Tumor Protein D53 (TPD53): Involvement in Malignant Transformation of Low-Malignant Oral Squamous Cell Carcinoma Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. Antibodies
2.3. RNA Isolation and Real-Time Quantitative Polymerase Chain Reaction (RT–qPCR)
2.4. Protein Extraction and Western Blotting
2.5. Gene Transfection
2.6. MTT Assay
2.7. Cell Cycle Assay
2.8. Anchorage-Independent Growth Assay
2.9. Cell Invasion Assay
2.10. Wound Healing Assay
2.11. Gelatin Zymography
2.12. Generation of Stable Clones
2.13. Mouse Xenograft Model
2.14. Immunohistochemistry
2.15. Statical Analysis
3. Results
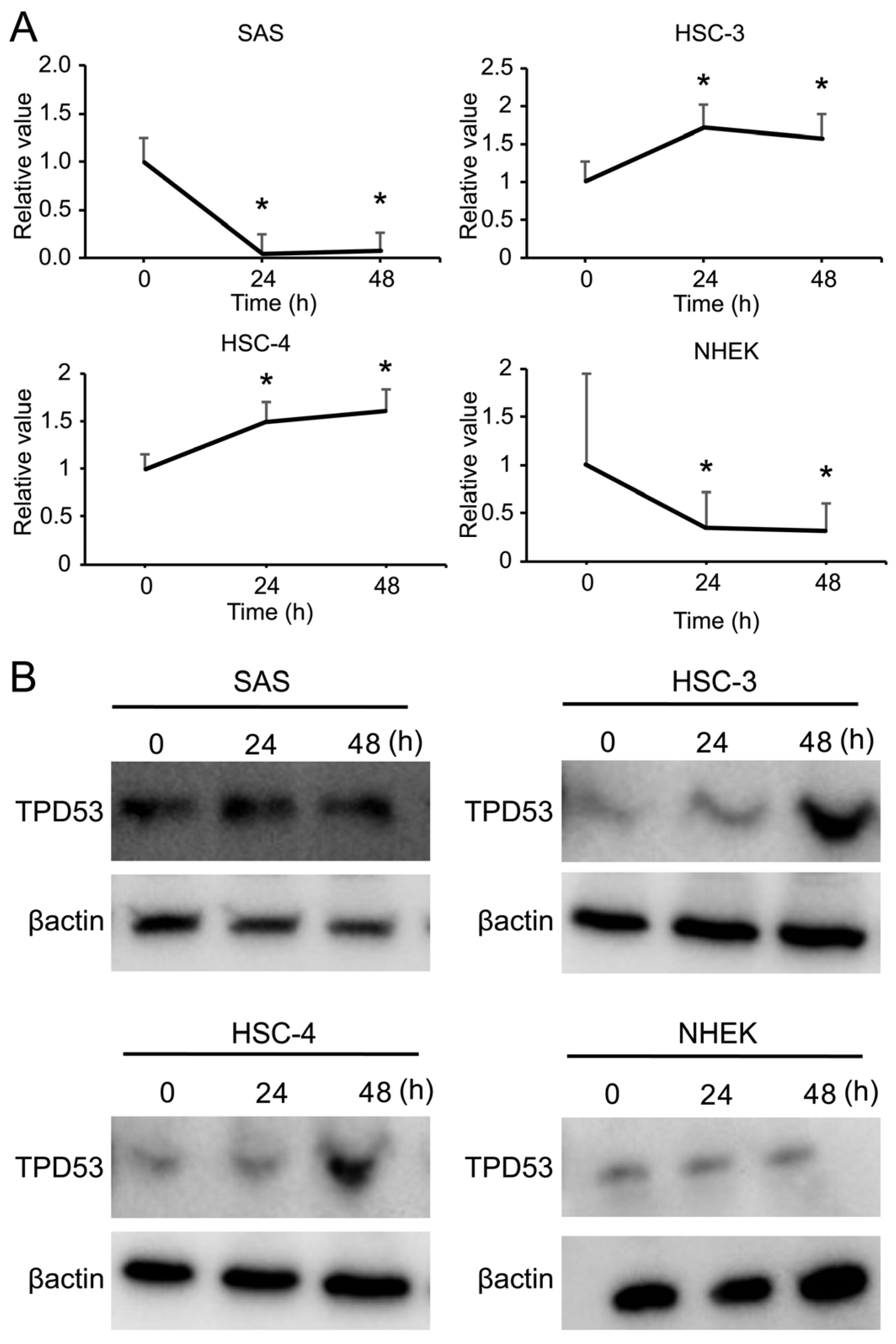
3.1. mRNA Expression of TPD53 Increased in Low-Malignant OSCC Cells in a Time-Dependent Manner
3.2. TPD53 Increased Cell Proliferation and Accelerated Cell Cycle Progression in Low-Malignant OSCC Cells
3.3. TPD53 Increased Cell Migration and Invasion
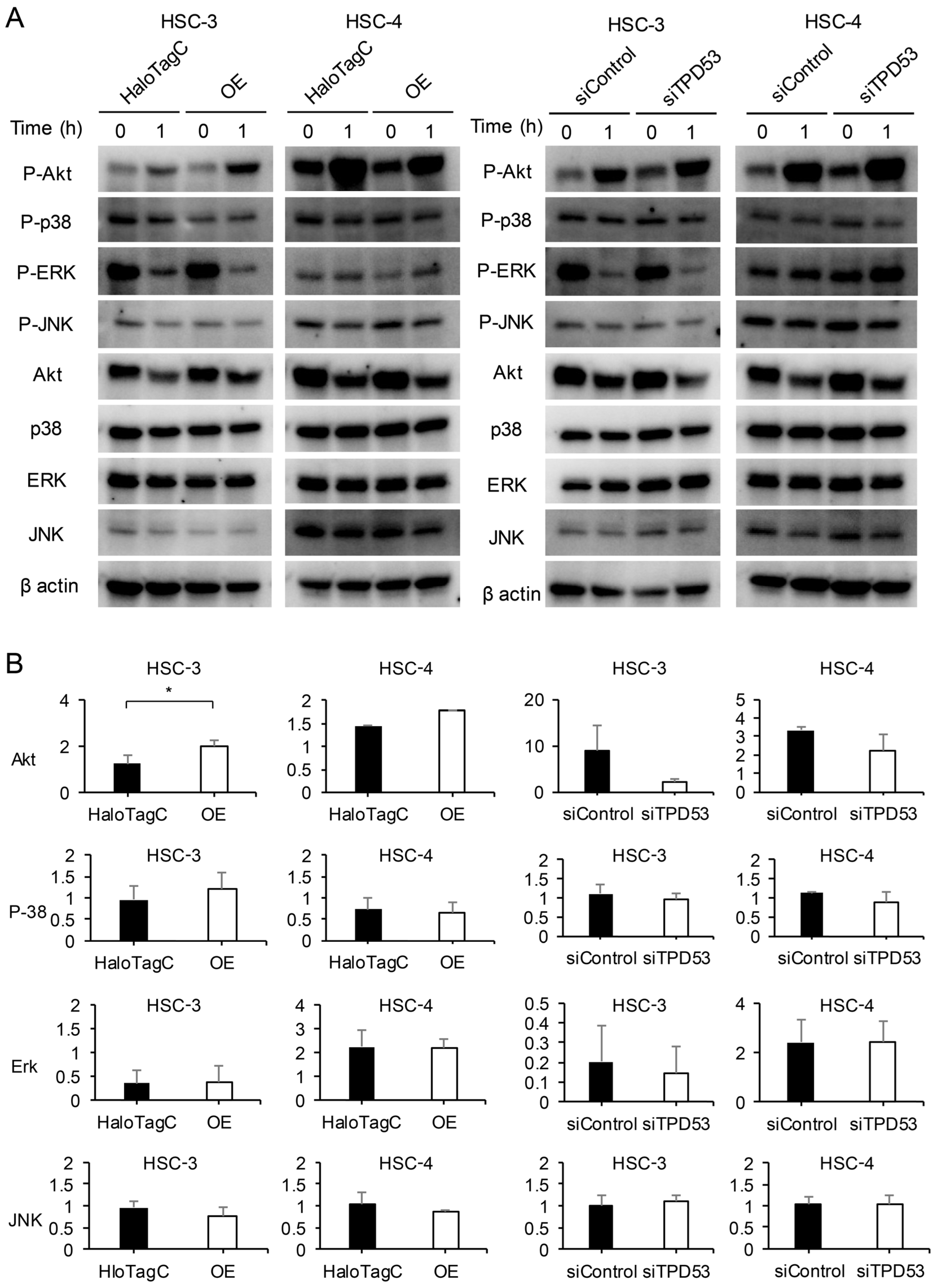
3.4. TPD53 Promoted Akt Signaling
3.5. TPD53 Enhanced the Growth of Xenograft HSC-3 in Nude Mice
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2019. CA Cancer J. Clin. 2019, 69, 7–34. [Google Scholar] [CrossRef] [PubMed]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Gharat, S.A.; Momin, M.; Bhavsar, C. Oral squamous cell carcinoma: Current treatment strategies and nanotechnology-based approaches for prevention and therapy. Crit. Rev. Ther. Drug Carrier Syst. 2016, 33, 363–400. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.W.; Park, Y.; Roh, J.L.; Cho, K.J.; Choi, S.H.; Nam, S.Y.; Kim, S.Y. Prognostic value of glucosylceramide synthase and P-glycoprotein expression in oral cavity cancer. Int. J. Clin. Oncol. 2016, 21, 883–889. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.; Rai, A.; Verma, A.K.; Alsahli, M.A.; Rahmani, A.H.; Almatroodi, S.A.; Alrumaihi, F.; Dev, K.; Sinha, A.; Sankhwar, S.; et al. Survival-based biomarker module identification associated with oral squamous cell carcinoma (OSCC). Biology 2021, 10, 760. [Google Scholar] [CrossRef]
- Gunardi, I.; Sufiawati, I.; Goenawan, H.; Herawati, D.M.D.; Lesmana, R.; Abdullah, A.G. Research trends in molecular biological studies on oral squamous cell carcinoma: A bibliometric analysis. Oncol. Rev. 2023, 17, 11585. [Google Scholar] [CrossRef]
- Byrne, J.A.; Mattei, M.G.; Basset, P. Definition of the tumor protein D52 (TPD52) gene family through cloning of D52 homologues in human (hD53) and mouse (mD52). Genomics 1996, 35, 523–532. [Google Scholar] [CrossRef]
- Wang, R.; Xu, J.; Saramäki, O.; Visakorpi, T.; Sutherland, W.M.; Zhou, J.; Sen, B.; Lim, S.D.; Mabjeesh, N.; Amin, M.; et al. PrLZ, a novel prostate-specific and androgen-responsive gene of the TPD52 family, amplified in chromosome 8q21.1 and overexpressed in human prostate cancer. Cancer Res. 2004, 64, 1589–1594. [Google Scholar] [CrossRef]
- Byrne, J.A.; Mattei, M.G.; Basset, P.; Gunning, P. Identification and in situ hybridization mapping of a mouse Tpd52l1 (D53) orthologue to chromosome 10A4-B2. Cytogenet. Cell Genet. 1998, 81, 199–201. [Google Scholar] [CrossRef]
- Byrne, J.A.; Nourse, C.R.; Basset, P.; Gunning, P. Identification of homo- and heteromeric interactions between members of the breast carcinoma-associated D52 protein family using the yeast two-hybrid system. Oncogene 1998, 16, 873–881. [Google Scholar] [CrossRef]
- Nourse, C.R.; Mattei, M.G.; Gunning, P.; Byrne, J.A. Cloning of a third member of the D52 gene family indicates alternative coding sequence usage in D52-like transcripts. Biochim. Biophys. Acta 1998, 1443, 155–168. [Google Scholar] [CrossRef] [PubMed]
- Cao, Q.; Chen, J.; Zhu, L.; Liu, Y.; Zhou, Z.; Sha, J.; Wang, S.; Li, J. A testis-specific and testis developmentally regulated tumor protein D52 (TPD52)-like protein TPD52L3/hD55 interacts with TPD52 family proteins. Biochem. Biophys. Res. Commun. 2006, 344, 798–806. [Google Scholar] [CrossRef] [PubMed]
- Byrne, J.A.; Tomasetto, C.; Garnier, J.M.; Rouyer, N.; Mattei, M.G.; Bellocq, J.P.; Rio, M.C.; Basset, P. A screening method to identify genes commonly overexpressed in carcinomas and the identification of a novel complementary DNA sequence. Cancer Res. 1995, 55, 2896–2903. [Google Scholar] [PubMed]
- Chen, S.L.; Maroulakou, I.G.; Green, J.E.; Romano-Spica, V.; Modi, W.; Lautenberger, J.; Bhat, N.K. Isolation and characterization of a novel gene expressed in multiple cancers. Oncogene 1996, 12, 741–751. [Google Scholar] [PubMed]
- Cheung, H.C.; Baggerly, K.A.; Tsavachidis, S.; Bachinski, L.L.; Neubauer, V.L.; Nixon, T.J.; Aldape, K.D.; Cote, G.J.; Krahe, R. Global analysis of aberrant pre-mRNA splicing in glioblastoma using exon expression arrays. BMC Genom. 2008, 9, 216. [Google Scholar] [CrossRef]
- Rubin, M.A.; Varambally, S.; Beroukhim, R.; Tomlins, S.A.; Rhodes, D.R.; Paris, P.L.; Hofer, M.D.; Storz-Schweizer, M.; Kuefer, R.; Fletcher, J.A.; et al. Overexpression, amplification, and androgen regulation of TPD52 in prostate cancer. Cancer Res. 2004, 64, 3814–3822. [Google Scholar] [CrossRef]
- Byrne, J.A.; Balleine, R.L.; Schoenberg Fejzo, M.; Mercieca, J.; Chiew, Y.E.; Livnat, Y.; St Heaps, L.; Peters, G.B.; Byth, K.; Karlan, B.Y.; et al. Tumor protein D52 (TPD52) is overexpressed and a gene amplification target in ovarian cancer. Int. J. Cancer 2005, 117, 1049–1054. [Google Scholar] [CrossRef]
- Byrne, J.A.; Maleki, S.; Hardy, J.R.; Gloss, B.S.; Murali, R.; Scurry, J.P.; Fanayan, S.; Emmanuel, C.; Hacker, N.F.; Sutherland, R.L.; et al. MAL2 and tumor protein D52 (TPD52) are frequently overexpressed in ovarian carcinoma, but differentially associated with histological subtype and patient outcome. BMC Cancer 2010, 10, 497. [Google Scholar] [CrossRef]
- Fejzo, M.S.; Dering, J.; Ginther, C.; Anderson, L.; Ramos, L.; Walsh, C.; Karlan, B.; Slamon, D.J. Comprehensive analysis of 20q13 genes in ovarian cancer identifies ADRM1 as amplification target. Genes Chromosomes Cancer 2008, 47, 873–883. [Google Scholar] [CrossRef]
- Petrova, D.T.; Asif, A.R.; Armstrong, V.W.; Dimova, I.; Toshev, S.; Yaramov, N.; Oellerich, M.; Toncheva, D. Expression of chloride intracellular channel protein 1 (CLIC1) and tumor protein D52 (TPD52) as potential biomarkers for colorectal cancer. Clin. Biochem. 2008, 41, 1224–1236. [Google Scholar] [CrossRef]
- Willems, A.; De Gendt, K.; Allemeersch, J.; Smith, L.B.; Welsh, M.; Swinnen, J.V.; Verhoeven, G. Early effects of Sertoli cell-selective androgen receptor ablation on testicular gene expression. Int. J. Androl. 2010, 33, 507–517. [Google Scholar] [CrossRef] [PubMed]
- Barbaric, D.; Byth, K.; Dalla-Pozza, L.; Byrne, J.A. Expression of tumor protein D52-like genes in childhood leukemia at diagnosis: Clinical and sample considerations. Leuk. Res. 2006, 30, 1355–1363. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.; Wilson, C.S.; Harvey, R.C.; Chen, I.M.; Murphy, M.H.; Atlas, S.R.; Bedrick, E.J.; Devidas, M.; Carroll, A.J.; Robinson, B.W.; et al. Gene expression profiles predictive of outcome and age in infant acute lymphoblastic leukemia: A Children’s Oncology Group study. Blood 2012, 119, 1872–1881. [Google Scholar] [CrossRef] [PubMed]
- Lewis, J.D.; Payton, L.A.; Whitford, J.G.; Byrne, J.A.; Smith, D.I.; Yang, L.; Bright, R.K. Induction of tumorigenesis and metastasis by the murine orthologue of tumor protein D52. Mol. Cancer Res. 2007, 5, 133–144. [Google Scholar] [CrossRef]
- Shehata, M.; Bièche, I.; Boutros, R.; Weidenhofer, J.; Fanayan, S.; Spalding, L.; Zeps, N.; Byth, K.; Bright, R.K.; Lidereau, R.; et al. Nonredundant functions for tumor protein D52-like proteins support specific targeting of TPD52. Clin. Cancer Res. 2008, 14, 5050–5060. [Google Scholar] [CrossRef]
- Ummanni, R.; Teller, S.; Junker, H.; Zimmermann, U.; Venz, S.; Scharf, C.; Giebel, J.; Walther, R. Altered expression of tumor protein D52 regulates apoptosis and migration of prostate cancer cells. FEBS J. 2008, 275, 5703–5713. [Google Scholar] [CrossRef]
- Zhang, D.; He, D.; Xue, Y.; Wang, R.; Wu, K.; Xie, H.; Zeng, J.; Wang, X.; Zhau, H.E.; Chung, L.W.; et al. PrLZ protects prostate cancer cells from apoptosis induced by androgen deprivation via the activation of Stat3/Bcl-2 pathway. Cancer Res. 2011, 71, 2193–2202. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, J.; Pang, B.; Liang, R.X.; Li, S.; Huang, P.T.; Wang, R.; Chung, L.W.; Zhau, H.E.; Huang, C.; et al. PC-1/PrLZ contributes to malignant progression in prostate cancer. Cancer Res. 2007, 67, 8906–8913. [Google Scholar] [CrossRef]
- Larocque, G.; Royle, S.J. Integrating intracellular nanovesicles into integrin trafficking pathways and beyond. Cell Mol. Life Sci. 2022, 79, 335. [Google Scholar] [CrossRef]
- Mukudai, Y.; Kondo, S.; Fujita, A.; Yoshihama, Y.; Shirota, T.; Shintani, S. Tumor protein D54 is a negative regulator of extracellular matrix-dependent migration and attachment in oral squamous cell carcinoma-derived cell lines. Cell Oncol. 2013, 36, 233–245. [Google Scholar] [CrossRef]
- Kato, K.; Mukudai, Y.; Motohashi, H.; Ito, C.; Kamoshida, S.; Shimane, T.; Kondo, S.; Shirota, T. Opposite effects of tumor protein D (TPD) 52 and TPD54 on oral squamous cell carcinoma cells. Int. J. Oncol. 2017, 50, 1634–1646. [Google Scholar] [CrossRef] [PubMed]
- Abe, Y.; Mukudai, Y.; Kurihara, M.; Houri, A.; Chikuda, J.; Yaso, A.; Kato, K.; Shimane, T.; Shirota, T. Tumor protein D52 is upregulated in oral squamous carcinoma cells under hypoxia in a hypoxia-inducible-factor-independent manner and is involved in cell death resistance. Cell Biosci. 2021, 11, 122. [Google Scholar] [CrossRef] [PubMed]
- Houri, A.; Mukudai, Y.; Abe, Y.; Watanabe, M.; Nara, M.; Miyamoto, S.; Kurihara, M.; Shimane, T.; Shirota, T. Suprabasin enhances the invasion, migration, and angiogenic ability of oral squamous cell carcinoma cells under hypoxic conditions. Oncol. Rep. 2023, 49, 83. [Google Scholar] [CrossRef] [PubMed]
- Momose, F.; Araida, T.; Negishi, A.; Ichijo, H.; Shioda, S.; Sasaki, S. Variant sublines with different metastatic potentials selected in nude mice from human oral squamous cell carcinomas. J. Oral. Pathol. Med. 1989, 18, 391–395. [Google Scholar] [CrossRef]
- Oshima, E.; Hayashi, Y.; Xie, Z.; Sato, H.; Hitomi, S.; Shibuta, I.; Urata, K.; Ni, J.; Iwata, K.; Shirota, T.; et al. M2 macrophage-derived cathepsin S promotes peripheral nerve regeneration via fibroblast-Schwann cell-signaling relay. J. Neuroinflammation 2023, 20, 258. [Google Scholar] [CrossRef]
- Nakamura, S.; Mukudai, Y.; Chikuda, J.; Zhang, M.; Shigemori, H.; Yazawa, K.; Kondo, S.; Shimane, T.; Shirota, T. Combinational anti-tumor effects of chemicals from Paeonia lutea leaf extract in oral squamous cell carcinoma cells. Anticancer. Res. 2021, 41, 6077–6086. [Google Scholar] [CrossRef]
- Chen, Y.; Lu, B.; Yang, Q.; Fearns, C.; Yates, J.R., 3rd; Lee, J.D. Combined integrin phosphoproteomic analyses and small interfering RNA--based functional screening identify key regulators for cancer cell adhesion and migration. Cancer Res. 2009, 69, 3713–3720. [Google Scholar] [CrossRef]
- Kurihara, M.; Mukudai, Y.; Watanabe, H.; Asakura, M.; Abe, Y.; Houri, A.; Chikuda, J.; Shimane, T.; Shirota, T. Autophagy prevents osteocyte cell death under hypoxic conditions. Cells Tissues Organs 2021, 210, 326–338. [Google Scholar] [CrossRef]
- Chen, H.; Pimienta, G.; Gu, Y.; Sun, X.; Hu, J.; Kim, M.S.; Chaerkady, R.; Gucek, M.; Cole, R.N.; Sukumar, S.; et al. Proteomic characterization of Her2/neu-overexpressing breast cancer cells. Proteomics 2010, 10, 3800–3810. [Google Scholar] [CrossRef]
- Crugliano, T.; Quaresima, B.; Gaspari, M.; Faniello, M.C.; Romeo, F.; Baudi, F.; Cuda, G.; Costanzo, F.; Venuta, S. Specific changes in the proteomic pattern produced by the BRCA1-Ser1841Asn missense mutation. Int. J. Biochem. Cell Biol. 2007, 39, 220–226. [Google Scholar] [CrossRef]
- Malek, R.L.; Irby, R.B.; Guo, Q.M.; Lee, K.; Wong, S.; He, M.; Tsai, J.; Frank, B.; Liu, E.T.; Quackenbush, J.; et al. Identification of Src transformation fingerprint in human colon cancer. Oncogene 2002, 21, 7256–7265. [Google Scholar] [CrossRef] [PubMed]
- Scanlan, M.J.; Gout, I.; Gordon, C.M.; Williamson, B.; Stockert, E.; Gure, A.O.; Jäger, D.; Chen, Y.T.; Mackay, A.; O’Hare, M.J.; et al. Humoral immunity to human breast cancer: Antigen definition and quantitative analysis of mRNA expression. Cancer Immun. 2001, 1, 4. [Google Scholar] [PubMed]
- Zhao, P.; Zhong, W.; Ying, X.; Yao, B.; Yuan, Z.; Fu, J.; Zhou, Z. Comparative proteomic analysis of anti-benzo(a)pyrene-7,8-dihydrodiol-9,10-epoxide-transformed and normal human bronchial epithelial G0/G1 cells. Chem. Biol. Interact. 2010, 186, 166–173. [Google Scholar] [CrossRef]
- Boutros, R.; Byrne, J.A. D53 (TPD52L1) is a cell cycle-regulated protein maximally expressed at the G2-M transition in breast cancer cells. Exp. Cell Res. 2005, 310, 152–165. [Google Scholar] [CrossRef] [PubMed]
- Boutros, R.; Fanayan, S.; Shehata, M.; Byrne, J.A. The tumor protein D52 family: Many pieces, many puzzles. Biochem. Biophys. Res. Commun. 2004, 325, 1115–1121. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Xu, J.; Mabjeesh, N.; Zhu, G.; Zhou, J.; Amin, M.; He, D.; Marshall, F.F.; Zhau, H.E.; Chung, L.W. PrLZ is expressed in normal prostate development and in human prostate cancer progression. Clin. Cancer Res. 2007, 13, 6040–6048. [Google Scholar] [CrossRef]
- Scheau, C.; Badarau, I.A.; Costache, R.; Caruntu, C.; Mihai, G.L.; Didilescu, A.C.; Constantin, C.; Neagu, M. The role of matrix metalloproteinases in the epithelial-mesenchymal transition of hepatocellular carcinoma. Anal. Cell. Pathol. 2019, 2019, 9423907. [Google Scholar] [CrossRef]
- Zanotelli, M.R.; Zhang, J.; Reinhart-King, C.A. Mechanoresponsive metabolism in cancer cell migration and metastasis. Cell Metab. 2021, 33, 1307–1321. [Google Scholar] [CrossRef]
- Di Nezza, L.A.; Jobling, T.; Salamonsen, L.A. Progestin suppresses matrix metalloproteinase production in endometrial cancer. Gynecol. Oncol. 2003, 89, 325–333. [Google Scholar] [CrossRef]
- Sasahira, T.; Kirita, T. Hallmarks of cancer-related newly prognostic factors of oral squamous cell carcinoma. Int. J. Mol. Sci. 2018, 19, 2413. [Google Scholar] [CrossRef]
- Aseervatham, J.; Ogbureke, K.U.E. Effects of DSPP and MMP20 silencing on adhesion, metastasis, angiogenesis, and epithelial-mesenchymal transition proteins in oral squamous cell carcinoma cells. Int. J. Mol. Sci. 2020, 21, 4734. [Google Scholar] [CrossRef] [PubMed]
- González-González, R.; Ortiz-Sarabia, G.; Molina-Frechero, N.; Salas-Pacheco, J.M.; Salas-Pacheco, S.M.; Lavalle-Carrasco, J.; López-Verdín, S.; Tremillo-Maldonado, O.; Bologna-Molina, R. Epithelial-mesenchymal transition associated with head and neck squamous cell carcinomas: A review. Cancers 2021, 13, 3027. [Google Scholar] [CrossRef] [PubMed]
- Boutros, R.; Bailey, A.M.; Wilson, S.H.; Byrne, J.A. Alternative splicing as a mechanism for regulating 14-3-3 binding: Interactions between hD53 (TPD52L1) and 14-3-3 proteins. J. Mol. Biol. 2003, 332, 675–687. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.L.; Zhang, X.K.; Halverson, D.O.; Byeon, M.K.; Schweinfest, C.W.; Ferris, D.K.; Bhat, N.K. Characterization of human N8 protein. Oncogene 1997, 15, 2577–2588. [Google Scholar] [CrossRef]
- Proux, V.; Provot, S.; Felder-Schmittbuhl, M.P.; Laugier, D.; Calothy, G.; Marx, M. Characterization of a leucine zipper-containing protein identified by retroviral insertion in avian neuroretina cells. J. Biol. Chem. 1996, 271, 30790–30797. [Google Scholar] [CrossRef]
- Proux-Gillardeaux, V.; Galli, T.; Callebaut, I.; Mikhailik, A.; Calothy, G.; Marx, M. D53 is a novel endosomal SNARE-binding protein that enhances interaction of syntaxin 1 with the synaptobrevin 2 complex in vitro. Biochem. J. 2003, 370, 213–221. [Google Scholar] [CrossRef]
- Sathasivam, P.; Bailey, A.M.; Crossley, M.; Byrne, J.A. The role of the coiled-coil motif in interactions mediated by TPD52. Biochem. Biophys. Res. Commun. 2001, 288, 56–61. [Google Scholar] [CrossRef]
- Wilson, S.H.; Bailey, A.M.; Nourse, C.R.; Mattei, M.G.; Byrne, J.A. Identification of MAL2, a novel member of the mal proteolipid family, though interactions with TPD52-like proteins in the yeast two-hybrid system. Genomics 2001, 76, 81–88. [Google Scholar] [CrossRef]
- Byrne, J.A.; Frost, S.; Chen, Y.; Bright, R.K. Tumor protein D52 (TPD52) and cancer—Oncogene understudy or understudied oncogene? Tumour Biol. 2014, 35, 7369–7382. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Watanabe, M.; Mukudai, Y.; Kindaichi, N.; Nara, M.; Yamada, K.; Abe, Y.; Houri, A.; Shimane, T.; Shirota, T. Tumor Protein D53 (TPD53): Involvement in Malignant Transformation of Low-Malignant Oral Squamous Cell Carcinoma Cells. Biomedicines 2024, 12, 2725. https://doi.org/10.3390/biomedicines12122725
Watanabe M, Mukudai Y, Kindaichi N, Nara M, Yamada K, Abe Y, Houri A, Shimane T, Shirota T. Tumor Protein D53 (TPD53): Involvement in Malignant Transformation of Low-Malignant Oral Squamous Cell Carcinoma Cells. Biomedicines. 2024; 12(12):2725. https://doi.org/10.3390/biomedicines12122725
Chicago/Turabian StyleWatanabe, Masataka, Yoshiki Mukudai, Nodoka Kindaichi, Maki Nara, Konomi Yamada, Yuzo Abe, Asami Houri, Toshikazu Shimane, and Tatsuo Shirota. 2024. "Tumor Protein D53 (TPD53): Involvement in Malignant Transformation of Low-Malignant Oral Squamous Cell Carcinoma Cells" Biomedicines 12, no. 12: 2725. https://doi.org/10.3390/biomedicines12122725
APA StyleWatanabe, M., Mukudai, Y., Kindaichi, N., Nara, M., Yamada, K., Abe, Y., Houri, A., Shimane, T., & Shirota, T. (2024). Tumor Protein D53 (TPD53): Involvement in Malignant Transformation of Low-Malignant Oral Squamous Cell Carcinoma Cells. Biomedicines, 12(12), 2725. https://doi.org/10.3390/biomedicines12122725






