Efficacy and Safety of Pazopanib in the Treatment of Thyroid Cancer: A Systematic Review
Abstract
1. Introduction
2. Materials and Methods
2.1. Eligibility Criteria
2.2. Information Sources
2.3. Search Strategy
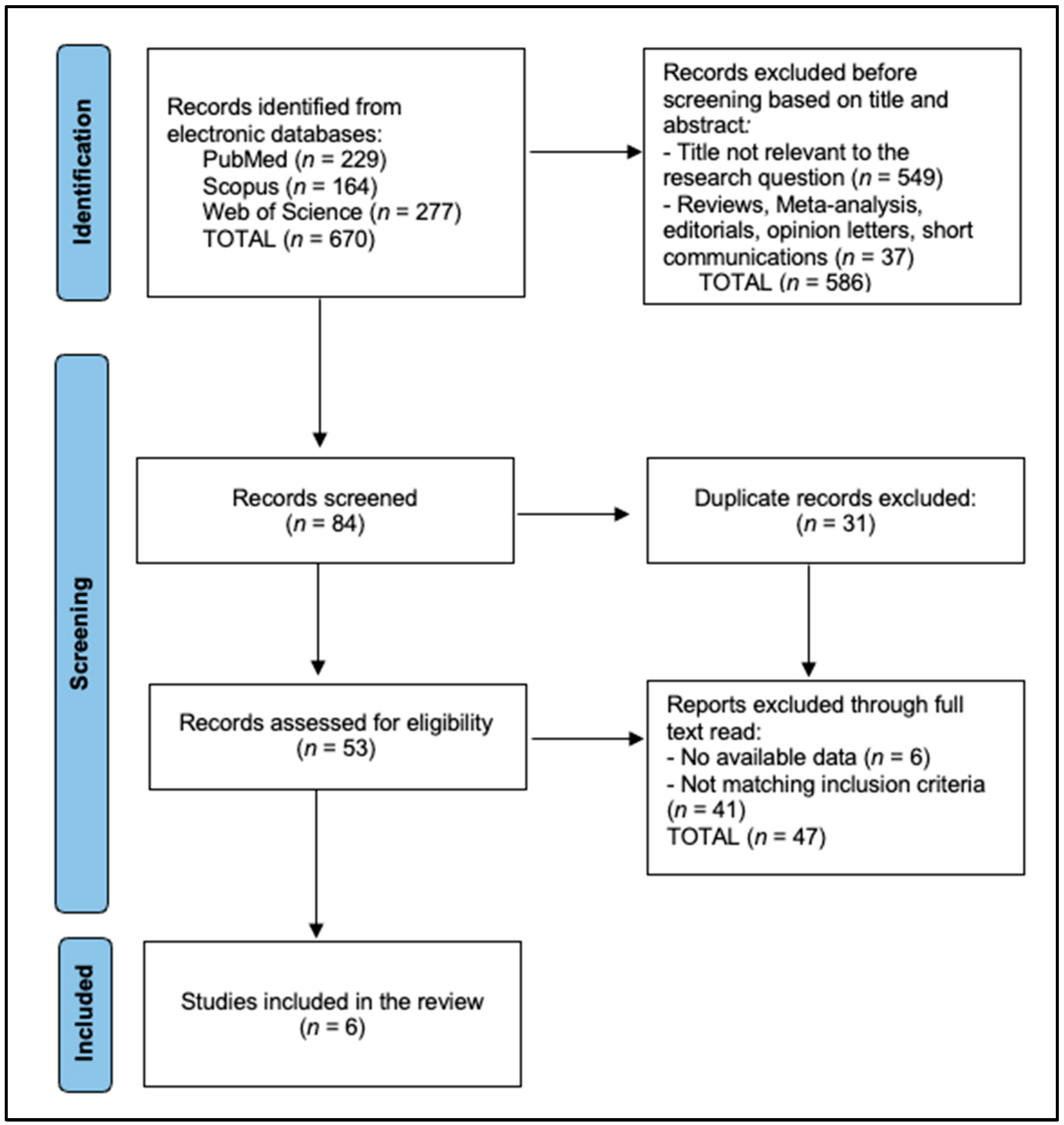
2.4. Selection Process
2.5. Data Collection and Quality Assessment
3. Results
3.1. Characteristics of Included Studies
3.2. Patient Demographics and Baseline Characteristics
3.3. Treatment Details and Efficacy Outcomes
3.4. Safety Profiles and Adverse Events
4. Discussion
4.1. Summary of Evidence
4.2. Limitations and Future Directions
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kitahara, C.M.; Farkas, D.K.; Jørgensen, J.O.L.; Cronin-Fenton, D.; Sørensen, H.T. Benign Thyroid Diseases and Risk of Thyroid Cancer: A Nationwide Cohort Study. J. Clin. Endocrinol. Metab. 2018, 103, 2216–2224. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sanabria, A.; Kowalski, L.P.; Shah, J.P.; Nixon, I.J.; Angelos, P.; Williams, M.D.; Rinaldo, A.; Ferlito, A. Growing incidence of thyroid carcinoma in recent years: Factors underlying overdiagnosis. Head Neck 2018, 40, 855–866. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hu, J.; Yuan, I.J.; Mirshahidi, S.; Simental, A.; Lee, S.C.; Yuan, X. Thyroid Carcinoma: Phenotypic Features, Underlying Biology and Potential Relevance for Targeting Therapy. Int. J. Mol. Sci. 2021, 22, 1950. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Singh, A.; Ham, J.; Po, J.W.; Niles, N.; Roberts, T.; Lee, C.S. The Genomic Landscape of Thyroid Cancer Tumourigenesis and Implications for Immunotherapy. Cells 2021, 10, 1082. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Stewart, L.A.; Kuo, J.H. Advancements in the treatment of differentiated thyroid cancer. Ther. Adv. Endocrinol. Metab. 2021, 12, 20420188211000251. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Coca-Pelaz, A.; Rodrigo, J.P.; Shah, J.P.; Nixon, I.J.; Hartl, D.M.; Robbins, K.T.; Kowalski, L.P.; Mäkitie, A.A.; Hamoir, M.; López, F.; et al. Recurrent Differentiated Thyroid Cancer: The Current Treatment Options. Cancers 2023, 15, 2692. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Dabelić, N.; Jukić, T.; Fröbe, A. Medullary Thyroid Cancer—Feature Review and Update on Systemic Treatment. Acta Clin. Croat. 2020, 59 (Suppl. S1), 50–59. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Xing, J.C.; Bishop, J.A.; Mathioudakis, N.; Agrawal, N.; Tufano, R.P. A large nonmetastatic anaplastic thyroid cancer with complete thyroidal confinement. Case Rep. Med. 2011, 2011, 583978. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Fagin, J.A.; Krishnamoorthy, G.P.; Landa, I. Pathogenesis of cancers derived from thyroid follicular cells. Nat. Rev. Cancer 2023, 23, 631–650. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Romei, C.; Elisei, R. RET/PTC Translocations and Clinico-Pathological Features in Human Papillary Thyroid Carcinoma. Front. Endocrinol. 2012, 3, 54. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bible, K.C.; Suman, V.J.; Molina, J.R.; Smallridge, R.C.; Maples, W.J.; Menefee, M.E.; Rubin, J.; Karlin, N.; Sideras, K.; Morris, J.C., 3rd; et al. A multicenter phase 2 trial of pazopanib in metastatic and progressive medullary thyroid carcinoma: MC057H. J. Clin. Endocrinol. Metab. 2014, 99, 1687–1693. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Priya, S.R.; Dravid, C.S.; Digumarti, R.; Dandekar, M. Targeted Therapy for Medullary Thyroid Cancer: A Review. Front. Oncol. 2017, 7, 238. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Alhejaily, A.G.; Alhuzim, O.; Alwelaie, Y. Anaplastic thyroid cancer: Pathogenesis, prognostic factors, and genetic landscape. Mol. Clin. Oncol. 2023, 19, 99. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lee, H.; Kim, S.Y.; Kim, S.M.; Chang, H.J.; Lee, Y.S.; Park, C.S.; Chang, H.S. Long-term survival of patients with anaplastic thyroid cancer after multimodal treatment. Transl. Cancer Res. 2020, 9, 5430–5436. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Niu, G.; Chen, X. Vascular endothelial growth factor as an anti-angiogenic target for cancer therapy. Curr. Drug Targets 2010, 11, 1000–1017. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhao, Y.; Adjei, A.A. Targeting Angiogenesis in Cancer Therapy: Moving Beyond Vascular Endothelial Growth Factor. Oncologist 2015, 20, 660–673. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cella, D.; Beaumont, J.L. Pazopanib in the treatment of advanced renal cell carcinoma. Ther. Adv. Urol. 2016, 8, 61–69. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Stamati, K.; Redondo, P.A.; Nyga, A.; Neves, J.B.; Tran, M.G.; Emberton, M.; Cheema, U.; Loizidou, M. The anti-angiogenic tyrosine kinase inhibitor Pazopanib kills cancer cells and disrupts endothelial networks in biomimetic three-dimensional renal tumoroids. J. Tissue Eng. 2020, 11, 2041731420920597. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Isham, C.R.; Bossou, A.R.; Negron, V.; Fisher, K.E.; Kumar, R.; Marlow, L.; Lingle, W.L.; Smallridge, R.C.; Sherman, E.J.; Suman, V.J.; et al. Pazopanib enhances paclitaxel-induced mitotic catastrophe in anaplastic thyroid cancer. Sci. Transl. Med. 2013, 5, 166ra3. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Higgins, J.P.; Altman, D.G.; Gøtzsche, P.C.; Jüni, P.; Moher, D.; Oxman, A.D.; Savovic, J.; Schulz, K.F.; Weeks, L.; Sterne, J.A.; et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011, 343, d5928. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chow, L.Q.; Santana-Davila, R.; Pantel, A.; Roth, M.; Anderson, L.N.; Failor, A.; Doot, R.; Mankoff, D. A phase I study of pazopanib in combination with escalating doses of 131I in patients with well-differentiated thyroid carcinoma borderline refractory to radioiodine. PLoS ONE 2017, 12, e0178325. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sherman, E.J.; Harris, J.; Bible, K.C.; Xia, P.; Ghossein, R.A.; Chung, C.H.; Riaz, N.; Gunn, G.B.; Foote, R.L.; Yom, S.S.; et al. Radiotherapy and paclitaxel plus pazopanib or placebo in anaplastic thyroid cancer (NRG/RTOG 0912): A randomised, double-blind, placebo-controlled, multicentre, phase 2 trial. Lancet Oncol. 2023, 24, 175–186. [Google Scholar] [CrossRef]
- de la Fouchardière, C.; Godbert, Y.; Dalban, C.; Illouz, F.; Wassermann, J.; Do Cao, C.; Bardet, S.; Zerdoud, S.; Chougnet, C.N.; Zalzali, M.; et al. Intermittent versus continuous administration of pazopanib in progressive radioiodine refractory thyroid carcinoma: Final results of the randomised, multicenter, open-label phase II trial PAZOTHYR. Eur. J. Cancer 2021, 157, 153–164. [Google Scholar] [CrossRef] [PubMed]
- Bible, K.C.; Suman, V.J.; Menefee, M.E.; Smallridge, R.C.; Molina, J.R.; Maples, W.J.; Karlin, N.J.; Traynor, A.M.; Kumar, P.; Goh, B.C.; et al. A multiinstitutional phase 2 trial of pazopanib monotherapy in advanced anaplastic thyroid cancer. J. Clin. Endocrinol. Metab. 2012, 97, 3179–3184. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bible, K.C.; Menefee, M.E.; Lin, C.J.; Millward, M.J.; Maples, W.J.; Goh, B.C.; Karlin, N.J.; Kane, M.A.; Adkins, D.R.; Molina, J.R.; et al. An International Phase 2 Study of Pazopanib in Progressive and Metastatic Thyroglobulin Antibody Negative Radioactive Iodine Refractory Differentiated Thyroid Cancer. Thyroid 2020, 30, 1254–1262. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ball, D.W.; Jin, N.; Xue, P.; Bhan, S.; Ahmed, S.R.; Rosen, D.M.; Schayowitz, A.; Clark, D.P.; Nelkin, B.D. Trametinib with and without pazopanib has potent preclinical activity in thyroid cancer. Oncol. Rep. 2015, 34, 2319–2324. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kurzrock, R.; Ball, D.W.; Zahurak, M.L.; Nelkin, B.D.; Subbiah, V.; Ahmed, S.; O’Connor, A.; Karunsena, E.; Parkinson, R.M.; Bishop, J.A.; et al. A Phase I Trial of the VEGF Receptor Tyrosine Kinase Inhibitor Pazopanib in Combination with the MEK Inhibitor Trametinib in Advanced Solid Tumors and Differentiated Thyroid Cancers. Clin. Cancer Res. 2019, 25, 5475–5484. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Milling, R.V.; Grimm, D.; Krüger, M.; Grosse, J.; Kopp, S.; Bauer, J.; Infanger, M.; Wehland, M. Pazopanib, Cabozantinib, and Vandetanib in the Treatment of Progressive Medullary Thyroid Cancer with a Special Focus on the Adverse Effects on Hypertension. Int. J. Mol. Sci. 2018, 19, 3258. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ferrari, S.M.; Elia, G.; Ragusa, F.; Paparo, S.R.; Mazzi, V.; Patrizio, A.; Piaggi, S.; Baldini, E.; Centanni, M.; La Motta, C.; et al. Antineoplastic Activity of Pazopanib in Anaplastic Thyroid Cancer in Primary Culture. Int. J. Mol. Sci. 2023, 24, 2398. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Fullmer, T.; Cabanillas, M.E.; Zafereo, M. Novel Therapeutics in Radioactive Iodine-Resistant Thyroid Cancer. Front. Endocrinol. 2021, 12, 720723. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hofmann, M.C.; Kunnimalaiyaan, M.; Wang, J.R.; Busaidy, N.L.; Sherman, S.I.; Lai, S.Y.; Zafereo, M.; Cabanillas, M.E. Molecular mechanisms of resistance to kinase inhibitors and redifferentiation in thyroid cancers. Endocr. Relat. Cancer 2022, 29, R173–R190. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ramos, A.; Sadeghi, S.; Tabatabaeian, H. Battling Chemoresistance in Cancer: Root Causes and Strategies to Uproot Them. Int. J. Mol. Sci. 2021, 22, 9451. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhao, H.; Liu, C.H.; Cao, Y.; Zhang, L.Y.; Zhao, Y.; Liu, Y.W.; Liu, H.F.; Lin, Y.S.; Li, X.Y. Survival prognostic factors for differentiated thyroid cancer patients with pulmonary metastases: A systematic review and meta-analysis. Front. Oncol. 2022, 12, 990154. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Pavlidis, E.T.; Galanis, I.N.; Pavlidis, T.E. Update on current diagnosis and management of anaplastic thyroid carcinoma. World J. Clin. Oncol. 2023, 14, 570–583. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
| Country | Year | Study Design | Sample Size | Study Quality | Study and Author |
|---|---|---|---|---|---|
| USA | 2010 | Multicenter Phase II Trial | 37 | High | Bible et al. [11] |
| USA | 2017 | Phase I Trial | 6 | Moderate | Chow et al. [21] |
| USA | 2023 | Randomized Phase II Trial | 71 | High | Sherman et al. [22] |
| France | 2021 | Randomized Phase II Trial (PAZOTHYR) | 100 | High | de la Fouchardière et al. [23] |
| USA | 2012 | Multicenter Phase II Trial | 15 | High | Bible et al. [24] |
| Multi-country | 2020 | International Phase II Trial | 60 | High | Bible et al. [25] |
| Sample Size | Mean Age (Years) | Gender (Male/Female) | Thyroid Cancer Type | Prior Treatments | Study and Author |
|---|---|---|---|---|---|
| 37 | Median 59 (range 25–82) | 17/20 | RAI-refractory DTC | Surgery, RAI, some prior MKIs | Bible et al. [11] |
| 6 | Mean 57.5 (range 37–72) | 4/2 | Well-differentiated thyroid carcinoma (WDTC) | Surgery, RAI, some prior TKIs | Chow et al. [21] |
| 89 (71 eligible) | Median 65 (IQR 58–68) | 34/37 | Anaplastic thyroid carcinoma (ATC) | Surgery, no prior systemic therapy for ATC | Sherman et al. [22] |
| 168 (100 randomized) | Median 67 (range 34–85) | 81/87 | RAI-refractory DTC | Surgery, RAI, some prior MKIs | de la Fouchardière et al. [23] |
| 15 | Median 66 (range 45–77) | 5/10 | Anaplastic thyroid carcinoma (ATC) | Surgery, radiation, prior systemic therapies | Bible et al. [24] |
| 60 | Median 60 (25th–75th percentile 51–69) | 33/27 | RAI-refractory DTC | Surgery, RAI, prior systemic therapies | Bible et al. [25] |
| Study and Author | Pazopanib Dosage | Treatment Duration | Efficacy Outcomes |
|---|---|---|---|
| Bible et al. [11] | 800 mg daily until progression or intolerability | Median PFS 11.7 months | Partial response rate of 49%; median OS not reached |
| Chow et al. [21] | 800 mg daily for 12 weeks | Median PFS 6.7 months | 4/5 (80%) achieved stable disease; no significant impact on iodine uptake |
| Sherman et al. [22] | 400 mg daily pre-IMRT, 300 mg during IMRT | Pazopanib arm median OS 5.7 months | No significant difference in OS between pazopanib and placebo arms |
| de la Fouchardière et al. [23] | 800 mg daily (continuous or intermittent) | Median TTF not significantly different | ORR 5% post-randomization; intermittent dosing not superior |
| Bible et al. [24] | 800 mg daily until progression or intolerability | Median PFS 2.1 months | No confirmed RECIST responses; minimal clinical activity |
| Bible et al. [25] | 800 mg daily until progression or intolerability | Median PFS 11.4 months | Partial response rate of 36.7%; median OS 2.6 years |
| Study and Author | Common Adverse Events | Grade 3–5 Adverse Events | Treatment Discontinuation Due to AEs |
|---|---|---|---|
| Bible et al. [11] | Hypertension (47%), Diarrhea (49%), Fatigue (51%) | Grade 3–4 AEs in 46%; one grade 5 AE (pulmonary hemorrhage) | 14% discontinued due to AEs |
| Chow et al. [21] | Fatigue (100%), Anorexia (83%), Diarrhea (67%), Hypertension (33%) | Grade 3–4 hematologic toxicity, fatigue, arrhythmia | Not specified |
| Sherman et al. [22] | Dysphagia, Radiation Dermatitis, ALT/AST Elevation, Oral Mucositis | Grade 3–5 AEs in 88.9% (pazopanib), 85.3% (placebo); one grade 5 AE (sepsis) in pazopanib arm | Not specified |
| de la Fouchardière et al. [23] | Hypertension (50%), Diarrhea (86%), AST/ALT Increase (44%) | Grade 3–4 AEs in 54% (before randomization); no significant difference between arms | 38% (IP), 34% (CP) experienced grade 3–4 AEs |
| Bible et al. [24] | Hypertension (53%), Fatigue (73%), Diarrhea (47%) | Grade 3–4 hypertension (13%), pharyngolaryngeal pain (13%); one death due to tumor hemorrhage | Treatment discontinued in some due to AEs |
| Bible et al. [25] | Hypertension (71.7%), Fatigue (78.3%), Diarrhea (75%) | Grade 3–5 hypertension (21.7%), fatigue (8.3%); two deaths possibly related | 10.3% discontinued due to AEs |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mederle, A.L.; Stana, L.G.; Ilie, A.C.; Borza, C.; Streian, C.G.; Nistor, D.; Cerbulescu, T.; Belovan, B.; Lascu, A. Efficacy and Safety of Pazopanib in the Treatment of Thyroid Cancer: A Systematic Review. Biomedicines 2024, 12, 2820. https://doi.org/10.3390/biomedicines12122820
Mederle AL, Stana LG, Ilie AC, Borza C, Streian CG, Nistor D, Cerbulescu T, Belovan B, Lascu A. Efficacy and Safety of Pazopanib in the Treatment of Thyroid Cancer: A Systematic Review. Biomedicines. 2024; 12(12):2820. https://doi.org/10.3390/biomedicines12122820
Chicago/Turabian StyleMederle, Alexandra Laura, Loredana Gabriela Stana, Adrian Cosmin Ilie, Claudia Borza, Caius Glad Streian, Daciana Nistor, Teodor Cerbulescu, Biliana Belovan, and Ana Lascu. 2024. "Efficacy and Safety of Pazopanib in the Treatment of Thyroid Cancer: A Systematic Review" Biomedicines 12, no. 12: 2820. https://doi.org/10.3390/biomedicines12122820
APA StyleMederle, A. L., Stana, L. G., Ilie, A. C., Borza, C., Streian, C. G., Nistor, D., Cerbulescu, T., Belovan, B., & Lascu, A. (2024). Efficacy and Safety of Pazopanib in the Treatment of Thyroid Cancer: A Systematic Review. Biomedicines, 12(12), 2820. https://doi.org/10.3390/biomedicines12122820






