The Promising Potency of Sodium–Glucose Cotransporter 2 Inhibitors in the Prevention of and as Treatment for Cognitive Impairment Among Type 2 Diabetes Patients
Abstract
1. Introduction
2. Potential Pathophysiological Pathways About Diabetic CI
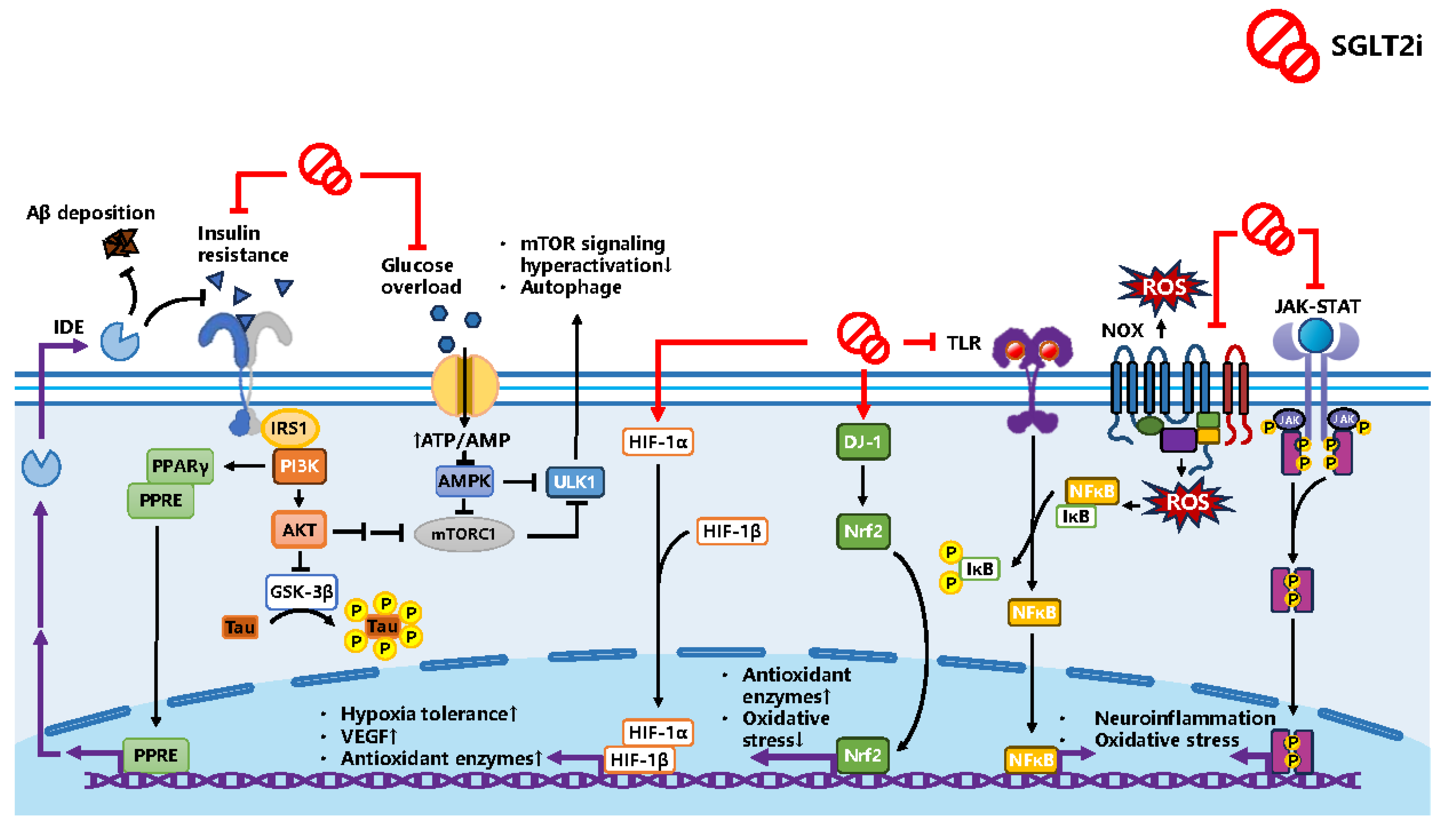
2.1. GSK3β: Impaired Insulin Signal Pathways for Insulin Resistance
2.2. IDE: Insulin Resistance and Aβ Protein
2.3. Neuroinflammation and Oxidative Stress
2.4. Cerebral Vascular Modification
3. SGLT2i Improving CI Among Patients with T2DM
3.1. Evidence from Primary and Secondary Analysis of Clinical Trials
3.1.1. Observational Clinical Trials and RCTs
| Author | Design | Population (Region) | Intervention | Versus | Outcome | Effect Size | Results (95%CI) | p for Results | |
|---|---|---|---|---|---|---|---|---|---|
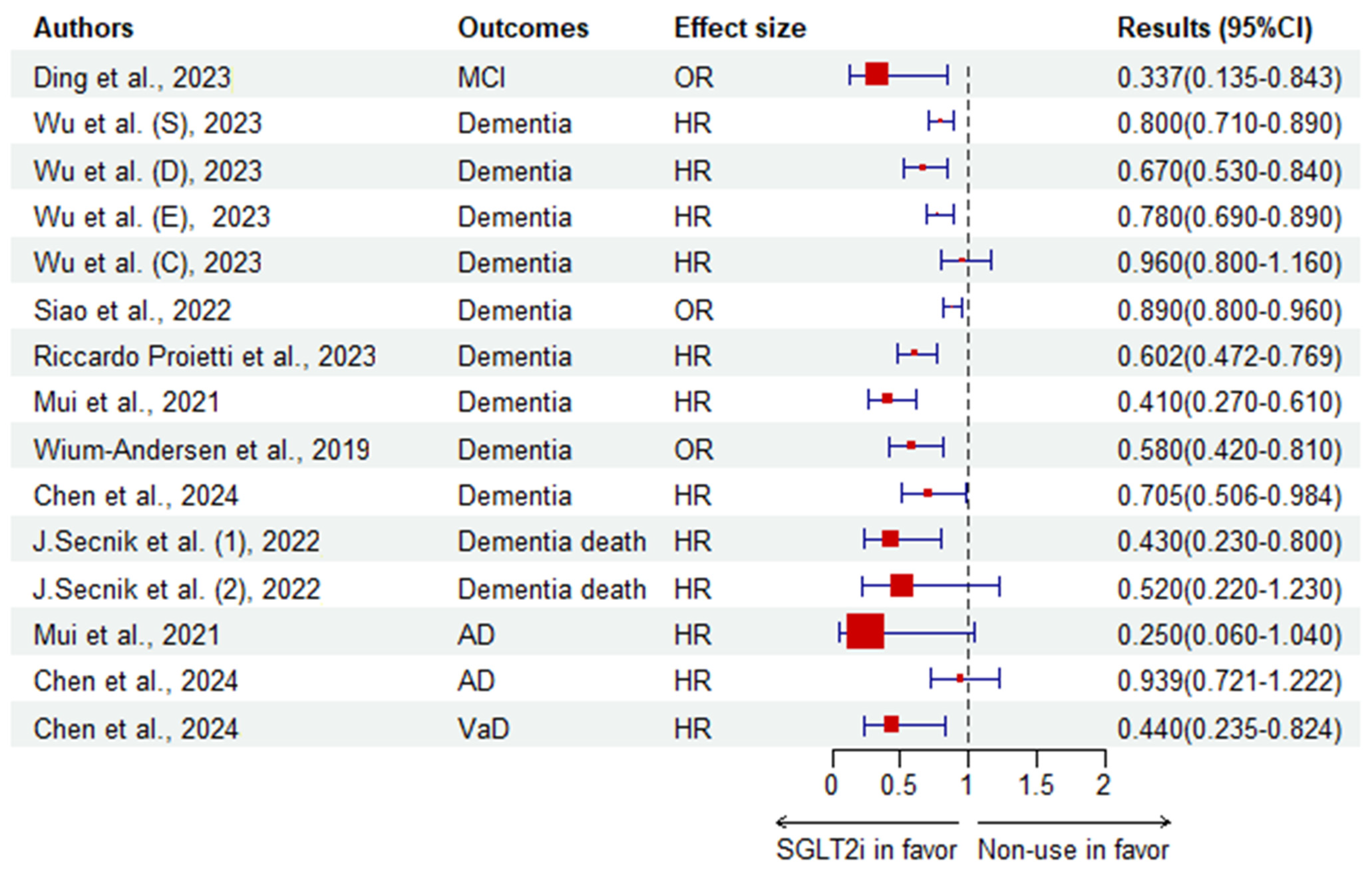
| Ding et al. [119]. | CS | 222 middle-aged and elderly patients with T2DM (China) | SGLT2i | Non-use | MCI | OR | 0.337 (0.135–0.843) | 0.020 | |
| Wium-Andersen et al. [122]. | N-CC | 11,619 dementia cases and 46,476 control all with T2DM (Denmark) | SGLT2i | Non-use | Dementia | OR | 0.58 (0.42–0.81) | ||
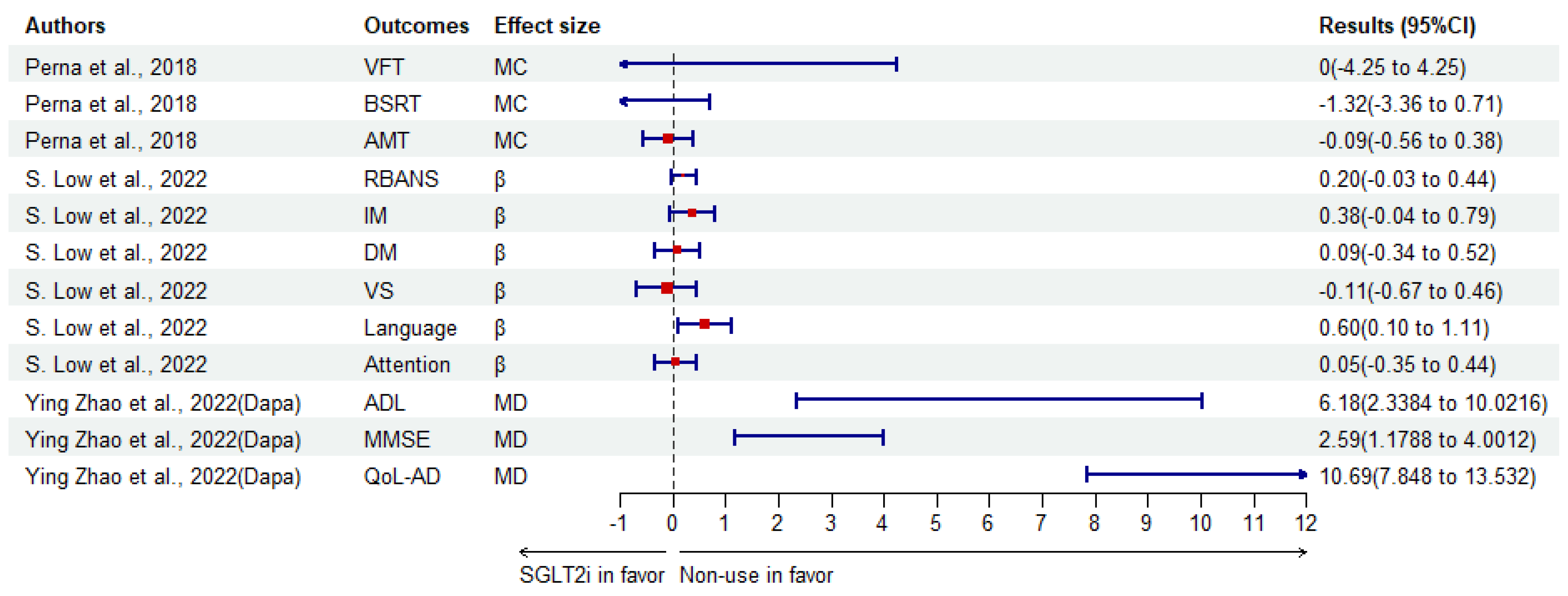
| S. Low et al. [123]. | C | 138 patients with T2DM (Singapore) | SGLT2i | Non-use | RBANS | β | 0.20 (−0.03–0.44) | 0.091 | |
| IM | 0.38 (−0.04–0.79) | 0.074 | |||||||
| DM | 0.09 (−0.34–0.52) | 0.677 | |||||||
| VS | −0.11 (−0.67–0.46) | 0.703 | |||||||
| Language | 0.60 (0.10–1.11) | 0.019 | |||||||
| Attention | 0.05 (−0.35–0.44) | 0.806 | |||||||
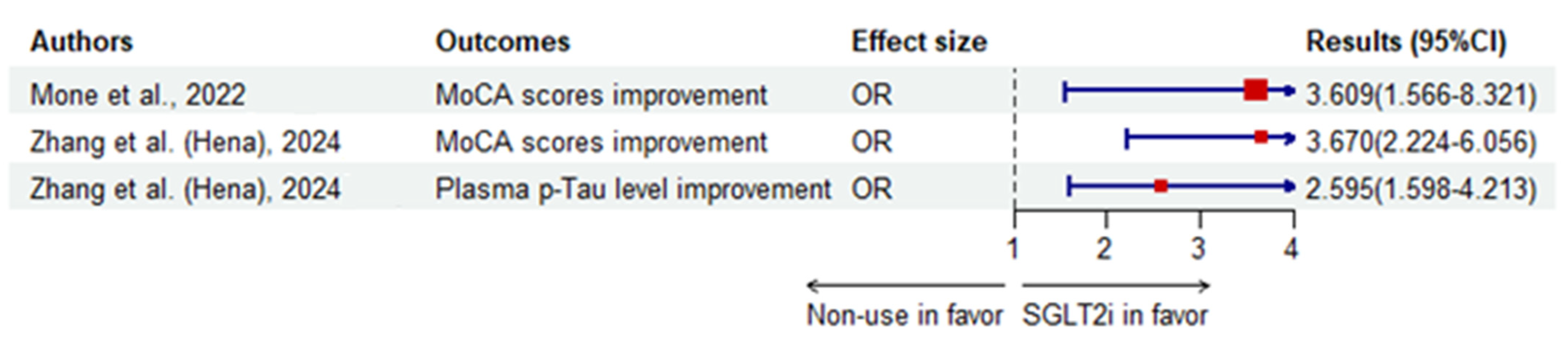
| Mone et al. [117]. | C | 162 Frail older adults > 65 years with T2DM and HFpEF (Italy) | Empagliflozin | Non-use | MoCA scores improvement | OR | 3.609 (1.566–8.321) | 0.003 | |
| P. Mone et al. [120]. | C | 166 frail elders with diabetes and CKD (USA) | Empagliflozin | Non-use | MoCA scores | MD | 1.9 (1.04) † | ||
| β | 0.794 (0.073–1.516) | 0.031 | |||||||
| Zhang et al. [124]. | C | 290 T2DM patients ≥ 45 years with MoCA ≤ 26 (henagliflozin, n = 135; non-use, n = 155) (China) | Henagliflozin | Non-use | MoCA scores improvement | OR | 3.670 (2.224–6.056) | <0.0001 | |
| Plasma p-Tau level improvement | 2.595 (1.598–4.213) | <0.0001 | |||||||
| Chen et al. [126]. | C | 2430 patients (age: 55–85 years) with AF and diabetes (Taiwan, China) | SGLT2i | Non-use | Dementia | HR | 0.705 (0.506–0.984) | ||
| AD | 0.939 (0.721–1.222) | ||||||||
| VaD | 0.440 (0.235–0.824) | ||||||||
| Other/mixed | 0.601 (0.296–1.220) | ||||||||
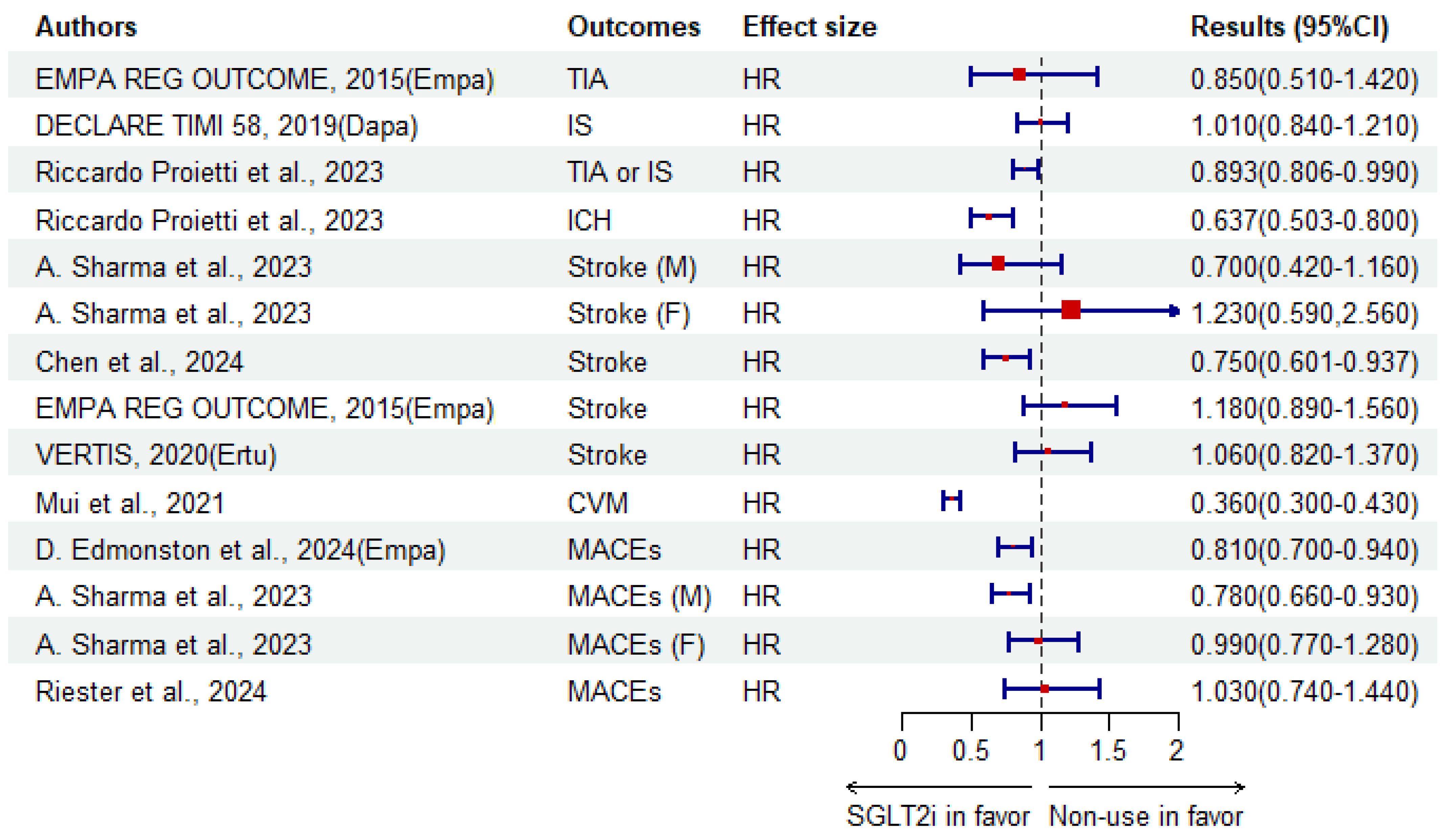
| Stroke | 0.750 (0.601–0.937) | ||||||||
| Riester et al. [133]. | C | 7710 elder aged ≥ 66 years with T2DM in nursing home (31.08% SGLT2i) (USA) | SGLT2i | GLP-1 RAs | MACE | HR | 1.03 (0.74–1.44) | ||
| A. Sharma et al. [129]. | C | 8026 patients (55.7% male) with T2DM at baseline (4231 SGLT2i users) (Australia) | SGLT2i | GLP-1 RAs | MACE | M | HR | 0.78 (0.66–0.93) | |
| F | 0.99 (0.77–1.28) | ||||||||
| Stroke | M | 0.70 (0.42–1.16) | |||||||
| F | 1.23 (0.59–2.56) | ||||||||
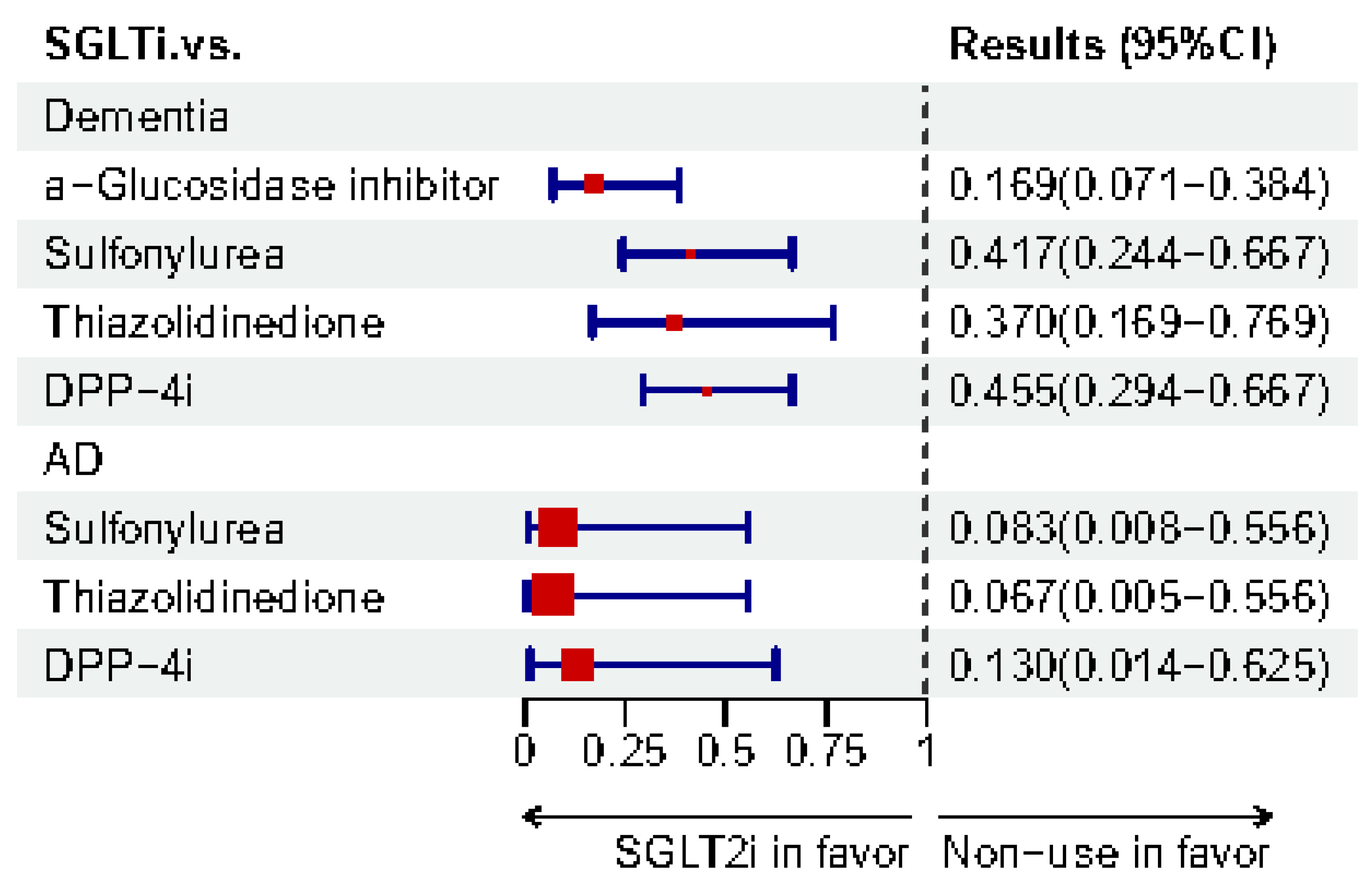
| Tang et al. [141]. | C | 35,458 new users aged ≥ 50 years of SGLT2i or SU with T2DM (USA) | SGLT2i | SU | Dementia | RD | −2.5% (−3.0%, −2.1%) | ||
| AD | −0.47% (−0.61%, −0.33%) | ||||||||
| VaD | −0.39% (−0.55%, −0.23%) | ||||||||
| Mui et al. [121]. | C | 51,460 T2DM users with a median age of 66.3 years (Hong Kong, China) | SGLT2i | DPP-4i | Dementia | HR | 0.41 (0.27–0.61) | <0.0001 | |
| AD | 0.25 (0.06–1.04) | 0.0546 | |||||||
| PD | 0.28 (0.09–0.91) | 0.0349 | |||||||
| CVM | 0.36 (0.30–0.43) | <0.0001 | |||||||
| D. Edmonston et al. [128]. | C | 62,197 adult patients with T2DM (USA) | Empagliflozin | DPP-4i | MACE | HR | 0.81 (0.70–0.94) | 0.007 | |
| Riccardo Proietti et al. [125]. | C | 89,356 patients aged ≥ 18 years with AF and T2DM (global) | Non-use | SGLT2i | IS or TIA | HR | 1.12 (1.01–1.24) | 0.029 | |
| ICH | 1.57 (1.25–1.99) | 0.001 | |||||||
| Dementia | 1.66 (1.30–2.12) | 0.001 | |||||||
| Wu et al. [116]. | C | 106,903 Residents of Ontario, Canada ≥ 66 years old free of dementia (Canada) | SGLT2i | DPP-4i | Dementia | HR | 0.80 (0.71–0.89) | ||
| Dapagliflozin | 0.67 (0.53–0.84) | ||||||||
| Empagliflozin | 0.78 (0.69–0.89) | ||||||||
| Canagliflozin | 0.96 (0.80–1.16) | ||||||||
| J.Secnik et al. [118]. | C | 132,402 subjects with diabetes at baseline (Sweden) | SGLT2i | Non-use | Dementia death | HR | 0.43 (0.23–0.80) | < 0.05 | |
| 0.52 (0.22–1.23) * | |||||||||
| Siao et al. [127]. | C | 976,972 patients with newly diagnosed T2DM (Taiwan, China) | SGLT2i | Non-use | Dementia | OR | 0.89 (0.82–0.96) | 0.0021 | |
| Perna et al. [134]. | RCT | 39 elder aged > 65 years subjects (women:16) with T2DM (Italy) | SGLT2i | Baseline | VFT | MC | 0 (−4.25–4.25) | 1 | |
| BSRT | −1.32 (−3.36–0.71) | 0.178 | |||||||
| AMT | −0.09 (−0.56–0.38) | 0.676 | |||||||
| Ying Zhao et al. [135]. | RCT | 96 patients with T2DM and MCI (China) | Dapagliflozin with CBT | Convention intervention | ADL | MD | 6.18 (1.96) † | <0.001 | |
| MMSE | 2.59 (0.72) † | <0.001 | |||||||
| QoL-AD | 10.69 (1.45) † | <0.001 | |||||||
| EMPA REG OUTCOME | RCT | 7028 adult patients with T2DM | Empagliflozin | Placebo | Stroke | HR | 1.18 (0.89–1.56) | 0.26 | |
| TIA | 0.85 (0.51–1.42) | 0.54 | |||||||
| VERTIS | RCT | 8246 patients aged ≥ 40 years with T2DM | Ertugliflozin | Placebo | Stroke | HR | 1.06 (0.82–1.37) | ||
| DECLARE TIMI 58 | RCT | 17,160 patients aged ≥ 40 years with T2DM | Dapagliflozin | Placebo | IS | HR | 1.01 (0.84–1.21) | ||
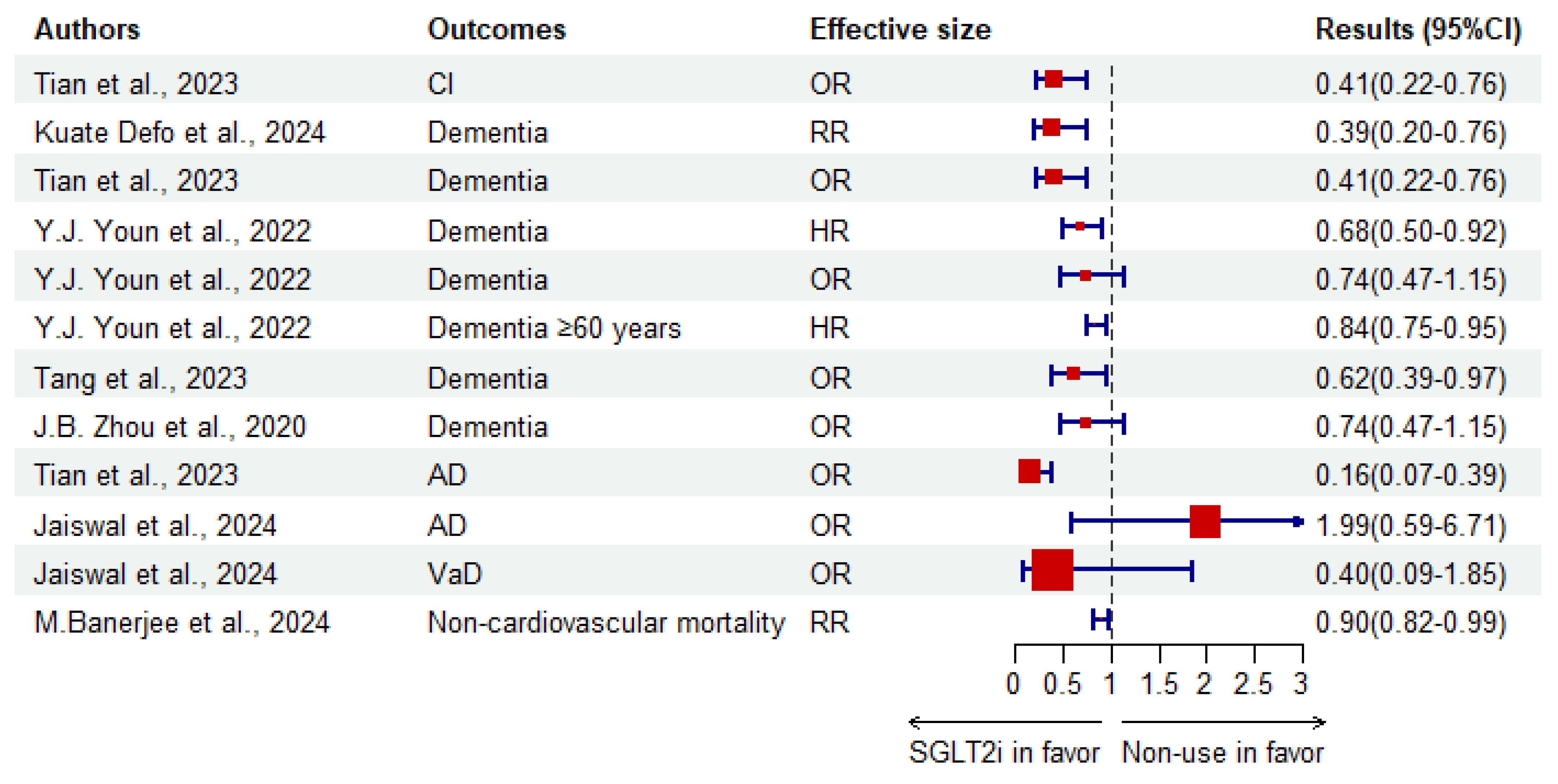
3.1.2. Evidence-Based Meta-Analyses
| Authors | Outcome | Effective Size | Results (95%CI) | I2 (%) | p for Heterogeneity | Studies Included (RCT/C/CS/CC) |
|---|---|---|---|---|---|---|
| Kuate Defo et al. [15]. | Dementia | RR | 0.39 (0.20–0.76) | 96.1 | 0.000 | 5 (0/2/1/2) |
| Tian et al. [20]. | CI | OR | 0.41 (0.22–0.76) | |||
| Dementia | 0.41 (0.22–0.76) | |||||
| AD | 0.16 (0.07–0.39) | |||||
| CI | SUCRA | 94.0% | 12 (3RCTs/9OTs) * | |||
| M.Banerjee et al. [139]. | Non-cardiovascular mortality | RR | 0.90 (0.82–0.99) | 0 | 0.75 | 8 (8/0/0/0) |
| Y.J. Youn et al. [136]. | Dementia | HR | 0.68 (0.50–0.92) | 87 | <0.001 | 4 (0/4/0/0) |
| OR | 0.74 (0.47–1.15) | 79 | 0.03 | 2 (0/0/0/2) | ||
| Dementia ≥ 60 years | HR | 0.84 (0.75–0.95) | 2 (0/2/0/0) | |||
| Cognitive function scores | SMD | 0.88 (0.32–1.44) | 3 (1/2/0/0) | |||
| Tang et al. [140]. | Dementia | RR | 0.62 (0.39–0.97) | 82.5 | 0.03 | 3 (0/1/0/2) |
| J.B. Zhou et al. [137]. | Dementia | OR | 0.74 (0.47–1.15) | 78.9 | 0.03 | 2 (0/0/0/2) |
| Jaiswal et al. [138]. | Dementia | OR | 1.37 (0.70–2.69) | 0 | 6 (6/0/0/0) | |
| AD | 1.99 (0.59–6.71) | 0 | 6 (6/0/0/0) | |||
| PD | 0.63 (0.25–1.61) | 0 | 9 (9/0/0/0) | |||
| VaD | 0.40 (0.09–1.85) | 0 | 4 (4/0/0/0) |
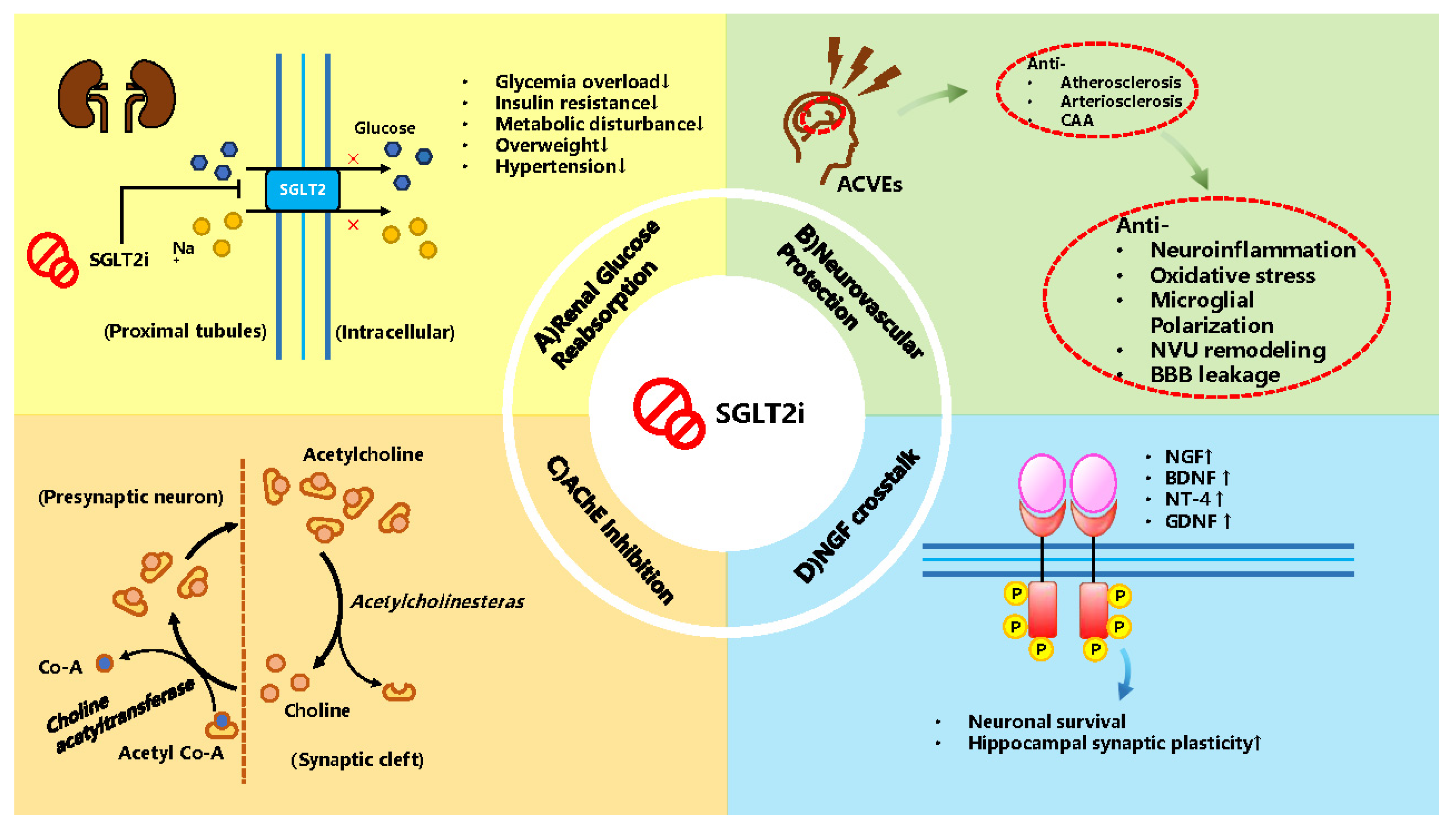
3.2. Potential Mechanism SGLT2i as a Treatment
3.2.1. Studied Mechanism in Animal Experiments
| Author | Model | Drugs | Control | Results | Mechanism |
|---|---|---|---|---|---|
| A.Y. Sim et al. [86]. | Male C57BL/6 mice fed with HFD and injected STZ | Empagliflozin, 25 mg/kg/day, orally | Vehicle or DPP-4i | Hippocampal-dependent cognitive functions↑; Hepatic lipid accumulation↓ and inflammation↓; Promoting glucose homeostasis | Restore brain insulin signaling; pTau and Aβ↓: IRS1/AKT/GSK3β pathways |
| pTau↓: ACE2/MasR↑ | |||||
| Iwona Piątkowska-Chmiel et al. [144]. | CD-1 male mice with administration of 20% aqueous fructose solution and injection of freshly prepared STZ solution | Empagliflozin, 10 mg/kg/day | Vehicle | Protective effects of cognition | Inflammatory cytokines↓ |
| BDNF↑ | |||||
| NT4↑ | |||||
| α-synuclein in prefrontal cortex↑ | |||||
| Dapagliflozin, 10 mg/kg/day | Vehicle | Protective effects of cognition | Inflammatory cytokines↓ | ||
| BDNF↑ | |||||
| HIF-1α↑ | |||||
| APP in hippocampus↑ | |||||
| T.Khan et al. [145]. | CLB57/6 mice fed with HFruD | Empagliflozin, 4.4 mg/kg/day | Normal saline (10 mL/kg) or RVS (1.5 mg/kg) | Attenuating memory deficit | NN↓, TBARS↓, SOD↑, catalase↑ |
| IL-1β, IL-6, TNF-α↓ | |||||
| TLR4-NFκB pathway↓ | |||||
| GSK3β/pTau↓, Aβ (1–40)↓, Aβ (1–42)↓ | |||||
| M.R Hayden et al. [146]. | Female db/db mice and wild control | Empagliflozin, 10 mg/kg/day | Ameliorating US remodeling of NVU | BM thickening↓, damage on BBB↓ | |
| Activated microglia invasion↓ | |||||
| Aberrant mitochondria remodeling↓ | |||||
| myelin remodeling↓ | |||||
| Axonal collapse↓ | |||||
| Myelin disarray↓ | |||||
| Jayarathne et al. [147]. | UM-HET3 mice | Canagliflozin, 14.4 mg/kg | Purina 5LG6 | Insulin sensitivity↑; locomotor activity↑; overall behavior function↑ | mTOR signaling↓ |
| Inflammatory response↓ | |||||
| microgliosis↓, astrogliosis↓ | |||||
| Hierro-Bujalance et al. [150]. | APP/PS1, db/db, APP/PS1 & db/db mice | Empagliflozin, 10 mg/kg | Learning and memory in AD, T2D and AD-T2D mice↑; Brain atrophy↓; Spontaneous bleeding↓ | amyloid pathology↓ | |
| Iba1+ burden↓ | |||||
| tau phosphorylation↓ |
3.2.2. Reviewed Mechanism
| Authors | Outcomes | Potential Mechanism | |
|---|---|---|---|
| F. Mancinetti et al. [152]. | LOAD and dementia | Direct effects | BP↓ |
| Cardiac function↑ | |||
| Weight↓ | |||
| Atherosclerotic plaque progression↓ | |||
| Indirect effects | Oxidative stress↓ | ||
| BDNF↑ | |||
| Aβ plaques↓ | |||
| AChE inhibition↑ | |||
| M.R. Rizzo et al. [153]. | Cognitive dysfucntion | Oxidative stress↓, NADPH oxidase↓ | |
| BDNF↑ | |||
| Insulin sensitivity↑ | |||
| Mitochondrial brain function↑ | |||
| Synaptic plasticity↑ | |||
| DJ-1/Nrf2 pathway | |||
| GDNF↑, PI3K/AKT/GSK3β (Ser9) pathway | |||
| NF-κB pathway↓, TNF-α levels↓ | |||
| Brain extra- and intracellular accumulation of Aβ and NFTs↓ | |||
| mTOR hyperactivation↓ | |||
| AChE inhibition | |||
| A. Pawlos et al. [154]. | Neuroprotection | Canagliflozin | Anti-inflammatory |
| Promoting M2 Macrophages Polarization | |||
| Oxidative Stress↓ | |||
| mTOR Signaling↓ | |||
| Dapagliflozin | Anti-epileptic Potential | ||
| CIMT Regression | |||
| Anti-inflammatory | |||
| NLRP3 Inflammasome↓ | |||
| Promoting M2 macrophage polarization | |||
| Oxidative Stress↓ | |||
| mTOR Signaling↓ | |||
| Empagliflozin | BDNF↑ | ||
| CIMT Regression | |||
| Anti-inflammatory | |||
| Blood–Brain Barrier protection | |||
| NLRP3 Inflammasome↓ | |||
| Promoting M2 macrophage polarization | |||
| Oxidative Stress↓ | |||
| Neurovascular unit remodeling | |||
| Cerebral ischemia/Reperfusion damage↓ | |||
| mTOR Signaling↓ | |||
| Ertugliflozin | Oxidative Stress↓ | ||
| mTOR Signaling↓ | |||
| Sotagliflozin | Oxidative Stress↓ | ||
| S.V. Birajdar et al. [159]. | Neurodegeneration | Insulin sensitivity↑ | |
| Oxidative Stress↓ | |||
| Neuronal damage↓ | |||
| G. Goodarzi et al. [160]. | AD | Empagliflozin | Oxidative Stress↓ |
| BDNF↑ | |||
| Infarct size | |||
| HIF-1α/VEGF↑ | |||
| Canagliflozin | AChE inhibition | ||
| Dapagliflozin | AChE inhibition | ||
| Hippocampal synaptic plasticity↑ | |||
| V. Chavda et al. [156]. | CVAs | Endothelia dysfunction↓ | |
| glycemia↓ | |||
| AGE superoxide↓ | |||
| C-peptide and NFκB↓ | |||
| Inflammation↓ | |||
| J Ariana Noel et al. [161]. | CI | AChE inhibition | |
| Oxidative Stress↓ | |||
| Inflammation (NFkB)↓ | |||
| mTOR pathway↓ | |||
| Mei et al. [162]. | CI | NLRP3 inflammasome↓ | |
| ROS dependent neuronal apoptosis | |||
| Cerebral Glu metabolism↑ | |||
| BDNF↑, NGF↑ | |||
| AMPK pathway↑ | |||
| CRP↓ | |||
| M.E Youssef et al. [163]. | CI | Dapagliflozin | DJ-1/Nrf2 pathway |
| GDNF↑ | |||
| PI3K/AKT/GSK3β pathway↑ | |||
| NFκB pathway↓, TNF↓ | |||
| AChE inhibition | |||
| Empagliflozin | inflammatory mediators↓ | ||
| oxidative stress↓ | |||
| p-tau↓ | |||
| Aβ↓ | |||
| Sim et al. [164]. | AD | Peripheral insulin sensitivity↑ | |
| Body weight↓ | |||
| Brain mitochondrial function↑ | |||
| Insulin signaling↑ | |||
| Cell death↓ | |||
| Synaptic plasticity↑ | |||
| mTOR inhibition | |||
| NLRP3 inflammasome-IL-1β modulation | |||
| Cerebral oxidative stress↓ | |||
| Riemma et al. [165]. | CI | M2 microglial polarization↑ | |
| JAK2/STAT1 pathway↓ | |||
| Free radicals↓, antioxidant enzymes↑ | |||
| AD | mTOR activity↓ | ||
| AChE inhibition, acetylcholine M1 receptor↑ | |||
| Michał Wiciński et al. [157]. | Ischemia-related cerebral damage | Empagliflozin | HIF-1α↑ |
| VEGF-A↑ | |||
| caspase-3↓ | |||
| Limiting infarct volume | |||
| Preventing US remodeling of NVU’s cell and myelin | |||
| Russell Esterline et al. [155]. | AD | mTOR pathway↓ | |
| AMPK/ULK1 pathway↑ | |||
4. Conclusions, Discussion and Perspectives
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| Terms | Full Name |
| DM | diabetes mellitus |
| T2DM | type 2 diabetes mellitus |
| IGT | impaired glucose tolerance |
| IFG | impaired fasting glucose |
| CI | cognitive impairment |
| MCI | mild cognitive impairment |
| AD | Alzheimer’s disease |
| VaD | vascular dementia |
| Aβ | amyloid β |
| NFTs | neurofibrillary tangles |
| CAA | cerebral amyloid angiopathy |
| OR | odds ratio |
| ADA | American Diabetes Association |
| MMSE | Mini-Mental State Examination |
| MoCA | Mini-Cog and Montreal Cognitive Assessment |
| SGLT2 | sodium–glucose cotransporter 2 |
| SGLT2i | sodium–glucose cotransporter 2 inhibitors |
| DPP-4i | dipeptidyl peptidase-4 inhibitors |
| SUs | sulfonylureas |
| TZDs | thiazolidinediones |
| GLP-1 RAs | glucagon-like peptide 1 receptor agonists |
| SUCRA | surface under the cumulative ranking curves |
| ACVEs | adverse cerebral vascular events |
| GSK3β | glucogen synthase kinase 3β |
| IR | insulin receptor |
| IRS | insulin receptor substrate |
| PI3K | phosphoinositide 3-kinase |
| AKT | protein kinase B (PKB) |
| BBB | blood–brain barrier |
| CNS | central neural system |
| IDE | insulin-degrading enzyme |
| APP | amyloid-β precursor protein |
| PPAR | peroxisome proliferators-activated receptors |
| NFκB | nuclear factor κ B |
| NO | nitric oxide |
| IL | interleukin |
| TNF | tumor necrosis factor |
| ROS | reactive oxygen species |
| NADPH | nicotinamide adenine dinucleotide phosphate |
| NOX2 | NADPH oxidase 2 |
| AGEs | advanced glycation end-products |
| HR | hazard ratio |
| HbA1c | glycosylated hemoglobin A1c |
| CRP | C-reactive protein |
| CKD | chronic kidney disease |
| HRQoL | health-related quality of life |
| RCTs | randomized control trials |
| HFpEF | heart failure with a preserved ejection fraction |
| 95%CI | 95% confidence interval |
| RBANS | Repeatable Battery for the Assessment of Neuropsychological Status |
| IS | ischemic stroke |
| TIA | transient ischemic attack |
| ICH | intracranial hemorrhage |
| MACEs | major adverse cardiovascular events |
| CBT | cognitive behavioral training |
| ADL | activity of daily living |
| MD | mean difference |
| SD | standard deviation |
| QOL-AD | the quality of life AD scores |
| C | cohort |
| CS | cross-sectional |
| N-CC | nested case-control |
| PD | Parkinson’s disease |
| CVM | cerebrovascular mortality |
| VFT | verbal fluency test |
| BRST | Babcock story recall test |
| AMT | attentive matrices test |
| IM | immediate memory |
| DM | delayed memory |
| VS | visuospatial/construction |
| MC | mean changes |
| RD | risk difference |
| SMD | standard mean difference |
| RR | risk ratio |
| AChE | acetylcholinesterase |
| AMPK | AMP-activated protein kinase |
| mTOR | mammalian target of rapamycin |
| HIF | hypoxia-inducible factor |
| NLRP3 | nucleotide-binding oligomerization domain, leucine-rich repeat and pyrin domain-containing 3 |
| ACVEs | adverse cerebral vascular events |
| NVU | neurovascular unit |
| NGF | nerve growth factor |
| BDNF | brain-derived neurotrophic factor |
| NT-4 | neurotrophins 4 |
| GDNF | glial cell-derived neurotrophic factor |
| PPRE | peroxisome proliferators reactive element |
| Nrf2 | nuclear factor-erythroid 2-related factor 2 |
| TLR | toll-like receptor |
| IκB | inhibitor of NFκB |
| JAK-STAT | Janus Kinase-signal transducer and activator of transcription |
| ACE2 | angiotensin-converting enzyme 2 |
| LOAD | Late-onset AD |
| VEGF-A | vascular endothelial growth factor A |
| IGF-1 | insulin-like growth factor-1 |
References
- Ahmad, E.; Lim, S.; Lamptey, R.; Webb, D.R.; Davies, M.J. Type 2 diabetes. Lancet 2022, 400, 1803–1820. [Google Scholar] [CrossRef] [PubMed]
- Alejandro, E.U.; Gregg, B.; Blandino-Rosano, M.; Cras-Méneur, C.; Bernal-Mizrachi, E. Natural history of β-cell adaptation and failure in type 2 diabetes. Mol. Asp. Med. 2015, 42, 19–41. [Google Scholar] [CrossRef]
- Narayan, K.M.V.; Kondal, D.; Chang, H.H.; Mohan, D.; Gujral, U.P.; Anjana, R.M.; Staimez, L.R.; Patel, S.A.; Ali, M.K.; Prabhakaran, D.; et al. Natural History of Type 2 Diabetes in Indians: Time to Progression. Diabetes Care 2024, 47, 858–863. [Google Scholar] [CrossRef]
- Global, regional, and national burden of diabetes from 1990 to 2021, with projections of prevalence to 2050: A systematic analysis for the Global Burden of Disease Study 2021. Lancet 2023, 402, 203–234. [CrossRef] [PubMed]
- Rooney, M.R.; Fang, M.; Ogurtsova, K.; Ozkan, B.; Echouffo-Tcheugui, J.B.; Boyko, E.J.; Magliano, D.J.; Selvin, E. Global Prevalence of Prediabetes. Diabetes Care 2023, 46, 1388–1394. [Google Scholar] [CrossRef]
- Sachdev, P.S.; Blacker, D.; Blazer, D.G.; Ganguli, M.; Jeste, D.V.; Paulsen, J.S.; Petersen, R.C. Classifying neurocognitive disorders: The DSM-5 approach. Nat. Rev. Neurol. 2014, 10, 634–642. [Google Scholar] [CrossRef]
- Langa, K.M.; Levine, D.A. The diagnosis and management of mild cognitive impairment: A clinical review. JAMA 2014, 312, 2551–2561. [Google Scholar] [CrossRef] [PubMed]
- Scheltens, P.; De Strooper, B.; Kivipelto, M.; Holstege, H.; Chételat, G.; Teunissen, C.E.; Cummings, J.; van der Flier, W.M. Alzheimer’s disease. Lancet 2021, 397, 1577–1590. [Google Scholar] [CrossRef]
- Deture, M.A.; Dickson, D.W. The neuropathological diagnosis of Alzheimer’s disease. Mol. Neurodegener. 2019, 14, 32. [Google Scholar] [CrossRef]
- O’Brien, J.T.; Thomas, A. Vascular dementia. Lancet 2015, 386, 1698–1706. [Google Scholar] [CrossRef]
- van der Flier, W.M.; Skoog, I.; Schneider, J.A.; Pantoni, L.; Mok, V.; Chen, C.L.H.; Scheltens, P. Vascular cognitive impairment. Nat. Rev. Dis. Primers 2018, 4, 18003. [Google Scholar] [CrossRef] [PubMed]
- Ott, A.; Stolk, R.P.; Hofman, A.; van Harskamp, F.; Grobbee, D.E.; Breteler, M.M. Association of diabetes mellitus and dementia: The Rotterdam Study. Diabetologia 1996, 39, 1392–1397. [Google Scholar] [CrossRef]
- Mukadam, N.; Wolters, F.J.; Walsh, S.; Wallace, L.; Brayne, C.; Matthews, F.E.; Sacuiu, S.; Skoog, I.; Seshadri, S.; Beiser, A.; et al. Changes in prevalence and incidence of dementia and risk factors for dementia: An analysis from cohort studies. Lancet Public Health 2024, 9, e443–e460. [Google Scholar] [CrossRef] [PubMed]
- Chekol Tassew, W.; Ferede, Y.A.; Zeleke, A.M. Cognitive impairment and associated factors among patients with diabetes mellitus in Africa: A systematic review and meta-analysis. Front. Endocrinol. 2024, 15, 1386600. [Google Scholar] [CrossRef] [PubMed]
- Kuate Defo, A.; Bakula, V.; Pisaturo, A.; Labos, C.; Wing, S.S.; Daskalopoulou, S.S. Diabetes, antidiabetic medications and risk of dementia: A systematic umbrella review and meta-analysis. Diabetes Obes. Metab. 2024, 26, 441–462. [Google Scholar] [CrossRef]
- Chen, Q.; Zhu, S.; Shang, J.; Fang, Q.; Xue, Q.; Hua, J. Trends in Cognitive Function Before and After Diabetes Onset: The China Health and Retirement Longitudinal Study. Neurology 2024, 102, e209165. [Google Scholar] [CrossRef] [PubMed]
- American Diabetes Association Professional Practice Committee. 13. Older Adults: Standards of Care in Diabetes-2024. Diabetes Care 2024, 47, S244–S257. [Google Scholar] [CrossRef]
- Brown, E.; Heerspink, H.J.L.; Cuthbertson, D.J.; Wilding, J.P.H. SGLT2 inhibitors and GLP-1 receptor agonists: Established and emerging indications. Lancet 2021, 398, 262–276. [Google Scholar] [CrossRef]
- Sunwoo, Y.; Park, J.; Choi, C.-Y.; Shin, S.; Choi, Y.J. Risk of Dementia and Alzheimer’s Disease Associated With Antidiabetics: A Bayesian Network Meta-Analysis. Am. J. Prev. Med. 2024, 67, 434–443. [Google Scholar] [CrossRef]
- Tian, S.; Jiang, J.; Wang, J.; Zhang, Z.; Miao, Y.; Ji, X.; Bi, Y. Comparison on cognitive outcomes of antidiabetic agents for type 2 diabetes: A systematic review and network meta-analysis. Diabetes Metab. Res. Rev. 2023, 39, e3673. [Google Scholar] [CrossRef]
- Haeusler, R.A.; McGraw, T.E.; Accili, D. Biochemical and cellular properties of insulin receptor signalling. Nat. Rev. Mol. Cell Biol. 2018, 19, 31–44. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Li, J.; Di, L.-J. Glycogen synthesis and beyond, a comprehensive review of GSK3 as a key regulator of metabolic pathways and a therapeutic target for treating metabolic diseases. Med. Res. Rev. 2022, 42, 946–982. [Google Scholar] [CrossRef] [PubMed]
- Scherer, T.; Sakamoto, K.; Buettner, C. Brain insulin signalling in metabolic homeostasis and disease. Nat. Rev. Endocrinol. 2021, 17, 468–483. [Google Scholar] [CrossRef]
- Coppari, R.; Bjørbæk, C. Leptin revisited: Its mechanism of action and potential for treating diabetes. Nat. Rev. Drug Discov. 2012, 11, 692–708. [Google Scholar] [CrossRef]
- Welters, H.J.; Kulkarni, R.N. Wnt signaling: Relevance to beta-cell biology and diabetes. Trends Endocrinol. Metab. 2008, 19, 349–355. [Google Scholar] [CrossRef]
- Meske, V.; Albert, F.; Ohm, T.G. Coupling of mammalian target of rapamycin with phosphoinositide 3-kinase signaling pathway regulates protein phosphatase 2A- and glycogen synthase kinase-3 -dependent phosphorylation of Tau. J. Biol. Chem. 2008, 283, 100–109. [Google Scholar] [CrossRef]
- Jolivalt, C.G.; Lee, C.A.; Beiswenger, K.K.; Smith, J.L.; Orlov, M.; Torrance, M.A.; Masliah, E. Defective insulin signaling pathway and increased glycogen synthase kinase-3 activity in the brain of diabetic mice: Parallels with Alzheimer’s disease and correction by insulin. J. Neurosci. Res. 2008, 86, 3265–3274. [Google Scholar] [CrossRef]
- Zhang, T.; Pan, B.-S.; Sun, G.-C.; Sun, X.; Sun, F.-Y. Diabetes synergistically exacerbates poststroke dementia and tau abnormality in brain. Neurochem. Int. 2010, 56, 955–961. [Google Scholar] [CrossRef] [PubMed]
- Qu, Z.; Jiao, Z.; Sun, X.; Zhao, Y.; Ren, J.; Xu, G. Effects of streptozotocin-induced diabetes on tau phosphorylation in the rat brain. Brain Res. 2011, 1383, 300–306. [Google Scholar] [CrossRef]
- Hu, S.H.; Jiang, T.; Yang, S.S.; Yang, Y. Pioglitazone ameliorates intracerebral insulin resistance and tau-protein hyperphosphorylation in rats with type 2 diabetes. Exp. Clin. Endocrinol. Diabetes 2013, 121, 220–224. [Google Scholar] [CrossRef]
- Yang, Y.; Ma, D.; Wang, Y.; Jiang, T.; Hu, S.; Zhang, M.; Yu, X.; Gong, C.-X. Intranasal insulin ameliorates tau hyperphosphorylation in a rat model of type 2 diabetes. J. Alzheimers Dis. 2013, 33, 329–338. [Google Scholar] [CrossRef] [PubMed]
- Gao, C.; Liu, Y.; Jiang, Y.; Ding, J.; Li, L. Geniposide ameliorates learning memory deficits, reduces tau phosphorylation and decreases apoptosis via GSK3β pathway in streptozotocin-induced alzheimer rat model. Brain Pathol. 2014, 24, 261–269. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Yang, Y.; Yuan, G.; Zhu, W.; Ma, D.; Hu, S. Exendin-4, a glucagon-like peptide-1 receptor agonist, reduces Alzheimer disease-associated tau hyperphosphorylation in the hippocampus of rats with type 2 diabetes. J. Investig. Med. 2015, 63, 267–272. [Google Scholar] [CrossRef]
- Ma, D.-L.; Chen, F.-Q.; Xu, W.-J.; Yue, W.-Z.; Yuan, G.; Yang, Y. Early intervention with glucagon-like peptide 1 analog liraglutide prevents tau hyperphosphorylation in diabetic db/db mice. J. Neurochem. 2015, 135, 301–308. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Yin, F.; Liu, J.; Liu, Z. Geniposide Attenuates the Phosphorylation of Tau Protein in Cellular and Insulin-deficient APP/PS1 Transgenic Mouse Model of Alzheimer’s Disease. Chem. Biol. Drug Des. 2016, 87, 409–418. [Google Scholar] [CrossRef]
- Wang, X.; Zhao, L. Calycosin ameliorates diabetes-induced cognitive impairments in rats by reducing oxidative stress via the PI3K/Akt/GSK-3β signaling pathway. Biochem. Biophys. Res. Commun. 2016, 473, 428–434. [Google Scholar] [CrossRef]
- Xu, W.; Liu, J.; Ma, D.; Yuan, G.; Lu, Y.; Yang, Y. Capsaicin reduces Alzheimer-associated tau changes in the hippocampus of type 2 diabetes rats. PLoS ONE 2017, 12, e0172477. [Google Scholar] [CrossRef]
- Ma, Q.-H.; Jiang, L.-F.; Mao, J.-L.; Xu, W.-X.; Huang, M. Vildagliptin prevents cognitive deficits and neuronal apoptosis in a rat model of Alzheimer’s disease. Mol. Med. Rep. 2018, 17, 4113–4119. [Google Scholar] [CrossRef]
- Zhou, M.; Chen, S.; Peng, P.; Gu, Z.; Yu, J.; Zhao, G.; Deng, Y. Dulaglutide ameliorates STZ induced AD-like impairment of learning and memory ability by modulating hyperphosphorylation of tau and NFs through GSK3β. Biochem. Biophys. Res. Commun. 2019, 511, 154–160. [Google Scholar] [CrossRef]
- Peng, X.; Shi, X.; Huang, J.; Zhang, S.; Yan, Y.; Ma, D.; Xu, W.; Xu, W.; Dong, K.; Tao, J.; et al. Exendin-4 Improves Cognitive Function of Diabetic Mice via Increasing Brain Insulin Synthesis. Curr. Alzheimer Res. 2021, 18, 546–557. [Google Scholar] [CrossRef]
- Latina, V.; Giacovazzo, G.; Calissano, P.; Atlante, A.; La Regina, F.; Malerba, F.; Dell’Aquila, M.; Stigliano, E.; Balzamino, B.O.; Micera, A.; et al. Tau Cleavage Contributes to Cognitive Dysfunction in Strepto-Zotocin-Induced Sporadic Alzheimer’s Disease (sAD) Mouse Model. Int. J. Mol. Sci. 2021, 22, 12158. [Google Scholar] [CrossRef] [PubMed]
- He, S.; Peng, W.-B.; Fu, X.-J.; Zhou, H.-L.; Wang, Z.-G. Deep Sea Water Alleviates Tau Phosphorylation and Cognitive Impairment via PI3K/Akt/GSK-3β Pathway. Mar. Biotechnol. 2022, 24, 68–81. [Google Scholar] [CrossRef]
- Zaki, M.O.; El-Desouky, S.; Elsherbiny, D.A.; Salama, M.; Azab, S.S. Glimepiride mitigates tauopathy and neuroinflammation in P301S transgenic mice: Role of AKT/GSK3β signaling. Inflammopharmacology 2022, 30, 1871–1890. [Google Scholar] [CrossRef]
- Jingxuan, L.; Litian, M.; Yanyang, T.; Jianfang, F. Knockdown of CLC-3 may improve cognitive impairment caused by diabetic encephalopathy. Diabetes Res. Clin. Pract. 2022, 190, 109970. [Google Scholar] [CrossRef]
- Chen, J.; Liu, Y.; Zhou, K.; Zhang, W.; Wen, B.; Xu, K.; Liu, Y.; Chen, L.; Huang, Y.; He, B.; et al. DISC1 inhibits GSK3β activity to prevent tau hyperphosphorylation under diabetic encephalopathy. Biofactors 2023, 49, 173–184. [Google Scholar] [CrossRef] [PubMed]
- Khoramipour, K.; Bejeshk, M.A.; Rajizadeh, M.A.; Najafipour, H.; Dehghan, P.; Farahmand, F. High-Intensity Interval Training Ameliorates Molecular Changes in the Hippocampus of Male Rats with the Diabetic Brain: The Role of Adiponectin. Mol. Neurobiol. 2023, 60, 3486–3495. [Google Scholar] [CrossRef] [PubMed]
- Moghazy, H.M.; Abdelhaliem, N.G.; Mohammed, S.A.; Hassan, A.; Abdelrahman, A. Liraglutide versus pramlintide in protecting against cognitive function impairment through affecting PI3K/AKT/GSK-3β/TTBK1 pathway and decreasing Tau hyperphosphorylation in high-fat diet- streptozocin rat model. Pflug. Arch. 2024, 476, 779–795. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.-W.; Xie, L.-Y.; Qi, M.-H.; Ren, S.; Wang, Y.-Q.; Hu, J.-N.; Wang, Z.; Tang, S.; Zhang, J.-T.; Li, W. Platycodin D Ameliorates Cognitive Impairment in Type 2 Diabetes Mellitus Mice via Regulating PI3K/Akt/GSK3β Signaling Pathway. J. Agric. Food Chem. 2024, 72, 12516–12528. [Google Scholar] [CrossRef]
- Rezaei, M.H.; Madadizadeh, E.; Aminaei, M.; Abbaspoor, M.; Schierbauer, J.; Moser, O.; Khoramipour, K.; Chamari, K. Leptin Signaling Could Mediate Hippocampal Decumulation of Beta-Amyloid and Tau Induced by High-Intensity Interval Training in Rats with Type 2 Diabetes. Cell Mol. Neurobiol. 2023, 43, 3465–3478. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, Z.; Mao, Y.; Li, B.; Zhu, Y.; Zhang, S.; Wang, S.; Jiang, Y.; Xu, N.; Xie, Y.; et al. NEAT1 regulates microtubule stabilization via FZD3/GSK3β/P-tau pathway in SH-SY5Y cells and APP/PS1 mice. Aging 2020, 12, 23233–23250. [Google Scholar] [CrossRef]
- Muneeb, M.; Mansou, S.M.; Saleh, S.; Mohammed, R.A. Vitamin D and rosuvastatin alleviate type-II diabetes-induced cognitive dysfunction by modulating neuroinflammation and canonical/noncanonical Wnt/β-catenin signaling. PLoS ONE 2022, 17, e0277457. [Google Scholar] [CrossRef] [PubMed]
- El-Safty, H.; Ismail, A.; Abdelsalam, R.M.; El-Sahar, A.E.; Saad, M.A. Dapagliflozin diminishes memory and cognition impairment in Streptozotocin induced diabetes through its effect on Wnt/β-Catenin and CREB pathway. Brain Res. Bull. 2022, 181, 109–120. [Google Scholar] [CrossRef] [PubMed]
- Kang, X.; Wang, D.; Zhang, L.; Huang, T.; Liu, S.; Feng, X.; Guo, Y.; Zhang, Z.; Wang, Z.; Ren, H.; et al. Exendin-4 ameliorates tau hyperphosphorylation and cognitive impairment in type 2 diabetes through acting on Wnt/β-catenin/NeuroD1 pathway. Mol. Med. 2023, 29, 118. [Google Scholar] [CrossRef] [PubMed]
- Zhao, F.; Guo, L.; Huang, T.; Liu, C.; Wu, D.; Fang, L.; Min, W. Interaction between the Neuroprotective and Hyperglycemia Mitigation Effects of Walnut-Derived Peptide LVRL via the Wnt3a/β-Catenin/GSK-3β Pathway in a Type 2 Diabetes Mellitus Model. J. Agric. Food Chem. 2024, 72, 16204–16220. [Google Scholar] [CrossRef] [PubMed]
- Dey, A.; Hao, S.; Wosiski-Kuhn, M.; Stranahan, A.M. Glucocorticoid-mediated activation of GSK3β promotes tau phosphorylation and impairs memory in type 2 diabetes. Neurobiol. Aging 2017, 57, 75–83. [Google Scholar] [CrossRef]
- Elahi, M.; Motoi, Y.; Shimonaka, S.; Ishida, Y.; Hioki, H.; Takanashi, M.; Ishiguro, K.; Imai, Y.; Hattori, N. High-fat diet-induced activation of SGK1 promotes Alzheimer’s disease-associated tau pathology. Hum. Mol. Genet. 2021, 30, 1693–1710. [Google Scholar] [CrossRef]
- Jack, C.R.; Andrews, J.S.; Beach, T.G.; Buracchio, T.; Dunn, B.; Graf, A.; Hansson, O.; Ho, C.; Jagust, W.; McDade, E.; et al. Revised criteria for diagnosis and staging of Alzheimer’s disease: Alzheimer’s Association Workgroup. Alzheimers Dement. 2024, 20, 5143–5169. [Google Scholar] [CrossRef]
- Deng, Y.; Wang, Z.; Wang, R.; Zhang, X.; Zhang, S.; Wu, Y.; Staufenbiel, M.; Cai, F.; Song, W. Amyloid-β protein (Aβ) Glu11 is the major β-secretase site of β-site amyloid-β precursor protein-cleaving enzyme 1(BACE1), and shifting the cleavage site to Aβ Asp1 contributes to Alzheimer pathogenesis. Eur. J. Neurosci. 2013, 37, 1962–1969. [Google Scholar] [CrossRef]
- Tian, Y.; Jing, G.; Zhang, M. Insulin-degrading enzyme: Roles and pathways in ameliorating cognitive impairment associated with Alzheimer’s disease and diabetes. Ageing Res. Rev. 2023, 90, 101999. [Google Scholar] [CrossRef]
- Kurochkin, I.V.; Guarnera, E.; Berezovsky, I.N. Insulin-Degrading Enzyme in the Fight against Alzheimer’s Disease. Trends Pharmacol. Sci. 2018, 39, 49–58. [Google Scholar] [CrossRef]
- Cordes, C.M.; Bennett, R.G.; Siford, G.L.; Hamel, F.G. Nitric oxide inhibits insulin-degrading enzyme activity and function through S-nitrosylation. Biochem. Pharmacol. 2009, 77, 1064–1073. [Google Scholar] [CrossRef] [PubMed]
- Cordes, C.M.; Bennett, R.G.; Siford, G.L.; Hamel, F.G. Redox regulation of insulin degradation by insulin-degrading enzyme. PLoS ONE 2011, 6, e18138. [Google Scholar] [CrossRef]
- Akhtar, M.W.; Sanz-Blasco, S.; Dolatabadi, N.; Parker, J.; Chon, K.; Lee, M.S.; Soussou, W.; McKercher, S.R.; Ambasudhan, R.; Nakamura, T.; et al. Elevated glucose and oligomeric β-amyloid disrupt synapses via a common pathway of aberrant protein S-nitrosylation. Nat. Commun. 2016, 7, 10242. [Google Scholar] [CrossRef] [PubMed]
- Kubis-Kubiak, A.; Wiatrak, B.; Piwowar, A. Hyper-glycemia and insulinemia induce morphological changes and modulate secretion of S100B, S100A8, amyloid β 1-40 and amyloid β 1-42, in a model of human dopaminergic neurons. Biomed. Pharmacother. 2022, 156, 113869. [Google Scholar] [CrossRef]
- Taubes, G. Neuroscience. Insulin insults may spur Alzheimer’s disease. Science 2003, 301, 40–41. [Google Scholar] [CrossRef]
- Farris, W.; Mansourian, S.; Chang, Y.; Lindsley, L.; Eckman, E.A.; Frosch, M.P.; Eckman, C.B.; Tanzi, R.E.; Selkoe, D.J.; Guenette, S. Insulin-degrading enzyme regulates the levels of insulin, amyloid beta-protein, and the beta-amyloid precursor protein intracellular domain in vivo. Proc. Natl. Acad. Sci. USA 2003, 100, 4162–4167. [Google Scholar] [CrossRef] [PubMed]
- Farris, W.; Mansourian, S.; Leissring, M.A.; Eckman, E.A.; Bertram, L.; Eckman, C.B.; Tanzi, R.E.; Selkoe, D.J. Partial loss-of-function mutations in insulin-degrading enzyme that induce diabetes also impair degradation of amyloid beta-protein. Am. J. Pathol. 2004, 164, 1425–1434. [Google Scholar] [CrossRef]
- Zhao, L.; Teter, B.; Morihara, T.; Lim, G.P.; Ambegaokar, S.S.; Ubeda, O.J.; Frautschy, S.A.; Cole, G.M. Insulin-degrading enzyme as a downstream target of insulin receptor signaling cascade: Implications for Alzheimer’s disease intervention. J. Neurosci. 2004, 24, 11120–11126. [Google Scholar] [CrossRef] [PubMed]
- Farris, W.; Leissring, M.A.; Hemming, M.L.; Chang, A.Y.; Selkoe, D.J. Alternative splicing of human insulin-degrading enzyme yields a novel isoform with a decreased ability to degrade insulin and amyloid beta-protein. Biochemistry 2005, 44, 6513–6525. [Google Scholar] [CrossRef]
- Im, H.; Manolopoulou, M.; Malito, E.; Shen, Y.; Zhao, J.; Neant-Fery, M.; Sun, C.-Y.; Meredith, S.C.; Sisodia, S.S.; Leissring, M.A.; et al. Structure of substrate-free human insulin-degrading enzyme (IDE) and biophysical analysis of ATP-induced conformational switch of IDE. J. Biol. Chem. 2007, 282, 25453–25463. [Google Scholar] [CrossRef]
- Zhao, J.; Li, L.; Leissring, M.A. Insulin-degrading enzyme is exported via an unconventional protein secretion pathway. Mol. Neurodegener. 2009, 4, 4. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Liu, L.; Lu, S.; Wang, D.; Liu, X.; Xie, L.; Wang, G. Impaired amyloid β-degrading enzymes in brain of streptozotocin-induced diabetic rats. J. Endocrinol. Investig. 2011, 34, 26–31. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Zhang, Y.; Deng, X.; Yin, F. Geniposide decreases the level of Aβ1-42 in the hippocampus of streptozotocin-induced diabetic rats. Acta Biochim. Biophys. Sin. 2013, 45, 787–791. [Google Scholar] [CrossRef]
- Mittal, K.; Mani, R.J.; Katare, D.P. Type 3 Diabetes: Cross Talk between Differentially Regulated Proteins of Type 2 Diabetes Mellitus and Alzheimer’s Disease. Sci. Rep. 2016, 6, 25589. [Google Scholar] [CrossRef]
- Song, M.K.; Bischoff, D.S.; Song, A.M.; Uyemura, K.; Yamaguchi, D.T. Metabolic relationship between diabetes and Alzheimer’s Disease affected by Cyclo(His-Pro) plus zinc treatment. BBA Clin. 2017, 7, 41–54. [Google Scholar] [CrossRef]
- Yang, S.; Chen, Z.; Cao, M.; Li, R.; Wang, Z.; Zhang, M. Pioglitazone ameliorates Aβ42 deposition in rats with diet-induced insulin resistance associated with AKT/GSK3β activation. Mol. Med. Rep. 2017, 15, 2588–2594. [Google Scholar] [CrossRef]
- Li, H.; Wu, J.; Zhu, L.; Sha, L.; Yang, S.; Wei, J.; Ji, L.; Tang, X.; Mao, K.; Cao, L.; et al. Insulin degrading enzyme contributes to the pathology in a mixed model of Type 2 diabetes and Alzheimer’s disease: Possible mechanisms of IDE in T2D and AD. Biosci. Rep. 2018, 38, BSR20170862. [Google Scholar] [CrossRef] [PubMed]
- Kazkayasi, I.; Burul-Bozkurt, N.; Ismail, M.-A.-M.; Merino-Serrais, P.; Pekiner, C.; Cedazo-Minguez, A.; Uma, S. Insulin deprivation decreases insulin degrading enzyme levels in primary cultured cortical neurons and in the cerebral cortex of rats with streptozotocin-induced diabetes. Pharmacol. Rep. 2018, 70, 677–683. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, P. Age-Related Increase of Insulin-Degrading Enzyme Is Inversely Correlated with Cognitive Function in APPswe/PS1dE9 Mice. Med. Sci. Monit. 2018, 24, 2446–2455. [Google Scholar] [CrossRef]
- Kang, J.Y.; Park, S.K.; Kim, J.M.; Park, S.B.; Yoo, S.K.; Han, H.J.; Kim, D.O.; Heo, H.J. 4,5-dicaffeyolquinic acid improves high-fat diet-induced cognitive dysfunction through the regulation of insulin degrading enzyme. J. Food Biochem. 2019, 43, e12855. [Google Scholar] [CrossRef]
- Hayrabedyan, S.; Todorova, K.; Spinelli, M.; Barnea, E.R.; Mueller, M. The core sequence of PIF competes for insulin/amyloid β in insulin degrading enzyme: Potential treatment for Alzheimer’s disease. Oncotarget 2018, 9, 33884–33895. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Li, H.; Cao, L.; Ren, Y.; Jiang, Y.; Xie, W.; Li, D. GLP-1 receptor regulates cell growth through regulating IDE expression level in Aβ1-42-treated PC12 cells. Biosci. Rep. 2018, 38, BSR20171284. [Google Scholar] [CrossRef]
- Fernández-de Frutos, M.; Galán-Chilet, I.; Goedeke, L.; Kim, B.; Pardo-Marqués, V.; Pérez-García, A.; Herrero, J.I.; Fernández-Hernando, C.; Kim, J.; Ramírez, C.M. MicroRNA 7 Impairs Insulin Signaling and Regulates Aβ Levels through Posttranscriptional Regulation of the Insulin Receptor Substrate 2, Insulin Receptor, Insulin-Degrading Enzyme, and Liver X Receptor Pathway. Mol. Cell Biol. 2019, 39, e00170-19. [Google Scholar] [CrossRef] [PubMed]
- Bosoi, C.R.; Vandal, M.; Tournissac, M.; Leclerc, M.; Fanet, H.; Mitchell, P.L.; Verreault, M.; Trottier, J.; Virgili, J.; Tremblay, C.; et al. High-Fat Diet Modulates Hepatic Amyloid β and Cerebrosterol Metabolism in the Triple Transgenic Mouse Model of Alzheimer’s Disease. Hepatol. Commun. 2021, 5, 446–460. [Google Scholar] [CrossRef]
- Martín-Martín, Y.; Pérez-García, A.; Torrecilla-Parra, M.; Fernández-de Frutos, M.; Pardo-Marqués, V.; Casarejos, M.J.; Busto, R.; Ramírez, C.M. New Insights on the Regulation of the Insulin-Degrading Enzyme: Role of microRNAs and RBPs. Cells 2022, 11, 2538. [Google Scholar] [CrossRef]
- Sim, A.Y.; Choi, D.H.; Kim, J.Y.; Kim, E.R.; Goh, A.R.; Lee, Y.-H.; Lee, J.E. SGLT2 and DPP4 inhibitors improve Alzheimer’s disease-like pathology and cognitive function through distinct mechanisms in a T2D-AD mouse model. Biomed. Pharmacother. 2023, 168, 115755. [Google Scholar] [CrossRef] [PubMed]
- Morelli, L.; Llovera, R.E.; Mathov, I.; Lue, L.-F.; Frangione, B.; Ghiso, J.; Castaño, E.M. Insulin-degrading enzyme in brain microvessels: Proteolysis of amyloid {beta} vasculotropic variants and reduced activity in cerebral amyloid angiopathy. J. Biol. Chem. 2004, 279, 56004–56013. [Google Scholar] [CrossRef]
- Inoue, Y.; Ueda, M.; Masuda, T.; Misumi, Y.; Yamashita, T.; Ando, Y. Memantine, a Noncompetitive N-Methyl-D-Aspartate Receptor Antagonist, Attenuates Cerebral Amyloid Angiopathy by Increasing Insulin-Degrading Enzyme Expression. Mol. Neurobiol. 2019, 56, 8573–8588. [Google Scholar] [CrossRef]
- Inoue, Y.; Masuda, T.; Misumi, Y.; Ando, Y.; Ueda, M. Metformin attenuates vascular pathology by increasing expression of insulin-degrading enzyme in a mixed model of cerebral amyloid angiopathy and type 2 diabetes mellitus. Neurosci. Lett. 2021, 762, 136136. [Google Scholar] [CrossRef]
- Devi, L.; Alldred, M.J.; Ginsberg, S.D.; Ohno, M. Mechanisms underlying insulin deficiency-induced acceleration of β-amyloidosis in a mouse model of Alzheimer’s disease. PLoS ONE 2012, 7, e32792. [Google Scholar] [CrossRef]
- Pandini, G.; Pace, V.; Copani, A.; Squatrito, S.; Milardi, D.; Vigneri, R. Insulin has multiple antiamyloidogenic effects on human neuronal cells. Endocrinology 2013, 154, 375–387. [Google Scholar] [CrossRef] [PubMed]
- Shingo, A.S.; Kanabayashi, T.; Kito, S.; Murase, T. Intracerebroventricular administration of an insulin analogue recovers STZ-induced cognitive decline in rats. Behav. Brain Res. 2013, 241, 105–111. [Google Scholar] [CrossRef]
- He, Z.; Han, S.; Zhu, H.; Hu, X.; Li, X.; Hou, C.; Wu, C.; Xie, Q.; Li, N.; Du, X.; et al. The Protective Effect of Vanadium on Cognitive Impairment and the Neuropathology of Alzheimer’s Disease in APPSwe/PS1dE9 Mice. Front. Mol. Neurosci. 2020, 13, 21. [Google Scholar] [CrossRef] [PubMed]
- Alafuzoff, I.; Aho, L.; Helisalmi, S.; Mannermaa, A.; Soininen, H. Beta-amyloid deposition in brains of subjects with diabetes. Neuropathol. Appl. Neurobiol. 2009, 35, 60–68. [Google Scholar] [CrossRef]
- Ramos-Rodriguez, J.J.; Ortiz-Barajas, O.; Gamero-Carrasco, C.; de la Rosa, P.R.; Infante-Garcia, C.; Zopeque-Garcia, N.; Lechuga-Sancho, A.M.; Garcia-Alloza, M. Prediabetes-induced vascular alterations exacerbate central pathology in APPswe/PS1dE9 mice. Psychoneuroendocrinology 2014, 48, 123–135. [Google Scholar] [CrossRef] [PubMed]
- Little, K.; Llorián-Salvador, M.; Scullion, S.; Hernández, C.; Simó-Servat, O.; Del Marco, A.; Bosma, E.; Vargas-Soria, M.; Carranza-Naval, M.J.; Van Bergen, T.; et al. Common pathways in dementia and diabetic retinopathy: Understanding the mechanisms of diabetes-related cognitive decline. Trends Endocrinol. Metab. 2022, 33, 50–71. [Google Scholar] [CrossRef]
- Song, X.; Zhu, Z.; Qian, X.; Liu, X.; Chen, S.; Tang, H. Multi-Omics Characterization of Type 2 Diabetes Mellitus-Induced Cognitive Impairment in the db/db Mouse Model. Molecules 2022, 27, 1904. [Google Scholar] [CrossRef]
- Cassano, V.; Leo, A.; Tallarico, M.; Nesci, V.; Cimellaro, A.; Fiorentino, T.V.; Citraro, R.; Hribal, M.L.; De Sarro, G.; Perticone, F.; et al. Metabolic and Cognitive Effects of Ranolazine in Type 2 Diabetes Mellitus: Data from an in vivo Model. Nutrients 2020, 12, 382. [Google Scholar] [CrossRef]
- Hui, Y.; Xu, Z.; Li, J.; Kuang, L.; Zhong, Y.; Tang, Y.; Wei, J.; Zhou, H.; Zheng, T. Nonenzymatic function of DPP4 promotes diabetes-associated cognitive dysfunction through IGF-2R/PKA/SP1/ERp29/IP3R2 pathway-mediated impairment of Treg function and M1 microglia polarization. Metabolism 2023, 138, 155340. [Google Scholar] [CrossRef]
- Fan, X.; Zhang, Y.; Song, Y.; Zhao, Y.; Xu, Y.; Guo, F.; Shao, M.; Ma, X.; Zhang, W.; Wei, F.; et al. Compound Danshen Dripping Pills moderate intestinal flora and the TLR4/MyD88/NF-κB signaling pathway in alleviating cognitive dysfunction in type 2 diabetic KK-Ay mice. Phytomedicine 2023, 111, 154656. [Google Scholar] [CrossRef]
- Li, Q.; Zhao, Y.; Guo, H.; Li, Q.; Yan, C.; Li, Y.; He, S.; Wang, N.; Wang, Q. Impaired lipophagy induced-microglial lipid droplets accumulation contributes to the buildup of TREM1 in diabetes-associated cognitive impairment. Autophagy 2023, 19, 2639–2656. [Google Scholar] [CrossRef]
- Li, Y.; Jiang, T.; Du, M.; He, S.; Huang, N.; Cheng, B.; Yan, C.; Tang, W.; Gao, W.; Guo, H.; et al. Ketohexokinase-dependent metabolism of cerebral endogenous fructose in microglia drives diabetes-associated cognitive dysfunction. Exp. Mol. Med. 2023, 55, 2417–2432. [Google Scholar] [CrossRef]
- Yao, Y.; Shi, J.; Zhang, C.; Gao, W.; Huang, N.; Liu, Y.; Yan, W.; Han, Y.; Zhou, W.; Kong, L. Pyruvate dehydrogenase kinase 1 protects against neuronal injury and memory loss in mouse models of diabetes. Cell Death Dis. 2023, 14, 722. [Google Scholar] [CrossRef]
- Wang, Z.; Cui, X.; Yan, W.; Liu, N.; Shang, J.; Yi, X.; Guo, T.; Wei, X.; Sun, Y.; Hu, H.; et al. Mollugin activates GLP-1R to improve cognitive dysfunction in type 2 diabetic mice. Life Sci. 2023, 331, 122026. [Google Scholar] [CrossRef]
- Poorgholam, P.; Yaghmaei, P.; Noureddini, M.; Hajebrahimi, Z. Artemisin and human endometrial-derived stem cells improve cognitive function and synaptic plasticity in a rat model of Alzheimer disease and diabetes. Metab. Brain Dis. 2023, 38, 1925–1936. [Google Scholar] [CrossRef]
- Cho, J.H.; Chae, C.W.; Lim, J.R.; Jung, Y.H.; Han, S.J.; Yoon, J.H.; Park, J.Y.; Han, H.J. Sodium butyrate ameliorates high glucose-suppressed neuronal mitophagy by restoring PRKN expression via inhibiting the RELA-HDAC8 complex. Autophagy 2024, 20, 1505–1522. [Google Scholar] [CrossRef]
- Dove, A.; Shang, Y.; Xu, W.; Grande, G.; Laukka, E.J.; Fratiglioni, L.; Marseglia, A. The impact of diabetes on cognitive impairment and its progression to dementia. Alzheimer’s Dement. 2021, 17, 1769–1778. [Google Scholar] [CrossRef]
- Filler, J.; Georgakis, M.K.; Dichgans, M. Risk factors for cognitive impairment and dementia after stroke: A systematic review and meta-analysis. Lancet Healthy Longev. 2024, 5, e31–e44. [Google Scholar] [CrossRef]
- Skrobot, O.A.; O’Brien, J.; Black, S.; Chen, C.; DeCarli, C.; Erkinjuntti, T.; Ford, G.A.; Kalaria, R.N.; Pantoni, L.; Pasquier, F.; et al. The Vascular Impairment of Cognition Classification Consensus Study. Alzheimers Dement. 2017, 13, 624–633. [Google Scholar] [CrossRef]
- American Diabetes Association Professional Practice Committee. 10. Cardiovascular Disease and Risk Management: Standards of Care in Diabetes-2024. Diabetes Care 2024, 47, S179–S218. [Google Scholar] [CrossRef]
- Blevins, B.L.; Vinters, H.V.; Love, S.; Wilcock, D.M.; Grinberg, L.T.; Schneider, J.A.; Kalaria, R.N.; Katsumata, Y.; Gold, B.T.; Wang, D.J.J.; et al. Brain arteriolosclerosis. Acta Neuropathol. 2021, 141, 1–24. [Google Scholar] [CrossRef]
- Wright, E.M. Glucose transport families SLC5 and SLC50. Mol. Aspects Med. 2013, 34, 183–196. [Google Scholar] [CrossRef]
- Ni, L.; Yuan, C.; Chen, G.; Zhang, C.; Wu, X. SGLT2i: Beyond the glucose-lowering effect. Cardiovasc. Diabetol. 2020, 19, 98. [Google Scholar] [CrossRef]
- Kintzoglanakis, K.; Diamantis, C.; Mariolis, A.; Paschou, S.A. Patient-important outcomes in type 2 diabetes: The paradigm of the sodium-glucose cotransporter-2 inhibitors and glucagon-like peptide-1 receptor agonists. Diabetes Vasc. Dis. Res. 2024, 21, 14791641241269743. [Google Scholar] [CrossRef]
- American Diabetes Association Professional Practice Committee. 9. Pharmacologic Approaches to Glycemic Treatment: Standards of Care in Diabetes-2024. Diabetes Care 2024, 47, S158–S178. [Google Scholar] [CrossRef]
- Wu, C.-Y.; Iskander, C.; Wang, C.; Xiong, L.Y.; Shah, B.R.; Edwards, J.D.; Kapral, M.K.; Herrmann, N.; Lanctôt, K.L.; Masellis, M.; et al. Association of Sodium-Glucose Cotransporter 2 Inhibitors With Time to Dementia: A Population-Based Cohort Study. Diabetes Care 2023, 46, 297–304. [Google Scholar] [CrossRef]
- Mone, P.; Lombardi, A.; Gambardella, J.; Pansini, A.; Macina, G.; Morgante, M.; Frullone, S.; Santulli, G. Empagliflozin Improves Cognitive Impairment in Frail Older Adults With Type 2 Diabetes and Heart Failure With Preserved Ejection Fraction. Diabetes Care 2022, 45, 1247–1251. [Google Scholar] [CrossRef]
- Secnik, J.; Xu, H.; Schwertner, E.; Hammar, N.; Alvarsson, M.; Winblad, B.; Eriksdotter, M.; Garcia-Ptacek, S.; Religa, D. Glucose-Lowering Medications and Post-Dementia Survival in Patients with Diabetes and Dementia. J. Alzheimers Dis. 2022, 86, 245–257. [Google Scholar] [CrossRef]
- Ding, J.; Shi, Q.; Tao, Q.; Su, H.; Du, Y.; Pan, T.; Zhong, X. Correlation between long-term glycemic variability and cognitive function in middle-aged and elderly patients with type 2 diabetes mellitus: A retrospective study. PeerJ 2023, 11, e16698. [Google Scholar] [CrossRef]
- Mone, P.; Guerra, G.; Lombardi, A.; Illario, M.; Pansini, A.; Marro, A.; Frullone, S.; Taurino, A.; Sorriento, D.; Verri, V.; et al. Effects of SGLT2 inhibition via empagliflozin on cognitive and physical impairment in frail diabetic elders with chronic kidney disease. Pharmacol. Res. 2024, 200, 107055. [Google Scholar] [CrossRef]
- Mui, J.V.; Zhou, J.; Lee, S.; Leung, K.S.K.; Lee, T.T.L.; Chou, O.H.I.; Tsang, S.L.; Wai, A.K.C.; Liu, T.; Wong, W.T.; et al. Sodium-Glucose Cotransporter 2 (SGLT2) Inhibitors vs. Dipeptidyl Peptidase-4 (DPP4) Inhibitors for New-Onset Dementia: A Propensity Score-Matched Population-Based Study With Competing Risk Analysis. Front. Cardiovasc. Med. 2021, 8, 747620. [Google Scholar] [CrossRef]
- Wium-Andersen, I.K.; Osler, M.; Jørgensen, M.B.; Rungby, J.; Wium-Andersen, M.K. Antidiabetic medication and risk of dementia in patients with type 2 diabetes: A nested case-control study. Eur. J. Endocrinol. 2019, 181, 499–507. [Google Scholar] [CrossRef]
- Low, S.; Goh, K.S.; Ng, T.P.; Moh, A.; Ang, S.F.; Wang, J.; Ang, K.; Tang, W.E.; Lim, Z.; Subramaniam, T.; et al. Association Between Use of Sodium-Glucose Co-Transporter-2 (SGLT2) Inhibitors and Cognitive Function in a Longitudinal Study of Patients with Type 2 Diabetes. J. Alzheimers Dis. 2022, 87, 635–642. [Google Scholar] [CrossRef]
- Zhang, S.; Wang, F.; Xie, L.; Xu, J.; Song, X.; Tao, J.; Chen, J.; Ma, D.; Yu, X.; Shi, X.; et al. Sodium-glucose cotransporter 2 inhibition through henagliflozin ameliorates cognitive impairment in patients with type 2 diabetes. J. Diabetes Investig. 2024, 15, 1596–1603. [Google Scholar] [CrossRef]
- Proietti, R.; Rivera-Caravaca, J.M.; López-Gálvez, R.; Harrison, S.L.; Marín, F.; Underhill, P.; Shantsila, E.; McDowell, G.; Vinciguerra, M.; Davies, R.; et al. Cerebrovascular, Cognitive and Cardiac Benefits of SGLT2 Inhibitors Therapy in Patients with Atrial Fibrillation and Type 2 Diabetes Mellitus: Results from a Global Federated Health Network Analysis. J. Clin. Med. 2023, 12, 2814. [Google Scholar] [CrossRef]
- Chen, Y.-Y.; Chang, H.-C.; Lin, Y.-J.; Chien, K.-L.; Hsieh, Y.-C.; Chung, F.-P.; Lin, C.-H.; Lip, G.Y.H.; Chen, S.-A. The impact of sodium-glucose co-transporter-2 inhibitors on dementia and cardiovascular events in diabetic patients with atrial fibrillation. Diabetes Metab. Res. Rev. 2024, 40, e3775. [Google Scholar] [CrossRef]
- Siao, W.-Z.; Lin, T.-K.; Huang, J.-Y.; Tsai, C.-F.; Jong, G.-P. The association between sodium-glucose cotransporter 2 inhibitors and incident dementia: A nationwide population-based longitudinal cohort study. Diabetes Vasc. Dis. Res. 2022, 19, 14791641221098168. [Google Scholar] [CrossRef]
- Edmonston, D.; Mulder, H.; Lydon, E.; Chiswell, K.; Lampron, Z.; Shay, C.; Marsolo, K.; Jones, W.S.; Butler, J.; Shah, R.C.; et al. Kidney and Cardiovascular Effectiveness of Empagliflozin Compared With Dipeptidyl Peptidase-4 Inhibitors in Patients With Type 2 Diabetes. Am. J. Cardiol. 2024, 221, 52–63. [Google Scholar] [CrossRef]
- Sharma, A.; Wood, S.; Bell, J.S.; De Blasio, M.J.; Ilomäki, J.; Ritchie, R.H. Sex differences in risk of cardiovascular events and mortality with sodium glucose co-transporter-2 inhibitors versus glucagon-like peptide 1 receptor agonists in Australians with type 2 diabetes: A population-based cohort study. Lancet Reg. Health West. Pac. 2023, 33, 100692. [Google Scholar] [CrossRef]
- Zinman, B.; Wanner, C.; Lachin, J.M.; Fitchett, D.; Bluhmki, E.; Hantel, S.; Mattheus, M.; Devins, T.; Johansen, O.E.; Woerle, H.J.; et al. Empagliflozin, Cardiovascular Outcomes, and Mortality in Type 2 Diabetes. N. Engl. J. Med. 2015, 373, 2117–2128. [Google Scholar] [CrossRef]
- Wiviott, S.D.; Raz, I.; Bonaca, M.P.; Mosenzon, O.; Kato, E.T.; Cahn, A.; Silverman, M.G.; Zelniker, T.A.; Kuder, J.F.; Murphy, S.A.; et al. Dapagliflozin and Cardiovascular Outcomes in Type 2 Diabetes. N. Engl. J. Med. 2019, 380, 347–357. [Google Scholar] [CrossRef]
- Cannon, C.P.; Pratley, R.; Dagogo-Jack, S.; Mancuso, J.; Huyck, S.; Masiukiewicz, U.; Charbonnel, B.; Frederich, R.; Gallo, S.; Cosentino, F.; et al. Cardiovascular Outcomes with Ertugliflozin in Type 2 Diabetes. N. Engl. J. Med. 2020, 383, 1425–1435. [Google Scholar] [CrossRef]
- Riester, M.R.; Zullo, A.R.; Joshi, R.; Daiello, L.A.; Hayes, K.N.; Ko, D.; Kim, D.H.; Munshi, M.; Berry, S.D. Comparative safety and cardiovascular effectiveness of sodium-glucose cotransporter-2 inhibitors and glucagon-like peptide-1 receptor agonists in nursing homes. Diabetes Obes. Metab. 2024, 26, 3403–3417. [Google Scholar] [CrossRef]
- Perna, S.; Mainardi, M.; Astrone, P.; Gozzer, C.; Biava, A.; Bacchio, R.; Spadaccini, D.; Solerte, S.B.; Rondanelli, M. 12-month effects of incretins versus SGLT2-Inhibitors on cognitive performance and metabolic profile. A randomized clinical trial in the elderly with Type-2 diabetes mellitus. Clin. Pharmacol. 2018, 10, 141–151. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhang, R.; Wang, S. Effect of Dapagliflozin Combined with Cognitive Behavior Training on Quality of Life and Cognitive Function in Elderly Patients with Type 2 Diabetes Mellitus Complicated with Mild Cognitive Impairment. Iran. J. Public Health 2022, 51, 1251–1258. [Google Scholar] [CrossRef]
- Youn, Y.J.; Kim, S.; Jeong, H.-J.; Ah, Y.-M.; Yu, Y.M. Sodium-glucose cotransporter-2 inhibitors and their potential role in dementia onset and cognitive function in patients with diabetes mellitus: A systematic review and meta-analysis. Front. Neuroendocrinol. 2024, 73, 101131. [Google Scholar] [CrossRef]
- Zhou, J.-B.; Tang, X.; Han, M.; Yang, J.; Simó, R. Impact of antidiabetic agents on dementia risk: A Bayesian network meta-analysis. Metabolism 2020, 109, 154265. [Google Scholar] [CrossRef]
- Jaiswal, V.; Mashkoor, Y.; Raj, N.; Rajak, K.; Jaiswal, A.; Fonarow, G.C. Association between SGLT2 Inhibitors and Risk of Dementia and Parkinson’s Disease: A Meta-Analysis of 12 Randomized Controlled Trials. Am. J. Med. 2024, 137, 1136–1141. [Google Scholar] [CrossRef]
- Banerjee, M.; Pal, R.; Maisnam, I.; Mukhopadhyay, S. GLP-1 receptor agonists, SGLT2 inhibitors and noncardiovascular mortality in type 2 diabetes: Insights from a meta-analysis. Diabetes Metab. Syndr. 2024, 18, 102943. [Google Scholar] [CrossRef]
- Tang, H.; Shao, H.; Shaaban, C.E.; Yang, K.; Brown, J.; Anton, S.; Wu, Y.; Bress, A.; Donahoo, W.T.; DeKosky, S.T.; et al. Newer glucose-lowering drugs and risk of dementia: A systematic review and meta-analysis of observational studies. J. Am. Geriatr. Soc. 2023, 71, 2096–2106. [Google Scholar] [CrossRef]
- Tang, H.; Donahoo, W.T.; Svensson, M.; Shaaban, C.E.; Smith, G.; Jaffee, M.S.; Huang, Y.; Hu, X.; Lu, Y.; Salloum, R.G.; et al. Heterogeneous treatment effects of sodium-glucose cotransporter 2 inhibitors on risk of dementia in people with type 2 diabetes: A population-based cohort study. Alzheimers Dement. 2024, 20, 5528–5539. [Google Scholar] [CrossRef]
- Cheng, H.; Zhang, Z.; Zhang, B.; Zhang, W.; Wang, J.; Ni, W.; Miao, Y.; Liu, J.; Bi, Y. Enhancement of Impaired Olfactory Neural Activation and Cognitive Capacity by Liraglutide, but Not Dapagliflozin or Acarbose, in Patients With Type 2 Diabetes: A 16-Week Randomized Parallel Comparative Study. Diabetes Care 2022, 45, 1201–1210. [Google Scholar] [CrossRef]
- Unno, K.; Taguchi, K.; Takagi, Y.; Hase, T.; Meguro, S.; Nakamura, Y. Mouse Models with SGLT2 Mutations: Toward Understanding the Role of SGLT2 beyond Glucose Reabsorption. Int. J. Mol. Sci. 2023, 24, 6278. [Google Scholar] [CrossRef]
- Piątkowska-Chmiel, I.; Herbet, M.; Gawrońska-Grzywacz, M.; Pawłowski, K.; Ostrowska-Leśko, M.; Dudka, J. Molecular and neural roles of sodium-glucose cotransporter 2 inhibitors in alleviating neurocognitive impairment in diabetic mice. Psychopharmacology 2023, 240, 983–1000. [Google Scholar] [CrossRef]
- Khan, T.; Khan, S.; Akhtar, M.; Ali, J.; Najmi, A.K. Empagliflozin nanoparticles attenuates type2 diabetes induced cognitive impairment via oxidative stress and inflammatory pathway in high fructose diet induced hyperglycemic mice. Neurochem. Int. 2021, 150, 105158. [Google Scholar] [CrossRef]
- Hayden, M.R.; Grant, D.G.; Aroor, A.R.; DeMarco, V.G. Empagliflozin Ameliorates Type 2 Diabetes-Induced Ultrastructural Remodeling of the Neurovascular Unit and Neuroglia in the Female db/db Mouse. Brain Sci. 2019, 9, 57. [Google Scholar] [CrossRef]
- Jayarathne, H.S.M.; Debarba, L.K.; Jaboro, J.J.; Ginsburg, B.C.; Miller, R.A.; Sadagurski, M. Neuroprotective effects of Canagliflozin: Lessons from aged genetically diverse UM-HET3 mice. Aging Cell 2022, 21, e13653. [Google Scholar] [CrossRef]
- Shaikh, S.; Rizvi, S.M.D.; Shakil, S.; Riyaz, S.; Biswas, D.; Jahan, R. Forxiga (dapagliflozin): Plausible role in the treatment of diabetes-associated neurological disorders. Biotechnol. Appl. Biochem. 2016, 63, 145–150. [Google Scholar] [CrossRef]
- Shakil, S. Molecular Interaction of Anti-Diabetic Drugs With Acetylcholinesterase and Sodium Glucose Co-Transporter 2. J. Cell Biochem. 2017, 118, 3855–3865. [Google Scholar] [CrossRef]
- Hierro-Bujalance, C.; Infante-Garcia, C.; Del Marco, A.; Herrera, M.; Carranza-Naval, M.J.; Suarez, J.; Alves-Martinez, P.; Lubian-Lopez, S.; Garcia-Alloza, M. Empagliflozin reduces vascular damage and cognitive impairment in a mixed murine model of Alzheimer’s disease and type 2 diabetes. Alzheimers Res. Ther. 2020, 12, 40. [Google Scholar] [CrossRef]
- O’Keefe, J.H.; Weidling, R.; O’Keefe, E.L.; Franco, W.G. SGLT inhibitors for improving Healthspan and lifespan. Prog. Cardiovasc. Dis. 2023, 81, 2–9. [Google Scholar] [CrossRef]
- Mancinetti, F.; Xenos, D.; De Fano, M.; Mazzieri, A.; Porcellati, F.; Boccardi, V.; Mecocci, P. Diabetes-Alzheimer’s connection in older age: SGLT2 inhibitors as promising modulators of disease pathways. Ageing Res. Rev. 2023, 90, 102018. [Google Scholar] [CrossRef]
- Rizzo, M.R.; Di Meo, I.; Polito, R.; Auriemma, M.C.; Gambardella, A.; di Mauro, G.; Capuano, A.; Paolisso, G. Cognitive impairment and type 2 diabetes mellitus: Focus of SGLT2 inhibitors treatment. Pharmacol. Res. 2022, 176, 106062. [Google Scholar] [CrossRef]
- Pawlos, A.; Broncel, M.; Woźniak, E.; Gorzelak-Pabiś, P. Neuroprotective Effect of SGLT2 Inhibitors. Molecules 2021, 26, 7213. [Google Scholar] [CrossRef]
- Esterline, R.; Oscarsson, J.; Burns, J. A role for sodium glucose cotransporter 2 inhibitors (SGLT2is) in the treatment of Alzheimer’s disease? Int. Rev. Neurobiol. 2020, 155, 113–140. [Google Scholar] [CrossRef]
- Chavda, V.; Vashi, R.; Patel, S. Cerebrovascular Complications of Diabetes: SGLT-2 Inhibitors as a Promising Future Therapeutics. Curr. Drug Targets 2021, 22, 1629–1636. [Google Scholar] [CrossRef]
- Wiciński, M.; Wódkiewicz, E.; Górski, K.; Walczak, M.; Malinowski, B. Perspective of SGLT2 Inhibition in Treatment of Conditions Connected to Neuronal Loss: Focus on Alzheimer’s Disease and Ischemia-Related Brain Injury. Pharmaceuticals 2020, 13, 379. [Google Scholar] [CrossRef]
- Avgerinos, K.I.; Mullins, R.J.; Vreones, M.; Mustapic, M.; Chen, Q.; Melvin, D.; Kapogiannis, D.; Egan, J.M. Empagliflozin Induced Ketosis, Upregulated IGF-1/Insulin Receptors and the Canonical Insulin Signaling Pathway in Neurons, and Decreased the Excitatory Neurotransmitter Glutamate in the Brain of Non-Diabetics. Cells 2022, 11, 3372. [Google Scholar] [CrossRef]
- Birajdar, S.V.; Mazahir, F.; Alam, M.I.; Kumar, A.; Yadav, A.K. Repurposing and clinical attributes of antidiabetic drugs for the treatment of neurodegenerative disorders. Eur. J. Pharmacol. 2023, 961, 176117. [Google Scholar] [CrossRef]
- Goodarzi, G.; Tehrani, S.S.; Fana, S.E.; Moradi-Sardareh, H.; Panahi, G.; Maniati, M.; Meshkani, R. Crosstalk between Alzheimer’s disease and diabetes: A focus on anti-diabetic drugs. Metab. Brain Dis. 2023, 38, 1769–1800. [Google Scholar] [CrossRef]
- Noel, J.A.; Hougen, I.; Sood, M.M. The Intersection of SGLT2 Inhibitors, Cognitive Impairment, and CKD. Front. Neurol. 2022, 13, 823569. [Google Scholar] [CrossRef] [PubMed]
- Mei, J.; Li, Y.; Niu, L.; Liang, R.; Tang, M.; Cai, Q.; Xu, J.; Zhang, D.; Yin, X.; Liu, X.; et al. SGLT2 inhibitors: A novel therapy for cognitive impairment via multifaceted effects on the nervous system. Transl. Neurodegener. 2024, 13, 41. [Google Scholar] [CrossRef] [PubMed]
- Youssef, M.E.; Yahya, G.; Popoviciu, M.S.; Cavalu, S.; Abd-Eldayem, M.A.; Saber, S. Unlocking the Full Potential of SGLT2 Inhibitors: Expanding Applications beyond Glycemic Control. Int. J. Mol. Sci. 2023, 24, 6039. [Google Scholar] [CrossRef] [PubMed]
- Sim, A.Y.; Barua, S.; Kim, J.Y.; Lee, Y.-H.; Lee, J.E. Role of DPP-4 and SGLT2 Inhibitors Connected to Alzheimer Disease in Type 2 Diabetes Mellitus. Front. Neurosci. 2021, 15, 708547. [Google Scholar] [CrossRef]
- Riemma, M.A.; Mele, E.; Donniacuo, M.; Telesca, M.; Bellocchio, G.; Castaldo, G.; Rossi, F.; De Angelis, A.; Cappetta, D.; Urbanek, K.; et al. Glucagon-like peptide-1 receptor agonists and sodium-glucose cotransporter 2 inhibitors, anti-diabetic drugs in heart failure and cognitive impairment: Potential mechanisms of the protective effects. Front. Pharmacol. 2024, 15, 1422740. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Liao, X.; Xu, J.; Yin, J.; Li, S.; Li, M.; Shi, X.; Zhang, S.; Li, C.; Xu, W.; et al. The Promising Potency of Sodium–Glucose Cotransporter 2 Inhibitors in the Prevention of and as Treatment for Cognitive Impairment Among Type 2 Diabetes Patients. Biomedicines 2024, 12, 2783. https://doi.org/10.3390/biomedicines12122783
Zhang Y, Liao X, Xu J, Yin J, Li S, Li M, Shi X, Zhang S, Li C, Xu W, et al. The Promising Potency of Sodium–Glucose Cotransporter 2 Inhibitors in the Prevention of and as Treatment for Cognitive Impairment Among Type 2 Diabetes Patients. Biomedicines. 2024; 12(12):2783. https://doi.org/10.3390/biomedicines12122783
Chicago/Turabian StyleZhang, Yibin, Xiaobin Liao, Jialu Xu, Jiaxin Yin, Shan Li, Mengni Li, Xiaoli Shi, Shujun Zhang, Chunyu Li, Weijie Xu, and et al. 2024. "The Promising Potency of Sodium–Glucose Cotransporter 2 Inhibitors in the Prevention of and as Treatment for Cognitive Impairment Among Type 2 Diabetes Patients" Biomedicines 12, no. 12: 2783. https://doi.org/10.3390/biomedicines12122783
APA StyleZhang, Y., Liao, X., Xu, J., Yin, J., Li, S., Li, M., Shi, X., Zhang, S., Li, C., Xu, W., Yu, X., & Yang, Y. (2024). The Promising Potency of Sodium–Glucose Cotransporter 2 Inhibitors in the Prevention of and as Treatment for Cognitive Impairment Among Type 2 Diabetes Patients. Biomedicines, 12(12), 2783. https://doi.org/10.3390/biomedicines12122783