The Prognostic Value of Preoperative Total Cholesterol in Surgically Treated Oral Cavity Cancer
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Cohort
2.2. Study Variables
2.3. Treatment and Follow-Up Plans
2.4. Statistical Analysis
3. Results
3.1. Patient Characteristics
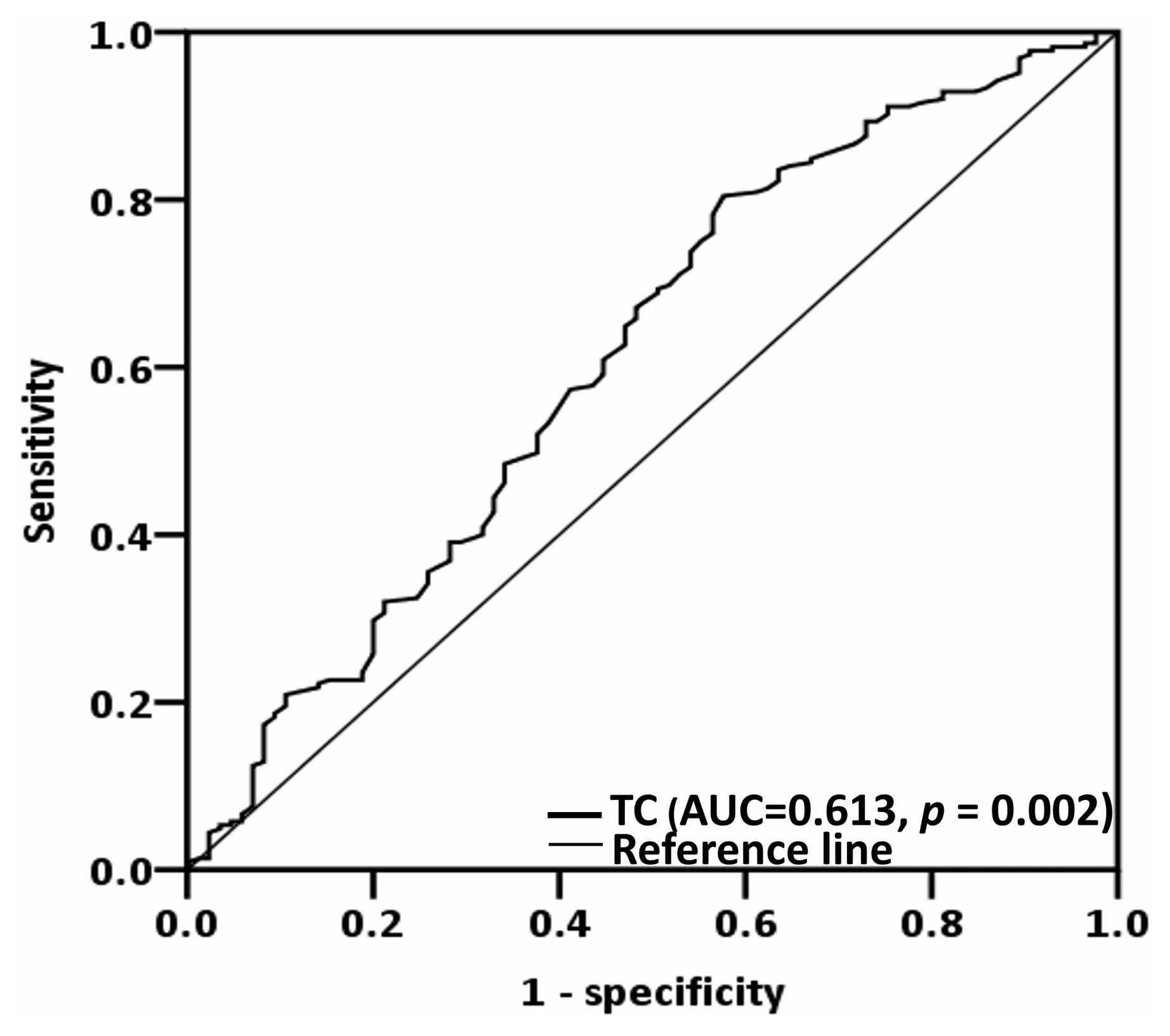
3.2. Determining the Optimal Total Cholesterol Cutoff
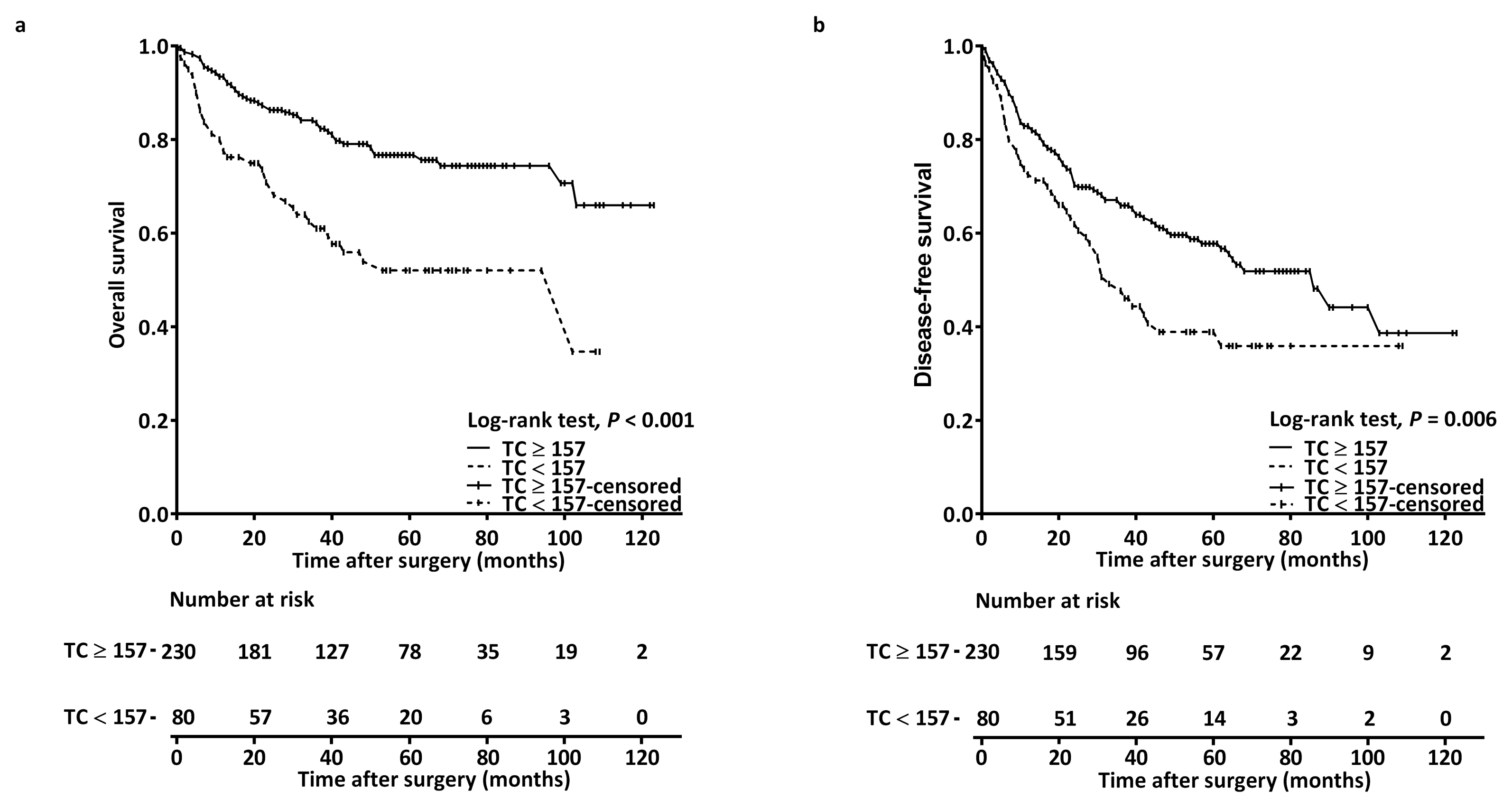
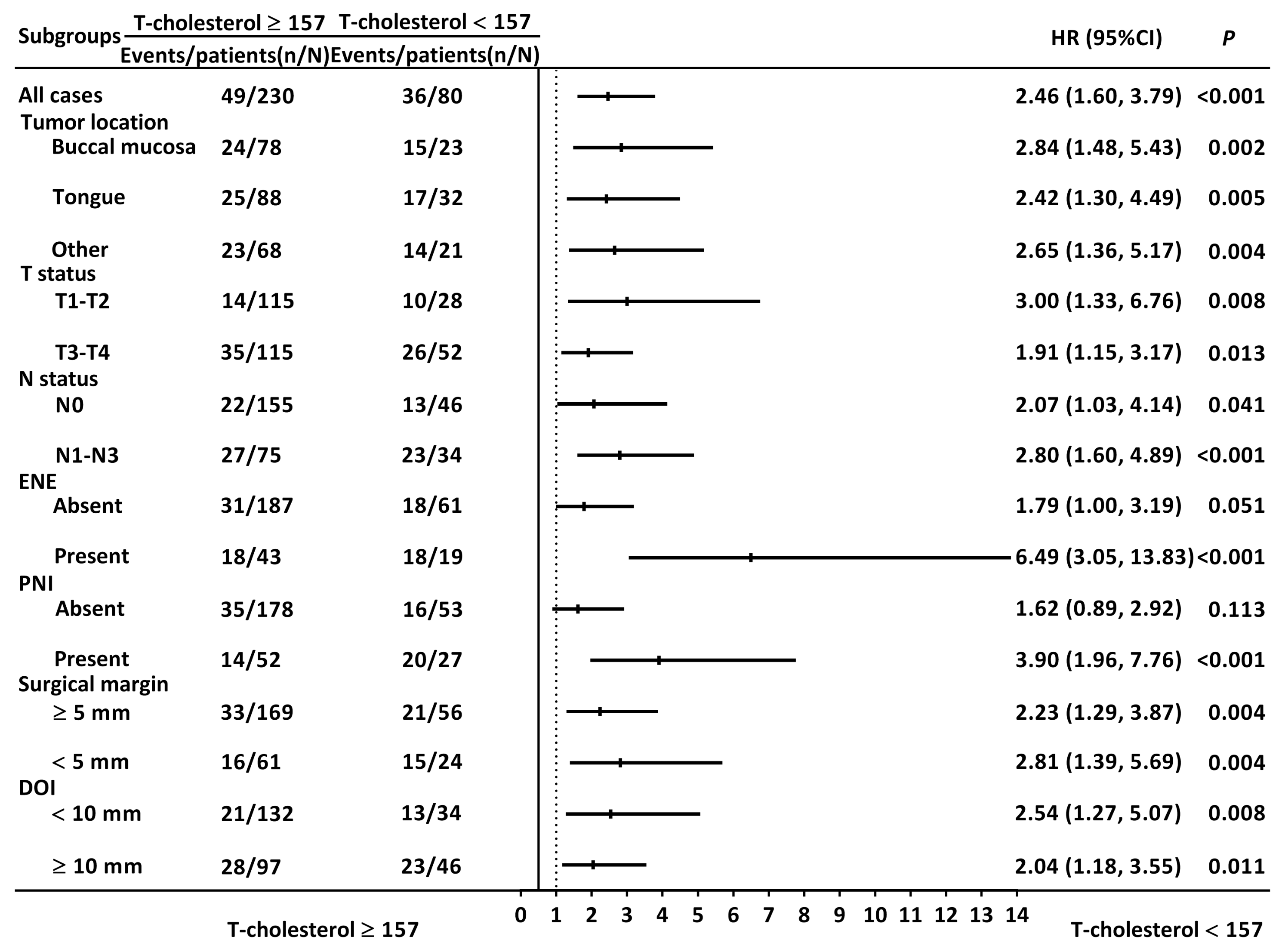
3.3. Prognostic Factors for Overall Survival
3.4. Prognostic Factors for Disease-Free Survival
3.5. Nomogram for Overall Survival Prediction
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Es-timates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Safi, A.-F.; Kauke, M.; Grandoch, A.; Nickenig, H.-J.; Zöller, J.E.; Kreppel, M. Analysis of clinicopathological risk factors for locoregional recurrence of oral squamous cell carcinoma–Retrospective analysis of 517 patients. J. Craniomaxillofac. Surg. 2017, 45, 1749–1753. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.Y.; Fan, K.H.; Lee, L.Y.; Hsueh, C.; Yang, L.Y.; Ng, S.H.; Wang, H.M.; Hsieh, C.H.; Lin, C.H.; Tsao, C.K.; et al. Precision Adjuvant Therapy Based on Detailed Pathologic Risk Factors for Resected Oral Cavity Squamous Cell Carcinoma: Long-Term Outcome Comparison of CGMH and NCCN Guide-lines. Int. J. Radiat. Oncol. Biol. Phys. 2020, 106, 916–925. [Google Scholar] [CrossRef] [PubMed]
- Lee, L.; Lin, C.; Cheng, N.; Tsai, C.; Hsueh, C.; Fan, K.; Wang, H.; Hsieh, C.; Ng, S.; Yeh, C.; et al. Poor tumor differentiation is an independent adverse prognostic variable in patients with locally advanced oral cavity cancer–Comparison with pathological risk factors according to the NCCN guidelines. Cancer Med. 2021, 10, 6627–6641. [Google Scholar] [CrossRef]
- Nair, D.; Mair, M.; Singhvi, H.; Mishra, A.; Nair, S.; Agrawal, J.; Chaturvedi, P. Perineural invasion: Independent prognostic factor in oral cancer that warrants adjuvant treatment. Head Neck 2018, 40, 1780–1787. [Google Scholar] [CrossRef]
- Huang, S.; Zhu, Y.; Cai, H.; Zhang, Y.; Hou, J. Impact of lymphovascular invasion in oral squamous cell carcinoma: A meta-analysis. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2021, 131, 319–328.e1. [Google Scholar] [CrossRef]
- Miao, X.; Wang, B.; Chen, K.; Ding, R.; Wu, J.; Pan, Y.; Ji, P.; Ye, B.; Xiang, M. Perspectives of lipid metabolism reprogramming in head and neck squamous cell carcinoma: An overview. Front. Oncol. 2022, 12, 1008361. [Google Scholar] [CrossRef]
- Kim, S.; Kim, G.; Cho, S.H.; Oh, R.; Kim, J.Y.; Lee, Y.-B.; Jin, S.-M.; Hur, K.Y.; Kim, J.H. Association between total cholesterol levels and all-cause mortality among newly diagnosed patients with cancer. Sci. Rep. 2024, 14, 58. [Google Scholar] [CrossRef]
- Wu, B.; Teng, L.; He, D.; Yu, D.-D.; Jiang, F. Dose-response relation between serum total cholesterol levels and overall cancer risk: Evidence from 12 prospective studies involving 1,926,275 participants. Int. J. Food Sci. Nutr. 2019, 70, 432–441. [Google Scholar] [CrossRef]
- de Martino, M.; Leitner, C.V.; Seemann, C.; Hofbauer, S.L.; Lucca, I.; Haitel, A.; Shariat, S.F.; Klatte, T. Preoperative serum cholesterol is an independent prognostic factor for patients with renal cell carcinoma (RCC). BJU Int. 2015, 115, 397–404. [Google Scholar] [CrossRef]
- Lin, F.; Zheng, R.; Yu, C.; Su, Y.; Yan, X.; Qu, F. Predictive role of serum cholesterol and triglycerides in cervical cancer survival. Int. J. Gynecol. Cancer 2021, 31, 171–176. [Google Scholar] [CrossRef] [PubMed]
- Ohno, Y.; Ohori, M.; Nakashima, J.; Okubo, H.; Satake, N.; Hashimoto, T.; Tachibana, M. Association between preoperative serum total cho-lesterol level and biochemical recurrence in prostate cancer patients who underwent radical prostatectomy. Mol. Clin. Oncol. 2016, 4, 1073–1077. [Google Scholar] [CrossRef] [PubMed]
- Shin, H.-J.; Roh, C.-K.; Son, S.-Y.; Hoon, H.; Han, S.-U. Prognostic value of hypocholesterolemia in patients with gastric cancer. Asian J. Surg. 2021, 44, 72–79. [Google Scholar] [CrossRef] [PubMed]
- Sok, M.; Ravnik, J.; Ravnik, M. Preoperative total serum cholesterol as a prognostic factor for survival in patients with resectable non-small-cell lung cancer. Wien. Klin. Wochenschr. 2009, 121, 314–317. [Google Scholar] [CrossRef]
- Wang, H.-X.; Ding, C.; Huang, J.-C.; Ma, Y.-W.; Lyu, S.-C.; Lang, R. Prognostic Value for Perioperative Serum Total Cholesterol Level on Postoperative Long-Term Prognosis of Pancreatic Cancer: A Retrospective Clinical Study. Diagnostics 2023, 13, 1402. [Google Scholar] [CrossRef]
- Chawda, J.G.; Jain, S.S.; Patel, H.R.; Chaduvula, N.; Patel, K. The relationship between serum lipid levels and the risk of oral cancer. Indian J. Med. Paediatr. Oncol. 2011, 32, 34–37. [Google Scholar] [CrossRef]
- Poorey, V.K.; Thakur, P. Alteration of Lipid Profile in Patients with Head and Neck Malignancy. Indian J. Otolaryngol. Head Neck Surg. 2016, 68, 135–140. [Google Scholar] [CrossRef]
- Wang, S.; Wang, L.; Li, H.; Zhang, J.; Peng, J.; Cheng, B.; Song, M.; Hu, Q. Correlation analysis of plasma lipid profiles and the prognosis of head and neck squamous cell carcinoma. Oral Dis. 2024, 30, 329–341. [Google Scholar] [CrossRef]
- Zhou, P.; Li, B.; Liu, B.; Chen, T.; Xiao, J. Prognostic role of serum total cholesterol and high-density lipoprotein cholesterol in cancer survivors: A systematic review and meta-analysis. Clin. Chim. Acta 2018, 477, 94–104. [Google Scholar] [CrossRef]
- Kao, H.-K.; Löfstrand, J.; Loh, C.Y.-Y.; Lao, W.W.-K.; Yi, J.-S.; Chang, Y.-L.; Chang, K.-P. Nomogram based on albumin and neutrophil-to-lymphocyte ratio for predicting the prognosis of patients with oral cavity squamous cell carcinoma. Sci. Rep. 2018, 8, 13081. [Google Scholar] [CrossRef]
- Husten, C.G. How should we define light or intermittent smoking? Does it matter? Nicotine Tob. Res. 2009, 11, 111–121. [Google Scholar] [CrossRef] [PubMed]
- Harrell, F.E., Jr.; Lee, K.L.; Mark, D.B. Multivariable prognostic models: Issues in developing models, evaluating assumptions and adequacy, and measuring and reducing errors. Stat. Med. 1996, 15, 361–387. [Google Scholar] [CrossRef]
- Ohno, Y.; Nakashima, J.; Nakagami, Y.; Gondo, T.; Ohori, M.; Hatano, T.; Tachibana, M. Clinical implications of preoperative serum total cholesterol in patients with clear cell renal cell carcinoma. Urology 2014, 83, 154–158. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Huang, D.; Zheng, H.; Cai, Q.; Guo, Z.; Wang, S. Preoperative serum total cholesterol is a predictor of prognosis in patients with renal cell carcinoma: A meta-analysis of observational studies. Int. Braz. J. Urol. 2020, 46, 158–168. [Google Scholar] [CrossRef]
- Chan, N.N.; Yamazaki, M.; Maruyama, S.; Abé, T.; Haga, K.; Kawaharada, M.; Izumi, K.; Kobayashi, T.; Tanuma, J.-I. Cholesterol Is a Regulator of CAV1 Localization and Cell Migration in Oral Squamous Cell Carcinoma. Int. J. Mol. Sci. 2023, 24, 6035. [Google Scholar] [CrossRef]
- Liu, S.; Wang, S.; Wang, Z. Identification of genetic mechanisms underlying lipid metabolism-mediated tumor immunity in head and neck squamous cell carcinoma. BMC Med. Genom. 2023, 16, 110. [Google Scholar] [CrossRef]
- Lu, M.; Hu, X.-H.; Li, Q.; Xiong, Y.; Hu, G.-J.; Xu, J.-J.; Zhao, X.-N.; Wei, X.-X.; Chang, C.C.; Liu, Y.-K.; et al. A specific cholesterol metabolic pathway is established in a subset of HCCs for tumor growth. J. Mol. Cell Biol. 2013, 5, 404–415. [Google Scholar] [CrossRef]
- Patel, P.; Shah, M.; Jha, F.; Raval, G.; Rawal, R.; Patel, M.; Patel, J.; Patel, D. Alterations in plasma lipid profile patterns in head and neck cancer and oral precancerous conditions. Indian J. Cancer 2004, 41, 25–31. [Google Scholar] [CrossRef]
- Acharya, S.; Rai, P.; Hallikeri, K.; Anehosur, V.; Kale, J. Serum lipid profile in oral squamous cell carcinoma: Alterations and asso-ciation with some clinicopathological parameters and tobacco use. Int. J. Oral Maxillofac. Surg. 2016, 45, 713–720. [Google Scholar] [CrossRef]
- Sai, S.K.; Babburi, S.; Deepthi, G.; Nandan, S.R.K.; Reddy, S.P.; Adusumilli, P. Lipid profile in oral potentially malignant disorders and oral squamous cell carcinoma—A prognostic view. J. Oral Maxillofac. Pathol. 2022, 26, 464–469. [Google Scholar] [CrossRef]
- Sherubin, E.; Kannan, K.; Kumar, D.; Joseph, I. Estimation of plasma lipids and its significance on histopathological grades in oral cancer: Prognostic significance an original research. J. Oral Maxillofac. Pathol. 2013, 17, 4–9. [Google Scholar] [CrossRef] [PubMed]
- Hashizume, M.; Yoshida, H.; Koike, N.; Suzuki, M.; Mihara, M. Overproduced interleukin 6 decreases blood lipid levels via up-regulation of very-low-density lipoprotein receptor. Ann. Rheum. Dis. 2010, 69, 741–746. [Google Scholar] [CrossRef] [PubMed]
- Schett, G. Physiological effects of modulating the interleukin-6 axis. Rheumatology 2018, 57, ii43–ii50. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Huang, X.; Meng, Z.; Dong, B.; Shiah, S.; Moore, D.D.; Huang, W. Significance and mechanism of CYP7a1 gene regulation during the acute phase of liver regeneration. Mol. Endocrinol. 2009, 23, 137–145. [Google Scholar] [CrossRef]
- Banka, C.L.; Yuan, T.; de Beer, M.C.; Kindy, M.; Curtiss, L.K.; de Beer, F.C. Serum amyloid A (SAA): Influence on HDL-mediated cellular cholesterol efflux. J. Lipid Res. 1995, 36, 1058–1065. [Google Scholar] [CrossRef]
- Gierens, H.; Nauck, M.; Roth, M.; Schinker, R.; Schurmann, C.; Scharnagl, H.; Neuhaus, G.; Wieland, H.; März, W. Interleukin-6 stimulates LDL receptor gene ex-pression via activation of sterol-responsive and Sp1 binding elements. Arterioscler. Thromb. Vasc. Biol. 2000, 20, 1777–1783. [Google Scholar] [CrossRef]
- Gurzov, E.N.; Stanley, W.J.; Pappas, E.G.; Thomas, H.E.; Gough, D.J. The JAK/STAT pathway in obesity and diabetes. FEBS J. 2016, 283, 3002–3015. [Google Scholar] [CrossRef]
- Huang, B.; Lang, X.; Li, X. The role of IL-6/JAK2/STAT3 signaling pathway in cancers. Front. Oncol. 2022, 12, 1023177. [Google Scholar] [CrossRef]
- Keidar, S.; Heinrich, R.; Kaplan, M.; Hayek, T.; Aviram, M. Angiotensin II administration to atherosclerotic mice increases mac-rophage uptake of oxidized ldl: A possible role for interleukin-6. Arterioscler. Thromb. Vasc. Biol. 2001, 21, 1464–1469. [Google Scholar] [CrossRef]
- Wang, Y.; Chang, S.; Tai, S.; Li, W.; Wang, L. Clinical significance of interleukin-6 and interleukin-6 receptor expressions in oral squamous cell carcinoma. Head Neck 2002, 24, 850–858. [Google Scholar] [CrossRef]
- Jinno, T.; Kawano, S.; Maruse, Y.; Matsubara, R.; Goto, Y.; Sakamoto, T.; Hashiguchi, Y.; Kaneko, N.; Tanaka, H.; Kitamura, R.; et al. Increased expression of interleukin-6 predicts poor response to chemoradiotherapy and unfavorable prognosis in oral squamous cell carcinoma. Oncol. Rep. 2015, 33, 2161–2168. [Google Scholar] [CrossRef] [PubMed]
- Datema, F.R.; Ferrier, M.B.; de Jong, R.J.B. Impact of severe malnutrition on short-term mortality and overall survival in head and neck cancer. Oral Oncol. 2011, 47, 910–914. [Google Scholar] [CrossRef] [PubMed]
- Yoshimura, T.; Suzuki, H.; Takayama, H.; Higashi, S.; Hirano, Y.; Tezuka, M.; Ishida, T.; Ishihata, K.; Amitani, M.; Amitani, H.; et al. Prognostic value of inflammatory biomarkers in aged patients with oral squamous cell carcinoma. Front. Pharmacol. 2022, 13, 996757. [Google Scholar] [CrossRef] [PubMed]
- Halimi, H.; Farjadian, S. Cholesterol: An important actor on the cancer immune scene. Front. Immunol. 2022, 13, 1057546. [Google Scholar] [CrossRef]
- Kidani, Y.; Bensinger, S.J. Modulating Cholesterol Homeostasis to Build a Better T Cell. Cell Metab. 2016, 23, 963–964. [Google Scholar] [CrossRef]
- Qin, W.-H.; Yang, Z.-S.; Li, M.; Chen, Y.; Zhao, X.-F.; Qin, Y.-Y.; Song, J.-Q.; Wang, B.-B.; Yuan, B.; Cui, X.-L.; et al. High Serum Levels of Cholesterol Increase Antitumor Functions of Nature Killer Cells and Reduce Growth of Liver Tumors in Mice. Gastroenterology 2020, 158, 1713–1727. [Google Scholar] [CrossRef]
- Muldoon, M.F.; Marslandb, A.; Flory, J.D.; Rabin, B.S.; Whiteside, T.L.; Manuck, S.B. Immune system differences in men with hypo- or hypercholesterolemia. Clin. Immunol. Immunopathol. 1997, 84, 145–149. [Google Scholar] [CrossRef]
- Yan, C.; Zheng, L.; Jiang, S.; Yang, H.; Guo, J.; Jiang, L.-Y.; Li, T.; Zhang, H.; Bai, Y.; Lou, Y.; et al. Exhaustion-associated cholesterol deficiency dampens the cytotoxic arm of antitumor immunity. Cancer Cell 2023, 41, 1276–1293.e11. [Google Scholar] [CrossRef]
- Dimitroulakos, J.; Marhin, W.H.; Tokunaga, J.; Irish, J.; Gullane, P.; Penn, L.Z.; Kamel-Reid, S. Microarray and biochemical analysis of lovas-tatin-induced apoptosis of squamous cell carcinomas. Neoplasia 2002, 4, 337–346. [Google Scholar] [CrossRef]
- Spampanato, C.; DE Maria, S.; Sarnataro, M.; Giordano, E.; Zanfardino, M.; Baiano, S.; Cartenì, M.; Morelli, F. Simvastatin inhibits cancer cell growth by inducing apoptosis correlated to activation of Bax and down-regulation of BCL-2 gene expression. Int. J. Oncol. 2012, 40, 935–941. [Google Scholar] [CrossRef]
- Kwon, M.; Nam, G.-H.; Jung, H.; Kim, S.A.; Kim, S.; Choi, Y.; Lee, Y.S.; Cho, H.J.; Kim, I.-S. Statin in combination with cisplatin makes favorable tumor-immune microenvironment for immunotherapy of head and neck squamous cell carcinoma. Cancer Lett. 2021, 522, 198–210. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.C.; Chen, W.M.; Shia, B.C.; Chen, M.; Wu, S.Y. The effect of statin medication on the incidence of oral squamous cell car-cinoma among betel-nut chewers. Head Neck 2023, 45, 1455–1467. [Google Scholar] [CrossRef] [PubMed]
- Spoerl, S.; Gerken, M.; Fischer, R.; Spoerl, S.; Kirschneck, C.; Wolf, S.; Taxis, J.; Ludwig, N.; Biermann, N.; Reichert, T.E.; et al. Statin Use Ameliorates Survival in Oral Squamous Cell Carcinoma—Data from a Population-Based Cohort Study Applying Propensity Score Matching. Biomedicines 2023, 11, 369. [Google Scholar] [CrossRef] [PubMed]
- Peters, S.A.; Singhateh, Y.; MacKay, D.; Huxley, R.R.; Woodward, M. Total cholesterol as a risk factor for coronary heart disease and stroke in women compared with men: A systematic review and meta-analysis. Atherosclerosis 2016, 248, 123–131. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.; Yu, H.; Kim, D.-K. The Risk of Ischemic and Hemorrhagic Stroke in Head and Neck Cancer: A Longitudinal Cohort Study. Cancers 2023, 15, 3503. [Google Scholar] [CrossRef]
- Nagele, P.; Rao, L.K.; Penta, M.; Kallogjeri, D.; Spitznagel, E.L.; Cavallone, L.F.; Nussenbaum, B.; Piccirillo, J.F. Postoperative myocardial injury after major head and neck cancer surgery. Head Neck 2011, 33, 1085–1091. [Google Scholar] [CrossRef]
- Liu, Y.; Coresh, J.; Eustace, J.A.; Longenecker, J.C.; Jaar, B.; Fink, N.E.; Tracy, R.P.; Powe, N.R.; Klag, M.J. Association between cholesterol level and mortality in dialysis patients: Role of inflammation and malnutrition. JAMA 2004, 291, 451–459. [Google Scholar] [CrossRef]

| Variables | Total, N (%) | Total Cholesterol ≥ 157 (n = 230) | Total Cholesterol < 157 (n = 80) | p |
|---|---|---|---|---|
| Age (years) | 0.010 a | |||
| <65 | 196 (63.2%) | 155 (67.4%) | 41 (51.3%) | |
| ≥65 | 114 (36.8%) | 75 (32.6%) | 39 (48.8%) | |
| Sex | 0.581 a | |||
| Men | 280 (90.3%) | 209 (90.9%) | 71 (88.8%) | |
| Women | 30 (9.7%) | 21 (9.1%) | 9 (11.3%) | |
| Tumor location | 0.282 a | |||
| Tongue | 120 (38.7%) | 95 (41.3%) | 25 (31.3%) | |
| Buccal mucosa | 101 (32.6%) | 72 (31.3%) | 29 (36.3%) | |
| Others | 89 (28.7%) | 63 (27.4%) | 26 (32.5%) | |
| Personal Habits * | 0.767 a | |||
| No exposure | 38 (12.3%) | 27 (11.7%) | 11 (13.8%) | |
| One exposure | 16 (5.2%) | 11 (4.8%) | 5 (6.3%) | |
| Two or more exposures | 256 (82.6%) | 192 (83.5%) | 64 (80.0%) | |
| AJCC stage | 0.110 a | |||
| I | 66 (21.3%) | 53 (23.0%) | 13 (16.3%) | |
| II | 43 (13.9%) | 36 (15.7%) | 7 (8.88%) | |
| III | 45 (14.5%) | 29 (12.6%) | 16 (20.0%) | |
| IV | 156 (50.3%) | 112 (48.7%) | 44 (55.0%) | |
| T status | 0.020 a | |||
| T1−T2 | 143 (46.1%) | 115 (50.0%) | 28 (35.0%) | |
| T3−T4 | 167 (53.9%) | 115 (50.0%) | 52 (65.0%) | |
| N status | 0.110 a | |||
| N0 | 201 (64.8%) | 155 (67.4%) | 46 (57.5%) | |
| N+ | 109 (35.2%) | 75 (32.6%) | 34 (42.5%) | |
| Presence of PNI | 0.049 a | |||
| No | 231 (74.5%) | 178 (77.4%) | 53 (66.3%) | |
| Yes | 79 (25.5%) | 52 (22.6%) | 27 (33.8%) | |
| Presence of ENE | 0.330 a | |||
| No | 248 (80.0%) | 187 (81.3%) | 61 (76.3%) | |
| Yes | 62 (20.0%) | 43 (18.7%) | 19 (23.8%) | |
| Presence of LVI | 0.183 a | |||
| No | 289 (93.2%) | 217 (94.3%) | 72 (90.0%) | |
| Yes | 21 (6.8%) | 13 (5.7%) | 8 (10.0%) | |
| Tumor grading | 0.296 a | |||
| W−D/M−D | 277 (89.4%) | 208 (90.4%) | 69 (86.3%) | |
| P−D | 33 (10.6%) | 22 (9.6%) | 11 (13.8%) | |
| Closest resection margin | 0.548 a | |||
| ≥5 mm | 225 (72.6%) | 169 (73.5%) | 56 (70.0%) | |
| <5 mm | 85 (27.4%) | 61 (26.5%) | 24 (30.0%) | |
| DOI ≥ 10 mm | 0.018 a | |||
| No | 167 (53.9%) | 133 (57.8%) | 34 (42.5%) | |
| Yes | 143 (46.1%) | 97 (42.2%) | 46 (57.5%) | |
| Treatment modality | 0.124 a | |||
| Surgery only | 149 (48.1%) | 118 (51.3%) | 31 (38.8%) | |
| Surgery then RT | 44 (14.2%) | 29 (12.6%) | 15 (18.8%) | |
| Surgery then CRT | 117 (37.7%) | 83 (36.1%) | 34 (42.5%) | |
| CCI | 0.112 a | |||
| 0 | 167 (53.9%) | 130 (56.5%) | 37 (46.3%) | |
| ≥1 | 143 (46.1%) | 100 (43.5%) | 43 (53.8%) | |
| WBC (×103/μL), median (IQR) | 7.80 (6.28−9.70) | 7.70 (6.20−9.50) | 8.10 (6.33−10.30) | 0.417 b |
| Cholesterol (mg/dL), median (IQR) | 179 (156−208) | 189 (176−221) | 140 (126−148) | <0.001 b |
| Variables | Univariable Analysis | Multivariable Analysis | |||
|---|---|---|---|---|---|
| HR (95% CI) | p | HR (95% CI) | p | ||
| Sex | Men vs. Women | 1.645 (0.715–3.783) | 0.241 | ||
| Age (years) | ≥65 vs. <65 | 0.763 (0.483–1.204) | 0.245 | ||
| AJCC stage | III–IV vs. I–II | 3.713 (2.056–6.708) | <0.001 | 2.405 (1.165–4.967) | 0.018 |
| Presence of PNI | Yes vs. no | 2.450 (1.585–3.789) | <0.001 | ||
| Presence of LVI | Yes vs. no | 3.886 (2.091–7.221) | <0.001 | 2.892 (1.507–5.551) | 0.001 |
| Tumor grading | P–D vs. W–D/M–D | 2.851 (1.669–4.870) | <0.001 | 2.111 (1.189–3.746) | 0.011 |
| Closest margin (mm) | <5 vs. ≥5 | 1.605 (1.031–2.498) | 0.036 | ||
| Adjuvant therapy | Yes vs. no | 3.090 (1.922–4.969) | <0.001 | ||
| CCI | ≥1 vs. 0 | 1.570 (1.023–2.410) | 0.039 | 1.623 (1.051–2.506) | 0.029 |
| Total cholesterol | <157 vs. ≥157 | 2.463 (1.601–3.792) | <0.001 | 2.114 (1.368–3.269) | 0.001 |
| Variables | Univariable Analysis | Multivariable Analysis | |||
|---|---|---|---|---|---|
| HR (95% CI) | p | HR (95% CI) | p | ||
| Sex | Men vs. Women | 1.509 (0.810–2.812) | 0.195 | ||
| Age (years) | ≥65 vs. <65 | 0.696 (0.485–1.018) | 0.053 | ||
| AJCC stage | III–IV vs. I–II | 2.126 (1.442–3.134) | <0.001 | 2.242 (1.391–3.615) | 0.001 |
| Presence of PNI | Yes vs. no | 1.359 (0.938–1.970) | 0.105 | ||
| Presence of LVI | Yes vs. no | 1.966 (1.083–3.568) | 0.026 | ||
| Tumor grading | P–D vs. W–D/M–D | 1.785 (1.109–2.873) | 0.017 | 1.732 (1.053–2.848) | 0.030 |
| Closest margin (mm) | <5 vs. ≥5 | 1.384 (0.971–1.972) | 0.072 | ||
| Adjuvant therapy | Yes vs. no | 1.526 (1.088–2.141) | 0.014 | ||
| CCI | ≥1 vs. 0 | 0.986 (0.704–1.380) | 0.934 | ||
| Total cholesterol | <157 vs. ≥157 | 1.630 (1.144–2.324) | 0.006 | 1.622 (1.127–2.333) | 0.009 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsai, Y.-T.; Tsai, M.-H.; Kudva, A.; Vito, A.D.; Lai, C.-H.; Liao, C.-T.; Kang, C.-J.; Tsai, Y.-H.; Hsu, C.-M.; Huang, E.I.; et al. The Prognostic Value of Preoperative Total Cholesterol in Surgically Treated Oral Cavity Cancer. Biomedicines 2024, 12, 2898. https://doi.org/10.3390/biomedicines12122898
Tsai Y-T, Tsai M-H, Kudva A, Vito AD, Lai C-H, Liao C-T, Kang C-J, Tsai Y-H, Hsu C-M, Huang EI, et al. The Prognostic Value of Preoperative Total Cholesterol in Surgically Treated Oral Cavity Cancer. Biomedicines. 2024; 12(12):2898. https://doi.org/10.3390/biomedicines12122898
Chicago/Turabian StyleTsai, Yao-Te, Ming-Hsien Tsai, Adarsh Kudva, Andrea De Vito, Chia-Hsuan Lai, Chun-Ta Liao, Chung-Jan Kang, Yuan-Hsiung Tsai, Cheng-Ming Hsu, Ethan I. Huang, and et al. 2024. "The Prognostic Value of Preoperative Total Cholesterol in Surgically Treated Oral Cavity Cancer" Biomedicines 12, no. 12: 2898. https://doi.org/10.3390/biomedicines12122898
APA StyleTsai, Y.-T., Tsai, M.-H., Kudva, A., Vito, A. D., Lai, C.-H., Liao, C.-T., Kang, C.-J., Tsai, Y.-H., Hsu, C.-M., Huang, E. I., Chang, G.-H., Tsai, M.-S., & Fang, K.-H. (2024). The Prognostic Value of Preoperative Total Cholesterol in Surgically Treated Oral Cavity Cancer. Biomedicines, 12(12), 2898. https://doi.org/10.3390/biomedicines12122898







