Biological and Cellular Effects of Percutaneous Electrolysis: A Systematic Review
Abstract
1. Introduction
2. Materials and Methods
2.1. Protocol and Registration
2.2. Information Sources and Search
2.3. Eligibility Criteria and Study Selection
2.4. Data Collection Process
2.5. Outcomes
2.6. Risk of Bias of Individual Studies
3. Results
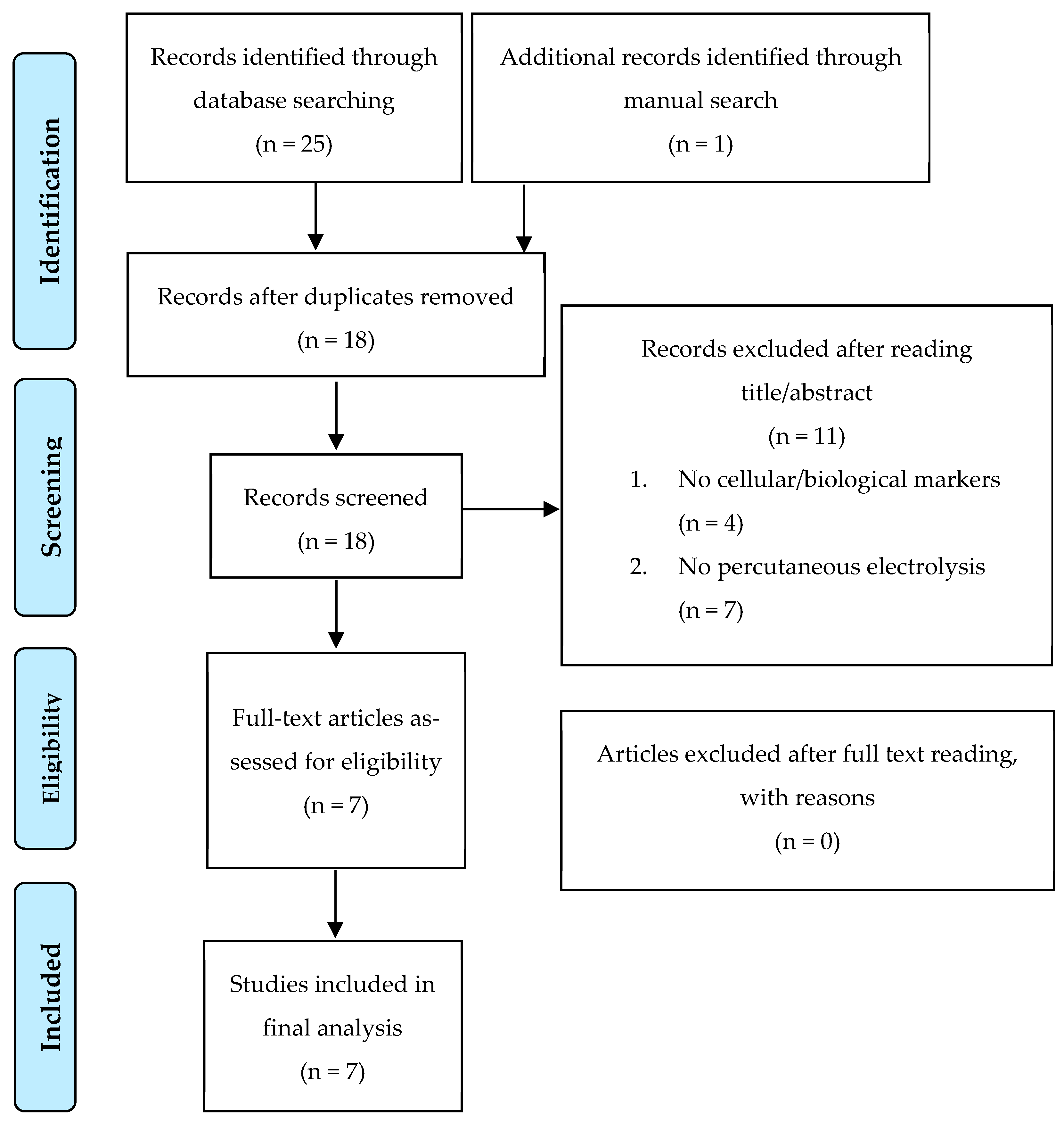
3.1. Search Strategy
3.2. Study Selection
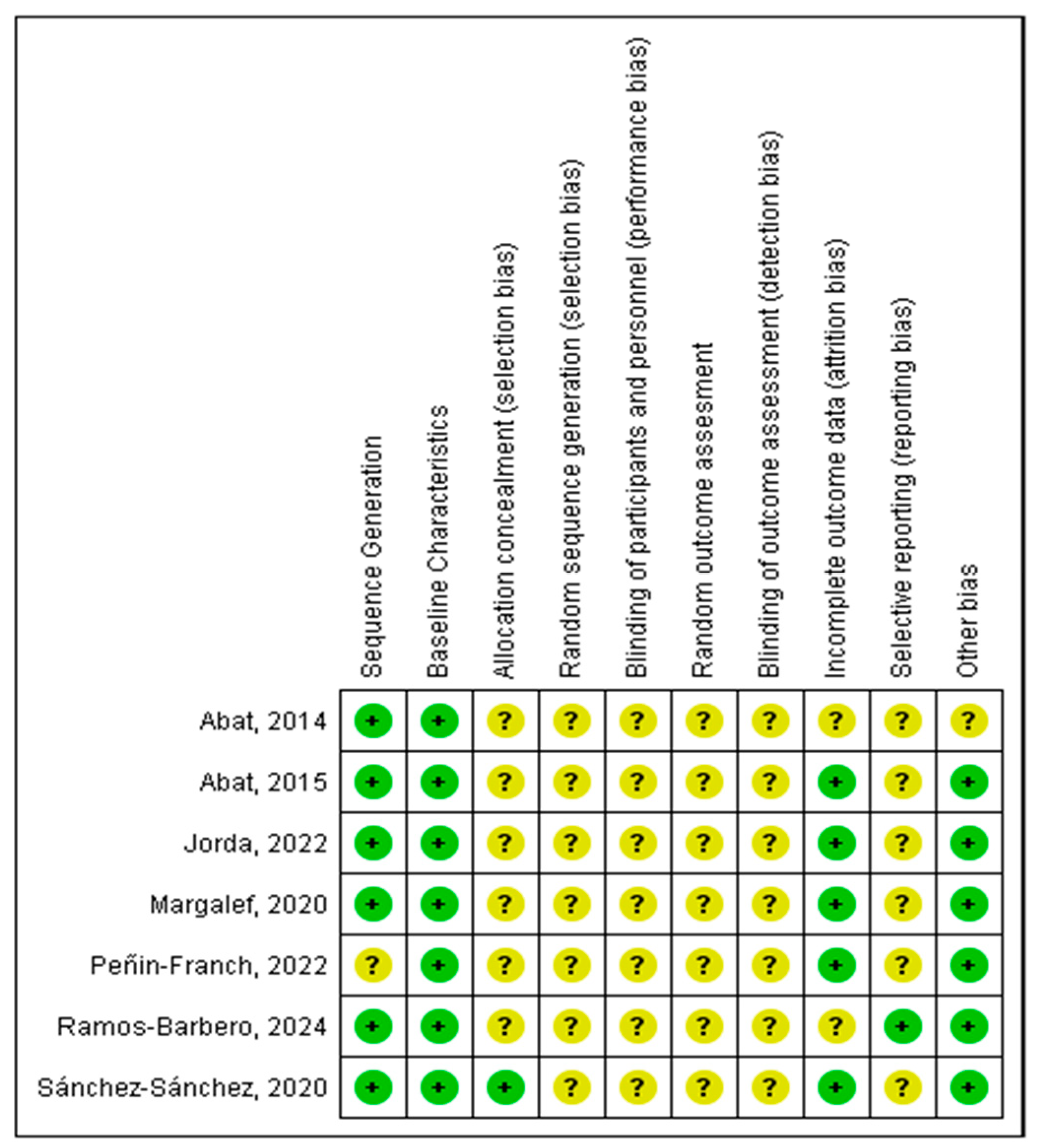
3.3. Risk of Bias of Individual Studies
3.4. Study Characteristics
4. Discussion
4.1. Inflammatory or Anti-Inflammatory Effect?
4.2. Cell Proliferation or Cell Death?
4.3. Extracellular Matrix and Tissue Remodeling
4.4. Clinical Implications
4.5. Limitations and Future Studies
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sánchez Ibañez, J.M. Clinical Course in the Treatment of Chronic Patellar Tendinopathy through Ultrasound Guided Percutaneous Electrolysis Intratissue (EPI®): Study of a Population Series of Cases in Sport. Ph.D. Thesis, Atlantic International University, Honolulu, HI, USA, 2009. [Google Scholar]
- Borrella-Andrés, S.; Malo-Urriés, M.; Pérez-Bellmunt, A.; Arias-Buría, J.L.; Rodríguez-Sanz, J.; Albarova-Corral, M.I.; González-Rueda, V.; Gallego-Sendarrubias, G.M.; Fernández-de-las-Peñas, C.; López-de-Celis, C. Application of Percutaneous Needle Electrolysis Does Not Elicit Temperature Changes: An In Vitro Cadaveric Study. Int. J. Environ. Res. Public Health 2022, 19, 15738. [Google Scholar] [CrossRef] [PubMed]
- García-Vidal, J.A.; Salinas, J.; Escolar-Reina, P.; Cuello, F.; Ortega, N.; de Dios Berná-Mestre, J.; López-Nicolás, M.; Valera-Garrido, F.; Medina-Mirapeix, F. Galvanic Current Dosage and Bacterial Concentration Are Determinants of the Bactericidal Effect of Percutaneous Needle Electrolysis: An in Vitro Study. Sci. Rep. 2021, 11, 18977. [Google Scholar] [CrossRef]
- Zhao, M. Electrical Fields in Wound Healing-An Overriding Signal That Directs Cell Migration. Semin. Cell Dev. Biol. 2009, 20, 674–682. [Google Scholar] [CrossRef] [PubMed]
- Margalef, R.; Minaya-Muñoz, F.; Valera-Garrido, F.; Bosque, M.; Santafé, M.M. Changes in PH as a Result of Galvanic Currents Used in Percutaneous Needle Electrolysis. Rev. Fisioter. Invasiva 2020, 3, 2–6. [Google Scholar] [CrossRef]
- Peñin-Franch, A.; García-Vidal, J.A.; Martínez, C.M.; Escolar-Reina, P.; Martínez-Ojeda, R.M.; Gómez, A.I.; Bueno, J.M.; Medina-Mirapeix, F.; Pelegrín, P. Galvanic Current Activates the NLRP3 Inflammasome to Promote Type I Collagen Production in Tendon. eLife 2022, 11, e73675. [Google Scholar] [CrossRef]
- Sánchez-sánchez, J.L.; Calderón-díez, L.; Herrero-turrión, J.; Méndez-sánchez, R.; Arias-buría, J.L.; Fernández-de-las-peñas, C. Changes in Gene Expression Associated with Collagen Regeneration and Remodeling of Extracellular Matrix after Percutaneous Electrolysis on Collagenase-Induced Achilles Tendinopathy in an Experimental Animal Model: A Pilot Study. J. Clin. Med. 2020, 9, 3316. [Google Scholar] [CrossRef]
- Abat, F.; Valles, S.L.; Gelber, P.E.; Polidori, F.; Jorda, A.; García-Herreros, S.; Monllau, J.C.; Sanchez-Ibáñez, J.M. An Experimental Study of Muscular Injury Repair in a Mouse Model of Notexin-Induced Lesion with EPI® Technique. BMC Sports Sci. Med. Rehabil. 2015, 7, 7. [Google Scholar] [CrossRef]
- Jorda, A.; Campos-Campos, J.; Aldasoro, C.; Colmena, C.; Aldasoro, M.; Alvarez, K.; Valles, S.L. Protective Action of Ultrasound-Guided Electrolysis Technique on the Muscle Damage Induced by Notexin in Rats. PLoS ONE 2022, 17, e0276634. [Google Scholar] [CrossRef]
- Ramos-Barbero, M.; Pérez-Jiménez, A.; Serrano-Carmona, S.; Mokhtari, K.; Lupiáñez, J.A.; Rufino-Palomares, E.E. The Efficacy of Intratissue Percutaneous Electrolysis (EPI®) and Nutritional Factors for the Treatment of Induced Tendinopathy in Wistar Rats: Hepatic Intermediary Metabolism Effects. Int. J. Mol. Sci. 2024, 25, 7315. [Google Scholar] [CrossRef]
- Abat, F.; Valles, S.L.; Gelber, P.E.; Polidori, F.; Stitik, T.P.; García-Herreros, S.; Monllau, J.C.; Sanchez-Ibánez, J.M. Molecular Repair Mechanisms Using the Intratissue Percutaneous Electrolysis Technique in Patellar Tendonitis. Rev. Esp. Cir. Ortop. Traumatol. 2014, 58, 201–205. [Google Scholar] [CrossRef]
- Verhagen, A.M.; Ekert, P.G.; Pakusch, M.; Silke, J.; Connolly, L.M.; Reid, G.E.; Moritz, R.L.; Simpson, R.J.; Vaux, D.L. Identification of DIABLO, a Mammalian Protein That Promotes Apoptosis by Binding to and Antagonizing IAP Proteins. Cell 2000, 102, 43–53. [Google Scholar] [CrossRef] [PubMed]
- Ghavami, S.; Hashemi, M.; Ande, S.R.; Yeganeh, B.; Xiao, W.; Eshraghi, M.; Bus, C.J.; Kadkhoda, K.; Wiechec, E.; Halayko, A.J.; et al. Apoptosis and Cancer: Mutations within Caspase Genes. J. Med. Genet. 2009, 46, 497–510. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Chiguano, G.F.; Navarro-Santana, M.J.; Cleland, J.A.; Arias-Buría, J.L.; Fernández-De-Las-Peñas, C.; Ortega-Santiago, R.; Plaza-Manzano, G. Effectiveness of Ultrasound-Guided Percutaneous Electrolysis for Musculoskeletal Pain: A Systematic Review and Meta-Analysis. Pain Med. 2021, 22, 1055–1071. [Google Scholar] [CrossRef] [PubMed]
- Augustyn, D.; Paez, A. The Effectiveness of Intratissue Percutaneous Electrolysis for the Treatment of Tendinopathy: A Systematic Review. S. Afr. J. Sports Med. 2022, 34, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Fakontis, C.; Iakovidis, P.; Lytras, D.; Kasimis, K.; Koutras, G.; Ntinou, S.R.; Kottaras, A.; Chatziprodromidou, I.P.; Chatzikonstantinou, P.; Apostolou, T. Efficacy of Percutaneous Needle Electrolysis versus Dry Needling in Musculoskeletal Pain: A Systematic Review and Meta-Analysis. J. Back Musculoskelet. Rehabil. 2023, 36, 1033–1046. [Google Scholar] [CrossRef]
- Sánchez-González, J.L.; Navarro-López, V.; Cañada-Sánchez, P.; Juárez-Vela, R.; de Viñaspre-Hernández, R.R.; Varela-Rodríguez, S. Efficacy of Different Intensities of Percutaneous Electrolysis for Musculoskeletal Pain: A Systematic Review and Meta-Analysis. Front. Med. 2023, 10, 1101447. [Google Scholar] [CrossRef]
- Asensio-Olea, L.; Leirós-Rodríguez, R.; Marqués-Sánchez, M.P.; de Carvalho, F.O.; Maciel, L.Y.S. Efficacy of Percutaneous Electrolysis for the Treatment of Tendinopathies: A Systematic Review and Meta-Analysis. Clin. Rehabil. 2023, 37, 747–759. [Google Scholar] [CrossRef]
- Ferreira, M.H.L.; Araujo, G.A.S.; De-La-Cruz-Torrez, B. Effectiveness of Percutaneous Needle Electrolysis to Reduce Pain in Tendinopathies: A Systematic Review with Meta-Analysis. J. Sport Rehabil. 2024, 33, 307–316. [Google Scholar] [CrossRef]
- Hooijmans, C.R.; Rovers, M.M.; De Vries, R.B.M.; Leenaars, M.; Ritskes-Hoitinga, M.; Langendam, M.W. SYRCLE’s Risk of Bias Tool for Animal Studies. BMC Med. Res. Methodol. 2014, 14, 43. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Altman, D.G.; Gøtzsche, P.C.; Jüni, P.; Moher, D.; Oxman, A.D.; Savović, J.; Schulz, K.F.; Weeks, L.; Sterne, J.A.C. The Cochrane Collaboration’s Tool for Assessing Risk of Bias in Randomised Trials. BMJ 2011, 343, d5928. [Google Scholar] [CrossRef]
- Cashin, A.G.; McAuley, J.H. Clinimetrics: Physiotherapy Evidence Database (PEDro) Scale. J. Physiother. 2020, 66, 59. [Google Scholar] [CrossRef] [PubMed]
- National Institutes of Health. Study Quality Assessment Tools. Available online: https://www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools (accessed on 15 October 2020).
- Ma, L.-L.; Wang, Y.-Y.; Yang, Z.-H.; Huang, D.; Weng, H.; Zeng, X.-T. Methodological Quality (Risk of Bias) Assessment Tools for Primary and Secondary Medical Studies: What Are They and Which Is Better? Mil. Med. Res. 2020, 7, 7. [Google Scholar] [CrossRef] [PubMed]
- Karki, R.; Kanneganti, T.D. The “Cytokine Storm”: Molecular Mechanisms and Therapeutic Prospects. Trends Immunol. 2021, 42, 681–705. [Google Scholar] [CrossRef] [PubMed]
- Jarczak, D.; Nierhaus, A. Cytokine Storm-Definition, Causes, and Implications. Int. J. Mol. Sci. 2022, 23, 11740. [Google Scholar] [CrossRef]
- Darrieutort-Laffite, C.; Blanchard, F.; Soslowsky, L.J.; Le Goff, B. Biology and Physiology of Tendon Healing. Jt. Bone Spine 2024, 91, 105696. [Google Scholar] [CrossRef]
- Ellis, I.M.; Schnabel, L.V.; Berglund, A.K. Defining the Profile: Characterizing Cytokines in Tendon Injury to Improve Clinical Therapy. J. Immunol. Regen. Med. 2022, 16, 100059. [Google Scholar] [CrossRef]
- Leong, N.L.; Kator, J.L.; Clemens, T.L.; James, A.; Enamoto-Iwamoto, M.; Jiang, J. Tendon and Ligament Healing and Current Approaches to Tendon and Ligament Regeneration. J. Orthop. Res. 2020, 38, 7–12. [Google Scholar] [CrossRef]
- Dueweke, J.J.; Awan, T.M.; Mendias, C.L. Regeneration of Skeletal Muscle After Eccentric Injury. J. Sport Rehabil. 2017, 26, 171–179. [Google Scholar] [CrossRef]
- Abat, F.; Diesel, W.J.; Gelber, P.E.; Polidori, F.; Monllau, J.C.; Sanchez-Ibañez, J.M. Effectiveness of the Intratissue Percutaneous Electrolysis (EPI®) Technique and Isoinertial Eccentric Exercise in the Treatment of Patellar Tendinopathy at Two Years Follow-Up. Muscles Ligaments Tendons J. 2014, 4, 188–193. [Google Scholar] [CrossRef]
- Margalef, R.; Valera-Garrido, F.; Minaya-Muñoz, F.; Bosque, M.; Ortiz, N.; Santafe, M.M. Percutaneous Needle Electrolysis Reverses Neurographic Signs of Nerve Entrapment by Induced Fibrosis in Mice. Evid. Based Complement. Altern. Med. 2020, 2020, 6615563. [Google Scholar] [CrossRef]
- Mattiussi, G.; Moreno, C. Treatment of Proximal Hamstring Tendinopathyrelated Sciatic Nerve Entrapment: Presentation of an Ultrasound-Guided “Intratissue Percutaneous Electrolysis” Application. Muscles Ligaments Tendons J. 2016, 6, 248–252. [Google Scholar] [CrossRef] [PubMed]
- Valera-Garrido, F.; Margalef, R.; Bosque, M.; Minaya-Muñoz, F.; Santafé, M.M. Percutaneous Needle Electrolysis Accelerates Functional Muscle Regeneration in Mice. Appl. Sci. 2022, 12, 10014. [Google Scholar] [CrossRef]
- Colmena, C. New Technique in Tendon Sport Recovery. Percutaneous Electrolysis Intratissue (EPI®). Int. J. Phys. Med. Rehabil. 2013, 1, 1000113. [Google Scholar] [CrossRef]
- García Naranjo, J.; Barroso Rosa, S.; Loro Ferrer, J.F.; Limiñana Cañal, J.M.; Suarez Hernández, E. A Novel Approach in the Treatment of Acute Whiplash Syndrome: Ultrasound-Guided Needle Percutaneous Electrolysis. A Randomized Controlled Trial. Orthop. Traumatol. Surg. Res. 2017, 103, 1229–1234. [Google Scholar] [CrossRef]
- Rodríguez-Huguet, M.; Góngora-Rodríguez, J.; Rodríguez-Huguet, P.; Ibañez-Vera, A.J.; Rodríguez-Almagro, D.; Martín-Valero, R.; Díaz-Fernández, Á.; Lomas-Vega, R. Effectiveness of Percutaneous Electrolysis in Supraspinatus Tendinopathy: A Single-Blinded Randomized Controlled Trial. J. Clin. Med. 2020, 9, 1837. [Google Scholar] [CrossRef]
- Valera-Garrido, F.; Minaya-Muñoz, F.; Medina-Mirapeix, F. Ultrasound-Guided Percutaneous Needle Electrolysis in Chronic Lateral Epicondylitis: Short-Term and Long-Term Results. Acupunct. Med. 2014, 32, 446–454. [Google Scholar] [CrossRef]
- Lopez-Martos, R.; Gonzalez-Perez, L.M.; Ruiz-Canela-Mendez, P.; Urresti-Lopez, F.J.; Gutierrez-Perez, J.L.; Infante-Cossio, P. Randomized, Double-Blind Study Comparing Percutaneous Electrolysis and Dry Needling for the Management of Temporomandibular Myofascial Pain. Med. Oral. Patol. Oral. Cir. Bucal 2018, 23, e454–e462. [Google Scholar] [CrossRef]
- Abat, F.; Gelber, P.E.; Polidori, F.; Monllau, J.C.; Sanchez-Ibañez, J.M. Clinical Results after Ultrasound-Guided Intratissue Percutaneous Electrolysis (EPI®) and Eccentric Exercise in the Treatment of Patellar Tendinopathy. Knee Surg. Sports Traumatol. Arthrosc. 2015, 23, 1046–1052. [Google Scholar] [CrossRef]
- Varela-Rodríguez, S.; Sánchez-Sánchez, J.L.; Velasco, E.; Delicado-Miralles, M.; Sánchez-González, J.L. Endogenous Pain Modulation in Response to a Single Session of Percutaneous Electrolysis in Healthy Population: A Double-Blinded Randomized Clinical Trial. J. Clin. Med. 2022, 11, 2889. [Google Scholar] [CrossRef]


| Population | Intervention | Outcomes |
|---|---|---|
| Rats | Percutaneous electrolysis | Physiological effects |
| Mice | EPI technique | Cellular response |
| Cell | Ultrasound-guided electrolysis | Metabolism |
| Human | Percutaneous needle electrolysis | Gene expression |
| Percutaneous galvanic electrolysis | Protein expression | |
| Intratissue percutaneous electrolysis | Cytokines | |
| Inflammatory response | ||
| Biological effects | ||
| Chemokine | ||
| Molecular effects | ||
| Cellular effects | ||
| Regeneration | ||
| Cell proliferation | ||
| Apoptosis | ||
| Oxidative stress | ||
| Interleukin | ||
| Angiogenesis |
| Pubmed Search Strategy |
| ((“percutaneous electrolysis” OR “EPI technique” OR “ultrasound-guided electrolysis” OR “percutaneous needle electrolysis” OR “percutaneous galvanic electrolysis” OR “Intratissue Percutaneous Electrolysis”) AND (“physiological effects”[Text Word] OR “cellular response”[Text Word] OR “Metabolism”[Text Word] OR “gene expression”[Text Word] OR “protein expression”[Text Word] OR “cytokines”[Text Word] OR “inflammatory response”[Text Word] OR “biological effects”[Text Word] OR “chemokine”[Text Word] OR “molecular effects”[Text Word] OR “cellular effects”[Text Word] OR “Regeneration”[Text Word] OR “cell proliferation”[Text Word] OR “apoptosis”[Text Word] OR “oxidative stress”[Text Word] OR “Interleukin”[Text Word] OR “angiogenesis”[Text Word]) AND (“rats”[Title/Abstract] OR “mice”[Title/Abstract] OR “cell” [Title/Abstract] OR “human”[Title/Abstract])) |
| Cochrane and Web of Science Search Strategy |
| (((“percutaneous electrolysis” OR “EPI technique” OR “ultrasound-guided electrolysis” OR “percutaneous needle electrolysis” OR “percutaneous galvanic electrolysis” OR “Intratissue Percutaneous Electrolysis”) AND (“physiological effects” OR “cellular response” OR “Metabolism” OR “gene expression” OR “protein expression” OR “cytokines” OR “inflammatory response” OR “biological effects” OR “chemokine” OR “molecular effects” OR “cellular effects” OR “Regeneration” OR “cell proliferation” OR “apoptosis” OR “oxidative stress” OR “Interleukin” OR “angiogenesis”) AND ((“rats” OR “mice” OR “cell” OR “human”)))) |
| Reference | Sample | Sample Gender | Age | Groups | Sample Characteristics | Percutaneous Electrolysis Intervention (miliAmperes:sec:impacts) | Treatment Frequency | Condition | Follow-Ups | Cellular and Biological Markers | Main Findings by Percutaneous Electrolysis Intervention |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Peñin-Franch (2022) [6] | Mice (n = At least 4 “Unclear”) | Male | 8–10 Weeks | -Control group -Dry needling group -Percutaneous electrolysis group | -NLRP3-deficient mice -Casp1/11-deficient mice -ASC-deficient mice (Pycard-/-) -Wild mice | 3:3:3 3:6:2 3:6:8 6:6:2 6:6:8 12:6:2 12:6:8 | Single | Chronic Tendon (Achilles) | 3–7–14–21 days post-electrolysis | Pro-Inflammatory Effects (IL-6; IL18; IL1β; IL1α; TNF α; Cxcl10; NLRP3; COX-2; polymorphonuclear cells M1) Anti-Inflammatory Effects (IL1rn; TGF β1; Arg1; Fizz1; Mrc1; Ym1; M2) Cell Death (LDH; Yo-Pro; Pycard; Casp1) Extracellular matrix and tissue remodeling (COL-I; COL-III) | Intervention: 12:6:2 Pro-Inflammatory Effects Significant increase in expression: (COX2; IL-6; TNFα). Significant increase in concentration: (IL1β; IL-18; NLRP3) Cell Death Significant increase in concentration: (LDH) |
| Intervention: 12:6:8 Cell Death Significant increase in concentration: (LDH; Yo-Pro) | |||||||||||
| Intervention: 6:6:8 and 3:6:8 Cell Death Significant increase in concentration: (Yo-Pro) | |||||||||||
| Intervention: 6:6:2 and 3:6:2 Pro-Inflammatory Effects Significant increase in concentration: (IL1β) | |||||||||||
| Intervention: 3:3:3 Pro-Inflammatory Effects Significant increase: (Polymorphonuclear cells M1) Significant increase in expression: (IL-6; IL1α; IL1β; Cxcl10; IL1rn; TGF β1) Extracellular matrix and tissue remodeling Significant increase in concentration: (COL-I) Significant decrease in concentration: (COL-III) | |||||||||||
| Sánchez-Sánchez (2020) [7] | Rats (n = 15) | Male | 8 Weeks | -Control group (n = 3) -Collagenase-confirming group (n = 3) -Collagenase control group (n = 3) -Collagenase percutaneous electrolysis group (n = 3) -Collagenase needling group (n = 3) | Sprague Dawley rats | 3:4:3 | 1 per week (3 weeks) | Chronic Tendon (Achilles) | 7 days after the last electrolysis session | Extracellular matrix and tissue remodeling (COX2; Col1a1; Col3a1; Mmp2; Mmp3; Mmp9; VEGF, Scx, B-act; Gapdh; Rpl19) | Intervention: 3:4:3 Extracellular matrix and tissue remodeling Significant increase: (COX2, Mmp9 y VEGF) |
| Jorda (2022) [9] | Rats (n = 20) | Female | 7 Months | -Control group (n = 5) -Notexin group (n = 5) -Percutaneous electrolysis group (n = 5) -Notexin percutaneous electrolysis group (n = 5) | Wistar rats | 6:5:4 | 2 times (at 7–11 days after Notexin) | Inflammatory Muscle (Quadriceps) | 3 days after the last electrolysis session | Pro-Inflammatory Effects (CCL3; CCL4; CCL5; CCR5; CCR8; NFkB) Anti-Inflammatory Effects (IL-6; IL-13; IL-10; CCL1; IkB) | Intervention: 6:5:4 Anti-Inflammatory Effects Significant increase: (IL-13; IL-10; CCL1; IkB) Pro-Inflammatory Effects Significant decrease: (IL-6; CCL3; CCL4; CCL5; CCR5; CCR8; NFkB) |
| Ramos-Barbero (2024) [10] | Rats (n = 24) | Male | Unclear | -Healthy control (n = 4) -Diseased control (n = 4) -Percutaneous electrolysis (n = 4) -Percutaneous electrolysis + hydroxytyrosol (n = 4) -Percutaneous electrolysis + maslinic acid (n = 4) -Percutaneous electrolysis with amino acids glycine and aspartate (n = 4). | Wistar rats | 3:4:1 | Single | Chronic Tendon (Achilles) | 13–26–40 days post-electrolysis | Anti-Inflammatory Effects (Metabolism Enzymes) (HK; PK; FBPase; LDH; G6PDH; CS; ME; HOAD; FAS;GDH; AST; ALT) | Intervention: 3:4:1 Anti-Inflammatory Effects (Metabolism Enzymes) Significant decrease: (CS; G6PDH; LDH; FBPase; T-HK; PK; ME; FAS; HOAD; ALT; AST) |
| Abat (2014) [11] | Rats (n = 24) | Female | 7 Months | -Control group (n = 6) -Collagenase-confirming group (n = 6) -Percutaneous electrolysis group 3mA (n = 6) -Percutaneous electrolysis group 6 mA (n = 6) | Sprague-Dawley | 3:4:3 6:4:3 | Single | Chronic Tendon (Patellar) | 3 days post-electrolysis | Cell Death (Cytochrome C; Smac/Diablo) Extracellular Matrix and Tissue Remodeling (VEGF; VEGFR; PPAR-γ/tubulin) | Intervention: 3:4:3 and 6:4:3 Cell Death Significant increase: (Cytochrome C; Smac/Diablo) Extracellular Matrix and Tissue Remodeling Significant increase: (VEGF; VEGFR; PPAR-γ/tubulin) |
| Abat (2015) [8] | Rats (n = 24) | Unclear | Unclear | -Control group (n = 6) -Notexin group 7 days (n = 6) -Notexin group 14 days (n = 6) -Notexin percutaneous electrolysis group (n = 6) | Sprague-Dawley | 3:5:4 | Single | Inflammatory Muscle (Quadriceps) | 7 days post-electrolysis | Pro-Inflammatory Effects (TNFα; IL-1B) Extracellular Matrix and Tissue Remodeling (VEGF; VEGFR; PPAR-γ/tubulin) | Intervention: 3:5:4 Pro-Inflammatory Effects Significant decrease: (TNFα; IL-1B) Extracellular Matrix and Tissue Remodeling Significant increase: (VEGF; VEGFR; PPAR-γ/tubulin) |
| Margalef (2020) [5] | Mice (n = 3) | Male | 5 Weeks | -Control group (n = 3 paws) -Percutaneous electrolysis group (n = 3 paws) | Unclear | 3:3:3 | Single | Gastrocnemius | Immediately post-electrolysis | pH | Intervention: 3:3:3 No changes |
| Reference | Condition | Biomarker | Pre-Intervention Mean (SD) | Post-Intervention Mean (SD) | |
|---|---|---|---|---|---|
| Peñin-Franch (2022) [6] | Chronic Tendon (Achilles) | Pro-Inflammatory Effects | Intervention: 12:6:2 | Intervention: 12:6:2 | |
| COX22ΔCT | 0.000 (0.000) | 0.012 (0.009) | |||
| IL-62ΔCT | 0.000 (0.000) | 0.133 (0.007) | |||
| TNFα2ΔCT | 0.002 (0.002) | 0.005 (0.003) | |||
| IL1β (pg/mL) | 8.641 (2.348) | 151.191 (58.308) | |||
| IL-18 (pg/mL) | 51.728 (22.983) | 227.518 (52.719) | |||
| NLRP32ΔCT | 4.530 (2.045) | 65.727 (4.603) | |||
| Cell Death | LDH (%) | 3.088 (0.551) | 7.850 (3.429) | ||
| Cell Death | Intervention: 12:6:8 | Intervention: 12:6:8 | |||
| LDH (%) | 3.088 (0.551) | 42.890 (13.831) | |||
| Yo-Pro (slope) | 18.287 (6.197) | 154.703 (37.134) | |||
| Pro-Inflammatory Effects | Intervention: 6:6:2 | Intervention: 6:6:2 | |||
| IL1β (pg/mL) | 8.641 (2.348) | 59.572 (13.631) | |||
| Cell Death | Intervention: 6:6:8 | Intervention: 6:6:8 | |||
| Yo-Pro (slope) | 18.287 (6.197) | 118.782 (45.521) | |||
| Cell Death | Intervention: 3:6:8 | Intervention: 3:6:8 | |||
| Yo-Pro (slope) | 18.287 (6.197) | 67.661 (30.408) | |||
| Pro-Inflammatory Effects | Intervention: 3:6:2 | Intervention: 3:6:2 | |||
| IL1β (pg/mL) | 8.641 (2.348) | 30.522 (18.482) | |||
| Pro-Inflammatory Effects | Intervention: 3:3:3 | Intervention: 3:3:3 | |||
| Polymorphonuclear cells M1 (nº) | 1.333 (2.016) | 10.708 (9.727) | |||
| IL-62ΔCT | 1.123 (0.569) | 4.105 (1.551) | |||
| IL1α2ΔCT | 0.000 (0.000) | 3.362-05 (2.569-05) | |||
| IL1β2ΔCT | 1.302 (1.142) | 21.529 (22.760) | |||
| Cxcl102ΔCT | 1.023 (0.243) | 4.782 (3.336) | |||
| IL1rn2ΔCT | 1.479 (1.667) | 4.533 (4.637) | |||
| TGF β1(Fold Change) | 0.158 (0.185) | 1.000 (0.397) | |||
| Extracellular matrix and tissue remodeling | COL-I (%) | 13.719 (7.307) | 26.083 (12.054) | ||
| COL-III (%) | 86.281 (7.307) | 73.901 (12.047) | |||
| Sánchez-Sánchez (2020) [7] | Chronic Tendon (Achilles) | Extracellular matrix and tissue remodeling | Intervention: 3:4:3 | Intervention: 3:4:3 | |
| COX22ΔCT | 0.044 (0.050) | 1.351 (0.706) | |||
| Mmp92ΔCT | 0.000 (0.000) | 8.564 (4.872) | |||
| VEGF2ΔCT | 0.109 (0.021) | 2.208 (0.135) | |||
| Jorda (2022) [9] | Inflammatory Muscle (Quadriceps) | Anti-Inflammatory Effects | Intervention: 6:5:4 | Intervention: 6:5:4 | |
| IL-13 (Pg/mL) | 11.499 (2.643) | 28.569 (3.571) | |||
| IL-10 (Pg/mL) | 35.829 (12.964) | 74.471 (12.839) | |||
| CCL12ΔCT | 0.898 (0.156) | 1.119 (0.117) | |||
| IkB (Arbitrary Units) | 0.447 (0.093) | 0.670 (0.064) | |||
| Pro-Inflammatory Effects | IL-6 (Pg/mL) | 84.401(8.643) | 67.109 (8.643) | ||
| CCL32ΔCT | 2.321 (0.339) | 1.770 (0.145) | |||
| CCL42ΔCT | 2.497 (0.267) | 1.843 (0.164) | |||
| CCL52ΔCT | 1.920 (0.465) | 1.310 (0.285) | |||
| CCR52ΔCT | 2.684 (0.291) | 1.453 (0.167) | |||
| CCR82ΔCT | 1.585 (0.110) | 1.174 (0.257) | |||
| NFkB (Arbitrary Units) | 1.190 (0.152) | 0.810 (0.067) | |||
| Ramos-Barbero (2024) [10] | Chronic Tendon (Achilles) | Anti-Inflammatory Effects (Metabolism Enzymes) | Intervention: 3:4:1 | Intervention: 3:4:1 | |
| CS (nmol/min/mg protein) | 7.840 (0.980) | 4.580 (0.360) | |||
| G6PDH (nmol/min/mg protein) | 25.570 (1.040) | 19.380 (1.740) | |||
| LDH (nmol/min/mg protein) | 4031.8 (269.1) | 3107.1 (282.3) | |||
| FBPase (nmol/min/mg protein) | 41.380 (7.600) | 35.910 (1.750) | |||
| T-HK (nmol/min/mg protein) | 2.260 (0.220) | 1.850 (0.080) | |||
| PK (nmol/min/mg protein) | 327.57 (40.600) | 283.03 (16.240) | |||
| ME (nmol/min/mg protein) | 3.970 (0.650) | 4.700 (0.280) | |||
| FAS (nmol/min/mg protein) | 1.440 (0.110) | 1.000 (0.040) | |||
| HOAD (nmol/min/mg protein) | 177.78 (10.330) | 132.37 (3.700) | |||
| ALT (nmol/min/mg protein) | 125.10 (9.530) | 113.68 (20.340) | |||
| AST (nmol/min/mg protein) | 1046.5 (100.50) | 722.68 (49.840) | |||
| Abat (2014) [11] | Chronic Tendon (Patellar) | Cell Death | Intervention: 3:4:3 | Intervention: 3:4:3 | |
| Cytochrome C (Relative Densitometry Unit) | 378.769 (78.842) | 412.145 (60.679) | |||
| Smac/Diablo (Relative Densitometry Unit) | 321.424 (12.856) | 1722.703 (49.281) | |||
| Extracellular Matrix and Tissue Remodeling | VEGF (Relative Densitometry Unit) | 19.028 (6.410) | 48.155 (6.993) | ||
| VEGFR (Relative Densitometry Unit) | 29.250 (0.573) | 85.592 (1.344) | |||
| PPAR-γ/tubulin (Relative Densitometry Unit) | 8.213 (1.006) | 8.883 (0.939) | |||
| Cell Death | Intervention: 6:4:3 | Intervention: 6:4:3 | |||
| Cytochrome C (Relative Densitometry Unit) | 378.769 (78.842) | 563.608 (42.422) | |||
| Smac/Diablo (Relative Densitometry Unit) | 321.424 (12.856) | 1474.160 (74.498) | |||
| Extracellular Matrix and Tissue Remodeling | VEGF (Relative Densitometry Unit) | 19.028 (6.410) | 42.522 (12.239) | ||
| PPAR-γ/tubulin (Relative Densitometry Unit) | 8.213 (1.006) | 13.107 (1.006) | |||
| Abat (2015) [8] | Inflammatory Muscle (Quadriceps) | Pro-Inflammatory Effects | Intervention: 3:5:4 | Intervention: 3:5:4 | |
| TNFα (Pg/mL) | 32.800 (3.100) | 16.200 (2.800) | |||
| IL-1β (Pg/mL) | 319.600 (13.50) | 120.200 (17.700) | |||
| Extracellular Matrix and Tissue Remodeling | VEGF (Relative Densitometry Unit) | 51.800 (6.700) | 85.035 (4.371) | ||
| VEGFR (Relative Densitometry Unit) | 38.500 (3.100) | 60.300 (4.900) | |||
| PPAR-γ/tubulin (Relative Densitometry Unit) | 23.000 (1.800) | 62.000 (6.100) | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodríguez-Sanz, J.; Rodríguez-Rodríguez, S.; López-de-Celis, C.; Malo-Urriés, M.; Pérez-Amodio, S.; Pérez-Antoñanzas, R.; Borrella-Andrés, S.; Albarova-Corral, I.; Mateos-Timoneda, M.Á. Biological and Cellular Effects of Percutaneous Electrolysis: A Systematic Review. Biomedicines 2024, 12, 2818. https://doi.org/10.3390/biomedicines12122818
Rodríguez-Sanz J, Rodríguez-Rodríguez S, López-de-Celis C, Malo-Urriés M, Pérez-Amodio S, Pérez-Antoñanzas R, Borrella-Andrés S, Albarova-Corral I, Mateos-Timoneda MÁ. Biological and Cellular Effects of Percutaneous Electrolysis: A Systematic Review. Biomedicines. 2024; 12(12):2818. https://doi.org/10.3390/biomedicines12122818
Chicago/Turabian StyleRodríguez-Sanz, Jacobo, Sergi Rodríguez-Rodríguez, Carlos López-de-Celis, Miguel Malo-Urriés, Soledad Pérez-Amodio, Román Pérez-Antoñanzas, Sergio Borrella-Andrés, Isabel Albarova-Corral, and Miguel Ángel Mateos-Timoneda. 2024. "Biological and Cellular Effects of Percutaneous Electrolysis: A Systematic Review" Biomedicines 12, no. 12: 2818. https://doi.org/10.3390/biomedicines12122818
APA StyleRodríguez-Sanz, J., Rodríguez-Rodríguez, S., López-de-Celis, C., Malo-Urriés, M., Pérez-Amodio, S., Pérez-Antoñanzas, R., Borrella-Andrés, S., Albarova-Corral, I., & Mateos-Timoneda, M. Á. (2024). Biological and Cellular Effects of Percutaneous Electrolysis: A Systematic Review. Biomedicines, 12(12), 2818. https://doi.org/10.3390/biomedicines12122818









