google Scholar] [CrossRef]
- Yoshihara, H.; Fukushima, T.; Hakuno, F.; Saeki, Y.; Tanaka, K.; Ito, A.; Yoshida, M.; Iemura, S.; Natsume, T.; Asano, T.; et al. Insulin/Insulin-like Growth Factor (IGF) Stimulation Abrogates an Association between a Deubiquitinating Enzyme USP7 and Insulin Receptor Substrates (IRSs) Followed by Proteasomal Degradation of IRSs. Biochem. Biophys. Res. Commun. 2012, 423, 122–127. [Google Scholar] [CrossRef]
- Vogt, M.; Classen, S.; Krause, A.K.; Peter, N.-J.; Petersen, C.; Rothkamm, K.; Borgmann, K.; Meyer, F. USP7 Deregulation Impairs S Phase Specific DNA Repair after Irradiation in Breast Cancer Cells. Biomedicines 2024, 12, 762. [Google Scholar] [CrossRef]
- Liu, X.; Lu, R.; Yang, Q.; He, J.; Huang, C.; Cao, Y.; Zhou, Z.; Huang, J.; Li, L.; Chen, R.; et al. USP7 reduces the level of nuclear DICER, impairing DNA damage response and promoting cancer progression. Mol. Oncol. 2024, 18, 170–189. [Google Scholar] [CrossRef]
- Pan, T.; Li, X.; Li, Y.; Tao, Z.; Yao, H.; Wu, Y.; Chen, G.; Zhang, K.; Zhou, Y.; Huang, Y. USP7 inhibition induces apoptosis in glioblastoma by enhancing ubiquitination of ARF4. Cancer Cell Int. 2021, 21, 508. [Google Scholar] [CrossRef]
- Zhang, B.; Li, J.; Wang, Y.; Liu, X.; Yang, X.; Liao, Z.; Deng, S.; Deng, Y.; Zhou, Z.; Tian, Y.; et al. Deubiquitinase USP7 stabilizes KDM5B and promotes tumor progression and cisplatin resistance in nasopharyngeal carcinoma through the ZBTB16/TOP2A axis. Cell Death Differ. 2024, 31, 309–321. [Google Scholar] [CrossRef] [PubMed]
- Shin, S.B.; Kim, C.H.; Jang, H.R.; Yim, H. Combination of Inhibitors of USP7 and PLK1 has a Strong Synergism against Paclitaxel Resistance. Int. J. Mol. Sci. 2020, 21, 8629. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Liu, Y.; Gao, Y.; Yuan, B.; Qi, X.; Fu, Y.; Zhu, Q.; Cao, T.; Zhang, S.; Yin, L.; et al. USP7 is a novel Deubiquitinase sustaining PLK1 protein stability and regulating chromosome alignment in mitosis. J. Exp. Clin. Cancer Res. 2019, 38, 468. [Google Scholar] [CrossRef] [PubMed]
- Xue, Q.; Yang, D.; Zhang, J.; Gan, P.; Lin, C.; Lu, Y.; Zhang, W.; Zhang, L.; Guang, X. USP7, negatively regulated by miR-409-5p, aggravates hypoxia-induced cardiomyocyte injury. APMIS 2021, 129, 152–162. [Google Scholar] [CrossRef]
- Lin, Y.-T.; Lin, J.; Liu, Y.-E.; Chen, Y.-C.; Liu, S.-T.; Hsu, K.-W.; Chen, D.-R.; Wu, H.-T. USP7 Induces Chemoresistance in Triple-Negative Breast Cancer via Deubiquitination and Stabilization of ABCB1. Cells 2022, 11, 3294. [Google Scholar] [CrossRef]
- He, Y.; Jiang, S.; Zhong, Y.; Wang, X.; Cui, Y.; Liang, J.; Sun, Y.; Zhu, Z.; Huang, Z.; Mao, X. USP7 promotes non-small-cell lung cancer cell glycolysis and survival by stabilizing and activating c-Abl. Clin. Transl. Med. 2023, 13, e1509. [Google Scholar] [CrossRef]
- Yang, Z.; Yu, W.; Xu, A.; Liu, B.; Jin, L.; Tao, H.; Wang, D. mTORC1 accelerates osteosarcoma progression via m(6)A-dependent stabilization of USP7 mRNA. Cell Death Discov. 2024, 10, 127. [Google Scholar] [CrossRef]
- Granieri, L.; Marocchi, F.; Melixetian, M.; Mohammadi, N.; Nicoli, P.; Cuomo, A.; Bonaldi, T.; Confalonieri, S.; Pisati, F.; Giardina, G.; et al. Targeting the USP7/RRM2 axis drives senescence and sensitizes melanoma cells to HDAC/LSD1 inhibitors. Cell Rep. 2022, 40, 111396. [Google Scholar] [CrossRef]
- Sakamoto, T.; Kuboki, S.; Furukawa, K.; Takayashiki, T.; Takano, S.; Yoshizumi, A.; Ohtsuka, M. TRIM27-USP7 complex promotes tumour progression via STAT3 activation in human hepatocellular carcinoma. Liver Int. 2023, 43, 194–207. [Google Scholar] [CrossRef]
- Bian, S.; Ni, W.; Zhu, M.; Zhang, X.; Qiang, Y.; Zhang, J.; Ni, Z.; Shen, Y.; Qiu, S.; Song, Q.; et al. Flap endonuclease 1 Facilitated Hepatocellular Carcinoma Progression by Enhancing USP7/MDM2-mediated P53 Inactivation. Int. J. Biol. Sci. 2022, 18, 1022–1038. [Google Scholar] [CrossRef]
- Su, D.; Ma, S.; Shan, L.; Wang, Y.; Wang, Y.; Cao, C.; Liu, B.; Yang, C.; Wang, L.; Tian, S.; et al. Ubiquitin-specific protease 7 sustains DNA damage response and promotes cervical carcinogenesis. J. Clin. Investig. 2018, 128, 4280–4296. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhang, B.; Feng, Z.; An, D.; Zhou, Z.; Wan, C.; Hu, Y.; Sun, Y.; Wang, Y.; Liu, X.; et al. Stabilization of KPNB1 by deubiquitinase USP7 promotes glioblastoma progression through the YBX1-NLGN3 axis. J. Exp. Clin. Cancer Res. 2024, 43, 28. [Google Scholar] [CrossRef] [PubMed]
- Hayal, T.B.; DoĞan, A.; Şişli, H.B.; Kiratli, B.; Şahin, F. Ubiquitin-specific protease 7 downregulation suppresses breast cancer in vitro. Turk. J. Biol. 2020, 44, 145–157. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Ma, S.; Song, N.; Li, X.; Liu, L.; Yang, S.; Ding, X.; Shan, L.; Zhou, X.; Su, D.; et al. Stabilization of histone demethylase PHF8 by USP7 promotes breast carcinogenesis. J. Clin. Investig. 2016, 126, 2205–2220. [Google Scholar] [CrossRef]
- Hu, T.; Zhang, J.; Sha, B.; Li, M.; Wang, L.; Zhang, Y.; Liu, X.; Dong, Z.; Liu, Z.; Li, P.; et al. Targeting the overexpressed USP7 inhibits esophageal squamous cell carcinoma cell growth by inducing NOXA-mediated apoptosis. Mol. Carcinog. 2019, 58, 42–54. [Google Scholar] [CrossRef]
- Gao, A.; Zhang, M.; Zhu, S.Q.; Zou, S.; Chen, H.; Li, X.; He, C.; Zhou, L.; Mei, Y.; Ding, W.; et al. DNA polymerase iota promotes EMT and metastasis of esophageal squamous cell carcinoma by interacting with USP7 to stabilize HIF-1α. Cell Death Dis. 2024, 15, 171. [Google Scholar] [CrossRef]
- Jurisic, A.; Sung, P.J.; Wappett, M.; Daubriac, J.; Lobb, I.T.; Kung, W.W.; Crawford, N.; Page, N.; Cassidy, E.; Feutren-Burton, S.; et al. USP7 inhibitors suppress tumour neoangiogenesis and promote synergy with immune checkpoint inhibitors by downregulating fibroblast VEGF. Clin. Transl. Med. 2024, 14, e1648. [Google Scholar] [CrossRef]
- Zhao, X.; Fu, J.; Hu, B.; Chen, L.; Wang, J.; Fang, J.; Ge, C.; Lin, H.; Pan, K.; Fu, L.; et al. Serine Metabolism Regulates YAP Activity Through USP7 in Colon Cancer. Front. Cell Dev. Biol. 2021, 9, 639111. [Google Scholar] [CrossRef]
- Pei, Y.; Fu, J.; Shi, Y.; Zhang, M.; Luo, G.; Luo, X.; Song, N.; Mi, T.; Yang, Y.; Li, J.; et al. Discovery of a Potent and Selective Degrader for USP7. Angew. Chem. Int. Ed. Engl. 2022, 61, e202204395. [Google Scholar] [CrossRef]
- Valles, G.J.; Bezsonova, I.; Woodgate, R.; Ashton, N.W. USP7 Is a Master Regulator of Genome Stability. Front. Cell Dev. Biol. 2020, 8, 717. [Google Scholar] [CrossRef]
- Ma, Y.; Adjemian, S.; Mattarollo, S.R.; Yamazaki, T.; Aymeric, L.; Yang, H.; Portela Catani, J.P.; Hannani, D.; Duret, H.; Steegh, K.; et al. Anticancer chemotherapy-induced intratumoral recruitment and differentiation of antigen-presenting cells. Immunity 2013, 38, 729–741. [Google Scholar] [CrossRef] [PubMed]
- Crusz, S.M.; Balkwill, F.R. Inflammation and cancer: Advances and new agents. Nat. Rev. Clin. Oncol. 2015, 12, 584–596. [Google Scholar] [CrossRef] [PubMed]
- Van Loosdregt, J.; Fleskens, V.; Fu, J.; Brenkman, A.B.; Bekker, C.P.J.; Pals, C.E.G.M.; Meerding, J.; Berkers, C.R.; Barbi, J.; Gröne, A.; et al. Stabilization of the Transcription Factor Foxp3 by the Deubiquitinase USP7 Increases Treg-Cell-Suppressive Capacity. Immunity 2013, 39, 259–271. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Wu, L.; Yan, G.; Chen, Y.; Zhou, M.; Wu, Y.; Li, Y. Inflammation and tumor progression: Signaling pathways and targeted intervention. Signal Transduct. Target. Ther. 2021, 6, 263. [Google Scholar] [CrossRef]
- De Marco Castro, E.; Calder, P.C.; Roche, H.M. β-1,3/1,6-Glucans and Immunity: State of the Art and Future Directions. Mol. Nutr. Food Res. 2021, 65, e1901071. [Google Scholar] [CrossRef]
- Chen, J.; Gao, L.; Wu, X.; Fan, Y.; Liu, M.; Peng, L.; Song, J.; Li, B.; Liu, A.; Bao, F. BCG-induced trained immunity: History, mechanisms and potential applications. J. Transl. Med. 2023, 21, 106. [Google Scholar] [CrossRef]
- Burger, E. Paracoccidioidomycosis Protective Immunity. J. Fungi 2021, 7, 137. [Google Scholar] [CrossRef]
- Abramiuk, M.; Grywalska, E.; Małkowska, P.; Sierawska, O.; Hrynkiewicz, R.; Niedźwiedzka-Rystwej, P. The Role of the Immune System in the Development of Endometriosis. Cells 2022, 11, 2028. [Google Scholar] [CrossRef]
- Palazón-Riquelme, P.; Worboys, J.D.; Green, J.; Valera, A.; Martín-Sánchez, F.; Pellegrini, C.; Brough, D.; López-Castejón, G. USP7 and USP47 Deubiquitinases Regulate NLRP3 Inflammasome Activation. EMBO Rep. 2018, 19, e44766. [Google Scholar] [CrossRef]
- Boyman, O.; Sprent, J. The role of interleukin-2 during homeostasis and activation of the immune system. Nat. Rev. Immunol. 2012, 12, 180–190. [Google Scholar] [CrossRef]
- Griffith, J.W.; Sokol, C.L.; Luster, A.D. Chemokines and chemokine receptors: Positioning cells for host defense and immunity. Annu. Rev. Immunol. 2014, 32, 659–702. [Google Scholar] [CrossRef] [PubMed]
- Colleran, A.; Collins, P.E.; O’Carroll, C.; Ahmed, A.; Mao, X.; McManus, B.; Kiely, P.A.; Burstein, E.; Carmody, R.J. Deubiquitination of NF-κB by Ubiquitin-Specific Protease-7 promotes transcription. Proc. Natl. Acad. Sci. USA 2013, 110, 618–623. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhang, Y.; Wang, S.; Dong, Q.Z.; Shen, Z.; Wang, W.; Tao, S.; Gu, C.; Liu, J.; Xie, Y.; et al. Prospero-related homeobox 1 drives angiogenesis of hepatocellular carcinoma through selectively activating interleukin-8 expression. Hepatology 2017, 66, 1894–1909. [Google Scholar] [CrossRef] [PubMed]
- Gan, Q.; Li, T.; Hu, B.; Lian, M.; Zheng, X. HSCARG inhibits activation of NF-kappaB by interacting with IkappaB kinase-beta. J. Cell Sci. 2009, 122, 4081–4088. [Google Scholar] [CrossRef]
- Li, T.; Guan, J.; Li, S.; Zhang, X.; Zheng, X. HSCARG downregulates NF-κB signaling by interacting with USP7 and inhibiting NEMO ubiquitination. Cell Death Dis. 2014, 5, e1229. [Google Scholar] [CrossRef]
- Xia, X.; Liao, Y.; Huang, C.; Liu, Y.; He, J.; Shao, Z.; Jiang, L.; Dou, Q.P.; Liu, J.; Huang, H. Deubiquitination and stabilization of estrogen receptor α by ubiquitin-specific protease 7 promotes breast tumorigenesis. Cancer Lett. 2019, 465, 118–128. [Google Scholar] [CrossRef]
- Lee, S.T.; Li, Z.; Wu, Z.; Aau, M.; Guan, P.; Karuturi, R.K.; Liou, Y.C.; Yu, Q. Context-specific regulation of NF-κB target gene expression by EZH2 in breast cancers. Mol. Cell 2011, 43, 798–810. [Google Scholar] [CrossRef]
- Su, D.; Wang, W.; Hou, Y.; Wang, L.; Yi, X.; Cao, C.; Wang, Y.; Gao, H.; Wang, Y.; Yang, C.; et al. Bimodal regulation of the PRC2 complex by USP7 underlies tumorigenesis. Nucleic Acids Res. 2021, 49, 4421–4440. [Google Scholar] [CrossRef]
- Duan, D.; Shang, M.; Han, Y.; Liu, J.; Liu, J.; Kong, S.H.; Hou, J.; Huang, B.; Lu, J.; Zhang, Y. EZH2-CCF-cGAS Axis Promotes Breast Cancer Metastasis. Int. J. Mol. Sci. 2022, 23, 1788. [Google Scholar] [CrossRef]

| Type of Cancer | Expression | Functions | References |
|---|---|---|---|
| Lung cancer | high expression | Promote proliferation, inhibit cell death, and promote glycolysis | [29,42,46] |
| Osteosarcoma | high expression | Promote metastasis | [30,47] |
| Oral squamous cell carcinoma | high expression | Promote proliferation, migration, and invasion; reduce apoptosis | [31] |
| Melanoma | high expression | Promote proliferation, migration, and invasion; reduce apoptosis and senescence | [33,48] |
| Liver cancer | high expression | Promote proliferation, migration, and invasion | [36,49,50] |
| Cervical cancer | high expression | Promote proliferation, migration, and invasion; enhance immune escape ability; inhibit cell death | [14,42,51] |
| Glioblastoma | high expression | Inhibit apoptosis; promote tumor growth and metastasis | [34,40,52] |
| Nasopharyngeal carcinoma | high expression | Promote tumor progression and cisplatin resistance | [41] |
| Prostate cancer | high expression | Inhibit cell death; promote proliferation | [42,43] |
| Breast cancer | high expression | Induce chemoresistance; promote tumor progression | [45,53,54] |
| Esophagus cancer | high expression | Promote tumor growth, EMT, and metastasis | [55,56] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, L.; Zhang, Y.; Yu, T.; Wu, H. The Role and Mechanism of Deubiquitinase USP7 in Tumor-Associated Inflammation. Biomedicines 2024, 12, 2734. https://doi.org/10.3390/biomedicines12122734
Wang L, Zhang Y, Yu T, Wu H. The Role and Mechanism of Deubiquitinase USP7 in Tumor-Associated Inflammation. Biomedicines. 2024; 12(12):2734. https://doi.org/10.3390/biomedicines12122734
Chicago/Turabian StyleWang, Luhong, Yong Zhang, Tao Yu, and Huijian Wu. 2024. "The Role and Mechanism of Deubiquitinase USP7 in Tumor-Associated Inflammation" Biomedicines 12, no. 12: 2734. https://doi.org/10.3390/biomedicines12122734
APA StyleWang, L., Zhang, Y., Yu, T., & Wu, H. (2024). The Role and Mechanism of Deubiquitinase USP7 in Tumor-Associated Inflammation. Biomedicines, 12(12), 2734. https://doi.org/10.3390/biomedicines12122734





