Haptic Technology: Exploring Its Underexplored Clinical Applications—A Systematic Review
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Strategy
2.2. Study Eligibility and Selection
2.3. Data Collection and Quality Assessment
2.4. Narrative-Qualitative Data Synthesis
3. Results
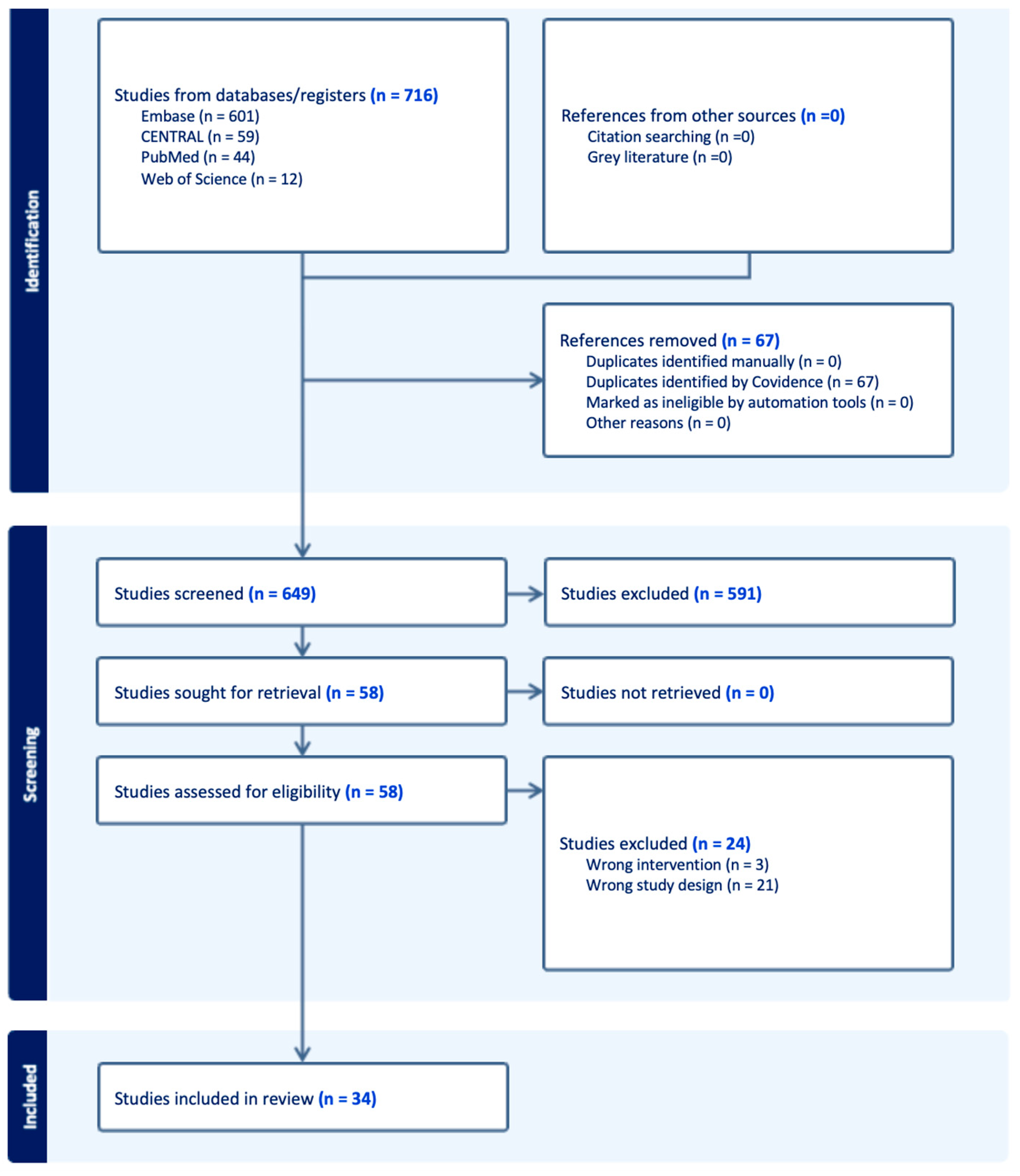
3.1. Study Selection
3.2. Evidence Summary
- (A)
- Clinical Outcomes.
- (B)
- Clinical Skills Training.
3.3. Market Trends in Haptic Technology Development
3.4. Risk of Bias
4. Discussion
4.1. Haptic Devices in Rehabilitation
4.2. Haptic Devices in Clinical Education and Simulation
4.3. Potential Applications and Research Recommendations
4.4. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Culmer, P.; Alazmani, A.; Mushtaq, F.; Cross, W.; Jayne, D. 15-Haptics in Surgical Robots. In Handbook of Robotic and Image-Guided Surgery; Abedin-Nasab, M.H., Ed.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 239–263. [Google Scholar]
- Stone, R.J. Haptic feedback: A brief history from telepresence to virtual reality. In Haptic Human-Computer Interaction; Brewster, S., Murray-Smith, R., Eds.; Springer: Berlin/Heidelberg, Germany, 2001; pp. 1–16. [Google Scholar]
- Adilkhanov, A.; Rubagotti, M.; Kappassov, Z. Haptic Devices: Wearability-Based Taxonomy and Literature Review. IEEE Access 2022, 10, 91923–91947. [Google Scholar] [CrossRef]
- Okamura, A.M. Haptic feedback in robot-assisted minimally invasive surgery. Curr. Opin. Urol. 2009, 19, 102–107. [Google Scholar] [CrossRef]
- Sreelakshmi, M.; Subash, T.D. Haptic Technology: A comprehensive review on its applications and future prospects. Mater. Today Proc. 2017, 4 Pt B, 4182–4187. [Google Scholar] [CrossRef]
- Lum, M.J.; Rosen, J.; King, H.; Friedman, D.C.; Lendvay, T.S.; Wright, A.S.; Sinanan, M.N.; Hannaford, B. Teleoperation in surgical robotics–network latency effects on surgical performance. In Proceedings of the 2009 Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Minneapolis, MN, USA, 3–6 September 2009; Volume 2009, pp. 6860–6863. [Google Scholar] [CrossRef]
- González, C.; Solanes, J.E.; Muñoz, A.; Gracia, L.; Girbés-Juan, V.; Tornero, J. Advanced teleoperation and control system for industrial robots based on augmented virtuality and haptic feedback. J. Manuf. Syst. 2021, 59, 283–298. [Google Scholar] [CrossRef]
- Tanwar, V.; Anand, V.; Aggarwal, P.; Kumar, M.; Kumar, G. Revolutionizing Military Training: A Comprehensive Review of Tactical and Technical Training through Augmented Reality, Virtual Reality, and Haptics. In Proceedings of the 2024 IEEE 9th International Conference for Convergence in Technology (I2CT), Pune, India, 5–7 April 2024. [Google Scholar] [CrossRef]
- Barbosa, F.; Mendes, D.; Rodrigues, R. Shape-A-Getti: A haptic device for getting multiple shapes using a simple actuator. Comput. Graph. 2023, 117, 42–50. [Google Scholar] [CrossRef]
- Garrofé, G.; Parés, C.; Gutiérrez, A.; Ruiz, C.; Serra, G.; Miralles, D. Virtual Haptic System for Shape Recognition Based on Local Curvatures. In Advances in Computer Graphics; Magnenat-Thalmann, N., Interrante, V., Thalmann, D., Papagiannakis, G., Sheng, B., Kim, J., Gavrilova, M., Eds.; Springer International Publishing: Cham, Switzerland, 2021; pp. 41–53. [Google Scholar]
- Van Wegen, M.; Herder, J.L.; Adelsberger, R.; Pastore-Wapp, M.; van Wegen, E.E.H.; Bohlhalter, S.; Nef, T.; Krack, P.; Vanbellingen, T. An Overview of Wearable Haptic Technologies and Their Performance in Virtual Object Exploration. Sensors 2023, 23, 1563. [Google Scholar] [CrossRef]
- Messaoudi, M.D.; Menelas, B.-A.J.; Mcheick, H. Review of Navigation Assistive Tools and Technologies for the Visually Impaired. Sensors 2022, 22, 7888. [Google Scholar] [CrossRef]
- Hayward, V.; Choksi, J.; Lanvin, G.; Ramstein, C. Design and Multi-Objective Optimization of a Linkage for a Haptic Interface. In Advances in Robot Kinematics and Computational Geometry; Springer: Berlin/Heidelberg, Germany, 1994. [Google Scholar] [CrossRef]
- Sigrist, R.; Rauter, G.; Riener, R.; Wolf, P. Augmented visual, auditory, haptic, and multimodal feedback in motor learning: A review. Psychon. Bull. Rev. 2013, 20, 21–53. [Google Scholar] [CrossRef] [PubMed]
- Grunwald, M.; Ettrich, C.; Krause, W.; Assmann, B.; Dähne, A.; Weiss, T.; Gertz, H.J. Haptic perception in anorexia nervosa before and after weight gain. J. Clin. Exp. Neuropsychol. 2001, 23, 520–529. [Google Scholar] [CrossRef] [PubMed]
- Fletcher, M.D.; Zgheib, J.; Perry, S.W. Sensitivity to haptic sound-localisation cues. Sci. Rep. 2021, 11, 312. [Google Scholar] [CrossRef] [PubMed]
- Rincon-Gonzalez, L.; Warren, J.P.; Meller, D.M.; Tillery, S.H. Haptic interaction of touch and proprioception: Implications for neuroprosthetics. IEEE Trans. Neural Syst. Rehabil. Eng. 2011, 19, 490–500. [Google Scholar] [CrossRef] [PubMed]
- Lackner, J.R.; DiZio, P. Vestibular, proprioceptive, and haptic contributions to spatial orientation. Annu. Rev. Psychol. 2005, 56, 115–147. [Google Scholar] [CrossRef]
- Sanfilippo, F.; Blazauskas, T.; Salvietti, G.; Ramos, I.; Vert, S.; Radianti, J.; Majchrzak, T.A.; Oliveira, D. A Perspective Review on Integrating VR/AR with Haptics into STEM Education for Multi-Sensory Learning. Robotics 2022, 11, 41. [Google Scholar] [CrossRef]
- Omata, S.; Murayama, Y.; Constantinou, C.E. Multi-sensory surgical support system incorporating, tactile, visual and auditory perception modalities. Stud. Health Technol. Inf. 2005, 111, 369–371. [Google Scholar]
- Steinberg, A.; Banerjee, P.; Drummond, J.; Zefran, M. Progress in the development of a haptic/virtual reality simulation program for scaling and root planning. J. Dent. Educ. 2003, 67, 161. [Google Scholar]
- Rhienmora, P.; Haddawy, P.; Dailey, M.; Khanal, P.; Suebnukarn, S. Development of a Dental Skills Training Simulator Using Virtual Realityand Haptic Device. NECTEC Tech. J. 2008, 8, 140–147. [Google Scholar]
- Judkins, T.N.; Oleynikov, D.; Stergiou, N. Objective evaluation of expert and novice performance during robotic surgical training tasks. Surg. Endosc. 2009, 23, 590–597. [Google Scholar] [CrossRef]
- El Rassi, I.; El Rassi, J.M. A review of haptic feedback in tele-operated robotic surgery. J. Med. Eng. Technol. 2020, 44, 247–254. [Google Scholar] [CrossRef]
- Suebnukarn, S.; Haddawy, P.; Dailey, M.; Cao, D. Interactive Segmentation and Three-Dimension Reconstruction for Cone-Beam Computed-Tomography Images. NECTEC Tech. J. 2008, 8, 154–161. [Google Scholar]
- Moix, T.; Ilic, D.; Bleuler, H.; Zoethout, J. A haptic device for guide wire in interventional radiology procedures. Stud. Health Technol. Inf. 2006, 119, 388–392. [Google Scholar]
- Motaharifar, M.; Norouzzadeh, A.; Abdi, P.; Iranfar, A.; Lotfi, F.; Moshiri, B.; Lashay, A.; Mohammadi, S.F.; Taghirad, H.D. Applications of Haptic Technology, Virtual Reality, and Artificial Intelligence in Medical Training During the COVID-19 Pandemic. Front. Robot. AI 2021, 8, 612949. [Google Scholar] [CrossRef]
- Choukou, M.-A.; Mbabaali, S.; Bani Hani, J.; Cooke, C. Haptic-Enabled Hand Rehabilitation in Stroke Patients: A Scoping Review. Appl. Sci. 2021, 11, 3712. [Google Scholar] [CrossRef]
- Gutiérrez, Á.; Sepúlveda-Muñoz, D.; Gil-Agudo, Á.; de los Reyes Guzmán, A. Serious Game Platform with Haptic Feedback and EMG Monitoring for Upper Limb Rehabilitation and Smoothness Quantification on Spinal Cord Injury Patients. Appl. Sci. 2020, 10, 963. [Google Scholar] [CrossRef]
- Che Me, R.; Biamonti, A.; Mohd Saad, M.R. Conceptual Design of Haptic-Feedback Navigation Device for Individuals with Alzheimer’s Disease. Stud. Health Technol. Inf. 2015, 217, 195–203. [Google Scholar]
- Fason, J.; Gudin, J.; Hurwitz, P. Improvement of Mental Health and Anxiety with Haptic Technology Patch Utilization: Interim Results from an Exploratory Study. Int. J. Psychiatry Res. 2024, 7, 1–8. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. Bmj 2021, 372, n71. [Google Scholar] [CrossRef]
- Sterne, J.A.C.; Savović, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A revised tool for assessing risk of bias in randomised trials. Bmj 2019, 366, l4898. [Google Scholar] [CrossRef]
- Popay, J.; Roberts, H.; Sowden, A.; Petticrew, M.; Arai, L.; Rodgers, M.; Britten, N.; Roen, K.; Duffy, S. Guidance on the Conduct of Narrative Synthesis in Systematic Reviews: A Product from the ESRC Methods Programme; Institute for Health Research: London, UK, 2006. [Google Scholar] [CrossRef]
- Ranzani, R.; Lambercy, O.; Metzger, J.C.; Califfi, A.; Regazzi, S.; Dinacci, D.; Petrillo, C.; Rossi, P.; Conti, F.M.; Gassert, R. Neurocognitive robot-assisted rehabilitation of hand function: A randomized control trial on motor recovery in subacute stroke. J. Neuroeng. Rehabil. 2020, 17, 115. [Google Scholar] [CrossRef]
- Ang, K.K.; Guan, C.; Phua, K.S.; Wang, C.; Zhou, L.; Tang, K.Y.; Ephraim Joseph, G.J.; Kuah, C.W.; Chua, K.S. Brain-computer interface-based robotic end effector system for wrist and hand rehabilitation: Results of a three-armed randomized controlled trial for chronic stroke. Front. Neuroeng. 2014, 7, 30. [Google Scholar] [CrossRef] [PubMed]
- Brokaw, E.B.; Murray, T.; Nef, T.; Lum, P.S. Retraining of interjoint arm coordination after stroke using robot-assisted time-independent functional training. J. Rehabil. Res. Dev. 2011, 48, 299–316. [Google Scholar] [CrossRef] [PubMed]
- Baur, K.; Speth, F.; Nagle, A.; Riener, R.; Klamroth-Marganska, V. Music meets robotics: A prospective randomized study on motivation during robot aided therapy. J. NeuroEngineering Rehabil. 2018, 15, 79. [Google Scholar] [CrossRef] [PubMed]
- Wuang, Y.P.; Huang, C.L.; Wu, C.S. Haptic Perception Training Programs on Fine Motor Control in Adolescents with Developmental Coordination Disorder: A Preliminary Study. J. Clin. Med. 2022, 11, 4755. [Google Scholar] [CrossRef]
- Bortone, I.; Barsotti, M.; Leonardis, D.; Crecchi, A.; Tozzini, A.; Bonfiglio, L.; Frisoli, A. Immersive Virtual Environments and Wearable Haptic Devices in rehabilitation of children with neuromotor impairments: A single-blind randomized controlled crossover pilot study. J. NeuroEngineering Rehabil. 2020, 17, 144. [Google Scholar] [CrossRef]
- Timmermans, A.A.; Lemmens, R.J.; Monfrance, M.; Geers, R.P.; Bakx, W.; Smeets, R.J.; Seelen, H.A. Effects of task-oriented robot training on arm function, activity, and quality of life in chronic stroke patients: A randomized controlled trial. J. Neuroeng. Rehabil. 2014, 11, 45. [Google Scholar] [CrossRef]
- Abdollahi, F.; Case Lazarro, E.D.; Listenberger, M.; Kenyon, R.V.; Kovic, M.; Bogey, R.A.; Hedeker, D.; Jovanovic, B.D.; Patton, J.L. Error augmentation enhancing arm recovery in individuals with chronic stroke: A randomized crossover design. Neurorehabil. Neural Repair. 2014, 28, 120–128. [Google Scholar] [CrossRef]
- Cameirão, M.S.; Badia, S.B.; Duarte, E.; Frisoli, A.; Verschure, P.F. The combined impact of virtual reality neurorehabilitation and its interfaces on upper extremity functional recovery in patients with chronic stroke. Stroke 2012, 43, 2720–2728. [Google Scholar] [CrossRef] [PubMed]
- Childs, S.; McVicker, Z.; Trombetta, R.; Awad, H.; Elfar, J.; Giordano, B. Patient-Specific 3-Dimensional Modeling and Its Use for Preoperative Counseling of Patients Undergoing Hip Arthroscopy. Orthop. J. Sports Med. 2018, 6, 2325967118794645. [Google Scholar] [CrossRef]
- Brown, J.D.; Paek, A.; Syed, M.; O’Malley, M.K.; Shewokis, P.A.; Contreras-Vidal, J.L.; Davis, A.J.; Gillespie, R.B. An exploration of grip force regulation with a low-impedance myoelectric prosthesis featuring referred haptic feedback. J. Neuroeng. Rehabil. 2015, 12, 104. [Google Scholar] [CrossRef]
- Hsu, H.Y.; Lin, C.W.; Lin, Y.C.; Wu, P.T.; Kato, H.; Su, F.C.; Kuo, L.C. Effects of vibrotactile-enhanced music-based intervention on sensorimotor control capacity in the hand of an aging brain: A pilot feasibility randomized crossover trial. BMC Geriatr. 2021, 21, 660. [Google Scholar] [CrossRef]
- Yoon, H.U.; Anil Kumar, N.; Hur, P. Synergistic Effects on the Elderly People’s Motor Control by Wearable Skin-Stretch Device Combined with Haptic Joystick. Front. Neurorobot. 2017, 11, 31. [Google Scholar] [CrossRef]
- Dobrushina, O.; Tamim, Y.; Wald, I.Y.; Maimon, A.; Amedi, A. Training interoceptive awareness with real-time haptic vs. visual heartbeat feedback. bioRxiv 2024. [Google Scholar] [CrossRef]
- Mueller, S.; Habermann, S.; Dudda, J.; Grunwald, M. Observation of own exploration movements impairs haptic spatial perception. Exp. Brain Res. 2013, 231, 415–423. [Google Scholar] [CrossRef]
- Rowland, D.; Davis, B.; Higgins, T.; Fey, A.M. Enhancing User Performance by Adaptively Changing Haptic Feedback Cues in a Fitts’s Law Task. IEEE Trans. Haptics 2024, 17, 92–99. [Google Scholar] [CrossRef]
- Zadravec, M.; Matjačić, Z. The influence of haptic support algorithm dynamics on the efficacy of motor learning. Zdr. Vestn. 2011, 80, 561–570. [Google Scholar]
- Chowriappa, A.; Raza, S.J.; Fazili, A.; Field, E.; Malito, C.; Samarasekera, D.; Shi, Y.; Ahmed, K.; Wilding, G.; Kaouk, J.; et al. Augmented-reality-based skills training for robot-assisted urethrovesical anastomosis: A multi-institutional randomised controlled trial. BJU Int. 2015, 115, 336–345. [Google Scholar] [CrossRef]
- Ström, P.; Hedman, L.; Särnå, L.; Kjellin, A.; Wredmark, T.; Felländer-Tsai, L. Early exposure to haptic feedback enhances performance in surgical simulator training: A prospective randomized crossover study in surgical residents. Surg. Endosc. 2006, 20, 1383–1388. [Google Scholar] [CrossRef]
- Hagelsteen, K.; Johansson, R.; Ekelund, M.; Bergenfelz, A.; Anderberg, M. Performance and perception of haptic feedback in a laparoscopic 3D virtual reality simulator. Minim. Invasive Ther. Allied. Technol. 2019, 28, 309–316. [Google Scholar] [CrossRef] [PubMed]
- Våpenstad, C.; Hofstad, E.F.; Bø, L.E.; Kuhry, E.; Johnsen, G.; Mårvik, R.; Langø, T.; Hernes, T.N. Lack of transfer of skills after virtual reality simulator training with haptic feedback. Minim. Invasive Ther. Allied. Technol. 2017, 26, 346–354. [Google Scholar] [CrossRef] [PubMed]
- Chao, C.; Chalouhi, G.E.; Bouhanna, P.; Ville, Y.; Dommergues, M. Randomized Clinical Trial of Virtual Reality Simulation Training for Transvaginal Gynecologic Ultrasound Skills. J. Ultrasound. Med. 2015, 34, 1663–1667. [Google Scholar] [CrossRef] [PubMed]
- Patel, A.; Koshy, N.; Ortega-Barnett, J.; Chan, H.C.; Kuo, Y.F.; Luciano, C.; Rizzi, S.; Matulyauskas, M.; Kania, P.; Banerjee, P.; et al. Neurosurgical tactile discrimination training with haptic-based virtual reality simulation. Neurol. Res. 2014, 36, 1035–1039. [Google Scholar] [CrossRef]
- Thompson, J.R.; Leonard, A.C.; Doarn, C.R.; Roesch, M.J.; Broderick, T.J. Limited value of haptics in virtual reality laparoscopic cholecystectomy training. Surg. Endosc. 2011, 25, 1107–1114. [Google Scholar] [CrossRef] [PubMed]
- Suebnukarn, S.; Haddawy, P.; Rhienmora, P.; Jittimanee, P.; Viratket, P. Augmented kinematic feedback from haptic virtual reality for dental skill acquisition. J. Dent. Educ. 2010, 74, 1357–1366. [Google Scholar] [CrossRef]
- Kantar, R.S.; Alfonso, A.R.; Ramly, E.P.; Cohen, O.; Rifkin, W.J.; Maliha, S.G.; Diaz-Siso, J.R.; Eisemann, B.S.; Saadeh, P.B.; Flores, R.L. Knowledge and Skills Acquisition by Plastic Surgery Residents through Digital Simulation Training: A Prospective, Randomized, Blinded Trial. Plast. Reconstr. Surg. 2020, 145, 184e–192e. [Google Scholar] [CrossRef] [PubMed]
- Lim, G.; Krohner, R.G.; Metro, D.G.; Rosario, B.L.; Jeong, J.H.; Sakai, T. Low-Fidelity Haptic Simulation Versus Mental Imagery Training for Epidural Anesthesia Technical Achievement in Novice Anesthesiology Residents: A Randomized Comparative Study. Anesth. Analg. 2016, 122, 1516–1523. [Google Scholar] [CrossRef]
- Salvador, M.A.; Arturo, M.M.; Fernando, P.E.; Dalia Danely, M.G.; Jorge, R.L.; Roberto, S.M.; José Rafael, R.B.; Jesús, T.J. Effects of Real Time Feedback on Novice’s Laparoscopic Learning Curve. J. Surg. Educ. 2024, 81, 1133–1153. [Google Scholar] [CrossRef]
- Postema, R.R.; van Gastel, L.A.; Hardon, S.F.; Bonjer, H.J.; Horeman, T. Haptic exploration improves performance of a laparoscopic training task. Surg. Endosc. 2021, 35, 4175–4182. [Google Scholar] [CrossRef]
- Alleblas, C.C.J.; Vleugels, M.P.H.; Coppus, S.; Nieboer, T.E. The effects of laparoscopic graspers with enhanced haptic feedback on applied forces: A randomized comparison with conventional graspers. Surg. Endosc. 2017, 31, 5411–5417. [Google Scholar] [CrossRef]
- Camp, C.L.; Martin, J.R.; Krych, A.J.; Taunton, M.J.; Spencer-Gardner, L.; Trousdale, R.T. Resection Technique Does Affect Resection Symmetry and Thickness of the Patella During Total Knee Arthroplasty: A Prospective Randomized Trial. J. Arthroplast. 2015, 30, 2110–2115. [Google Scholar] [CrossRef] [PubMed]
- Chang, S.H.; Yu, N.Y. Visual and Haptic Perception Training to Improve Handwriting Skills in Children With Dysgraphia. Am. J. Occup. Ther. 2017, 71, 7102220030.p1–7102220030.p10. [Google Scholar] [CrossRef]
- Demain, S.; Metcalf, C.D.; Merrett, G.V.; Zheng, D.; Cunningham, S. A narrative review on haptic devices: Relating the physiology and psychophysical properties of the hand to devices for rehabilitation in central nervous system disorders. Disabil. Rehabil. Assist. Technol. 2013, 8, 181–189. [Google Scholar] [CrossRef]
- Basalp, E.; Wolf, P.; Marchal-Crespo, L. Haptic Training: Which Types Facilitate (re)Learning of Which Motor Task and for Whom? Answers by a Review. IEEE Trans. Haptics 2021, 14, 722–739. [Google Scholar] [CrossRef] [PubMed]
- Hirano, M.; Sakurada, M.; Furuya, S. Overcoming the ceiling effects of experts’ motor expertise through active haptic training. Sci. Adv. 2020, 6, eabd2558. [Google Scholar] [CrossRef] [PubMed]
- Feygin, D.; Keehner, M.; Tendick, R. Haptic guidance: Experimental evaluation of a haptic training method for a perceptual motor skill. In Proceedings of the IEEE 10th Symposium on Haptic Interfaces for Virtual Environment and Teleoperator Systems (HAPTICS 2002), Orlando, FL, USA, 24–25 March 2002; pp. 40–47. [Google Scholar]
- Leleve, A.; McDaniel, T.; Rossa, C. Haptic Training Simulation. Front. Virtual Real. 2020, 1. [Google Scholar] [CrossRef]
- Kappers, A.M.L.; Bergmann Tiest, W.M. Haptic perception. Wiley Interdiscip. Rev. Cogn. Sci. 2013, 4, 357–374. [Google Scholar] [CrossRef]
- Ankarali, M.M.; Tutkun Sen, H.; De, A.; Okamura, A.M.; Cowan, N.J. Haptic feedback enhances rhythmic motor control by reducing variability, not improving convergence rate. J. Neurophysiol. 2014, 111, 1286–1299. [Google Scholar] [CrossRef]
- Wang, D.; Li, T.; Yang, G.; Zhang, Y. Effects of concurrent and delayed visual feedback on motor memory consolidation. IEEE Trans. Haptics 2017, 10, 350–357. [Google Scholar] [CrossRef] [PubMed]
- Van der Meijden, O.A.J.; Schijven, M.P. The value of haptic feedback in conventional and robot-assisted minimal invasive surgery and virtual reality training: A current review. Surg. Endosc. 2009, 23, 1180–1190. [Google Scholar] [CrossRef]
- Singapogu, R.; Burg, T.; Burg, K.J.L.; Smith, D.E.; Eckenrode, A.H. A perspective on the role and utility of haptic feedback in laparoscopic skills training. Crit. Rev. Biomed. Eng. 2014, 42. [Google Scholar] [CrossRef] [PubMed]
- Meling, T.R.; Meling, T.R. The impact of surgical simulation on patient outcomes: A systematic review and meta-analysis. Neurosurg. Rev. 2021, 44, 843–854. [Google Scholar] [CrossRef]
- Rangarajan, K.; Davis, H.; Pucher, P.H. Systematic review of virtual haptics in surgical simulation: A valid educational tool? J. Surg. Educ. 2020, 77, 337–347. [Google Scholar] [CrossRef]
- Vasseur, D.; Ipakchian Askari, S.; Suijkerbuijk, S.; Nap, H.H.; W, I.J. Sensory, Affective, and Social Experiences with Haptic Devices in Intramural Care Practice. Nurs. Rep. 2024, 14, 230–253. [Google Scholar] [CrossRef] [PubMed]
- Richard, G.; Pietrzak, T.; Argelaguet, F.; Lécuyer, A.; Casiez, G. Studying the role of haptic feedback on virtual embodiment in a drawing task. Front. Virtual Real. 2021, 1, 573167. [Google Scholar] [CrossRef]
- Repperger, D.W.; Phillips, C.A. Haptic devices as a paradigm to enhance learning/function--theory and empirical studies. Disabil. Rehabil. Assist. Technol. 2010, 5, 97–107. [Google Scholar] [CrossRef]
- Gentaz, E.; Rossetti, Y. Is haptic perception continuous with cognition? Behav. Brain Sci. 1999, 22, 378–379. [Google Scholar] [CrossRef]
- Cheung, K.L.; Tunik, E.; Adamovich, S.V.; Boyd, L.A. Neuroplasticity and Virtual Reality. In Virtual Reality for Physical and Motor Rehabilitation; Weiss, P.L., Keshner, E.A., Levin, M.F., Eds.; Springer: New York: New York, NY, USA, 2014; pp. 5–24. [Google Scholar]
- Volz, M.S.; Suarez-Contreras, V.; Mendonca, M.E.; Pinheiro, F.S.; Merabet, L.B.; Fregni, F. Effects of sensory behavioral tasks on pain threshold and cortical excitability. PLoS ONE 2013, 8, e52968. [Google Scholar] [CrossRef]
- Davis, R.L.; Orta Martinez, M.; Schneider, O.; MacLean, K.E.; Okamura, A.M.; Blikstein, P. The Haptic Bridge: Towards a Theory for Haptic-Supported Learning; ACM: New York, NY, USA, 2017; pp. 51–60. [Google Scholar]
- Sun, F.; Lu, Q.; Hao, M.; Wu, Y.; Li, Y.; Liu, L.; Li, L.; Wang, Y.; Zhang, T. An artificial neuromorphic somatosensory system with spatio-temporal tactile perception and feedback functions. NPJ Flex. Electron. 2022, 6, 72. [Google Scholar] [CrossRef]
- Weiss, E.J.; Flanders, M. Somatosensory comparison during haptic tracing. Cereb Cortex 2011, 21, 425–434. [Google Scholar] [CrossRef] [PubMed]
- Kaklanis, N.; Votis, K.; Tzovaras, D. Adding Haptic Feedback to Web Applications Towards Improving End-Users’ Cognitive Capabilities; IEEE: New York, NY, USA, 2015; pp. 245–249. [Google Scholar]
- Kitada, R. The brain network for haptic object recogniton. In Pervasive Haptics: Science, Design, and Application; Springer: Berlin/Heidelberg, Germany, 2016; pp. 21–37. [Google Scholar]
- Millar, S. Network models for haptic perception. Infant Behav. Dev. 2005, 28, 250–265. [Google Scholar] [CrossRef]
- Zhang, S.; Wang, D.; Afzal, N.; Zhang, Y.; Wu, R. Rhythmic haptic stimuli improve short-term attention. IEEE Trans. Haptics 2016, 9, 437–442. [Google Scholar] [CrossRef]
- List, A.; Iordanescu, L.; Grabowecky, M.; Suzuki, S. Haptic guidance of overt visual attention. Atten. Percept. Psychophys. 2014, 76, 2221–2228. [Google Scholar] [CrossRef]
- Wang, D.; Zhang, Y.; Yang, X.; Yang, G.; Yang, Y. Force control tasks with pure haptic feedback promote short-term focused attention. IEEE Trans. Haptics 2014, 7, 467–476. [Google Scholar] [CrossRef] [PubMed]
- Ottink, L.; Buimer, H.; van Raalte, B.; Doeller, C.F.; van der Geest, T.M.; van Wezel, R.J.A. Cognitive map formation supported by auditory, haptic, and multimodal information in persons with blindness. Neurosci. Biobehav. Rev. 2022, 140, 104797. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Shen, G. Haptic Sensing and Feedback Techniques toward Virtual Reality. Research 2024, 7, 0333. [Google Scholar] [CrossRef] [PubMed]
- Konczak, J.; Sciutti, A.; Avanzino, L.; Squeri, V.; Gori, M.; Masia, L.; Abbruzzese, G.; Sandini, G. Parkinson’s disease accelerates age-related decline in haptic perception by altering somatosensory integration. Brain 2012, 135 Pt 11, 3371–3379. [Google Scholar] [CrossRef]
- Solaro, C.; Cattaneo, D.; Basteris, A.; Carpinella, I.; De Luca, A.; Mueller, M.; Bertoni, R.; Ferrarin, M.; Sanguineti, V. Haptic vs sensorimotor training in the treatment of upper limb dysfunction in multiple sclerosis: A multi-center, randomised controlled trial. J. Neurol. Sci. 2020, 412, 116743. [Google Scholar] [CrossRef]
| Company | Foundation | Location | Technology Description |
|---|---|---|---|
| Immersion Corporation | 1993 | USA | Haptic technology for medical simulation and training. |
| Haption | 2001 | FR | Real-time haptic interfaces for surgical training and diagnostics. |
| Synaptics Incorporated | 1986 | USA | Provides touch, display, and biometrics technology, crucial for medical devices that require sophisticated haptic feedback. |
| Microchip Technology Inc. | 1989 | USA | Designs essential components like microcontrollers and integrated circuits for haptic feedback systems in medical devices. |
| ON Semiconductor Corporation | 1999 | USA | Develops sensors and controllers for energy-efficient haptic solutions in clinical environments. |
| Ultraleap Holdings Ltd. | 2013 | UK | Touchless haptic technology and hand tracking for virtual reality applications in healthcare. |
| DOT | 2014 | KR | Develops innovative haptic technology, primarily focused on creating accessible devices for the visually impaired. |
| Johnson Electric Holdings Limited | 1959 | HK | Engineers motion subsystems and haptic components for use in various medical devices. |
| 3d Systems Corporation | 1986 | USA | 3D printing and additive manufacturing technologies, including haptic devices for medical modeling. |
| SMK Corporation | 1925 | JP | Manufactures electronic components, including those for haptic interfaces used in clinical instruments. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pacheco-Barrios, K.; Ortega-Márquez, J.; Fregni, F. Haptic Technology: Exploring Its Underexplored Clinical Applications—A Systematic Review. Biomedicines 2024, 12, 2802. https://doi.org/10.3390/biomedicines12122802
Pacheco-Barrios K, Ortega-Márquez J, Fregni F. Haptic Technology: Exploring Its Underexplored Clinical Applications—A Systematic Review. Biomedicines. 2024; 12(12):2802. https://doi.org/10.3390/biomedicines12122802
Chicago/Turabian StylePacheco-Barrios, Kevin, Jorge Ortega-Márquez, and Felipe Fregni. 2024. "Haptic Technology: Exploring Its Underexplored Clinical Applications—A Systematic Review" Biomedicines 12, no. 12: 2802. https://doi.org/10.3390/biomedicines12122802
APA StylePacheco-Barrios, K., Ortega-Márquez, J., & Fregni, F. (2024). Haptic Technology: Exploring Its Underexplored Clinical Applications—A Systematic Review. Biomedicines, 12(12), 2802. https://doi.org/10.3390/biomedicines12122802







