State of Knowledge About Thyroid Cancers in the Era of COVID-19—A Narrative Review
Abstract
1. Introduction
2. Description of TCs
2.1. Papillary Cancer
2.2. Follicular Thyroid Carcinoma
2.3. Poorly Differentiated Thyroid Carcinoma
2.4. Anaplastic Thyroid Carcinoma
2.5. Medullary Thyroid Carcinoma
2.6. Other Malignant TCs
3. The Effect of COVID-19 on the Thyroid Gland
3.1. Impact of the COVID-19 Pandemic on Mortality Due to TC
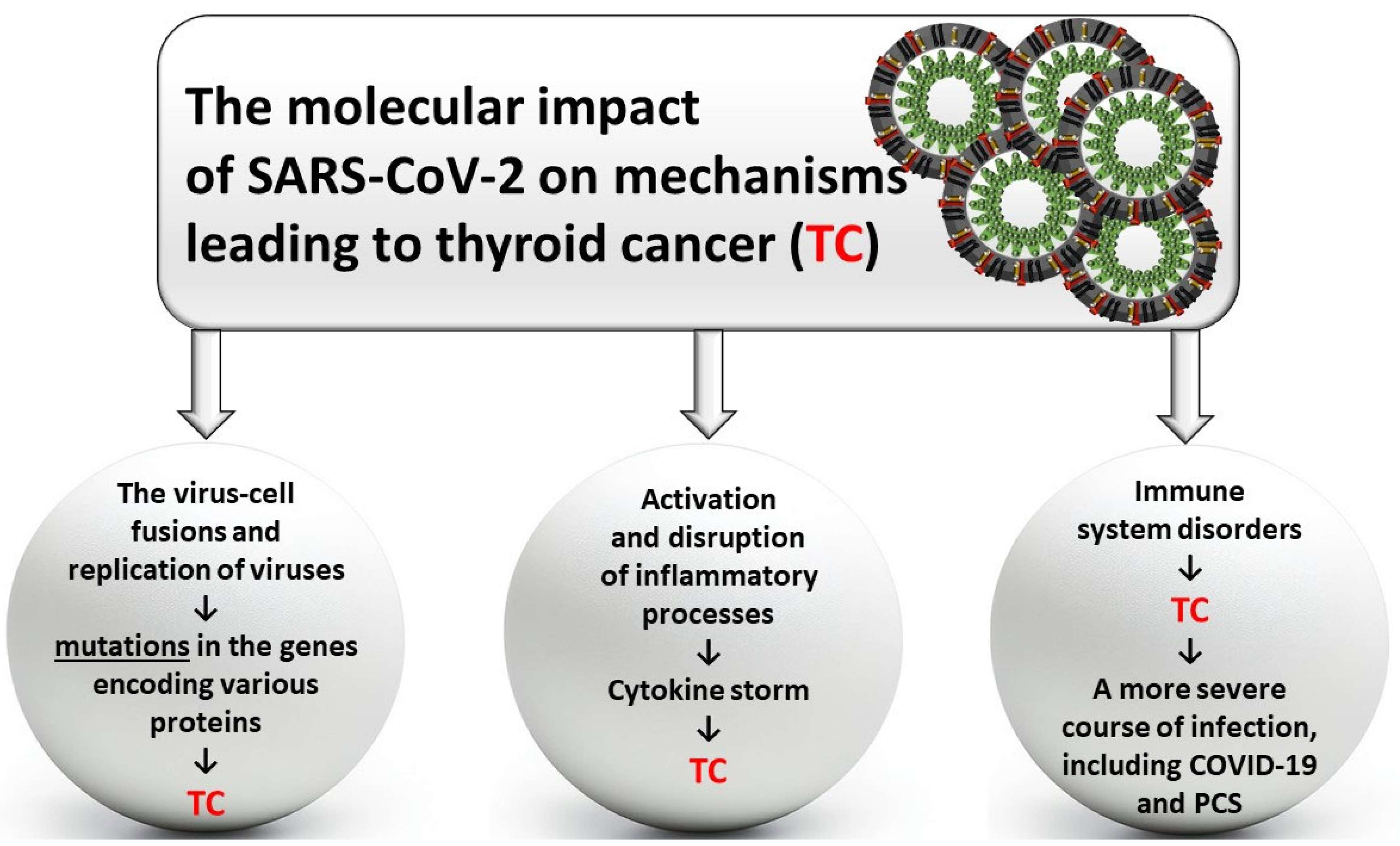
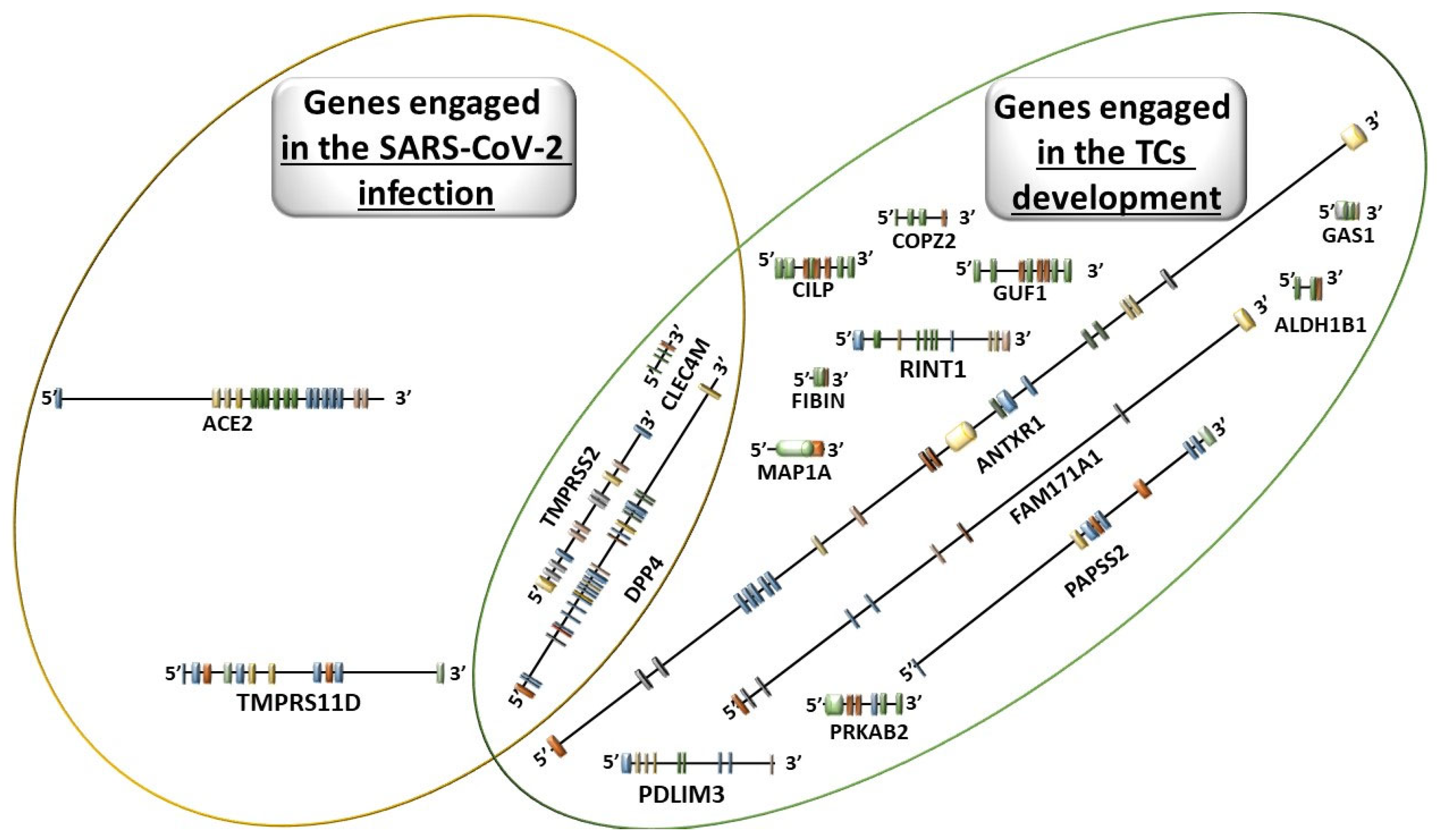
3.2. Molecular Disorders Induced by SARS-CoV-2
3.3. The Impact of COVID-19 and PCS on Thyroid Dysfunction
3.4. The Impact of Medications Used in the Treatment of Thyroid Diseases (Including Thyroid Cancer) in COVID-19
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
References
- Kakudo, K.; Bychkov, A.; Bai, Y.; Li, Y.; Liu, Z.; Jung, C.K. The new 4th edition World Health Organization classification for thyroid tumors, Asian perspectives. Pathol. Int. 2018, 68, 641–664. [Google Scholar] [CrossRef] [PubMed]
- Al Rasheed, M.R.H.; Xu, B. Molecular Alterations in Thyroid Carcinoma. Surg. Pathol. Clin. 2019, 12, 921–930. [Google Scholar] [CrossRef] [PubMed]
- Bronowicka-Szydełko, A.; Kotyra, Ł.; Lewandowski, Ł.; Gamian, A.; Kustrzeba-Wójcicka, I. Role of Advanced Glycation End-Products and Other Ligands for AGE Receptors in Thyroid Cancer Progression. J. Clin. Med. 2021, 10, 4084. [Google Scholar] [CrossRef] [PubMed]
- Takano, T. Natural history of thyroid cancer [Review]. Endocr. J. 2017, 64, 237–244. [Google Scholar] [CrossRef] [PubMed]
- Hlozek, J.; Pekova, B.; Rotnágl, J.; Holý, R.; Astl, J. Genetic Changes in Thyroid Cancers and the Importance of Their Preoperative Detection in Relation to the General Treatment and Determination of the Extent of Surgical Intervention—A Review. Biomedicines 2022, 10, 1515. [Google Scholar] [CrossRef]
- Baughn, L.B.; Sharma, N.; Elhaik, E.; Sekulic, A.; Bryce, A.H.; Fonseca, R. Targeting TMPRSS2 in SARS-CoV-2 Infection. Mayo Clin. Proc. 2020, 95, 1989–1999. [Google Scholar] [CrossRef]
- Gudima, G.; Kofiadi, I.; Shilovskiy, I.; Kudlay, D.; Khaitov, M. Antiviral Therapy of COVID-19. Int. J. Mol. Sci. 2023, 24, 8867. [Google Scholar] [CrossRef]
- Ibrahimpasic, T.; Ghossein, R.; Shah, J.P.; Ganly, I. Poorly Differentiated Carcinoma of the Thyroid Gland: Current Status and Future Prospects. Thyroid 2019, 29, 311–321. [Google Scholar] [CrossRef]
- Tsou, P.; Wu, C.-J. Mapping Driver Mutations to Histopathological Subtypes in Papillary Thyroid Carcinoma: Applying a Deep Convolutional Neural Network. J. Clin. Med. 2019, 8, 1675. [Google Scholar] [CrossRef]
- Ravella, L.; Lopez, J.; Descotes, F.; Giai, J.; Lapras, V.; Denier, M.-L.; Borson-Chazot, F.; Lifante, J.-C.; Decaussin-Petrucci, M. Preoperative Role of RAS or BRAF K601E in the Guidance of Surgery for Indeterminate Thyroid Nodules. World J. Surg. 2020, 44, 2264–2271. [Google Scholar] [CrossRef]
- Sahin, M.; Haymana, C.; Demirci, I.; Tasci, I.; Rıfat, E.; Unluturk, U.; Satman, I.; Demir, T.; Cakal, E.; Ata, N.; et al. The Clinical Outcomes of COVID-19 Infection in Patients with a History of Thyroid Cancer: A Nationwide Study. Clin. Endocrinol. 2021, 95, 628–637. [Google Scholar] [CrossRef] [PubMed]
- Hinnebusch, A.G.; Lorsch, J.R. The mechanism of eukaryotic translation initiation: New insights and challenges. Cold Spring Harb. Perspect. Biol. 2012, 4, a011544. [Google Scholar] [CrossRef] [PubMed]
- Krishnamoorthy, G.P.; Davidson, N.R.; Leach, S.D.; Zhao, Z.; Lowe, S.W.; Lee, G.; Landa, I.; Nagarajah, J.; Saqcena, M.; Singh, K.; et al. EIF1AX and RAS Mutations Cooperate to Drive Thyroid Tumorigenesis Through ATF4 and C-MYC. Cancer Discov. 2019, 9, 264–281. [Google Scholar] [CrossRef] [PubMed]
- Abe, I.; King-Yin Lam, A. Anaplastic Thyroid Carcinoma: Current Issues in Genomics and Therapeutics. Curr. Oncol. Rep. 2021, 23, 31. [Google Scholar] [CrossRef] [PubMed]
- Yoo, S.-K.; Song, Y.S.; Lee, E.K.; Hwang, J.; Kim, H.H.; Jung, G.; Kim, Y.A.; Kim, S.-J.; Cho, S.W.; Won, J.-K.; et al. Integrative Analysis of Genomic and Transcriptomic Characteristics Associated with Progression of Aggressive Thyroid Cancer. Nat. Commun. 2019, 10, 2764. [Google Scholar] [CrossRef]
- Li, Y.; Guo, L.; Ying, S.; Feng, G.H.; Zhang, Y. Transcriptional repression of p21 by EIF1AX promotes the proliferation of breast cancer cells. Cell Prolif. 2020, 53, e12903. [Google Scholar] [CrossRef]
- Fruman, D.A.; Chiu, H.; Hopkins, B.D.; Bagrodia, S.; Cantley, L.C.; Abraham, R.T. The PI3K Pathway in Human Disease. Cell 2017, 170, 605–635. [Google Scholar] [CrossRef]
- García-Rostán, G.; Costa, A.M.; Pereira-Castro, I.; Salvatore, G.; Hernandez, R.; Hermsem, M.J.A.; Herrero, A.; Fusco, A.; Cameselle-Teijeiro, J.; Santoro, M. Mutation of the PIK3CA Gene in Anaplastic Thyroid Cancer. Cancer Res. 2005, 65, 10199–10207. [Google Scholar] [CrossRef]
- Gammon, A.; Jasperson, K.; Champine, M. Genetic Basis of Cowden Syndrome and Its Implications for Clinical Practice and Risk Management. Appl. Clin. Genet. 2016, 9, 83–92. [Google Scholar] [CrossRef]
- Mirshahi, U.L.; Kim, J.; Best, A.F.; Chen, Z.E.; Hu, Y.; Haley, J.S.; Golden, A.; Stahl, R.; Manickam, K.; Carr, A.G.; et al. A Genome-First Approach to Characterize DICER1 Pathogenic Variant Prevalence, Penetrance, and Phenotype. JAMA Netw. Open 2021, 4, e210112. [Google Scholar] [CrossRef]
- Calebiro, D.; Grassi, E.S.; Eszlinger, M.; Ronchi, C.L.; Godbole, A.; Bathon, K.; Guizzardi, F.; De Filippis, T.; Krohn, K.; Jaeschke, H.; et al. Recurrent EZH1 Mutations Are a Second Hit in Autonomous Thyroid Adenomas. J. Clin. Investig. 2016, 126, 3383–3388. [Google Scholar] [CrossRef] [PubMed]
- Bouchard, J.J.; Otero, J.H.; Scott, D.C.; Szulc, E.; Martin, E.W.; Sabri, N.; Granata, D.; Marzahn, M.R.; Lindorff-Larsen, K.; Salvatella, X.; et al. Cancer Mutations of the Tumor Suppressor SPOP Disrupt the formation of Active, Phase-Separated Compartments. Mol. Cell 2018, 72, 19–36.e8. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.; Fried, J.; Li, Y.; Hu, L.; Gao, M.; Zhang, S.; Xu, B. Functional Roles of Speckle-Type Poz (SPOP) Protein in Genomic Stability. J. Cancer 2018, 9, 3257–3262. [Google Scholar] [CrossRef]
- Miasaki, F.Y.; Fuziwara, C.S.; de Carvalho, G.A.; Kimura, E.T. Genetic Mutations and Variants in the Susceptibility of Familial Non-Medullary Thyroid Cancer. Genes 2020, 11, 1364. [Google Scholar] [CrossRef]
- Haugen, B.R.; Alexander, E.K.; Bible, K.C.; Doherty, G.M.; Mandel, S.J.; Nikiforov, Y.E.; Pacini, F.; Randolph, G.W.; Sawka, A.M.; Schlumberger, M.; et al. 2015 American Thyroid Association Management Guidelines for Adult Patients with Thyroid Nodules and Differentiated Thyroid Cancer: The American Thyroid Association Guidelines Task force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid 2016, 26, 1–133. [Google Scholar] [CrossRef]
- Donehower, L.A.; Soussi, T.; Korkut, A.; Liu, Y.; Schultz, A.; Cardenas, M.; Li, X.; Babur, O.; Hsu, T.K.; Lichtarge, O.; et al. Integrated Analysis of TP53 Gene and Pathway Alterations in the Cancer Genome Atlas. Cell Rep. 2019, 28, 1370–1384.e5. [Google Scholar] [CrossRef]
- Valvo, V.; Nucera, C. Coding Molecular Determinants of Thyroid Cancer Development and Progression. Endocrinol. Metab. Clin. N. Am. 2019, 48, 37–59. [Google Scholar] [CrossRef]
- Dratwa, M.; Wysoczańska, B.; Łacina, P.; Kubik, T.; Bogunia-Kubik, K. TERT—Regulation and Roles in Cancer formation. Front. Immunol. 2020, 11, 589929. [Google Scholar] [CrossRef]
- Santoro, M.; Moccia, M.; Federico, G.; Carlomagno, F. RET Gene Fusions in Malignancies of the Thyroid and Other Tissues. Genes 2020, 11, 424. [Google Scholar] [CrossRef]
- Skálová, A.; Ptáková, N.; Santana, T.; Agaimy, A.; Ihrler, S.; Uro-Coste, E.; Thompson, L.D.R.; Bishop, J.A.; Baněčkova, M.; Rupp, N.J.; et al. NCOA4-RET and TRIM27-RET Are Characteristic Gene Fusions in Salivary Intraductal Carcinoma, Including Invasive and Metastatic Tumors: Is “Intraductal” Correct? Am. J. Surg. Pathol. 2019, 43, 1303–1313. [Google Scholar] [CrossRef]
- Raman, P.; Koenig, R.J. PAX8-PPARγ Fusion Protein in Thyroid Carcinoma. Nat. Rev. Endocrinol. 2014, 10, 616–623. [Google Scholar] [CrossRef]
- Nikiforova, M.N.; Lynch, R.A.; Biddinger, P.W.; Alexander, E.K.; Dorn, G.W.; Tallini, G.; Kroll, T.G.; Nikiforov, Y.E. RAS Point Mutations and PAX8-PPARγ Rearrangement in Thyroid Tumors: Evidence for Distinct Molecular Pathways in Thyroid Follicular Carcinoma. J. Clin. Endocrinol. Metab. 2003, 88, 2318–2326. [Google Scholar] [CrossRef] [PubMed]
- Di Magliano, M.P.; Di Lauro, R.; Zannini, M. Pax8 Has a Key Role in Thyroid Cell Differentiation. Proc. Natl. Acad. Sci. USA 2000, 97, 13144–13149. [Google Scholar] [CrossRef] [PubMed]
- Cameselle-Teijeiro, J.M.; Peteiro-González, D.; Caneiro-Gómez, J.; Sánchez-Ares, M.; Abdulkader, I.; Eloy, C.; Melo, M.; Amendoeira, I.; Soares, P.; Sobrinho-Simões, M. Cribriform-Morular Variant of Thyroid Carcinoma: A Neoplasm with Distinctive Phenotype Associated with the Activation of the WNT/Β-Catenin Pathway. Mod. Pathol. 2018, 31, 1168–1179. [Google Scholar] [CrossRef] [PubMed]
- Amendoeira, I.; Maia, T.; Sobrinho-Simões, M. Non-invasive Follicular Thyroid Neoplasm with Papillary-Like Nuclear Features (NIFTP): Impact on the Reclassification of Thyroid Nodules. Endocr.-Relat. Cancer 2018, 25, R247–R258. [Google Scholar] [CrossRef] [PubMed]
- Deng, X.; Wu, B.; Xiao, K.; Kang, J.; Xie, J.; Zhang, X.; Fan, Y. MiR-146b-5p Promotes Metastasis and Induces Epithelial-Mesenchymal Transition in Thyroid Cancer by Targeting ZNRF3. Cell. Physiol. Biochem. 2015, 35, 71–82. [Google Scholar] [CrossRef]
- Giordano, T.J. Follicular Cell Thyroid Neoplasia: Insights from Genomics and the Cancer Genome Atlas Research Network. Curr. Opin. Oncol. 2016, 28, 1–4. [Google Scholar] [CrossRef]
- Gonzalez-Gonzalez, R.; Bologna-Molina, R.; Carreon-Burciaga, R.G.; Gómezpalacio-Gastelum, M.; Molina-Frechero, N.; Salazar-Rodríguez, S. Papillary Thyroid Carcinoma: Differential Diagnosis and Prognostic Values of Its Different Variants: Review of the Literature. ISRN Oncol. 2011, 2011, 915925. [Google Scholar] [CrossRef]
- Zhu, Z.; Ciampi, R.; Nikiforova, M.N.; Gandhi, M.; Nikiforov, Y.E. Prevalence of RET/PTC Rearrangements in Thyroid Papillary Carcinomas: Effects of the Detection Methods and Genetic Heterogeneity. J. Clin. Endocrinol. Metab. 2006, 91, 3603–3610. [Google Scholar] [CrossRef]
- Nath, M.C.; Erickson, L.A. Aggressive Variants of Papillary Thyroid Carcinoma: Hobnail, Tall Cell, Columnar, and Solid. Adv. Anat. Pathol. 2018, 25, 172–179. [Google Scholar] [CrossRef]
- Kaliszewski, K.; Diakowska, D.; Wojtczak, B.; Rudnicki, J. Cancer Screening Activity Results in Overdiagnosis and Overtreatment of Papillary Thyroid Cancer: A 10-Year Experience at a Single Institution. PLoS ONE 2020, 15, e0236257. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Z.L.; Shen, C.T.; Sun, Z.K.; Wei, W.J.; Zhang, X.Y.; Song, H.J.; Luo, Q.Y. Circulating Long Non-Coding RNAs Act as Biomarkers for Predicting 131 I Uptake and Mortality in Papillary Thyroid Cancer Patients with Lung Metastases. Cell. Physiol. Biochem. 2016, 40, 1377–1390. [Google Scholar] [CrossRef] [PubMed]
- Levy, R.A.; Hui, V.W.; Sood, R.; Fish, S.; Markowitz, A.J.; Wong, R.J.; Guillem, J.G. Cribriform-Morular Variant of Papillary Thyroid Carcinoma: An Indication to Screen for Occult FAP. Fam. Cancer 2014, 13, 547–551. [Google Scholar] [CrossRef] [PubMed]
- Laforga, J.B.; Pedro, T.; Gasent, J.M. Pulmonary Metastasis of Cribriform-Morular Variant of Thyroid Carcinoma Mimicking Primary Adenocarcinoma of the Lung: A Potential Pitfall. Diagn. Cytopathol. 2020, 48, 78–81. [Google Scholar] [CrossRef]
- Rogers, W.A.; Craig, W.L.; Entwistle, V.A. Ethical issues raised by thyroid cancer overdiagnosis: A matter for public health? Bioethics 2017, 31, 590–598. [Google Scholar] [CrossRef]
- Pusztaszeri, M.P.; Krane, J.F.; Cibas, E.S.; Daniels, G.; Faquin, W.C. FNAB of benign thyroid nodules with papillary hyperplasia: A cytological and histological evaluation. Cancer Cytopathol. 2014, 122, 666–677. [Google Scholar] [CrossRef]
- Sherman, S.I. Thyroid Carcinoma. Lancet 2003, 361, 501–511. [Google Scholar] [CrossRef]
- Coca-Pelaz, A.; Shah, J.P.; Hernandez-Prera, J.C.; Ghossein, R.A.; Rodrigo, J.P.; Hartl, D.M.; Olsen, K.D.; Shaha, A.R.; Zafereo, M.; Suarez, C.; et al. Papillary Thyroid Cancer-Aggressive Variants and Impact on Management: A Narrative Review. Adv. Ther. 2020, 37, 3112–3128. [Google Scholar] [CrossRef]
- Kaliszewski, K.; Diakowska, D.; Rzeszutko, M.; Wojtczak, B.; Rudnicki, J. The Correlation of Age with Prognosis of Atypia of Undetermined Significance and Follicular Lesion of Undetermined Significance in Thyroid Nodules. Cancer Manag. Res. 2021, 13, 3101–3111. [Google Scholar] [CrossRef]
- Basolo, F.; Macerola, E.; Poma, A.M.; Torregrossa, L. The 5th Edition of WHO Classification of Tumors of Endocrine Organs: Changes in the Diagnosis of Follicular-Derived Thyroid Carcinoma. Endocrine 2023, 80, 470–476. [Google Scholar] [CrossRef]
- Hawasli, A.; Rizzo, P.; Khoury, H.; McCaffrey, J.L. Can Fine-Needle Aspiration Biopsy of Thyroid Nodule Help in Determining the Extent of Surgery in Follicular and Hurthle Cell Neoplasm at a Community Teaching Institution? Am. Surg. 2002, 68, 907–910. [Google Scholar] [CrossRef]
- Tian, P.; Du, W.; Liu, X.; Ding, Y.; Zhang, Z.; Li, J.; Wang, Y. Ultrasonographic Characteristics of Thyroid Nodule Rupture After Microwave Ablation. Medicine 2021, 100, e25070. [Google Scholar] [CrossRef] [PubMed]
- McCaffrey, J.C. Aerodigestive Tract Invasion by Well-Differentiated Thyroid Carcinoma: Diagnosis, Management, Prognosis, and Biology. Laryngoscope 2006, 116, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Gupta, N.; Dasyam, A.K.; Carty, S.E.; Nikiforova, M.N.; Ohori, N.P.; Armstrong, M.; Yip, L.; LeBeau, S.O.; McCoy, K.L.; Coyne, C.; et al. RAS Mutations in Thyroid FNA Specimens Are Highly Predictive of Predominantly Low-Risk Follicular-Pattern Cancers. J. Clin. Endocrinol. Metab. 2013, 98, E914–E922. [Google Scholar] [CrossRef] [PubMed]
- Kahn, N.F.; Perzin, K.H. Follicular Carcinoma of the Thyroid: An Evaluation of the Histologic Criteria Used for Diagnosis. Pathol. Annu. 1983, 18 Pt 1, 221–253. [Google Scholar]
- O’Neill, J.P.; Shaha, A.R. Anaplastic thyroid cancer. Oral Oncol. 2013, 49, 702–706. [Google Scholar] [CrossRef]
- Zheng, X.; Jia, B.; Tian, X.T.; Song, X.; Wu, M.L.; Kong, Q.Y.; Li, H.; Liu, J. Correlation of reactive oxygen species levels with resveratrol sensitivities of anaplastic thyroid cancer cells. Oxid. Med. Cell. Longev. 2018, 2018, 6235417. [Google Scholar] [CrossRef]
- Thyroid Gland Undifferentiated (Anaplastic) Carcinoma (Concept Id: C0238461)—MedGen—NCBI. Available online: https://www.ncbi.nlm.nih.gov/medgen/116064 (accessed on 10 March 2021).
- Yu, X.M.; Jaskula-Sztul, R.; Ahmed, K.; Harrison, A.D.; Kunnimalaiyaan, M.; Chen, H. Resveratrol Induces differentiation markers expression in anaplastic thyroid carcinoma via activation of Notch1 signaling and suppresses cell growth. Mol. Cancer Ther. 2013, 12, 1276–1287. [Google Scholar] [CrossRef]
- Sobrinho-Simões, M.; Máximo, V.; Rocha, A.S.; Trovisco, V.; Castro, P.; Preto, A.; Lima, J.; Soares, P. Intragenic Mutations in Thyroid Cancer. Endocrinol. Metab. Clin. N. Am. 2008, 37, 333–362. [Google Scholar] [CrossRef]
- Lai, W.A.; Hang, J.F.; Liu, C.Y.; Bai, Y.; Liu, Z.; Gu, H.; Hong, S.W.; Pyo, J.Y.; Jung, C.K.; Kakudo, K.; et al. PAX8 expression in anaplastic thyroid carcinoma is less than those reported in early studies: A multi-institutional study of 182 cases using the monoclonal antibody MRQ-50. Virchows Arch. 2020, 476, 431–437. [Google Scholar] [CrossRef]
- Yin, Y.; Hong, S.; Yu, S.; Huang, Y.; Chen, S.; Liu, Y.; Zhang, Q.; Li, Y.; Xiao, H. MiR-195 Inhibits Tumor Growth and Metastasis in Papillary Thyroid Carcinoma Cell Lines by Targeting CCND1 and FGF2. Int. J. Endocrinol. 2017, 2017, 6180425. [Google Scholar] [CrossRef]
- Gertner, M.E.; Kebebew, E. Multiple endocrine neoplasia type 2. Curr. Treat. Options Oncol. 2004, 5, 315–325. [Google Scholar] [CrossRef] [PubMed]
- Gimm, O. Thyroid Cancer. Cancer Lett. 2001, 163, 143–156. [Google Scholar] [CrossRef] [PubMed]
- Al-Rawi, M.; Wheeler, M.H. Medullary Thyroid Carcinoma—Update and Present Management Controversies. Ann. R. Coll. Surg. Engl. 2006, 88, 433–438. [Google Scholar] [CrossRef] [PubMed]
- Kebebew, E.; Ituarte, P.H.G.; Siperstein, A.E.; Duh, Q.Y.; Clark, O.H. Medullary Thyroid Carcinoma: Clinical Characteristics, Treatment, Prognostic Factors, and a Comparison of Staging Systems. Cancer 2000, 88, 1139–1148. [Google Scholar] [CrossRef]
- Clayman, G.L.; El-Baradie, T.S. Medullary thyroid cancer. Otolaryngol. Clin. N. Am. 2003, 36, 91–105. [Google Scholar] [CrossRef]
- Viola, D.; Elisei, R. Management of Medullary Thyroid Cancer. Endocrinol. Metab. Clin. N. Am. 2019, 48, 285–301. [Google Scholar] [CrossRef]
- Hoff, A.O.; Hoff, P.M. Medullary Thyroid Carcinoma. Hematol./Oncol. Clin. N. Am. 2007, 21, 475–488. [Google Scholar] [CrossRef]
- Greco, C.; Brigante, G.; Taliani, E.; Corrado, S.; Simoni, M.; Madeo, B. Concomitant Medullary Thyroid Carcinoma with Paraganglioma-Like Pattern and Papillary Thyroid Carcinoma. Endocrinol. Diabetes Metab. Case Rep. 2019, 2019, 19-0094. [Google Scholar] [CrossRef]
- Matias-Guiu, X. Mixed Medullary and Follicular Carcinoma of the Thyroid: On the Search for Its Histogenesis. Am. J. Pathol. 1999, 155, 1413–1418. [Google Scholar] [CrossRef]
- Owen, C.; Constantinidou, A.; Miah, A.B.; Thway, K.; Fisher, C.; Benson, C.; Zaidi, S.; Messiou, C.; Van Der Graaf, W.T.A.; Jones, R.L. Synovial Sarcoma of the Thyroid Gland, Diagnostic Pitfalls and Clinical Management. Anticancer Res. 2018, 38, 5275–5282. [Google Scholar] [CrossRef]
- Cho, J.K.; Woo, S.H.; Park, J.; Kim, M.J.; Jeong, H.S. Primary Squamous Cell Carcinomas in the Thyroid Gland: An Individual Participant Data Meta-Analysis. Cancer Med. 2014, 3, 1396–1403. [Google Scholar] [CrossRef] [PubMed]
- Son, D.H.; Roh, J.L.; Cho, K.J. Combined Squamous Cell Carcinoma and Follicular Carcinoma of the Thyroid. Korean J. Pathol. 2014, 48, 418–422. [Google Scholar] [CrossRef] [PubMed]
- Raggio, B.; Barr, J.; Ghandour, Z.; Friedlander, P. Primary Squamous Cell Carcinoma of the Thyroid. Ochsner J. 2019, 19, 290–292. [Google Scholar] [CrossRef] [PubMed]
- Vrinceanu, D.; Dumitru, M.; Marinescu, A.; Serboiu, C.; Musat, G.; Radulescu, M.; Popa-Cherecheanu, M.; Ciornei, C.; Manole, F. Management of Giant Thyroid Tumors in Patients with Multiple Comorbidities in a Tertiary Head and Neck Surgery Center. Biomedicines 2024, 12, 2204. [Google Scholar] [CrossRef]
- Chablani, S.V.; Sabra, M.M. Thyroid Cancer and Telemedicine During the COVID-19 Pandemic. J. Endocr. Soc. 2021, 5, bvab059. [Google Scholar] [CrossRef]
- Medas, F.; Ansaldo, G.L.; Avenia, N.; Basili, G.; Bononi, M.; Bove, A.; Carcoforo, P.; Casaril, A.; Cavallaro, G.; Conzo, G.; et al. Impact of the COVID-19 Pandemic on Surgery for Thyroid Cancer in Italy: Nationwide Retrospective Study. Br. J. Surg. 2021, 108, E166–E167. [Google Scholar] [CrossRef]
- Zhang, D.; Fu, Y.; Zhou, L.; Liang, N.; Wang, T.; Del Rio, P.; Rausei, S.; Boni, L.; Park, D.; Jafari, J.; et al. Thyroid Surgery During Coronavirus-19 Pandemic Phases I, II and III: Lessons Learned in China, South Korea, Iran and Italy. J. Endocrinol. Investig. 2021, 44, 1065–1073. [Google Scholar] [CrossRef]
- Tsang, V.H.M.; Gild, M.; Glover, A.; Clifton-Bligh, R.; Robinson, B.G. Thyroid Cancer in the Age of COVID-19. Endocr.-Relat. Cancer 2020, 27, R407–R416. [Google Scholar] [CrossRef]
- Duntas, L.H.; Jonklaas, J. COVID-19 and Thyroid Diseases: A Bidirectional Impact. J. Endocr. Soc. 2021, 5, bvab076. [Google Scholar] [CrossRef]
- Forner, D.; Murnaghan, S.; Porter, G.; Mason, R.J.; Hong, P.; Taylor, S.M.; Bentley, J.; Hirsch, G.; Noel, C.W.; Rigby, M.H.; et al. Psychosocial Distress in Adult Patients Awaiting Cancer Surgery during the COVID-19 Pandemic. Curr. Oncol. 2021, 28, 1867–1878. [Google Scholar] [CrossRef]
- Nocini, R.; Lippi, G.; Mattiuzzi, C. No Impact of COVID-19 Pandemic on Early Mortality for Thyroid Cancer in the US. Comment on Lee et al. Impact of the COVID-19 Pandemic on Thyroid Cancer Surgery. Curr. Oncol. 2024, 31, 6267–6269. [Google Scholar] [CrossRef] [PubMed]
- Anupiya, F.; Doshi, P.K.; Vora, N.; Parekh, B.; Soundarrajan, S.; Kasagga, A.; Iffath, F.; Ahmed, M. Disparities in the Place of Death for Patients With Malignant Neoplasms of the Thyroid Gland. Cureus 2024, 16, e55506. [Google Scholar] [CrossRef]
- Kathuria-Prakash, N.; Mosaferi, T.; Xie, M.; Antrim, L.; Angell, T.E.; In, G.K.; Su, M.A.; Lechner, M.G. COVID-19 Outcomes of Patients with Differentiated Thyroid Cancer: A Multicenter Los Angeles Cohort Study. Endocr. Pract. 2021, 27, 90–94. [Google Scholar] [CrossRef] [PubMed]
- Shahzad, H.; Khokhar, A.J.; Ibrahim, S.; U Farooq, M.; Rashid, R.; U Raheem, H.; Singh, G. High Procalcitonin Does Not Always Indicate a Bacterial Infection. Cureus 2024, 16, e72274. [Google Scholar] [CrossRef] [PubMed]
- Saha, A.; Mukhopadhyay, M.; Paul, S.; Bera, A.; Bandyopadhyay, T. Incidental diagnosis of medullary thyroid carcinoma due to persistently elevated procalcitonin in a patient with COVID-19 pneumonia: A case report. World J. Clin. Cases 2022, 10, 7171–7177. [Google Scholar] [CrossRef]
- Gianotti, L.; D’Agnano, S.; Pettiti, G.; Tassone, F.; Giraudo, G.; Lauro, C.; Lauria, G.; Del Bono, V.; Borretta, G. Persistence of Elevated Procalcitonin in a Patient with Coronavirus Disease 2019 Uncovered a Diagnosis of Medullary Thyroid Carcinoma. AACE Clin. Case Rep. 2021, 7, 288–292. [Google Scholar] [CrossRef]
- Sira, L.; Balogh, Z.; Vitális, E.; Kovács, D.; Győry, F.; Molnár, C.; Bodor, M.; Nagy, E.V. Case Report: Medullary Thyroid Cancer Workup Initiated by Unexpectedly High Procalcitonin Level-Endocrine Training Saves Life in the COVID-19 Unit. Front. Endocrinol. 2021, 12, 727320. [Google Scholar] [CrossRef]
- Ashrafi, S.; Hatami, H.; Bidhendi-Yarandi, R.; Panahi, M.H. The Prevalence of Thyroid Disorders in COVID-19 Patients: A Systematic Review and Meta-Analysis. BMC Endocr. Disord. 2024, 24, 5. [Google Scholar] [CrossRef]
- Lui, D.T.W.; Lee, C.H.; Chow, W.S.; Lee, A.C.H.; Tam, A.R.; Fong, C.H.Y.; Law, C.Y.; Leung, E.K.H.; To, K.K.W.; Tan, K.C.B.; et al. Thyroid Dysfunction in Relation to Immune Profile, Disease Status and Outcome in 191 Patients with COVID-19. J. Clin. Endocrinol. Metab. 2021, 106, E926–E935. [Google Scholar] [CrossRef]
- Liang, W.; Guan, W.; Chen, R.; Wang, W.; Li, J.; Xu, K.; Li, C.; Ai, Q.; Lu, W.; Liang, H.; et al. Cancer Patients in SARS-Cov-2 Infection: A Nationwide Analysis in China. Lancet Oncol. 2020, 21, 335. [Google Scholar] [CrossRef]
- Dworakowska, D.; Morley, S.; Mulholland, N.; Grossman, A.B. COVID-19 related thyroiditis: A novel disease entity? Clin. Endocrinol. 2021, 95, 369–377. [Google Scholar] [CrossRef]
- Raman, B.; Bluemke, D.A.; Lüscher, T.F.; Neubauer, S. Long COVID: Post-Acute Sequelae of COVID-19 with A Cardiovascular Focus. Eur. Heart J. 2022, 43, 1157–1172. [Google Scholar] [CrossRef] [PubMed]
- Abramczyk, U.; Nowaczyński, M.; Słomczyński, A.; Wojnicz, P.; Zatyka, P.; Kuzan, A. Consequences of COVID-19 for the Pancreas. Int. J. Mol. Sci. 2022, 23, 864. [Google Scholar] [CrossRef] [PubMed]
- Bronowicka-Szydełko, A.; Gostomska-Pampuch, K.; Kuzan, A.; Pietkiewicz, J.; Krzystek-Korpacka, M.; Gamian, A. Effect of Advanced Glycation End-Products in A Wide Range of Medical Problems Including COVID-19. Adv. Med. Sci. 2024, 69, 36–50. [Google Scholar] [CrossRef] [PubMed]
- Bansal, R.; Gubbi, S.; Koch, C.A. COVID-19 and Chronic Fatigue Syndrome: An Endocrine Perspective. J. Clin. Transl. Endocrinol. 2022, 27, 100284. [Google Scholar] [CrossRef]
- Sudre, C.H.; Murray, B.; Varsavsky, T.; Graham, M.S.; Penfold, R.S.; Bowyer, R.C.; Pujol, J.C.; Klaser, K.; Antonelli, M.; Canas, L.S.; et al. Attributes and Predictors of Long COVID. Nat. Med. 2021, 27, 626–631. [Google Scholar] [CrossRef]
- Raveendran, A.V.; Misra, A. Post COVID-19 Syndrome (“Long COVID”) and Diabetes: Challenges in Diagnosis and Management. Diabetes Metab. Syndr. 2021, 15, 102235. [Google Scholar] [CrossRef]
- Fiodorenko-Dumas, Z.; Dumas, I.; Rabczynski, M.; Małecki, R.; Adamiec, R.; Paprocka-Borowicz, M. Lack of Evidence of the Correlation Between Plasma Asymmetrical Dimethylarginine Correlation and IMT in Type 2 Diabetic Patients with Chronic Vascular Complication. Acta Biochim. Pol. 2021, 68, 143–149. [Google Scholar] [CrossRef]
- Fiodorenko-Dumas, Z.; Dumas, I.; Mastej, K.; Adamiec, R. Physical Activity—Related Changes in ADMA and Vwf Levels in Patients with Type 2 Diabetes: A Preliminary Study. Adv. Clin. Exp. Med. 2017, 26, 601–608. [Google Scholar] [CrossRef]
- Korompoki, E.; Gavriatopoulou, M.; Hicklen, R.S.; Stathopoulos, I.N.; Kastritis, E.; Fotiou, D.; Stamatelopoulos, K.; Terpos, E.; Kotanidou, A.; Hagberg, C.A.; et al. Epidemiology and Organ Specific Sequelae of Post-Acute COVID19: A Narrative Review. J. Infect. 2021, 83, 1–16. [Google Scholar] [CrossRef]
- Qu, N.; Hui, Z.; Shen, Z.; Kan, C.; Hou, N.; Sun, X.; Han, F. Thyroid Cancer and COVID-19: Prospects for Therapeutic Approaches and Drug Development. Front. Endocrinol. 2022, 3, 873027. [Google Scholar] [CrossRef]
- Li, M.-Y.; Li, L.; Zhang, Y.; Wang, X.-S. Expression of the SARS-Cov-2 Cell Receptor Gene ACE2 in a Wide Variety of Human Tissues. Infect. Dis. Poverty 2020, 9, 23–29. [Google Scholar] [CrossRef] [PubMed]
- Bronowicka-Szydełko, A.; Lewandowski, Ł.; Lubieniecki, P.; Adamiec-Mroczek, J.; Doroszko, A.; Trocha, M.; Kujawa, K.; Matera-Witkiewicz, A.; Rabczyński, M.; Kuźnik, E.; et al. Pre-Hospital Oxygen Therapy and Saturation Variability in COVID-19 Patients with and without Glucose Metabolism Disorders: Part of the COLOS Study. Sci. Rep. 2024, 14, 19286. [Google Scholar] [CrossRef]
- Lewandowski, Ł.; Bronowicka-Szydełko, A.; Rabczyński, M.; Bednarska-Chabowska, D.; Adamiec-Mroczek, J.; Doroszko, A.; Trocha, M.; Kujawa, K.; Matera-Witkiewicz, A.; Kuźnik, E.; et al. Insulin and Metformin Administration: Unravelling the Multifaceted Association with Mortality across Various Clinical Settings Considering Type 2 Diabetes Mellitus and COVID-19. Biomedicines 2024, 12, 605. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.I.; Thomas, C.; O’Connor, H.; Dowker, F.; Horgan, L.; Khan, M.A. Impact of the COVID-19 Pandemic on the Management of Gallbladder, Biliary Tract, and Pancreatic Diseases. Cureus 2023, 15, e43473. [Google Scholar] [CrossRef]
- Paleczny, B.; Seredyński, R.; Wyciszkiewicz, M.; Nowicka-Czudak, A.; Łopusiewicz, W.; Adamiec, D.; Wiecha, S.; Mroczek, D.; Chmura, P.; Konefał, M.; et al. Low Ventilatory Responsiveness To Transient Hypoxia Or Breath-Holding Predicts Fast Marathon Performance in Healthy Middle-Aged and Older Men. Sci. Rep. 2021, 11, 10255. [Google Scholar] [CrossRef]
- Seredyński, R.; Pawłowska-Seredyńska, K.; Ponikowska, B.; Paleczny, B. Acute Effects of Increased Gut Microbial Fermentation on the Hypoxic Ventilatory Response in Humans. Exp. Physiol. 2021, 106, 748–758. [Google Scholar] [CrossRef]
- Pergolizzi, J.; LeQuang, J.A.K.; Breve, F.; Magnusson, P.M.; Varrassi, G. Exploring the Implications of New-Onset Diabetes in COVID-19: A Narrative Review. Cureus 2023, 15, e33319. [Google Scholar] [CrossRef]
- Lam-Chung, C.E.; Elizondo Ochoa, Á.; Segura, K.Y.; Silva-Serrano, J.; Tusié Luna, M.T.; Almeda-Valdes, P. Differentiating Among Type 1, Type 2 Diabetes, and MODY: Raising Awareness About the Clinical Implementation of Genetic Testing in Latin America. AACE Clin. Case Rep. 2020, 7, 138–140. [Google Scholar] [CrossRef]
- Chudek, J.; Kolonko, A.; Ziaja, J.; Francuz, T.; Kamińska, D.; Owczarek, A.J.; Kuczera, P.; Kujawa-Szewieczek, A.; Kusztal, M.; Kowalik, A.P.; et al. Beneficial Effect of Successful Simultaneous Pancreas-Kidney Transplantation on Plasma Profile of Metalloproteinases in Type 1 Diabetes Mellitus Patients. J. Clin. Med. 2021, 10, 3800. [Google Scholar] [CrossRef]
- Salmi, H.; Heinonen, S.; Hästbacka, J.; Lääper, M.; Rautiainen, P.; Miettinen, P.J.; Vapalahti, O.; Hepojoki, J.; Knip, M. New-Onset Type 1 Diabetes in Finnish Children During the COVID-19 Pandemic. Arch. Dis. Child. 2022, 107, 180–185. [Google Scholar] [CrossRef]
- Rahmati, M.; Yon, D.K.; Lee, S.W.; Udeh, R.; McEVoy, M.; Kim, M.S.; Gyasi, R.M.; Oh, H.; Sánchez, G.F.L.; Jacob, L.; et al. New-Onset Type 1 Diabetes in Children and Adolescents As Postacute Sequelae of SARS-Cov-2 Infection: A Systematic Review and Meta-Analysis of Cohort Studies. J. Med. Virol. 2023, 95, e28833. [Google Scholar] [CrossRef] [PubMed]
- Rabczyński, M.; Chwałek, S.; Adamiec-Mroczek, J.; Lewandowski, Ł.; Trocha, M.; Nowak, B.; Misiuk-Hojło, M.; Bednarska-Chabowska, D.; Kuźnik, E.; Lubieniecki, P.; et al. Neutrophil Elastase, Neuron-Specific Enolase, and S100B Protein as Potential Markers of Long-Term Complications Caused by COVID-19 in Patients with Type 2 Diabetes Mellitus (T2DM) and Advanced Stage of Diabetic Nephropathy (NfT2DM)-Observational Studies. Int. J. Mol. Sci. 2024, 25, 11791. [Google Scholar] [CrossRef] [PubMed]
- Darvishi, M.; Nazer, M.R.; Shahali, H.; Nouri, M. Association of Thyroid Dysfunction and COVID-19: A Systematic Review And Meta-Analysis. Front. Endocrinol. 2022, 13, 947594. [Google Scholar] [CrossRef]
- Facchiano, A.; Facchiano, F.; Facchiano, A. An Investigation into the Molecular Basis of Cancer Comorbidities in Coronavirus Infection. FEBS Open Bio 2020, 10, 2363–2374. [Google Scholar] [CrossRef] [PubMed]
- Șandru, F.; Carsote, M.; Petca, R.C.; Gheorghisan-Galateanu, A.A.; Petca, A.; Valea, A.; Dumitrașcu, M.C. COVID-19-Related Thyroid Conditions (Review). Exp. Ther. Med. 2021, 22, 756. [Google Scholar] [CrossRef]
- Hu, A.; Cao, F.-F.; Lu, D.; Yang, L.-Y. The Role of T2E Mediated CBF-1/RBP-Jκ Signaling in Metastatic Thyroid Cancer. Am. J. Transl. Res. 2021, 13, 7610–7621. [Google Scholar]
- Kuzan, A.; Królewicz, E.; Nowakowska, K.; Stach, K.; Kaliszewski, K.; Domosławski, P.; Kotyra, Ł.; Gamian, A.; Kustrzeba-Wójcicka, I. Contribution of Glycation and Oxidative Stress to Thyroid Gland Pathology—A Pilot Study. Biomolecules 2021, 11, 557. [Google Scholar] [CrossRef]
- Rossini, A.; Cassibba, S.; Perticone, F.; Benatti, S.V.; Venturelli, S.; Carioli, G.; Ghirardi, A.; Rizzi, M.; Barbui, T.; Trevisan, R.; et al. Increased Prevalence of Autoimmune Thyroid Disease After COVID-19: A Single-Center, Prospective Study. Front. Endocrinol. 2023, 14, 1126683. [Google Scholar] [CrossRef]
- Dai, M.; Liu, D.; Liu, M.; Zhou, F.; Li, G.; Chen, Z.; Zhang, Z.; You, H.; Wu, M.; Zheng, Q.; et al. Patients with Cancer Appear More Vulnerable to SARS-CoV-2: A Multicenter Study during the COVID-19 Outbreak. Cancer Discov. 2020, 10, 783–791. [Google Scholar] [CrossRef]
- Han, T.; Kang, J.; Li, G.; Ge, J.; Gu, J. Analysis of 2019-nCoV receptor ACE2 expression in different tissues and its significance study. Ann. Transl. Med. 2020, 8, 1077. [Google Scholar] [CrossRef]
- Murugan, A.K.; Alzahrani, A.S. SARS-CoV-2: Emerging Role in the Pathogenesis of Various Thyroid Diseases. J. Inflamm. Res. 2021, 14, 6191–6221. [Google Scholar] [CrossRef]
- Pramono, L.A. COVID-19 and Thyroid Diseases: How the Pandemic Situation Affects Thyroid Disease Patients. J. ASEAN Fed. Endocr. Soc. 2020, 35, 155–157. [Google Scholar] [CrossRef] [PubMed]
- Giovanella, L.; Ruggeri, R.M.; Ovčariček, P.P.; Campenni, A.; Treglia, G.; Deandreis, D. Prevalence of Thyroid Dysfunction in Patients with COVID-19: A Systematic Review. Clin. Transl. Imaging 2021, 9, 233–240. [Google Scholar] [CrossRef] [PubMed]
- Clausen, C.L.; Rasmussen, A.K.; Johannsen, T.H.; Hilsted, L.M.; Skakkebæk, N.E.; Szecsi, P.B.; Pedersen, L.; Benfield, T.; Juul, A. Thyroid Function in COVID-19 and the Association with Cytokine Levels and Mortality. Endocr. Connect. 2021, 10, 1234–1242. [Google Scholar] [CrossRef] [PubMed]
- Attiq, A.; Afzal, S.; Wahab, H.A.; Ahmad, W.; Kandeel, M.; Almofti, Y.A.; Alameen, A.O.; Wu, Y.S. Cytokine Storm-induced Thyroid Dysfunction in COVID-19: Insights into Pathogenesis and Therapeutic Approaches. Drug Des. Dev. Ther. 2024, 18, 4215–4240. [Google Scholar] [CrossRef]
- Aslani, M.; Mortazavi-Jahromi, S.S.; Mirshafiey, A. Cytokine Storm in the Pathophysiology of COVID-19: Possible Functional Disturbances of miRNAs. Int. Immunopharmacol. 2021, 101 Pt A, 108172. [Google Scholar] [CrossRef]
- Speer, G.; Somogyi, P. Thyroid Complications of SARS and Coronavirus Disease 2019 (COVID-19). Endocr. J. 2021, 68, 129–136. [Google Scholar] [CrossRef]
- Feghali, K.; Atallah, J.; Norman, C. Manifestations of thyroid disease post COVID-19 illness: Report of Hashimoto thyroiditis, Graves’ disease, and subacute thyroiditis. J. Clin. Transl. Endocrinol. Case Rep. 2021, 22, 100094. [Google Scholar] [CrossRef]
- Lee, I.-C.; Huo, T.-I.; Huang, Y.-H. Gastrointestinal and liver manifestations in patients with COVID-19. J. Chin. Med. Assoc. 2020, 83, 521–523. [Google Scholar] [CrossRef]
- Sun, L.; Surya, S.; Le, A.N.; Desai, H.; Doucette, A.; Gabriel, P.; Ritchie, M.D.; Rader, D.; Maillard, I.; Bange, E.; et al. Rates of COVID-19-Related Outcomes in Cancer Compared with Noncancer Patients. JNCI Cancer Spectr. 2021, 5, Pkaa120. [Google Scholar] [CrossRef]
- Davis, P.J.; Lin, H.-Y.; Hercbergs, A.; Keating, K.A.; Mousa, S.A. Coronaviruses and Integrin αvβ3: Does Thyroid Hormone Modify the Relationship? Endocr. Res. 2020, 45, 210–215. [Google Scholar] [CrossRef]
- Prete, A.; Falcone, M.; Bottici, V.; Giani, C.; Tiseo, G.; Agate, L.; Matrone, A.; Cappagli, V.; Valerio, L.; Lorusso, L.; et al. Thyroid cancer and COVID-19: Experience at one single thyroid disease referral center. Endocrine 2021, 72, 332–339. [Google Scholar] [CrossRef] [PubMed]
- Smulever, A.; Abelleira, E.; Bueno, F.; Pitoia, F. Thyroid Cancer in the Era of COVID-19. Endocrine 2020, 70, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Rifat, M.A.; Arefin, M.K.; Fakir, A.Y.; Rumi, S.N.F.; Osmany, H.Q.; Roctim, H.R.; Chowdhury, N.; Habib, S.H.R.; Chowdhury, I.A.; Basu, K.C. COVID-19 Pandemic and Thyroid Cancer Treatment in Perspective of Bangladesh. J. Dhaka Med. Coll. 2020, 29, 178–181. [Google Scholar] [CrossRef]
- Zhang, C.; Jin, H.; Wen, Y.F.; Yin, G. Efficacy of COVID-19 Treatments: A Bayesian Network Meta-Analysis of Randomized Controlled Trials. Front. Public Health 2021, 9, 729559. [Google Scholar] [CrossRef] [PubMed]
- Schlesinger, N.; Firestein, B.L.; Brunetti, L. Colchicine in COVID-19: An Old Drug, New Use. Curr. Pharmacol. Rep. 2020, 6, 137–145. [Google Scholar] [CrossRef]
- Zhang, L.; Yang, Z.; Granieri, L.; Pasculescu, A.; Datti, A.; Asa, S.L.; Xu, Z.; Ezzat, S. High-Throughput Drug Library Screening Identifies Colchicine as A Thyroid Cancer Inhibitor. Oncotarget 2016, 7, 19948–19959. [Google Scholar] [CrossRef]
- Puhl, A.C.; Gomes, G.F.; Damasceno, S.; Fritch, E.J.; Levi, J.A.; Johnson, N.J.; Scholle, F.; Premkumar, L.; Hurst, B.L.; LeeMontiel, F. Vandetanib Reduces Inflammatory Cytokines and Ameliorates COVID-19 in Infected Mice. bioRxiv Prepr. 2021. [Google Scholar] [CrossRef]
- Elisei, R.; Schlumberger, M.J.; Müller, S.P.; Schöffski, P.; Brose, M.S.; Shah, M.H.; Licitra, L.; Jarzab, B.; Medvedev, V.; Kreissl, M.C.; et al. Cabozantinib in Progressive Medullary Thyroid Cancer. J. Clin. Oncol. 2013, 31, 3639–3646. [Google Scholar] [CrossRef]
- Miller, K.; Bergmann, L.; Doehn, C.; Grünwald, V.; Gschwend, J.E.; Ivanyi, P.; Keilholz, U.; Kuczyk, M.A. Interdisciplinary Recommendations for the Treatment of Advanced Metastatic Renal Cell Carcinoma. Aktuelle Urol. 2020, 51, 572–581. [Google Scholar] [CrossRef]
- Brose, M.S.; Nutting, C.M.; Jarzab, B.; Elisei, R.; Siena, S.; Bastholt, L.; de la Fouchardiere, C.; Pacini, F.; Paschke, R.; Shong, Y.K.; et al. Sorafenib in radioactive iodine-refractory, locally advanced or metastatic differentiated thyroid cancer: A randomised, double-blind, phase 3 trial. Lancet 2014, 384, 319–328. [Google Scholar] [CrossRef]
- Gupta-Abramson, V.; Troxel, A.B.; Nellore, A.; Puttaswamy, K.; Redlinger, M.; Ransone, K.; Mandel, S.J.; Flaherty, K.T.; Loevner, L.A.; O’Dwyer, P.J.; et al. Phase II Trial of Sorafenib in Advanced Thyroid Cancer. J. Clin. Oncol. 2008, 26, 4714–4719. [Google Scholar] [CrossRef]
- Kloos, R.T.; Ringel, M.D.; Knopp, M.V.; Hall, N.C.; King, M.; Stevens, R.; Liang, J.; Wakely, P.E., Jr.; Vasko, V.V.; Saji, M.; et al. Phase II Trial of Sorafenib in Metastatic Thyroid Cancer. J. Clin. Oncol. 2009, 27, 1675–1684. [Google Scholar] [CrossRef] [PubMed]
- Lam, E.T.; Ringel, M.D.; Kloos, R.T.; Prior, T.W.; Knopp, M.V.; Liang, J.; Sammet, S.; Hall, N.C.; Wakely, P.E., Jr.; Vasko, V.V.; et al. Phase II Clinical Trial of Sorafenib in Metastatic Medullary Thyroid Cancer. J. Clin. Oncol. 2010, 28, 2323–2330. [Google Scholar] [CrossRef]
- Kim, S.; Yazici, Y.D.; Calzada, G.; Wang, Z.-Y.; N Younes, M.; A Jasser, S.; El-Naggar, A.K.; Myers, J.N. Sorafenib Inhibits the Angiogenesis and Growth of Orthotopic Anaplastic Thyroid Carcinoma Xenografts in Nude Mice. Mol. Cancer Ther. 2007, 6, 1785–1792. [Google Scholar] [CrossRef] [PubMed]
- Ito, Y.; Onoda, N.; Ito, K.-I.; Sugitani, I.; Takahashi, S.; Yamaguchi, I.; Kabu, K.; Tsukada, K. Sorafenib in Japanese Patients with Locally Advanced or Metastatic Medullary Thyroid Carcinoma and Anaplastic Thyroid Carcinoma. Thyroid 2017, 27, 1142–1148. [Google Scholar] [CrossRef] [PubMed]
- Taguchi, Y.-H.; Turki, T. A New Advanced in Silico Drug Discovery Method for Novel Coronavirus (SARS-CoV-2) with Tensor Decomposition-Based Unsupervised Feature Extraction. PLoS ONE 2020, 15, e0238907. [Google Scholar] [CrossRef]
- Locantore, P.; Del Gatto, V.; Corsello, A.; Pontecorvi, A. Lenvatinib Treatment for Thyroid Cancer in COVID Era: Safety in A Patient with Lung Metastases and SARS-Cov-2 Infection. Anticancer Drugs 2021, 32, 1116–1117. [Google Scholar] [CrossRef]
- Matsui, J.; Funahashi, Y.; Uenaka, T.; Watanabe, T.; Tsuruoka, A.; Asada, M. Multi-Kinase Inhibitor E7080 Suppresses Lymph Node and Lung Metastases of Human Mammary Breast Tumor MDA-MB-231 Via Inhibition of Vascular Endothelial Growth Factor-Receptor (VEGF-R) 2 and VEGF-R3 kinase. Clin. Cancer Res. 2008, 14, 5459–5465. [Google Scholar] [CrossRef]
- Puszkiel, A.; Noé, G.; Bellesoeur, A.; Kramkimel, N.; Paludetto, M.N.; Schoemann, A.T.; Vidal, M.; Goldwasser, F.; Chatelut, E.; Blanchet, B. Clinical Pharmacokinetics and Pharmacodynamics of Dabrafenib. Clin. Pharmacokinet. 2019, 58, 451–467. [Google Scholar] [CrossRef]
- Islam, T.; Rahman, M.R.; Aydin, B.; Beklen, H.; Arga, K.Y.; Shahjaman, M. Integrative Transcriptomics Analysis of Lung Epithelial Cells and Identification of Repurposable Drug Candidates for COVID-19. Eur. J. Pharmacol. 2020, 887, 173594. [Google Scholar] [CrossRef]
- Wan, W.; Zhu, S.; Li, S.; Shang, W.; Zhang, R.; Li, H.; Liu, W.; Xiao, G.; Peng, K.; Zhang, L. High-Throughput Screening of an FDA-Approved Drug Library Identifies Inhibitors against Arenaviruses and SARS-CoV-2. ACS Infect. Dis. 2021, 7, 1409–1422. [Google Scholar] [CrossRef]
- Chtita, S.; Belhassan, A.; Aouidate, A.; Belaidi, S.; Bouachrine, M.; Lakhlifi, T. Discovery of Potent SARS-CoV-2 Inhibitors from Approved Antiviral Drugs via Docking and Virtual Screening. Comb. Chem. High Throughput Screen. 2021, 24, 441–454. [Google Scholar] [CrossRef] [PubMed]
- Markham, A. Selpercatinib: First Approval. Drugs 2020, 80, 1119–1124. [Google Scholar] [CrossRef] [PubMed]
- Jozaghi, Y.; Zafereo, M.; Williams, M.D.; Gule-Monroe, M.K.; Wang, J.; Grubbs, E.G.; Vaporciyan, A.; Hu, M.I.; Busaidy, N.; Dadu, R.; et al. Neoadjuvant Selpercatinib for Advanced Medullary Thyroid Cancer. Head Neck 2021, 43, E7–E12. [Google Scholar] [CrossRef] [PubMed]
- Al-Zaqri, N.; Pooventhiran, T.; Alharthi, F.A.; Bhattacharyya, U.; Thomas, R. Structural Investigations, quantum mechanical studies on proton and metal affinity and biological activity predictions of selpercatinib. J. Mol. Liq. 2021, 325, 114765. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.V.; Macke, L.A.; Colton, B.S.; Imran, S.S.; Christiansen, J.; Chow-Maneval, E.; Hornby, Z.; Multani, P.S. Response to Entrectinib in Differentiated Thyroid Cancer with a ROS1 Fusion. JCO Precis. Oncol. 2017, 1, PO.17.00105. [Google Scholar] [CrossRef]
- El-Aarag, S.A.; Mahmoud, A.; ElHefnawi, M. Identifying Potential Novel Insights for COVID-19 Pathogenesis and Therapeutics Using An Integrated Bioinformatics Analysis of Host Transcriptome. Int. J. Biol. Macromol. 2022, 194, 770–780. [Google Scholar] [CrossRef]
- Peralta-Garcia, A.; Torrens-Fontanals, M.; Stepniewski, T.M.; Grau-Expósito, J.; Perea, D.; Ayinampudi, V.; Waldhoer, M.; Zimmermann, M.; Buzón, M.J.; Genescà, M.; et al. Entrectinib-A SARS-CoV-2 Inhibitor in Human Lung Tissue (HLT) Cells. Int. J. Mol. Sci. 2021, 22, 13592. [Google Scholar] [CrossRef]
- Federman, N.; McDermott, R. Larotrectinib, A Highly Selective Tropomyosin Receptor Kinase (TRK) Inhibitor for the Treatment of TRK Fusion Cancer. Expert. Rev. Clin. Pharmacol. 2019, 10, 931–939. [Google Scholar] [CrossRef]
- Groussin, L.; Clerc, J.; Huillard, O. Larotrectinib-Enhanced Radioactive Iodine Uptake in Advanced Thyroid Cancer. N. Engl. J. Med. 2020, 383, 1686–1687. [Google Scholar] [CrossRef]
- Abhithaj, J.; Dileep, F.; Sharanya, C.S.; Arun, K.G.; Sadasivan, C.; Jayadevi Variyar, E. Repurposing simeprevir, calpain inhibitor IV and a cathepsin F inhibitor against SARS-CoV-2 and insights into their interactions with Mpro. Biomol. Struct. Dyn. 2022, 40, 325–336. [Google Scholar] [CrossRef]
- Jang, S.; Atkins, M.B. Treatment of BRAF-Mutant Melanoma: The Role of Vemurafenib and Other Therapies. Clin. Pharmacol. Ther. 2014, 95, 24–31. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Greer, R.A.; Song, Y.; Praveen, H.; Song, Y. In Silico Identification of Available Drugs Targeting Cell Surface Bip to Disrupt SARS-Cov-2 Binding and Replication: Drug Repurposing Approach. Eur. J. Pharm. Sci. 2021, 160, 105771. [Google Scholar] [CrossRef] [PubMed]
- Miyamoto, S.; Kakutani, S.; Sato, Y.; Hanashi, A.; Kinoshita, Y.; Ishikawa, A. Drug Review: Pazopanib. Jpn. J. Clin. Oncol. 2018, 48, 503–513. [Google Scholar] [CrossRef] [PubMed]
- Bible, K.C.; Suman, V.J.; Molina, J.R.; Smallridge, R.C.; Maples, W.J.; Menefee, M.E.; Rubin, J.; Sideras, K.; Morris, J.C.; McIver, B.; et al. Endocrine Malignancies Disease Oriented Group; Mayo Clinic Cancer Center; Mayo Phase 2 Consortium. Efficacy of Pazopanib in Progressive, Radioiodine-Refractory, Metastatic Differentiated Thyroid Cancers: Results of A Phase 2 Consortium Study. Lancet Oncol. 2010, 11, 962–972. [Google Scholar] [CrossRef]
- Bible, K.C.; Suman, V.J.; Molina, J.R.; Smallridge, R.C.; Maples, W.J.; Menefee, M.E.; Rubin, J.; Karlin, N.; Sideras, K.; Morris, J.C., III; et al. Endocrine Malignancies Disease Oriented Group, Mayo Clinic Cancer Center, and the Mayo Phase 2 Consortium. A multicenter phase 2 trial of pazopanib in metastatic and progressive medullary thyroid carcinoma: MC057H. J. Clin. Endocrinol. Metab. 2014, 99, 1687–1693. [Google Scholar] [CrossRef]
- Carr, L.L.; Mankoff, D.A.; Goulart, B.H.; Eaton, K.D.; Capell, P.T.; Kell, E.M.; Bauman, J.E.; Martins, R.G. Phase II Study of Daily Sunitinib in FDG-PET-Positive, Iodine-Refractory Differentiated Thyroid Cancer and Metastatic Medullary Carcinoma of the Thyroid with Functional Imaging Correlation. Clin. Cancer Res. 2010, 16, 5260–5268. [Google Scholar] [CrossRef]
- Gao, Y.; Liu, P.; Shi, R. Anlotinib as a molecular targeted therapy for tumors. Oncol. Lett. 2020, 20, 1001–1014. [Google Scholar] [CrossRef]
- Capdevila, J.; Trigo, J.M.; Aller, J.; Manzano, H.L.; Adrián, S.G.; Llopis, C.Z.; Reig, O.; Bohn, U.; Y Cajal, T.R.; Duran-Poveda, M.; et al. Axitinib Treatment in Advanced RAI-Resistant Differentiated Thyroid Cancer (DTC) and Refractory Medullary Thyroid Cancer (MTC). Eur. J. Endocrinol. 2017, 177, 309–317. [Google Scholar] [CrossRef]
- Cohen, E.E.W.; Rosen, L.S.; Vokes, E.E.; Kies, M.S.; Forastiere, A.A.; Worden, F.P.; Kane, M.A.; Sherman, E.; Kim, S.; Bycott, P.; et al. Axitinib Is an Active Treatment for All Histologic Subtypes of Advanced Thyroid Cancer: Results from a Phase II Study. J. Clin. Oncol. 2008, 26, 4708–4713. [Google Scholar] [CrossRef]
- Lim, S.M.; Chung, W.Y.; Nam, K.-H.; Kang, S.-W.; Lim, J.Y.; Kim, H.-G.; Shin, S.H.; Sun, J.-M.; Kim, S.-G.; Kim, J.-H.; et al. An Open Label, Multicenter, Phase II Study of Dovitinib in Advanced Thyroid Cancer. Eur. J. Cancer 2015, 51, 1588–1595. [Google Scholar] [CrossRef]
- Stebbing, J.; Phelan, A.; Griffin, I.; Tucker, C.; Oechsle, O.; Smith, D.; Richardson, P. COVID-19: Combining Antiviral and Anti-inflammatory Treatments. Lancet Infect. Dis. 2020, 20, 400–402. [Google Scholar] [CrossRef] [PubMed]
- Girgis, A.S.; Panda, S.S.; Srour, A.M.; Abdelnaser, A.; Nasr, S.; Moatasim, Y.; Kutkat, O.; El Taweel, A.; Kandeil, A.; Mostafa, A.; et al. 3-Alkenyl-2-oxindoles: Synthesis, Antiproliferative and Antiviral Properties Against SARS-CoV-2. Bioorg. Chem. 2021, 114, 105131. [Google Scholar] [CrossRef] [PubMed]
- Hasskarl, J. Everolimus. Recent Results Cancer Res. 2018, 211, 101–123. [Google Scholar] [CrossRef] [PubMed]
- Roskoski Jr, R. Properties of FDA-Approved Small Molecule Protein Kinase Inhibitors: A 2020 Update. Pharmacol. Res. 2020, 152, 104609. [Google Scholar] [CrossRef]
- Lim, S.M.; Chang, H.; Yoon, M.J.; Hong, Y.K.; Kim, H.; Chung, W.Y.; Park, C.S.; Nam, K.H.; Kang, S.W.; Kim, M.K.; et al. A Multicenter, Phase II Trial of Everolimus in Locally Advanced or Metastatic Thyroid Cancer of All Histologic Subtypes. Ann. Oncol. 2013, 24, 3089–3094. [Google Scholar] [CrossRef]
- Hanna, G.J.; Busaidy, N.L.; Chau, N.G.; Wirth, L.J.; Barletta, J.A.; Calles, A.; Haddad, R.I.; Kraft, S.; Cabanillas, M.E.; Rabinowits, G.; et al. Genomic Correlates of Response to Everolimus in Aggressive Radioiodine-refractory Thyroid Cancer: A Phase II Study. Clin. Cancer Res. 2018, 24, 1546–1553. [Google Scholar] [CrossRef]
- Procaccini, C.; De Rosa, V.; Galgani, M.; Abanni, L.; Calì, G.; Porcellini, A.; Carbone, F.; Fontana, S.; Horvath, T.L.; La Cava, A.; et al. An Oscillatory Switch in Mtor Kinase Activity Sets Regulatory T Cell Responsiveness. Immunity 2010, 33, 929–941. [Google Scholar] [CrossRef]
- Terrazzano, G.; Rubino, V.; Palatucci, A.T.; Giovazzino, A.; Carriero, F.; Ruggiero, G. An Open Question: Is It Rational to Inhibit the mTor-Dependent Pathway as COVID-19 Therapy? Front. Pharmacol. 2020, 11, 856. [Google Scholar] [CrossRef]
- Kwok, G.; Yau, T.C.C.; Chiu, J.W.; Tse, E.; Kwong, Y.-L. Pembrolizumab (Keytruda). Hum. Vaccines Immunother. 2016, 12, 2777–2789. [Google Scholar] [CrossRef]
- Mehnert, J.M.; Varga, A.; Brose, M.S.; Aggarwal, R.R.; Lin, C.C.; Prawira, A.; de Braud, F.; Tamura, K.; Doi, T.; Piha-Paul, S.A.; et al. Safety and Antitumor Activity of the Anti-PD-1 Antibody Pembrolizumab in Patients with Advanced, PD-L1-Positive Papillary or Follicular Thyroid Cancer. BMC Cancer 2019, 19, 196. [Google Scholar] [CrossRef]
- Naing, A.; Gainor, J.F.; Gelderblom, H.; Forde, P.M.; Butler, M.O.; Lin, C.C.; Sharma, S.; Ochoa de Olza, M.; Varga, A.; Taylor, M.; et al. A First-in-Human Phase 1 Dose Escalation Study of Spartalizumab (PDR001), An Anti-PD-1 Antibody, in Patients with Advanced Solid Tumors. J. Immunother. Cancer 2020, 8, e000530. [Google Scholar] [CrossRef] [PubMed]
- Capdevila, J.; Wirth, L.J.; Ernst, T.; Aix, S.P.; Lin, C.C.; Ramlau, R.; Butler, M.O.; Delord, J.P.; Gelderblom, H.; Ascierto, P.A.; et al. PD-1 Blockade in Anaplastic Thyroid Carcinoma. J. Clin. Oncol. 2020, 38, 2620–2627. [Google Scholar] [CrossRef] [PubMed]
- Loretelli, C.; Abdelsalam, A.; D’Addio, F.; Ben Nasr, M.; Assi, E.; Usuelli, V.; Maestroni, A.; Seelam, A.J.; Ippolito, E.; Di Maggio, S.; et al. PD-1 blockade counteracts post-COVID-19 immune abnormalities and stimulates the anti-SARS-CoV-2 immune response. JCI Insight 2021, 6, e146701. [Google Scholar] [CrossRef] [PubMed]
| Protein That Is Mutated | Role of Protein Encoded by Gene | Oncogenes Diagnosed in Thyroid Cancers | Etiology of Gene Mutation | Consequences of the Mutation | Types of Thyroid Cancers with the Mutation (Frequency Within TC, %) | Kinds of Cancer with the Particular Mutation |
|---|---|---|---|---|---|---|
| BRAF | Encodes serine-threonine kinase of B-Raf proteins engaged in sending signals inside cells and in managing cell growth. | BRAFV600E BRAFK601E | The point mutation: valine (V) is substituted by glutamic acid (E) at amino acid 600 of B-RAF. The point mutation: lysine (L) is substituted by glutamic acid (E) at amino acid 601 of B-RAF. | Tumor driven by BRAFV600E induces mitogen-activated protein kinase MAPK-signaling (does not respond to the negative feedback from ERK to RAF). BRAFV600E alters methylation (as a consequence of expression of numerous genes), promoting PTC tumorigenesis. Activation of the MAPK pathways. | PTC (80%), ATC FTC | Colorectal cancer [8,9] Thyroid adenoma [10] |
| RAS | GTPase is involved in regulating cell division, differentiation and apoptosis. They code p21 protein, engaged in cell growth, proliferation, and differentiation. | NRAS HRAS KRAS | Mutations occur in codons 12, 13 (KRAS), and 61 (HRAS and NRAS) of RAS gene. | Tumors driven by RAS decrease MAPK-signaling (responding to the negative ERK feedback). Translation of altered p21 protein—uncontrolled cell proliferation. | FTC (30–45%), PDTC (20–40%), ATC (10–20%), DTC, MTC | Lung, pancreatic, colorectal cancer [11] |
| EIF1A | Eukaryotic translation initiation factor 1A. It is a component of the 43S pre-initiation complex (PIC) required for the binding of the 43S complex to the 5′ end of capped RNA (controlling the initiation of protein synthesis). Encoded on human chromosomes X and Y (EIF1AX, EIF1AY). | EIF1AX (EIF1AX-A113splice in TC) | Mutations in exons numbers 2, 5 and 6. | EIF1AX mutations is highly associated with RAS mutations. The increase or altered function of proteins owing alteration for specific substitutions in the C- and N-terminal tails, e.g., the promotion of the G1/S phase transition through the transcriptional repression of p21. The cellular mechanisms caused by this mutation are poorly understood. | ATC (11–33%), PDTC (11%), FTC (2.5–7.4%), PTC (1–2.3%) | Uveal melanomas, breast, ovarian cancers, Benign thyroid adenomas [12,13,14,15,16] |
| PI3K | Phosphatidylinositol 3-kinase, the component of the PTEN/PI3K/AKT pathway, regulates cell cycle progression, cell survival, adhesion, motility and spreading, angiogenesis, glucose homeostasis, and cell size and organ size control. | PIK3CA | Mutation in exons numbers 9 and 20; amplification of catalytic subunit of p110alpha of PI3K. | Activation of the PTEN/PI3K/AKT pathway. | ATC (23%), FTC (8%), PTC (1%) | Colorectal, gastric, breast, ovarian cancers, and high-grade brain tumors [17,18] |
| PTEN | The phosphatase and tensin homolog, a key negative regulator of the PI3K/mTOR pathway. | PTEN/PHTS | The inactivation of the PTEN gene by epigenetic mutation caused by aberrant methylation or gene deletion. | Leads to a cluster of tissue overgrowth syndromes termed PTEN hamartoma tumor syndrome (PHTS). | PTC (1.5–5%), FTC (1–4.5%) | Breast, ovarian, endometrial, colon, prostate cancer, and glioblastomas [17,19] |
| DICER1 | Ribonuclease III involved in cleaving double stranded pre-microRNAs into mature miRNAs. | DICER1 | This mutation affects the metal ion-binding capacity of the RNAse domain, interfering with the catalytic site of the enzyme, reducing the production of 5p miRNAs. | Changes in post-transcriptional regulation of gene expression. Germline mutations have been associated with a familial tumor susceptibility syndrome. | FTC (7–10%) | Lungs, kidneys, ovaries cancers, multinodular goiter (MNG), Sertoli–Leydig cell tumors (SLCT), cystic nephroma, pleuropulmonary blastoma, cancer of cervical embryonal, rhabdomyosarcoma, and Wilms tumor [20] |
| EZH1 | Enhancer of zeste homolog 1—a member of the Polycomb group protein complex, components for prevention of cancer stem cell development. | EZH1Q571R EZH1Y642F EZH1M349L | The point mutation called hot-spot mutation (c.1712A>G; p.Gln571Arg) in the enhancer of zeste homolog 1. | EZH1 are associated with alterations in cAMP pathway genes. | FTC: 3–5.9% | Uveal melanoma [21] |
| SPOP | Speckle-Type Poz Protein, an E3 ubiquitin ligase adaptor protein. SPOP interacts with CUL3 during ubiquitination of substrates. | SPOPSCR3 SPOPAR SPOPERA | Missense mutations, loss of expression. | SPOP mutants promote the error-prone non-homologous end joining (NHEJ) pathway. | PTC: | Prostate, liver, and endometrium cancer [22,23] |
| p53 | p53 drives DNA repair, arrests, cell-cycle, senescence, and apoptosis when it is phosphorylated by DNA damage response (DDR) kinases. It acts a key failsafe mechanism of cellular anti-cancer defenses. | TP53 | Mutations in 5–9 exons of gene encoding Tp53. | Disorders in mechanisms of cellular anti-cancer defenses. | ATC: 58–80%, PDTC: 26% FTC: 11% PTC: 3.5% | Breast, brain, adreno cortical cancers, central nervous system tumors, and sarcomas [24,25,26] |
| TERT | Encodes the reverse transcriptase (subunit of the telomerase complex), elongating the telomere portion of chromosomes (adds repeated sequences). Its expression and activity are strongly increased in cancer cells (usually is absent or low). | TERTC228T TERTC250T | Point mutations. | Ensures chromosomal stability (maintains telomere length), leads cancer cells to senescence. | PTC: 7–22% FTC: 14–17% DTC: 10% ATC: 70%, PDTC: 40% | Various types of cancer [25,27,28] |
| FGF | fibroblast growth factor | |||||
| receptors for TSH | ||||||
| Chromosomal Rearragments | ||||||
| RET | Rearranged during Transfection (RET)— the tyrosine kinase, is composed by an extracellular (EC), a transmembrane (TM), and an intracellular (IC) portion. The functional tyrosine-kinase receptor (RTK) of glial cell line-derived neurotrophic factor (GDNF), neurturin (NRT), artemin (ART), and persephin (PSF) growth factors. These growth factors bind to GFRs (GDNF family receptor), forming a complex that mediates RET dimerization and activation. | ACBD5-RET, AFAP1L2-RET, AKAP13-RET, ANKRD26-RET, RET/PTC1, DLG5-RET, ERC1-RET, FKBP15-RET, RET/PTC5, HOOK3-RET, KIAA1468-RET, RET/PTC8, RET/PTC3, PCM1-RET, RET/PTC2, RUFY2-RET, SPECC1L-RET, TBL1XR1-RET, SQSTM1-RET, RET/PTC6, TRIM27-RET, RET/PTC7, UEVLD-RET, TFG-RET, RET/PTC1, RET/PTC3, PPFIBP2-RET, MYH13-RET | Gene amplification, fusion, single base substitutions (or small insertions), deletions either in RET sequences. | It triggers signaling along the MAPK pathway, leading to an uncontrolled proliferation. It activates the transcription of the RET tyrosine-kinase domain. | PTC PDTC MTC | Lymphoma, cholangiocarcinoma, lung adenocarcinoma, breast invasive carcinoma, spindle cell tumor of soft tissues, colorectal carcinoma, stomach adenocarcinoma, spitzoid neoplasms, invasive mucinous lung adenocarcinoma, infantile myofibromatosis, intraductal carcinoma of the salivary gland, lipofibromatosis, and lipofibromatosis-like neuronal tumors [29,30] |
| PAX8 PPARγ | PAX8—one of the paired box transcription factor, necessary for thyroid development, drives the expression of genes encoding thyroglobulin, thyroid peroxidase, and the sodium iodide symporter. PPARγ, the nuclear receptor of transcription factors, regulates adipogenesis and modulates lipid metabolism and insulin sensitivity. PPARγ has anti-inflammatory activity (a tumor suppressor). | PAX8/PPARG | The PAX8/PPARG rearrangement— a translocation between chromosomal regions 2q13 and 3p25.60—results in a fusion transcript: the sequence of PAX8 (2q13) is fused in frame with the exons of PPARγ1 (3p25). The PAX8 promoter (highly active in thyroid follicular cells) drives the expression of the fusion transcript of PPFP. | Production of fusion protein PAX8/PPARγ (PPFG). PPFG acts as dominant negative inhibitor of wild-type PPARγ and/or as a unique transcriptional activator of subsets of PPARγ and PAX8-responsive genes. | FTC (30–35%) PTC (17.6%) | Non-invasive follicular thyroid neoplasm with papillary-like nuclear features [31,32,33,34,35] |
| Medication | The Impact of the Drug on the Course of COVID-19 |
|---|---|
| Levothyroxine (T4) (Euthyrox, Letrox and others) | The drug is routinely used in patients with postoperative hypothyroidism. Similar to endogenous thyroid hormones, T4 stimulates the immune system to defend against viruses (T-cell activation, secretion of IFN-γ and cytokines, regulation of chemotaxis, and phagocytosis) [105]. T4 is believed to influence the fusion of SARS-CoV-2 with target cells and activate human platelets. Consequently, patients taking high doses of T4 are at a higher risk of COVID-19 infection and have a poorer prognosis, including potential thrombotic complications [134]. |
| Colchicine | Colchicine is an anti-inflammatory drug used to treat various conditions, including gout and recurrent pericarditis; it reduces neutrophil chemotaxis, inhibits inflammasome signaling, and decreases cytokine production. It has been reported to lower mortality risk and improve clinical outcomes in COVID-19 patients. Zhang identifies colchicine as an inhibitor of thyroid cancer [138,139,140]. |
| Vandetanib | Belonging to the multikinase inhibitors, Vandetanib is an FDA-approved drug for the treatment of advanced medullary thyroid cancer (MTC). It selectively blocks the epidermal growth factor receptor (EGFR), vascular endothelial growth factor receptors (VEGFR2/3), and RET tyrosine kinase. Like other multikinase inhibitors (MKIs), it suppresses cytokine secretion, making it potentially useful in the treatment of COVID-19. Studies on animal models and cell cultures have demonstrated that Vandetanib significantly reduces inflammatory cytokine levels and immune cell infiltration in the lungs, suggesting its potential application in COVID-19 treatment [141]. |
| Cabozantinib | Cabozantinib belongs to the class of multikinase inhibitors. It is aimed at inhibiting VEGFR, c-MET, and RET; it is an FDA-approved drug for the treatment of progressive and symptomatic MTC and has a strong antiangiogenic effect [142]. Although cabozantinib exhibits strong anti-inflammatory activity, caution is suggested when using it for the treatment of COVID-19 or PCS [143]. |
| Sorafenib | Sorafenib belongs to the class of multikinase inhibitors. It is targeted at inhibiting VEGFR1–3, PDGFR, and RET. It is approved for the treatment of RAI-resistant DTC, with proven efficacy in treating metastatic thyroid cancer, including PTC and RAI-resistant DTC [144,145,146], as well as in the treatment of MTC [147]. However, its efficacy in treating ATC is debated [148,149]. Sorafenib has been shown to exhibit antiviral activity. Additionally, it interacts with genes whose expression is induced by SARS-CoV-2, making it a potential candidate for the treatment of COVID-19 [150]. However, it may interact with other drugs used in COVID-19, and its use should be immediately discontinued in cases of hypokalemia and fever [151]. |
| Lenwatynib Dabrafenib in combination with trametinib | Lenvatinib, like other MKIs, targets tumor angiogenesis [152]. It is aimed at inhibiting VEGFR1–3, FGFR1–4, PDGFR, and RET. Single patient studies involving DTC and COVID-19 show no contraindications for continuing treatment despite viral infection [151]. Both drugs have been approved by the FDA for the treatment of ATC with the BRAFV600E gene mutation (dabrafenib is a selective inhibitor of the BRAFV600E mutant kinase and belongs to MKIs, while trametinib is a mitogen-activated protein kinase inhibitor) [153]. Computational analysis has shown that dabrafenib exhibits antiviral properties against SARS-CoV-2 [154], and this suggestion was confirmed in cell studies—dabrafenib inhibited SARS-CoV-2 replication [155]. There are also studies suggesting the effectiveness of trametinib in treating COVID-19 [156]. |
| Selperkatynib | Selpercatinib (a selective RET receptor inhibitor) belongs to the MKIs and has been approved by the FDA for the treatment of MTC with RET mutations [157,158]. Computational studies with elements of quantum mechanics suggest that this drug may strongly bind to SARS-CoV-2 proteins, thereby mimicking their functions, which could have therapeutic significance [159]. |
| Entrectinib | Entrectinib belongs to the MKIs, specifically to the new inhibitors of anaplastic lymphoma kinase, c-ros oncogene 1 (ROS1) kinase. Bioinformatics analysis indicates the potential significance of entrectinib in the treatment of COVID-19 [160,161]. Additionally, entrectinib has shown antiviral activity against SARS-CoV-2 in human lung tissue [162]. |
| Larotrectinib | Larotrectinib is a selective NTRK inhibitor belonging to the MKIs, approved by the FDA for the treatment of solid tumors with NTRK gene fusions [163]. Its efficacy has been demonstrated in the treatment of PTC [164]. Similar to entrectinib, its potential properties against COVID-19 have also been shown [165]. |
| Vemurafenib | Vemurafenib is an MKI, a selective inhibitor of the mutated serine-threonine kinase BRAF, and strongly inhibits ERK phosphorylation [166]. In silico analysis showed that this drug inhibits virus binding and replication by blocking the substrate-binding domain (which triggers apoptosis in infected cells), making it a candidate for COVID-19 treatment [167]. |
| Pazopanib, sunitynib, anlotinib, aksytynib i dovitinib | These are MKIs belonging to antiangiogenic creatine kinase inhibitors, showing efficacy in the treatment of thyroid cancer (TC) by inhibiting VEGFR1–3, PDGFR, FGFR, and RET [168]. The efficacy of pazopanib has been demonstrated in the treatment of patients with DTC or MTC [169,170,171], anlotinib in the treatment of MTC, even with metastases [172,173], and axitinib shows significant anticancer activity in all histological subtypes of advanced TC [174]. Dovitinib shows potential therapeutic significance in the treatment of locally advanced or metastatic DTC and MTC [175]. Given that these inhibitors clearly influence the reduction of Ebola virus infectivity and hepatitis C inflammation, it is possible that they may also affect SARS-CoV-2, as confirmed by studies [176,177]. It is most likely that pazopanib disrupts the binding of SARS-CoV-2 to immunoglobulins associated with the cell surface, which may make it effective in the treatment of COVID-19 [167]. |
| Everolimus | Everolimus belongs to the MKIs and is an inhibitor of the serine-threonine kinase signaling pathway mTOR [178]. It has been approved by the FDA for the treatment of HER2(-) breast cancer and pancreatic neuroendocrine tumors [179]. Phase II studies have confirmed its therapeutic significance in locally advanced or metastatic thyroid cancer (TC) [180], such as in patients with DTC, MTC, and ATC [181]. It is hypothesized that everolimus, by inhibiting mTOR, may be effective in the treatment of COVID-19 by reducing conventional T cell proliferation, decreasing the cytokine storm, and maintaining the growth and activity of regulatory T cells [182,183]. |
| Pembrolizumab Spartalizumab | Pembrolizumab is a humanized monoclonal antibody (anti-PD-1) approved by the FDA for the treatment of advanced melanoma and non-small cell lung cancer [184]. Clinical studies have shown that pembrolizumab also has anticancer effects in several patients with advanced DTC [185]. Spartalizumab is a humanized monoclonal immunoglobulin-4 antibody that blocks interactions with PD-L1 and PD-L2 [186], and its therapeutic effects have been demonstrated in ATC [187]. Analysis of the immune profiles of COVID-19 survivors showed that PD-1 blockade normalizes the immune phenotype and restores T cell functions, making pembrolizumab and spartalizumab effective in the treatment of COVID-19 [188]. |
| Corticosteroids | Corticosteroids are commonly used in patients with brain metastases or extensive lung metastases during I131 treatment; there is a suspicion that, similar to previous coronavirus pandemics (MERS-CoV and SARS), corticosteroids may be associated with worse outcomes in patients [80]. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bronowicka-Szydełko, A.; Rabczyński, M.; Dumas, I.; Fiodorenko-Dumas, Ż.; Wojtczak, B.; Kotyra, Ł.; Kustrzeba-Wójcicka, I.; Lewandowski, Ł.; Ponikowska, B.; Kuzan, A.; et al. State of Knowledge About Thyroid Cancers in the Era of COVID-19—A Narrative Review. Biomedicines 2024, 12, 2829. https://doi.org/10.3390/biomedicines12122829
Bronowicka-Szydełko A, Rabczyński M, Dumas I, Fiodorenko-Dumas Ż, Wojtczak B, Kotyra Ł, Kustrzeba-Wójcicka I, Lewandowski Ł, Ponikowska B, Kuzan A, et al. State of Knowledge About Thyroid Cancers in the Era of COVID-19—A Narrative Review. Biomedicines. 2024; 12(12):2829. https://doi.org/10.3390/biomedicines12122829
Chicago/Turabian StyleBronowicka-Szydełko, Agnieszka, Maciej Rabczyński, Ilias Dumas, Żanna Fiodorenko-Dumas, Beata Wojtczak, Łukasz Kotyra, Irena Kustrzeba-Wójcicka, Łukasz Lewandowski, Beata Ponikowska, Aleksandra Kuzan, and et al. 2024. "State of Knowledge About Thyroid Cancers in the Era of COVID-19—A Narrative Review" Biomedicines 12, no. 12: 2829. https://doi.org/10.3390/biomedicines12122829
APA StyleBronowicka-Szydełko, A., Rabczyński, M., Dumas, I., Fiodorenko-Dumas, Ż., Wojtczak, B., Kotyra, Ł., Kustrzeba-Wójcicka, I., Lewandowski, Ł., Ponikowska, B., Kuzan, A., Kluz, J., Gamian, A., & Madziarska, K. (2024). State of Knowledge About Thyroid Cancers in the Era of COVID-19—A Narrative Review. Biomedicines, 12(12), 2829. https://doi.org/10.3390/biomedicines12122829







