Sex-Dependent Differences in the Elemental Composition of Internal Organs Determined via Total Reflection X-Ray Fluorescence and Inductively Coupled Plasma Optical Emission Spectroscopy
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Sample Preparation
2.3. Apparatus and Measurement Conditions
2.4. Quantification and Data Analysis
- Cx—concentration of the element x in the liquid organ sample [μg/g],
- CIS—concentration of the internal standard in the liquid sample [μg/g],
- Nx—net pulse number for the element x in the sample spectrum [cts],
- NIS—net pulse number for the internal standard in the sample spectrum [cts],
- sx—relative sensitivity for the element x.
- Cxj—concentration of the element x in the organ subsample j [μg/g],
- NBGxj—area of the background under the Kα line of the element x in the spectrum of subsample j [cts],
- Nxj—net peak area of the Kα line of the element x in the spectrum of subsample j [cts].
- BECy—background equivalent concentration of the element x [μg/g],
- RSD0y—relative standard deviation for the blank of the element x [a.u.].
3. Results
3.1. Validation of Experimental Methods
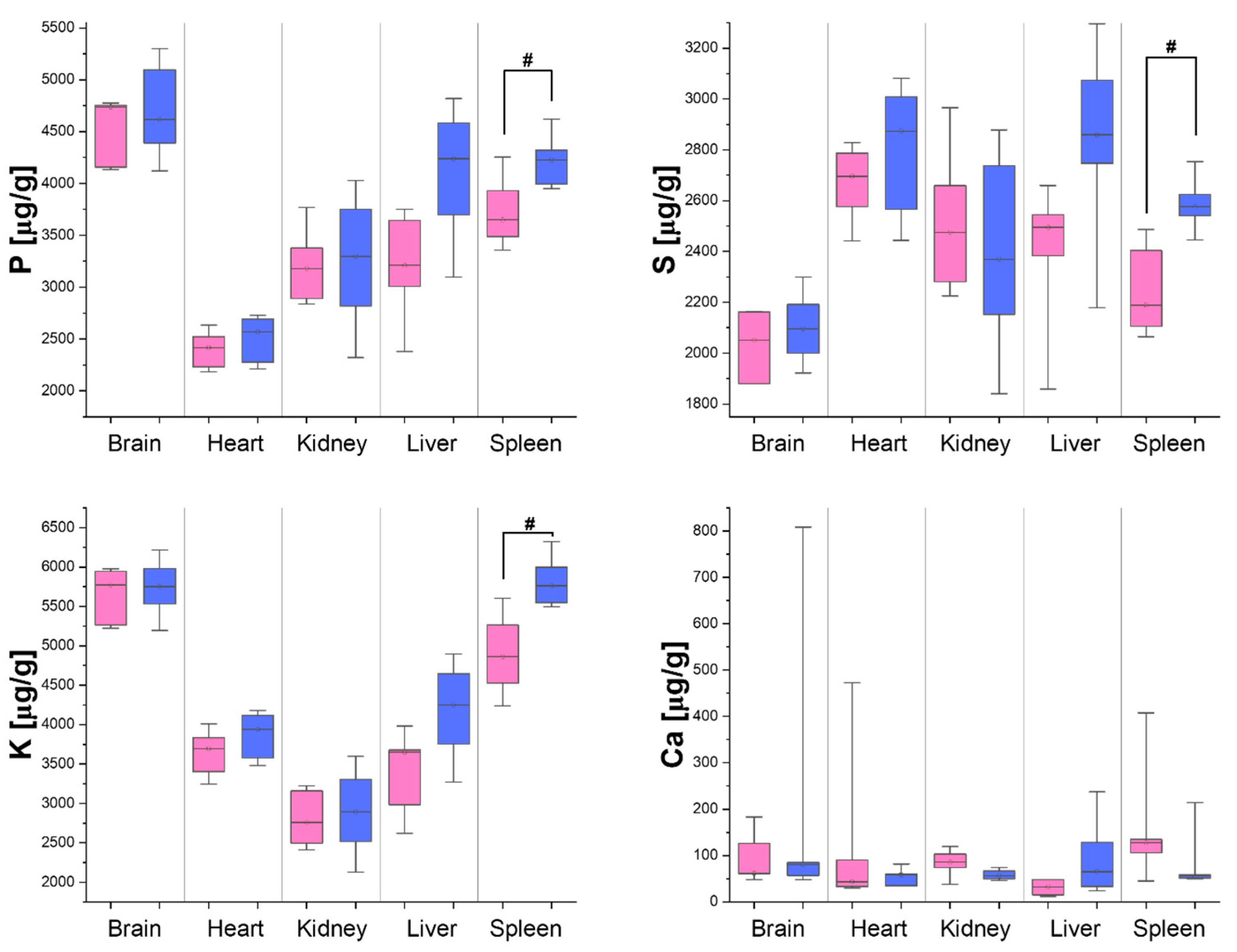
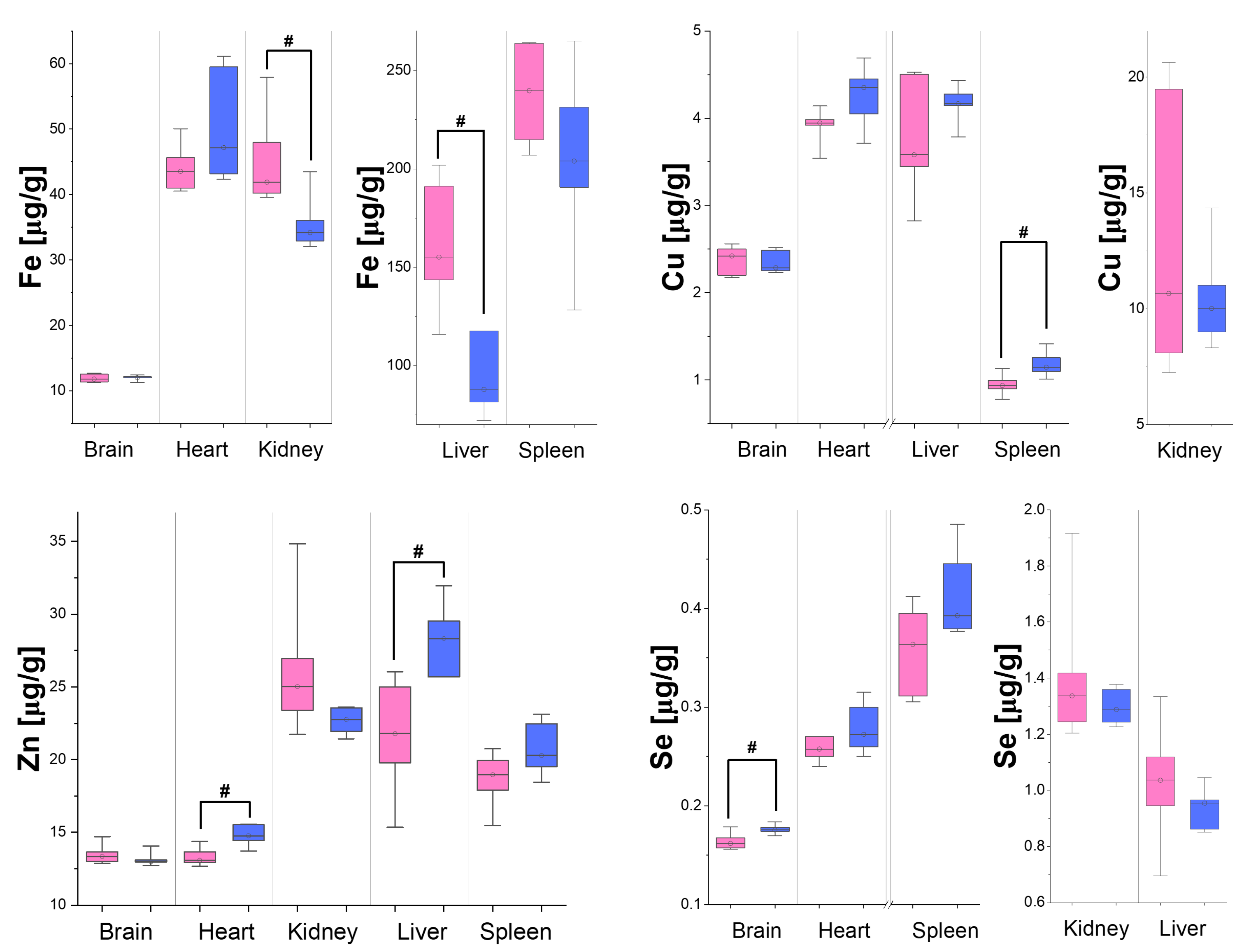
3.2. Differences in Organ Element Composition Between Male and Female Rats
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| Element/Tissue | Concentration of Element in Tissue [µg/g] | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Brain | Heart | Kidneys | Liver | Spleen | Literature | |||||||
| M | F | M | F | M | F | M | F | M | F | M | F | |
| P | 2519–6110 | - | 2035–2970 | - | 2529–4250 | 3280–6034 | 2981–5750 | 3380–6542 | 3185–5460 | 6997–7439 | [48,49] | [53,54,55] |
| S | 1312–2030 | - | 2490–2529 | - | 2290–2331 | 3924–4386 | 2351–2980 | 2390–4192 | 1957–2490 | 3705–4476 | [48,49] | [53,54,55] |
| K | 3092–6100 | - | 2768–3470 | - | 2473–2920 | 2580–5668 | 3205–5440 | 3000–7294 | 4045–5290 | 7865–9098 | [48,49,50] | [53,55] |
| Ca | 33–730 | - | 26–112 | - | 55–120 | 72–291 | 30–1300 | 32.3–159.0 | 33–2070 | 124–236 | [48,49,50] | [53,56] |
| Fe | 11.4–20.6 | - | 57–75 | 445–674 | 40–45 | 103–513 | 71–137 | 278–1023 | 185–547 | 592–818 | [48,49,51] | [53,54,55,56] |
| Cu | 1.94–3.78 | - | 4.49–9.04 | 18.4–21.6 | 6.37–8.84 | 16.0–48.7 | 3.16–8.37 | 5.4–17.0 | 1.10–2.34 | 3.03–4.09 | [48,49,52] | [53,54,55,56] |
| Zn | 10.8–50.0 | - | 14.6–25.4 | 62.6–69.1 | 18.9–32.0 | 29.0–80.4 | 29.4–125.0 | 32.3–103.0 | 20.4–159.0 | 30–57 | [48,49,52] | [53,54,55,56] |
| Se | 0.127–0.193 | - | 0.364–0.430 | - | 1.40–1.90 | 2.5–3.1 | 0.59–1.60 | 2.32–3.23 | 0.42–0.54 | 0.64–0.81 | [48,50,51] | [53,54,55,56] |
References
- Barré-Sinoussi, F.; Montagutelli, X. Animal models are essential to biological research: Issues and perspectives. Future Sci. OA 2015, 1, FSO63. [Google Scholar] [CrossRef] [PubMed]
- Phillips, N.L.H.; Roth, T.L. Animal Models and Their Contribution to Our Understanding of the Relationship Between Environments, Epigenetic Modifications, and Behavior. Genes 2019, 10, 47. [Google Scholar] [CrossRef] [PubMed]
- Domínguez-Oliva, A.; Hernández-Ávalos, I.; Martínez-Burnes, J.; Olmos-Hernández, A.; Verduzco-Mendoza, A.; Mota-Rojas, D. The Importance of Animal Models in Biomedical Research: Current Insights and Applications. Animals 2023, 13, 1223. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, P.; Roy, S.; Ghosh, D.; Nandi, S.K. Role of animal models in biomedical research: A review. Lab. Anim. Res. 2022, 38, 18. [Google Scholar] [CrossRef]
- Karlsson Lind, L.; Rydberg, D.M.; Schenck-Gustafsson, K. Sex and gender differences in drug treatment: Experiences from the knowledge database Janusmed Sex and Gender. Biol. Sex. Differ. 2023, 14, 28. [Google Scholar] [CrossRef]
- Klein, S.L.; Roberts, C.W. Sex and Gender Differences in Infection and Treatments for Infectious Diseases, 1st ed.; Springer International Publishing: Cham, Switzerland, 2015; pp. 1–29. [Google Scholar]
- Regitz-Zagrosek, V. Sex and gender differences in health. EMBO Rep. 2012, 13, 596–603. [Google Scholar] [CrossRef]
- Gao, Z.; Xiong, J.; Chen, Z.; Deng, X.; Xu, Z.; Sun, A.; Fan, Y. Gender differences of morphological and hemodynamic characteristics of abdominal aortic aneurysm. Biol. Sex Differ. 2020, 11, 41. [Google Scholar] [CrossRef]
- Yan, H.; Wu, W.; Chang, X.; Xia, M.; Ma, S.; Wang, L.; Gao, J. Gender differences in the efficacy of pioglitazone treatment in nonalcoholic fatty liver disease patients with abnormal glucose metabolism. Biol. Sex Differ. 2021, 12, 1. [Google Scholar] [CrossRef]
- Strack, C.; Behrens, G.; Sag, S.; Mohr, M.; Zeller, J.; Lahmann, C.; Hubauer, U.; Loew, T.; Maier, L.; Fischer, M.; et al. Gender differences in cardiometabolic health and disease in a cross-sectional observational obesity study. Biol. Sex Differ. 2022, 13, 8. [Google Scholar] [CrossRef]
- Lewis, D.A.; Kamon, E.; Hodgson, J.L. Physiological differences between genders Implications for sports conditioning. Sports Med. 1986, 3, 357–369. [Google Scholar] [CrossRef]
- Harms, C.A. Does gender affect pulmonary function and exercise capacity? Respir. Physiol. Neurobiol. 2006, 151, 124–131. [Google Scholar] [CrossRef] [PubMed]
- Raghavan, D.; Varkey, A.; Bartter, T. Chronic obstructive pulmonary disease: The impact of gender. Curr. Opin. Pulm. Med. 2017, 23, 117–123. [Google Scholar] [CrossRef] [PubMed]
- Robles, P.G.; Brooks, D.; Goldstein, R.; Salbach, N.; Mathur, S. Gender-associated differences in pulmonary rehabilitation outcomes in people with chronic obstructive pulmonary disease: A systematic review. J. Cardiopulm. Rehabil. Prev. 2014, 34, 87–97. [Google Scholar] [CrossRef] [PubMed]
- Blum, A. Gender differences in vascular ageing and in coronary artery disease pathophysiology. QJM Int. J. Med. 2023, 116, 745–749. [Google Scholar] [CrossRef]
- Schramm, K.; Rochon, P.J. Gender Differences in Peripheral Vascular Disease. Semin. Interv. Radiol. 2018, 35, 9–16. [Google Scholar] [CrossRef]
- Pabbidi, M.R.; Kuppusamy, M.; Didion, S.P.; Sanapureddy, P.; Reed, J.T.; Sontakke, S.P. Sex differences in the vascular function and related mechanisms: Role of 17β-estradiol. Am. J. Physiology. Heart Circ. Physiol. 2018, 315, H1499–H1518. [Google Scholar] [CrossRef]
- Cain, K.C.; Jarrett, M.E.; Burr, R.L.; Rosen, S.; Hertig, V.L.; Heitkemper, M.M. Gender differences in gastrointestinal, psychological, and somatic symptoms in irritable bowel syndrome. Dig. Dis. Sci. 2009, 54, 1542–1549. [Google Scholar] [CrossRef]
- Narayanan, S.P.; Anderson, B.; Bharucha, A.E. Sex- and Gender-Related Differences in Common Functional Gastroenterologic Disorders. Mayo Clin. Proc. 2021, 96, 1071–1089. [Google Scholar] [CrossRef]
- Kim, N. Sex/Gender Differences in the Gastrointestinal Diseases. In Sex/Gender-Specific Medicine in the Gastrointestinal Diseases; Kim, N., Ed.; Springer: Singapore, 2022. [Google Scholar] [CrossRef]
- Karlstadt, R.G.; Hogan, D.L.; Foxx-Orenstein, A. Principles of Gender-Specific Medicine: Normal Physiology of the Gastrointestinal Tract and Gender Differences; Elsevier Inc.: Amsterdam, The Netherlands, 2007; pp. 377–396. [Google Scholar] [CrossRef]
- Trincot, C.E.; Caron, K.M. Lymphatic Function and Dysfunction in the Context of Sex Differences. ACS Pharmacol. Transl. Sci. 2019, 2, 311–324. [Google Scholar] [CrossRef]
- Dill-Garlow, R.; Chen, K.E.; Walker, A.M. Sex Differences in Mouse Popliteal Lymph Nodes. Sci. Rep. 2019, 9, 965. [Google Scholar] [CrossRef]
- Ortona, E.; Pierdominici, M.; Rider, V. Editorial: Sex Hormones and Gender Differences in Immune Responses. Front. Immunol. 2019, 10, 1076. [Google Scholar] [CrossRef] [PubMed]
- Klein, S.; Flanagan, K. Sex differences in immune responses. Nat. Rev. Immunol. 2016, 16, 626–638. [Google Scholar] [CrossRef] [PubMed]
- Arnold, C.G.; Libby, A.; Vest, A. Immune mechanisms associated with sex-based differences in severe COVID-19 clinical outcomes. Biol. Sex Differ. 2022, 13, 7. [Google Scholar] [CrossRef]
- Kelly, S.J.; Ostrowski, N.L.; Wilson, M.A. Gender differences in brain and behavior: Hormonal and neural bases. Pharmacol. Biochem. Behav. 1999, 64, 655–664. [Google Scholar] [CrossRef] [PubMed]
- Derntl, B.; Finkelmeyer, A.; Eickhoff, S.; Kellermann, T.; Falkenberg, D.I.; Schneider, F.; Habel, U. Multidimensional assessment of empathic abilities: Neural correlates and gender differences. Psychoneuroendocrinology 2010, 35, 67–82. [Google Scholar] [CrossRef]
- Chao, A.M.; Loughead, J.; Bakizada, Z.M.; Hopkins, C.M.; Geliebter, A.; Gur, R.C.; Wadden, T.A. Sex/gender differences in neural correlates of food stimuli: A systematic review of functional neuroimaging studies. Obes. Rev. 2017, 18, 687–699. [Google Scholar] [CrossRef]
- Gilsanz, V.; Kovanlikaya, A.; Costin, G.; Roe, T.F.; Sayre, J.; Kaufman, F. Differential effect of gender on the sizes of the bones in the axial and appendicular skeletons. J. Clin. Endocrinol. Metab. 1997, 82, 1603–1607. [Google Scholar] [CrossRef]
- Nieves, J.W.; Formica, C.; Ruffing, J.; Zion, M.; Garrett, P.; Lindsay, R.; Cosman, F. Males have larger skeletal size and bone mass than females, despite comparable body size. J. Bone Miner. Res. 2005, 20, 529–535. [Google Scholar] [CrossRef]
- Lang, T.F. The bone-muscle relationship in men and women. J. Osteoporos. 2011, 2011, 702735. [Google Scholar] [CrossRef]
- Planeta, K.; Setkowicz, Z.; Janik-Olchawa, N.; Matusiak, K.; Ryszawy, D.; Drozdz, A.; Janeczko, K.; Ostachowicz, B.; Chwiej, J. Comparison of Elemental Anomalies Following Implantation of Different Cell Lines of Glioblastoma Multiforme in the Rat Brain: A Total Reflection X-ray Fluorescence Spectroscopy Study. ACS Chem. Neurosci. 2020, 11, 4447–4459. [Google Scholar] [CrossRef]
- Drozdz, A.; Matusiak, K.; Setkowicz, Z.; Ciarach, M.; Janeczko, K.; Sandt, C.; Borondics, F.; Horak, D.; Babic, M.; Chwiej, J. FTIR microspectroscopy revealed biochemical changes in liver and kidneys as a result of exposure to low dose of iron oxide nanoparticles. Spectrochim. Acta. Part A Mol. Biomol. Spectrosc. 2020, 236, 118355. [Google Scholar] [CrossRef] [PubMed]
- Wilk, A.; Setkowicz, Z.; Banas, D.; Fernández-Ruiz, R.; Marguí, E.; Matusiak, K.; Wrobel, P.; Wudarczyk-Mocko, J.; Janik-Olchawa, N.; Chwiej, J. Glioblastoma multiforme influence on the elemental homeostasis of the distant organs: The results of inter-comparison study carried out with TXRF method. Sci. Rep. 2024, 14, 1254. [Google Scholar] [CrossRef] [PubMed]
- Planeta, K.; Setkowicz, Z.; Czyzycki, M.; Janik-Olchawa, N.; Ryszawy, D.; Janeczko, K.; Simon, R.; Baumbach, T.; Chwiej, J. Altered Elemental Distribution in Male Rat Brain Tissue as a Predictor of Glioblastoma Multiforme Growth-Studies Using SR-XRF Microscopy. Int. J. Mol. Sci. 2022, 23, 703. [Google Scholar] [CrossRef] [PubMed]
- Rugiel, M.; Drozdz, A.; Matusiak, K.; Setkowicz, Z.; Klodowski, K.; Chwiej, J. Organ Metallome Processed with Chemometric Methods Enable the Determination of Elements that May Serve as Markers of Exposure to Iron Oxide Nanoparticles in Male Rats. Biol. Trace Elem. Res. 2020, 198, 602–616. [Google Scholar] [CrossRef]
- Matusiak, K.; Drozdz, A.; Setkowicz, Z.; Kubala-Kukus, A.; Stabrawa, I.; Ciarach, M.; Janeczko, K.; Horak, D.; Babic, M.; Chwiej, J. Intravenously administered d-mannitol-coated maghemite nanoparticles cause elemental anomalies in selected rat organs. Met. Integr. Biometal Sci. 2020, 12, 1811–1821. [Google Scholar] [CrossRef]
- Skoczeń, A.; Matusiak, K.; Setkowicz, Z.; Kubala-Kukuś, A.; Stabrawa, I.; Ciarach, M.; Janeczko, K.; Chwiej, J. Low Doses of Polyethylene Glycol Coated Iron Oxide Nanoparticles Cause Significant Elemental Changes within Main Organs. Chem. Res. Toxicol. 2018, 31, 876–884. [Google Scholar] [CrossRef] [PubMed]
- Chwiej, J.; Palczynska, M.; Skoczen, A.; Janeczko, K.; Cieslak, J.; Simon, R.; Setkowicz, Z. Elemental changes of hippocampal formation occurring during postnatal brain development. J. Trace Elem. Med. Biol. 2018, 49, 1–7. [Google Scholar] [CrossRef]
- Chwiej, J.; Patulska, A.; Skoczen, A.; Janeczko, K.; Ciarach, M.; Simon, R.; Setkowicz, Z. Elemental changes in the hippocampal formation following two different formulas of ketogenic diet: An X-ray fluorescence microscopy study. J. Biol. Inorg. Chem. JBIC 2015, 20, 1277–1286. [Google Scholar] [CrossRef][Green Version]
- Baptista, M.; Figueiredo, C.; Azevedo, O.M.; Pina Rodrigues, M.T.; Costa, T.; Santos, M.T.; Queiroz, N.; Rosa, R.; Raimundo, J. Tissue and gender-related differences in the elemental composition of juvenile ocean sunfish (Mola spp.). Chemosphere 2021, 272, 129131. [Google Scholar] [CrossRef]
- da Silva Júnior, F.J.T.M.; Ribeiro, J.D.N.; da Silva, H.L.A.; da Silva Carneiro, C.; de Jesus, E.F.O.; de Araújo, U.B.; Lazzarini, S.M.; Souza, A.R.; Simões, J.S.; Lopes, R.T.; et al. Study of inorganic elements in different organs and tissues of Amazonian manatee (Trichechus inunguis) from Brazil. Environ. Sci. Pollut. Res. Int. 2022, 29, 30486–30495. [Google Scholar] [CrossRef]
- Selvaggi, R.; Pallottini, M.; Caldaroni, B.; Dörr, A.J.M.; Magara, G.; Gravina, P.; Grispoldi, L.; Cenci-Goga, B.; Goretti, E.; La Porta, G.; et al. Sex and seasonal differences in metal accumulation of selected tissues in red swamp crayfish from Lake Trasimeno (Umbria, Italy). Environ. Sci. Pollut. Res. Int. 2023, 30, 6234–6244. [Google Scholar] [CrossRef] [PubMed]
- van Beest, F.M.; Schmidt, N.M.; Frederiksen, M.L.; Krogh, A.K.H.; Petersen, H.H.; Hansson, S.V. Direct and Indirect Linkages Between Trace Element Status and Health Indicators—A Multi-tissue Case-Study of Two Deer Species in Denmark. Biol. Trace Elem. Res. 2024, 202, 3623–3638. [Google Scholar] [CrossRef] [PubMed]
- Wenting, E.; Siepel, H.; Christerus, M.; Jansen, P.A. Ionomic Variation Among Tissues in Fallow Deer (Dama dama) by Sex and Age. Biol. Trace Elem. Res. 2024, 202, 965–979. [Google Scholar] [CrossRef] [PubMed]
- Brodbeck, B.E.; Lyons, K.; Miller, N.R.; Mohan, J.A. Sex influences elemental variation in the mineralized vertebrae cartilage of round stingray (Urobatis halleri). Mar. Biol. 2023, 170, 117. [Google Scholar] [CrossRef]
- Planeta, K.; Kubala-Kukus, A.; Drozdz, A.; Matusiak, K.; Setkowicz, Z.; Chwiej, J. The assessment of the usability of selected instrumental techniques for the elemental analysis of biomedical samples. Scienific. Rep. 2021, 11, 3704. [Google Scholar] [CrossRef]
- Leblondel, G.; Mauras, Y.; Allain, P. Tissue distribution of some elements in rats. Biol. Trace Elem. Res. 1986, 10, 327–333. [Google Scholar] [CrossRef]
- Gélinas, Y.; Youla, M.; Béliveau, R.; Schmit, J.P. Multi-element analysis of biological tissues by inductively coupled plasma mass spectrometry: Healthy Sprague Dawley rats. Anal. Chim. Acta 1992, 269, 115–122. [Google Scholar] [CrossRef]
- Yiin, S.J.; Chern, C.L.; Sheu, J.Y.; Lin, T.H. Cadmium-induced liver, heart, and spleen lipid peroxidation in rats and protection by selenium. Biol. Trace Elem. Res. 2000, 78, 219–230. [Google Scholar] [CrossRef]
- Cui, X.; Okayasu, R. Arsenic accumulation, elimination, and interaction with copper, zinc and manganese in liver and kidney of rats. Food Chem. Toxicol. 2008, 46, 3646–3650. [Google Scholar] [CrossRef]
- Shimamura, T.; Iijima, S.; Hirayama, M.; Iwashita, M.; Akiyama, S.; Takaku, Y.; Yumoto, S. Age-related effects of major and trace element concentrations in rat liver and their mutual relationships. J. Trace Elem. Med. Biol. 2013, 27, 286–294. [Google Scholar] [CrossRef]
- Dos Santos, L.R.S.S.R.; de Freitas Santos, A., Jr.; das Graças Andrade Korn, M. Effects of furosemide administration on the concentration of essential and toxic elements in Wistar rats by inductively coupled plasma optical emission spectrometry. J. Trace Elem. Med. Biol. 2018, 48, 25–29. [Google Scholar] [CrossRef] [PubMed]
- Kawon, K.; Rugiel, M.; Setkowicz, Z.; Matusiak, K.; Kubala-Kukus, A.; Stabrawa, I.; Szary, K.; Rauk, Z.; Chwiej, J. Ketogenic diet influence on the elemental homeostasis of internal organs is gender dependent. Sci. Rep. 2023, 13, 18448. [Google Scholar] [CrossRef] [PubMed]
- Shimamura, T.; Iijima, S.; Hirayama, M.; Iwashita, M.; Akiyama, S.; Takaku, Y.; Yumoto, S. The concentrations of major and trace elements in rat kidney: Aging effects and mutual relationships. J. Trace Elem. Med. Biol. 2013, 27, 12–20. [Google Scholar] [CrossRef]
- Stoica, G.; O’Leary, M. In vitro malignant transformation of in vivo ENU-induced rat ovarian Sertoli cell tumor (adenoma). J. Cancer Res. Clin. Oncol. 1988, 114, 142–148. [Google Scholar] [CrossRef] [PubMed]
- Hornung, R.; Major, A.L.; McHale, M.; Liaw, L.H.; Sabiniano, L.A.; Tromberg, B.J.; Berns, M.W.; Tadir, Y. In vivo detection of metastatic ovarian cancer by means of 5-aminolevulinic acid-induced fluorescence in a rat model. J. Am. Assoc. Gynecol. Laparosc. 1998, 5, 141–148. [Google Scholar] [CrossRef]
- Wachnik, A.; Biró, G.; Gergely, A.; Nagy, K.; Gaál, O.; Antal, M. Sex dependent differences in trace element levels in rat tissues. Die Nahr. 1988, 32, 999–1001. [Google Scholar] [CrossRef]
- Szoboszlai, N.; Polgári, Z.; Mihucz, V.G.; Záray, G. Recent trends in total reflection X-ray fluorescence spectrometry for biological applications. Anal. Chim. Acta 2009, 633, 1–18. [Google Scholar] [CrossRef]
- Szalóki, I.; Záray, G.; Szoboszlai, N. The Use of X-Ray Techniques in Medical Research. In Analytical Techniques for Clinical Chemistry: Methods and Applications; John Wiley & Sons: Hoboken, NJ, USA, 2012; pp. 595–623. [Google Scholar] [CrossRef]
- Fernández-Ruiz, R. TXRF biological application review: TXRF spectrometry in the bioanalytical sciences: A brief review. X-Ray Spectrom. 2022, 51, 279–293. [Google Scholar] [CrossRef]
- Haschke, M.; Flock, J.; Haller, M. X-Ray Fluorescence Spectroscopy for Laboratory Applications. Anal. Bioanal. Chem. 2021, 413, 6455–6456. [Google Scholar] [CrossRef]
- Fabry, L.; Pahlke, S.; Beckhoff, B. Total-Reflection X-Ray Fluorescence (TXRF) Analysis. In Surface and Thin Film Analysis: A Compendium of Principles, Instrumentation, and Applications, 2nd ed.; Wiley: Weinheim, Germany, 2011; pp. 265–292. [Google Scholar] [CrossRef]
- Streli, C.; Wobrauschek, P.; Aiginger, H. Light Element Analysis with TXRF. Adv. X-Ray Anal. 1991, 35, 947–952. [Google Scholar] [CrossRef]
- Streli, C.; Bauer, V.; Wobrauschek, P. Recent Developments in Txrf of Light Elements. Adv. X-Ray Anal. 1995, 39, 771–779. [Google Scholar] [CrossRef]
- Prost, J.; Wobrauschek, P.; Streli, C. Comparison of different excitation modes for the analysis of light elements with a TXRF vacuum chamber. Powder Diffr. 2015, 30, 93–98. [Google Scholar] [CrossRef]
- Galvão Novaes, C.; Almeida Bezerra, M.; Galvão Paranhos da Silva, E.; Pinto dos Santos, A.M.; Lago da Silva Romão, I.; Honorato Santos Neto, J. A review of multivariate designs applied to the optimization of methods based on inductively coupled plasma optical emission spectrometry (ICP OES). Microchem. J. 2016, 128, 331–346. [Google Scholar] [CrossRef]
- Khan, K.F. Application, principle and operation of ICP-OES in pharmaceutical analysis. Pharma Innov. J. 2019, 8, 281–282. [Google Scholar]
- Khan, S.R.; Sharma, B.; Chawla, P.A. Inductively Coupled Plasma Optical Emission Spectrometry (ICP-OES): A Powerful Analytical Technique for Elemental Analysis. Food Anal. Methods 2022, 15, 666–688. [Google Scholar] [CrossRef]
- Sneddon, J.; Vincent, M.D. ICP-OES and ICP-MS for the Determination of Metals: Application to Oysters. Anal. Lett. 2008, 41, 1291–1303. [Google Scholar] [CrossRef]
- Onufrak, S.J.; Bellasi, A.; Cardarelli, F.; Vaccarino, V.; Muntner, P.; Shaw, L.J.; Raggi, P. Investigation of gender heterogeneity in the associations of serum phosphorus with incident coronary artery disease and all-cause mortality. Am. J. Epidemiol. 2009, 169, 67–77. [Google Scholar] [CrossRef] [PubMed]
- Carrillo-López, N.; Román-García, P.; Rodríguez-Rebollar, A.; Fernández-Martín, J.L.; Naves-Díaz, M.; Cannata-Andía, J.B. Indirect regulation of PTH by estrogens may require FGF23. J. Am. Soc. Nephrol. JASN 2009, 20, 2009–2017. [Google Scholar] [CrossRef]
- Takasugi, S.; Shioyama, M.; Kitade, M.; Nagata, M.; Yamaji, T. Involvement of estrogen in phosphorus-induced nephrocalcinosis through fibroblast growth factor 23. Sci. Rep. 2020, 10, 4864. [Google Scholar] [CrossRef]
- Jüppner, H. Phosphate and FGF-23. Kidney international. Supplement 2011, 79, S24–S27. [Google Scholar] [CrossRef]
- Parasoglou, P.; Osorio, R.S.; Khegai, O.; Kovbasyuk, Z.; Miller, M.; Ho, A.; Dehkharghani, S.; Wisniewski, T.; Convit, A.; Mosconi, L.; et al. Phosphorus metabolism in the brain of cognitively normal midlife individuals at risk for Alzheimer’s disease. Neuroimage. Rep. 2022, 2, 100121. [Google Scholar] [CrossRef] [PubMed]
- Park, J.C.; Han, S.H.; Byun, M.S.; Yi, D.; Lee, J.H.; Park, K.; Lee, D.Y.; Mook-Jung, I. Low Serum Phosphorus Correlates with Cerebral Aβ Deposition in Cognitively Impaired Subjects: Results from the KBASE Study. Front. Aging Neurosci. 2017, 9, 362. [Google Scholar] [CrossRef] [PubMed]
- Nimni, M.E.; Han, B.; Cordoba, F. Are we getting enough sulfur in our diet? Nutr. Metab. 2007, 4, 24. [Google Scholar] [CrossRef] [PubMed]
- Melnik, A. Relations between sex hormones levels and sulfur amino acids and hydrogen sulfide in blood. Rep. Vinnytsia Natl. Med. Univ. 2017, 21, 6–8. Available online: https://reports-vnmedical.com.ua/index.php/journal/article/view/60 (accessed on 31 August 2024).
- Škovierová, H.; Vidomanová, E.; Mahmood, S.; Sopková, J.; Drgová, A.; Červeňová, T.; Halašová, E.; Lehotský, J. The Molecular and Cellular Effect of Homocysteine Metabolism Imbalance on Human Health. Int. J. Mol. Sci. 2016, 17, 1733. [Google Scholar] [CrossRef]
- Calil, C.M.; Lima, P.O.; Bernardes, C.F.; Groppo, F.C.; Bado, F.; Marcondes, F.K. Influence of gender and menstrual cycle on volatile sulphur compounds production. Arch. Oral Biol. 2008, 53, 1107–1112. [Google Scholar] [CrossRef]
- Udensi, U.K.; Tchounwou, P.B. Potassium Homeostasis, Oxidative Stress, and Human Disease. Int. J. Clin. Exp. Physiol. 2017, 4, 111–122. [Google Scholar] [CrossRef]
- Rossi, G.P.; Caroccia, B.; Seccia, T.M. Role of estrogen receptors in modulating aldosterone biosynthesis and blood pressure. Steroids 2019, 152, 108486. [Google Scholar] [CrossRef]
- Parham, W.A.; Mehdirad, A.A.; Biermann, K.M.; Fredman, C.S. Hyperkalemia revisited. Tex. Heart Inst. J. 2006, 33, 40–47. [Google Scholar]
- Lanham-New, S.A.; Lambert, H.; Frassetto, L. Potassium. Adv. Nutr. 2012, 3, 820–821. [Google Scholar] [CrossRef]
- Cheung, W.Y. Calcium and Cell Function; Academic Press: Cambridge, MA, USA, 2013; Volume 5. [Google Scholar]
- Cormick, G.; Belizán, J.M. Calcium Intake and Health. Nutrients 2019, 11, 1606. [Google Scholar] [CrossRef] [PubMed]
- de Faria, L.L.; Babler, F.; Ferreira, L.C.; de Noronha Junior, O.A.; Marsolla, F.L.; Ferreira, D.L. Soft tissue calcifications: A pictorial essay. Radiol. Bras. 2020, 53, 337–344. [Google Scholar] [CrossRef] [PubMed]
- Gallagher, J.C.; Riggs, B.L.; DeLuca, H.F. Effect of estrogen on calcium absorption and serum vitamin D metabolites in postmenopausal osteoporosis. J. Clin. Endocrinol. Metab. 1980, 51, 1359–1364. [Google Scholar] [CrossRef] [PubMed]
- Zheng, W.; Shi, M.; You, S.E.; Ji, H.; Roesch, D.M. Estrogens contribute to a sex difference in plasma potassium concentration: A mechanism for regulation of adrenal angiotensin receptors. Gend. Med. 2006, 3, 43–53. [Google Scholar] [CrossRef] [PubMed]
- Abbaspour, N.; Hurrell, R.; Kelishadi, R. Review on iron and its importance for human health. J. Res. Med. Sci. Off. J. Isfahan Univ. Med. Sci. 2014, 19, 164–174. [Google Scholar]
- Ward, D.M.; Kaplan, J. Ferroportin-mediated iron transport: Expression and regulation. Biochim. Biophys. Acta 2012, 1823, 1426–1433. [Google Scholar] [CrossRef]
- Harrison-Findik, D.D. Gender-related variations in iron metabolism and liver diseases. World J. Hepatol. 2010, 2, 302–310. [Google Scholar] [CrossRef]
- Drakesmith, H.; Nemeth, E.; Ganz, T. Ironing out Ferroportin. Cell Metab. 2015, 22, 777–787. [Google Scholar] [CrossRef]
- Chen, J.; Jiang, Y.; Shi, H.; Peng, Y.; Fan, X.; Li, C. The molecular mechanisms of copper metabolism and its roles in human diseases. Pflug. Arch. Eur. J. Physiol. 2020, 472, 1415–1429. [Google Scholar] [CrossRef]
- Ruiz, L.M.; Libedinsky, A.; Elorza, A.A. Role of Copper on Mitochondrial Function and Metabolism. Front. Mol. Biosci. 2021, 8, 711227. [Google Scholar] [CrossRef]
- Quinn, J.F.; Harris, C.; Kaye, J.A.; Lind, B.; Carter, R.; Anekonda, T.; Ralle, M. Gender Effects on Plasma and Brain Copper. Int. J. Alzheimer’s Dis. 2011, 2011, 150916. [Google Scholar] [CrossRef] [PubMed]
- Carruthers, M.E.; Hobbs, C.B.; Warren, R.L. Raised serum copper and caeruloplasmin levels in subjects taking oral contraceptives. J. Clin. Pathol. 1966, 19, 498–500. [Google Scholar] [CrossRef] [PubMed]
- Mehta, S.W.; Eikum, R. Effect of estrogen on serum and tissue levels of copper and zinc. Adv. Exp. Med. Biol. 1989, 258, 155–162. [Google Scholar] [CrossRef]
- Garner, T.B.; Hester, J.M.; Carothers, A.; Diaz, F.J. Role of zinc in female reproduction. Biol. Reprod. 2021, 104, 976–994. [Google Scholar] [CrossRef]
- Roohani, N.; Hurrell, R.; Kelishadi, R.; Schulin, R. Zinc and its importance for human health: An integrative review. J. Res. Med. Sci. Off. J. Isfahan Univ. Med. Sci. 2013, 18, 144–157. [Google Scholar]
- Rea, I.M. Sex and age changes in serum zinc levels. Nutr. Res. 1987, 9, 121–125. [Google Scholar] [CrossRef]
- Seale, L.A.; Ogawa-Wong, A.N.; Berry, M.J. Sexual Dimorphism In Selenium Metabolism And Selenoproteins. Free Radic. Biol. Med. 2018, 127, 198–205. [Google Scholar] [CrossRef]
- Schomburg, L.; Schweizer, U. Hierarchical regulation of selenoprotein expression and sex-specific effects of selenium. Biochim. Biophys. Acta 2009, 1790, 1453–1462. [Google Scholar] [CrossRef] [PubMed]
- Prasad, A.S.; Mantzoros, C.S.; Beck, F.W.; Hess, J.W.; Brewer, G.J. Zinc status and serum testosterone levels of healthy adults. Nutrition 1996, 12, 344–348. [Google Scholar] [CrossRef]
- Mirnamniha, M.; Faroughi, F.; Tahmasbpour, E.; Ebrahimi, P.; Beigi Harchegani, A. An overview on role of some trace elements in human reproductive health, sperm function and fertilization process. Rev. Environ. Health 2019, 34, 339–348. [Google Scholar] [CrossRef]
| Element | Certified Value [µg/g] * | Measured Value [µg/g] |
|---|---|---|
| P | 690–1120 | 778 |
| S | 6000–7000 | 6384 |
| K | 2100–2700 | 2790 |
| Ca | 226–332 | 322 |
| Fe | 2200–2500 | 3147 |
| Cu | 3.7–4.8 | 5.17 |
| Zn | 12–14 | 26.16 |
| Se | 0.15–0.31 | 0.48 |
| Element | Intra-Day Precision [%] * | Inter-Day Precision [%] |
|---|---|---|
| P | 1.19 | 0.89 |
| S | 0.96 | 1.08 |
| K | 1.04 | 2.38 |
| Ca | 2.13 | 0.98 |
| Fe | 0.42 | 0.35 |
| Cu | 1.58 | 1.37 |
| Zn | 3.36 | 1.65 |
| Se | 4.44 | 2.91 |
| Element | Brain | Heart | Kidneys | Liver | Spleen |
|---|---|---|---|---|---|
| Fe | 0.0551 (0.0020) | 0.0647 (0.0036) | 0.0695 (0.0036) | 0.0756 (0.0031) | 0.1351 (0.0057) |
| Cu | 0.0335 (0.0014) | 0.0380 (0.0021) | 0.0450 (0.0027) | 0.0369 (0.0015) | 0.0562 (0.0024) |
| Zn | 0.0425 (0.0017) | 0.0513 (0.0030) | 0.0640 (0.0037) | 0.0502 (0.0020) | 0.0697 (0.0028) |
| Se | 0.01848 (0.00072) | 0.0195 (0.0011) | 0.0226 (0.0014) | 0.01936 (0.00077) | 0.0304 (0.0012) |
| Element | LOD (SD) [μg/g] |
|---|---|
| P | 0.0217 (0.0045) |
| S | 0.059 (0.015) |
| K | 0.0242 (0.0087) |
| Ca | 0.0144 (0.0038) |
| Tissue/Element | P | S | K | Ca | Fe | Cu | Zn | Se |
|---|---|---|---|---|---|---|---|---|
| Brain | - | - | - | - | - | - | - | ↑ |
| Heart | - | - | - | - | - | - | ↑ | - |
| Kidneys | - | - | - | - | ↓ | - | - | - |
| Liver | - | - | - | - | ↓ | - | ↑ | - |
| Spleen | ↑ | ↑ | ↑ | - | - | ↑ | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wilk, A.; Setkowicz, Z.; Matusiak, K.; Margui Grabulosa, E.; Rugiel, M.; Kasprzyk, P.; Drozdz, A.; Chwiej, J. Sex-Dependent Differences in the Elemental Composition of Internal Organs Determined via Total Reflection X-Ray Fluorescence and Inductively Coupled Plasma Optical Emission Spectroscopy. Biomedicines 2024, 12, 2774. https://doi.org/10.3390/biomedicines12122774
Wilk A, Setkowicz Z, Matusiak K, Margui Grabulosa E, Rugiel M, Kasprzyk P, Drozdz A, Chwiej J. Sex-Dependent Differences in the Elemental Composition of Internal Organs Determined via Total Reflection X-Ray Fluorescence and Inductively Coupled Plasma Optical Emission Spectroscopy. Biomedicines. 2024; 12(12):2774. https://doi.org/10.3390/biomedicines12122774
Chicago/Turabian StyleWilk, Aleksandra, Zuzanna Setkowicz, Katarzyna Matusiak, Eva Margui Grabulosa, Marzena Rugiel, Paula Kasprzyk, Agnieszka Drozdz, and Joanna Chwiej. 2024. "Sex-Dependent Differences in the Elemental Composition of Internal Organs Determined via Total Reflection X-Ray Fluorescence and Inductively Coupled Plasma Optical Emission Spectroscopy" Biomedicines 12, no. 12: 2774. https://doi.org/10.3390/biomedicines12122774
APA StyleWilk, A., Setkowicz, Z., Matusiak, K., Margui Grabulosa, E., Rugiel, M., Kasprzyk, P., Drozdz, A., & Chwiej, J. (2024). Sex-Dependent Differences in the Elemental Composition of Internal Organs Determined via Total Reflection X-Ray Fluorescence and Inductively Coupled Plasma Optical Emission Spectroscopy. Biomedicines, 12(12), 2774. https://doi.org/10.3390/biomedicines12122774







