Microbiota and Cytokine Modulation: Innovations in Enhancing Anticancer Immunity and Personalized Cancer Therapies
Abstract
1. Exploring the Influence of Gut Microbiota on Anticancer Immunity
1.1. Cellular Aspects of Gut Microbiota on Anticancer Immunity
1.2. Humoral Aspects of Gut Microbiota on Anticancer Immunity
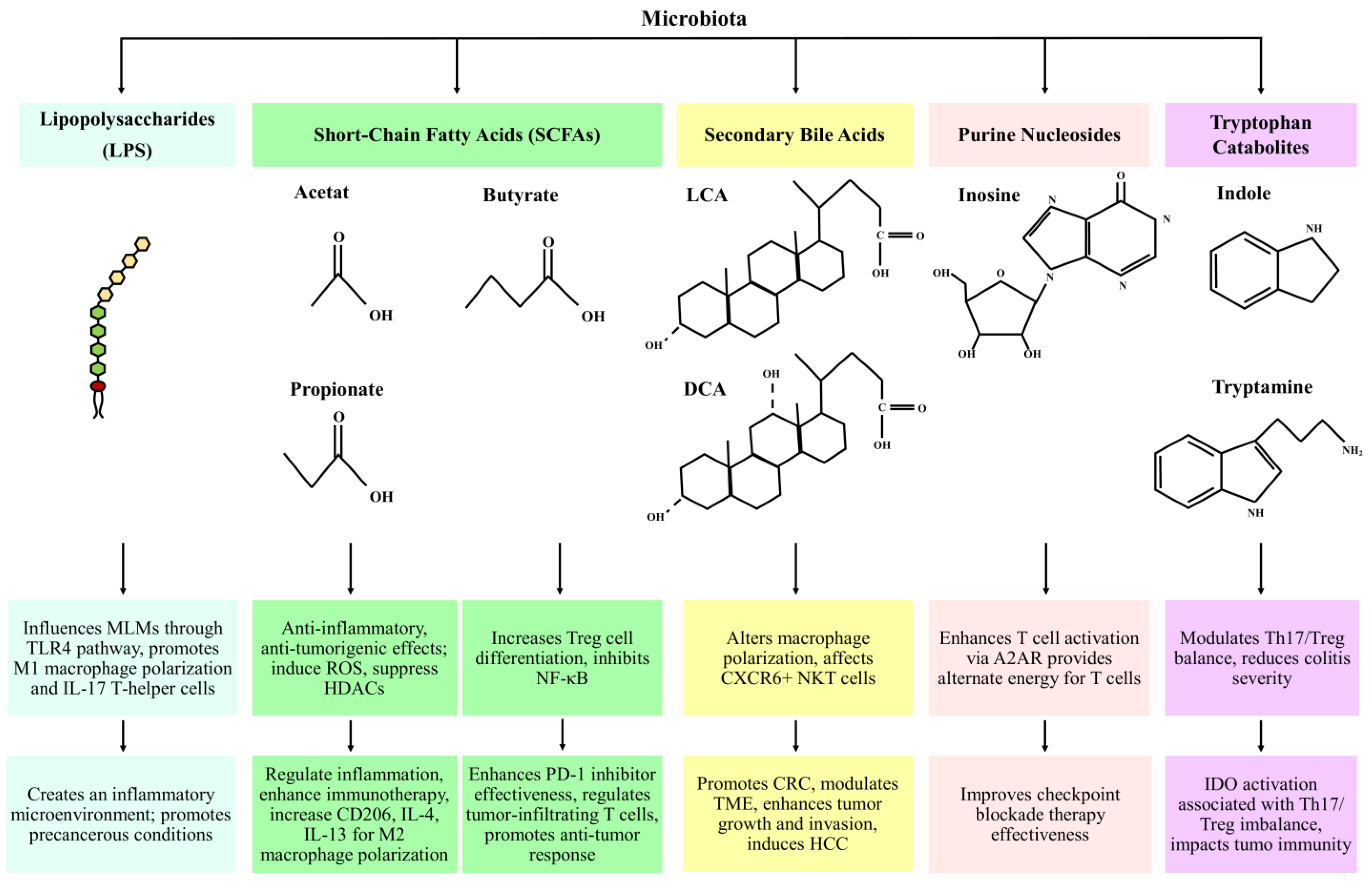
2. Microbial Metabolites as Mediators of Host Immune Responses in Cancer
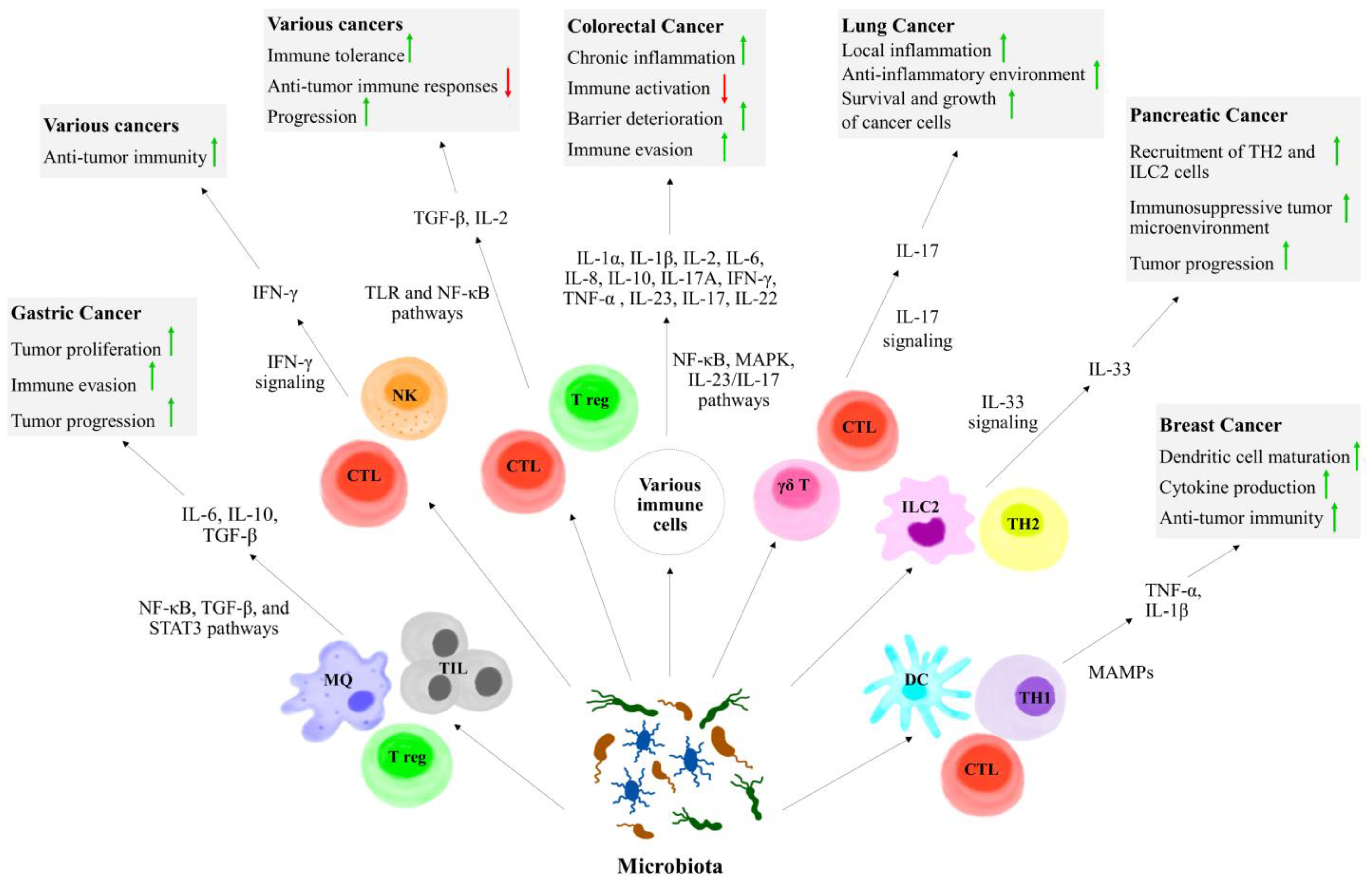
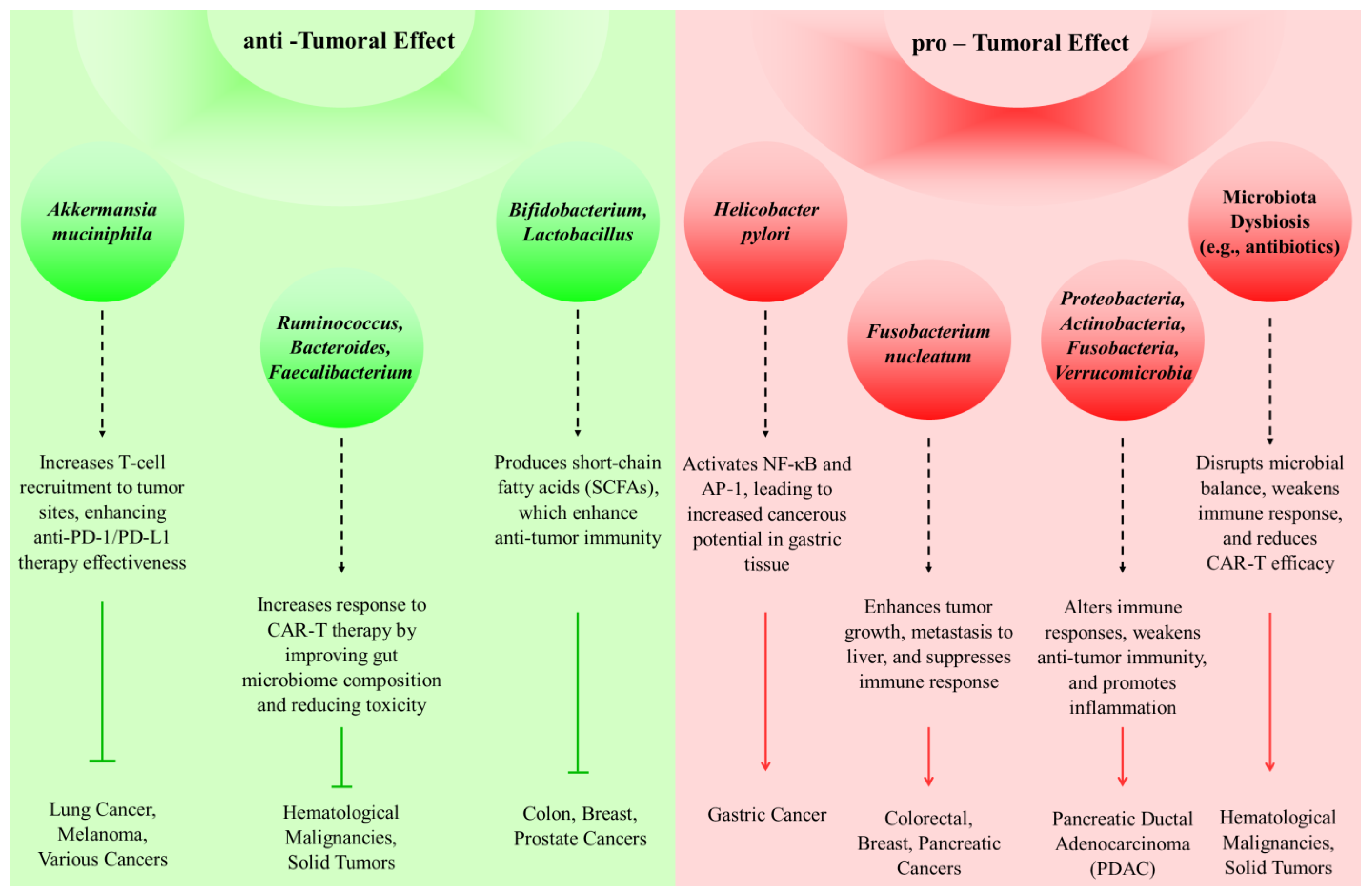
3. Immunomodulatory Roles of the Microbiome in Cancer Defense Mechanisms
4. Influence of Microbiota on Chemotherapy
4.1. Influence of Microbiota on Toxicity of Chemotherapy
4.2. Influence of Microbiota on Efficacy of Chemotherapy
5. The Role of Microbiota in Cytokine Modulation and Anticancer Immunity
6. Microbiome Profiling and Cytokine Interactions: Innovations and Applications in Personalized Cancer Therapies
7. Strategies for Enhancing Antitumor Immune Responses
8. The Gut–Immune Axis: Insights into Cancer Immunotherapy
9. Targeting the Tumor Microbiome: Implications for Immunotherapy Efficacy
10. Therapeutic Modulation of Gut Microbiota in Cancer Immunotherapy: Mechanisms, Clinical Advances, and Future Directions
11. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zhao, L.-Y.; Mei, J.-X.; Yu, G.; Lei, L.; Zhang, W.-H.; Liu, K.; Chen, X.-L.; Kołat, D.; Yang, K.; Hu, J.-K. Role of the gut microbiota in anticancer therapy: From molecular mechanisms to clinical applications. Signal Transduct. Target. Ther. 2023, 8, 201. [Google Scholar] [CrossRef] [PubMed]
- Al-Ishaq, R.K.; Koklesova, L.; Kubatka, P.; Büsselberg, D. Immunomodulation by gut microbiome on gastrointestinal cancers: Focusing on colorectal cancer. Cancers 2022, 14, 2140. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Q.; Lin, Y.; Ma, Y.; Li, X.; Liang, J.; Chen, Z.; Liu, K.; Huang, Y.; Luo, H.; Huang, R. Exploring the emerging role of the gut microbiota and tumor microenvironment in cancer immunotherapy. Front. Immunol. 2020, 11, 612202. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Deng, X.; Chen, T. Exploring the modulatory effects of gut microbiota in anti-cancer therapy. Front. Oncol. 2021, 11, 644454. [Google Scholar] [CrossRef]
- Zhang, L.; Yu, L. The role of the microscopic world: Exploring the role and potential of intratumoral microbiota in cancer immunotherapy. Medicine 2024, 103, e38078. [Google Scholar] [CrossRef] [PubMed]
- Patel, J.; Crawford, J.M. Microbiota-Regulated Outcomes of Human Cancer Immunotherapy via the PD-1/PD-L1 Axis. Biochemistry 2018, 57, 901–903. [Google Scholar] [CrossRef] [PubMed]
- Keen, E.C.; Crofts, T.S.; Dantas, G. Checkpoint Checkmate: Microbiota Modulation of Cancer Immunotherapy. Clin. Chem. 2018, 64, 1280–1283. [Google Scholar] [CrossRef]
- Liu, J.; Tan, Y.; Cheng, H.; Zhang, D.; Feng, W.; Peng, C. Functions of Gut Microbiota Metabolites, Current Status and Future Perspectives. Aging Dis. 2022, 13, 1106–1126. [Google Scholar] [CrossRef]
- Zhou, C.-B.; Zhou, Y.-L.; Fang, J.-Y. Gut microbiota in cancer immune response and immunotherapy. Trends Cancer 2021, 7, 647–660. [Google Scholar] [CrossRef]
- He, D.; Li, X.; An, R.; Wang, L.; Wang, Y.; Zheng, S.; Chen, X.; Wang, X. Response to PD-1-Based Immunotherapy for Non-Small Cell Lung Cancer Altered by Gut Microbiota. Oncol. Ther. 2021, 9, 647–657. [Google Scholar] [CrossRef]
- Del Giudice, T.; Staropoli, N.; Tassone, P.; Tagliaferri, P.; Barbieri, V. Gut Microbiota Are a Novel Source of Biomarkers for Immunotherapy in Non-Small-Cell Lung Cancer (NSCLC). Cancers 2024, 16, 1806. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Yang, G.; Tian, Y.; Zhang, Q.; Liu, Z.; Xin, Y. The role of the gut microbiota in gastric cancer: The immunoregulation and immunotherapy. Front. Immunol. 2023, 14, 1183331. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Li, L.; Xu, C.; Wang, Y.; Wang, Z.; Chen, M.; Jiang, Z.; Pan, J.; Yang, C.; Li, X.; et al. Cross-talk between the gut microbiota and monocyte-like macrophages mediates an inflammatory response to promote colitis-associated tumourigenesis. Gut 2020, 70, 1495–1506. [Google Scholar] [CrossRef] [PubMed]
- Hodgkinson, K.; El Abbar, F.; Dobranowski, P.; Manoogian, J.; Butcher, J.; Figeys, D.; Mack, D.; Stintzi, A. Butyrate’s role in human health and the current progress towards its clinical application to treat gastrointestinal disease. Clin. Nutr. 2022, 42, 61–75. [Google Scholar] [CrossRef] [PubMed]
- Busbee, P.B.; Menzel, L.; Alrafas, H.R.; Dopkins, N.; Becker, W.; Miranda, K.; Tang, C.; Chatterjee, S.; Singh, U.; Nagarkatti, M.; et al. Indole-3-carbinol prevents colitis and associated microbial dysbiosis in an IL-22-dependent manner. J. Clin. Investig. 2020, 5, 127551. [Google Scholar] [CrossRef]
- Liang, L.; Liu, L.; Zhou, W.; Yang, C.; Mai, G.; Li, H.; Chen, Y. Gut microbiota-derived butyrate regulates gut mucus barrier repair by activating the macrophage/WNT/ERK signaling pathway. Clin. Sci. 2022, 136, 291–307. [Google Scholar] [CrossRef]
- Nomura, M.; Nagatomo, R.; Doi, K.; Shimizu, J.; Baba, K.; Saito, T.; Matsumoto, S.; Inoue, K.; Muto, M. Association of Short-Chain Fatty Acids in the Gut Microbiome With Clinical Response to Treatment With Nivolumab or Pembrolizumab in Patients With Solid Cancer Tumors. JAMA Netw. Open 2020, 3, e202895. [Google Scholar] [CrossRef]
- Coutzac, C.; Jouniaux, J.M.; Paci, A.; Schmidt, J.; Mallardo, D.; Seck, A.; Asvatourian, V.; Cassard, L.X.; Saulnier, P.; Lacroix, L.; et al. Systemic short chain fatty acids limit antitumor effect of CTLA-4 blockade in hosts with cancer. Nat. Commun. 2020, 11, 2168. [Google Scholar] [CrossRef]
- Rossi, T.; Vergara, D.; Fanini, F.; Maffia, M.; Bravaccini, S.; Pirini, F. Microbiota-Derived Metabolites in Tumor Progression and Metastasis. Int. J. Mol. Sci. 2020, 21, 5786. [Google Scholar] [CrossRef]
- Arshad, T.; Mansur, F.; Palek, R.; Manzoor, S.; Liska, V. A double edged sword role of interleukin-22 in wound healing and tissue regeneration. Front. Immunol. 2020, 11, 2148. [Google Scholar] [CrossRef]
- Kuroda, T.; Kitadai, Y.; Tanaka, S.; Yang, X.; Mukaida, N.; Yoshihara, M.; Chayama, K. Monocyte chemoattractant protein-1 transfection induces angiogenesis and tumorigenesis of gastric carcinoma in nude mice via macrophage recruitment. Clin. Cancer Res. 2005, 11, 7629–7636. [Google Scholar] [CrossRef] [PubMed]
- Cao, H.; Xu, M.; Dong, W.; Deng, B.; Wang, S.; Zhang, Y.; Wang, S.; Luo, S.; Wang, W.; Qi, Y.; et al. Secondary bile acid-induced dysbiosis promotes intestinal carcinogenesis. Int. J. Cancer 2017, 140, 2545–2556. [Google Scholar] [CrossRef]
- Cheng, K.; Raufman, J.P. Bile acid-induced proliferation of a human colon cancer cell line is mediated by transactivation of epidermal growth factor receptors. Biochem. Pharmacol. 2005, 70, 1035–1047. [Google Scholar] [CrossRef] [PubMed]
- Yoshimoto, S.; Loo, T.M.; Atarashi, K.; Kanda, H.; Sato, S.; Oyadomari, S.; Iwakura, Y.; Oshima, K.; Morita, H.; Hattori, M.; et al. Obesity-induced gut microbial metabolite promotes liver cancer through senescence secretome. Nature 2013, 499, 97–101. [Google Scholar] [CrossRef] [PubMed]
- Mao, B.; Guo, W.; Tang, X.; Zhang, Q.; Yang, B.; Zhao, J.; Cui, S.; Zhang, H. Inosine Pretreatment Attenuates LPS-Induced Lung Injury through Regulating the TLR4/MyD88/NF-κB Signaling Pathway In Vivo. Nutrients 2022, 14, 2830. [Google Scholar] [CrossRef] [PubMed]
- Favre, D.; Mold, J.; Hunt, P.W.; Kanwar, B.; Loke, P.N.; Seu, L.; Barbour, J.D.; Lowe, M.M.; Jayawardene, A.; Aweeka, F.; et al. Tryptophan catabolism by indoleamine 2,3-dioxygenase 1 alters the balance of TH17 to regulatory T cells in HIV disease. Sci. Transl. Med. 2010, 2, 32ra36. [Google Scholar] [CrossRef]
- Zhou, Y.; Li, L.; Yu, Z.; Gu, X.; Pan, R.; Li, Q.; Yuan, C.; Cai, F.; Zhu, Y.; Cui, Y. Dermatophagoides pteronyssinus allergen Der p 22: Cloning, expression, IgE-binding in asthmatic children, and immunogenicity. Pediatr. Allergy Immunol. 2022, 33, e13835. [Google Scholar] [CrossRef]
- Jain, T.; Sharma, P.; Are, A.C.; Vickers, S.M.; Dudeja, V. New insights into the cancer–microbiome–immune axis: Decrypting a decade of discoveries. Front. Immunol. 2021, 12, 622064. [Google Scholar] [CrossRef]
- Cazzaniga, M.; Zonzini, G.B.; Di Pierro, F.; Palazzi, C.M.; Cardinali, M.; Bertuccioli, A. Influence of the microbiota on the effectiveness and toxicity of oncological therapies, with a focus on chemotherapy. Pathol. Oncol. Res. 2023, 29, 1611300. [Google Scholar] [CrossRef]
- Daillère, R.; Vétizou, M.; Waldschmitt, N.; Yamazaki, T.; Isnard, C.; Poirier-Colame, V.; Duong, C.P.; Flament, C.; Lepage, P.; Roberti, M.P. Enterococcus hirae and Barnesiella intestinihominis facilitate cyclophosphamide-induced therapeutic immunomodulatory effects. Immunity 2016, 45, 931–943. [Google Scholar] [CrossRef]
- Shin, Y.; Han, S.; Kwon, J.; Ju, S.; Choi, T.G.; Kang, I.; Kim, S.S. Roles of Short-Chain Fatty Acids in Inflammatory Bowel Disease. Nutrients 2023, 15, 4466. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Hou, X.; Wang, H.; Du, H.; Liu, Y. Influence of Gut Microbiota-Mediated Immune Regulation on Response to Chemotherapy. Pharmaceuticals 2024, 17, 604. [Google Scholar] [CrossRef]
- Amabebe, E.; Kumar, A.; Tatiparthy, M.; Kammala, A.K.; Taylor, B.D.; Menon, R. Cargo exchange between human and bacterial extracellular vesicles in gestational tissues: A new paradigm in communication and immune development. Extracell. Vesicles Circ. Nucleic Acids 2024, 5, 297–328. [Google Scholar] [CrossRef]
- Xu, Y.; Du, H.; Chen, Y.; Ma, C.; Zhang, Q.; Li, H.; Xie, Z.; Hong, Y. Targeting the gut microbiota to alleviate chemotherapy-induced toxicity in cancer. Crit. Rev. Microbiol. 2024, 50, 564–580. [Google Scholar] [CrossRef] [PubMed]
- Lehr, K.; Nikitina, D.; Vilchez-Vargas, R.; Steponaitiene, R.; Thon, C.; Skieceviciene, J.; Schanze, D.; Zenker, M.; Malfertheiner, P.; Kupcinskas, J. Microbial composition of tumorous and adjacent gastric tissue is associated with prognosis of gastric cancer. Sci. Rep. 2023, 13, 4640. [Google Scholar] [CrossRef] [PubMed]
- Lyu, Z.; Xin, M.; Oyston, D.R.; Xue, T.; Kang, H.; Wang, X.; Wang, Z.; Li, Q. Cause and consequence of heterogeneity in human mesenchymal stem cells: Challenges in clinical application. Pathol.-Res. Pract. 2024, 260, 155354. [Google Scholar] [CrossRef]
- Tian, L.; Li, Y.; Chang, R.; Zhang, P.; Zhang, J.; Huo, L. Lentiviral vector-mediated IL-9 overexpression stimulates cell proliferation by targeting c-myc and cyclin D1 in colitis-associated cancer. Oncol. Lett. 2019, 17, 175–182. [Google Scholar] [CrossRef]
- Trinchieri, G. Cancer immunity: Lessons from infectious diseases. J. Infect. Dis. 2015, 212, S67–S73. [Google Scholar] [CrossRef]
- Orberg, E.T.; Fan, H.; Tam, A.J.; Dejea, C.M.; Shields, C.D.; Wu, S.; Chung, L.; Finard, B.B.; Wu, X.; Fathi, P. The myeloid immune signature of enterotoxigenic Bacteroides fragilis-induced murine colon tumorigenesis. Mucosal Immunol. 2017, 10, 421–433. [Google Scholar] [CrossRef]
- Taur, Y.; Jenq, R.R.; Perales, M.-A.; Littmann, E.R.; Morjaria, S.; Ling, L.; No, D.; Gobourne, A.; Viale, A.; Dahi, P.B. The effects of intestinal tract bacterial diversity on mortality following allogeneic hematopoietic stem cell transplantation. Blood J. Am. Soc. Hematol. 2014, 124, 1174–1182. [Google Scholar] [CrossRef]
- Wang, M.; Yu, F.; Li, P. Intratumor microbiota in cancer pathogenesis and immunity: From mechanisms of action to therapeutic opportunities. Front. Immunol. 2023, 14, 1269054. [Google Scholar] [CrossRef] [PubMed]
- Lombardo, E.; Alvarez-Barrientos, A.; Maroto, B.; Boscá, L.; Knaus, U.G. TLR4-mediated survival of macrophages is MyD88 dependent and requires TNF-α autocrine signalling. J. Immunol. 2007, 178, 3731–3739. [Google Scholar] [CrossRef]
- Lu, Y.; Yuan, X.; Wang, M.; He, Z.; Li, H.; Wang, J.; Li, Q. Gut microbiota influence immunotherapy responses: Mechanisms and therapeutic strategies. J. Hematol. Oncol. 2022, 15, 47. [Google Scholar] [CrossRef] [PubMed]
- Sivan, A.; Corrales, L.; Hubert, N.; Williams, J.B.; Aquino-Michaels, K.; Earley, Z.M.; Benyamin, F.W.; Man Lei, Y.; Jabri, B.; Alegre, M.-L. Commensal Bifidobacterium promotes antitumor immunity and facilitates anti–PD-L1 efficacy. Science 2015, 350, 1084–1089. [Google Scholar] [CrossRef] [PubMed]
- Iida, N.; Dzutsev, A.; Stewart, C.A.; Smith, L.; Bouladoux, N.; Weingarten, R.A.; Molina, D.A.; Salcedo, R.; Back, T.; Cramer, S. Commensal bacteria control cancer response to therapy by modulating the tumor microenvironment. Science 2013, 342, 967–970. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Zhou, Y.; Liao, L.; Tan, H.; Li, Y.; Li, Z.; Zhou, B.; Bao, M.; He, B. Pharmacokinetics effects of chuanxiong rhizoma on warfarin in pseudo germ-free rats. Front. Pharmacol. 2023, 13, 1022567. [Google Scholar] [CrossRef]
- Kirchberger, S.; Royston, D.J.; Boulard, O.; Thornton, E.; Franchini, F.; Szabady, R.L.; Harrison, O.; Powrie, F. Innate lymphoid cells sustain colon cancer through production of interleukin-22 in a mouse model. J. Exp. Med. 2013, 210, 917–931. [Google Scholar] [CrossRef]
- Zhao, L.; Liao, M.; Li, L.; Chen, L.; Zhang, T.; Li, R. Cadmium activates the innate immune system through the AIM2 inflammasome. Chem.-Biol. Interact. 2024, 399, 111122. [Google Scholar] [CrossRef]
- Zhu, Z.; Gu, Y.; Zeng, C.; Yang, M.; Yu, H.; Chen, H.; Zhang, B.; Cai, H. Olanzapine-induced lipid disturbances: A potential mechanism through the gut microbiota-brain axis. Front. Pharmacol. 2022, 13, 897926. [Google Scholar] [CrossRef]
- Vétizou, M.; Pitt, J.M.; Daillère, R.; Lepage, P.; Waldschmitt, N.; Flament, C.; Rusakiewicz, S.; Routy, B.; Roberti, M.P.; Duong, C.P.; et al. Anticancer immunotherapy by CTLA-4 blockade relies on the gut microbiota. Science 2015, 350, 1079–1084. [Google Scholar] [CrossRef]
- Paulos, C.M.; Wrzesinski, C.; Kaiser, A.; Hinrichs, C.S.; Chieppa, M.; Cassard, L.; Palmer, D.C.; Boni, A.; Muranski, P.; Yu, Z.; et al. Microbial translocation augments the function of adoptively transferred self/tumor-specific CD8+ T cells via TLR4 signaling. J. Clin. Investig. 2007, 117, 2197–2204. [Google Scholar] [CrossRef] [PubMed]
- Sun, D.; Li, X.; Nie, S.; Liu, J.; Wang, S. Disorders of cancer metabolism: The therapeutic potential of cannabinoids. Biomed. Pharmacother. 2023, 157, 113993. [Google Scholar] [CrossRef] [PubMed]
- Monemi, M.; Garrosi, L.; Mirzaei, S.; Farhadi, B.; Disfani, R.A.; Zabihi, M.R.; Akhoondian, M.; Vajargah, P.G.; Khorshid, A.; Karkhah, S. Identification of proteins’ expression pathway and the effective miRNAs for the treatment of human papillomavirus-induced cervical cancer: In-silico analyses-experimental research. Ann. Med. Surg. 2024, 86, 5784–5792. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, J.L.; Mallevaey, T.; Scott-Browne, J.; Gapin, L. CD1d-restricted iNKT cells, the ‘Swiss-Army knife’ of the immune system. Curr. Opin. Immunol. 2008, 20, 358–368. [Google Scholar] [CrossRef]
- Saqib, U.; Sarkar, S.; Suk, K.; Mohammad, O.; Baig, M.S.; Savai, R. Phytochemicals as modulators of M1-M2 macrophages in inflammation. Oncotarget 2018, 9, 17937–17950. [Google Scholar] [CrossRef]
- Liu, S.; Li, X.; Xie, Q.; Zhang, S.; Liang, X.; Li, S.; Zhang, P. Identification of a lncRNA/circRNA-miRNA-mRNA network in Nasopharyngeal Carcinoma by deep sequencing and bioinformatics analysis. J. Cancer 2024, 15, 1916. [Google Scholar] [CrossRef]
- Yang, J.; Zhou, X.; Liu, X.; Ling, Z.; Ji, F. Role of the Gastric Microbiome in Gastric Cancer: From Carcinogenesis to Treatment. Front. Microbiol. 2021, 12, 641322. [Google Scholar] [CrossRef]
- Takayama, S.; Takahashi, H.; Matsuo, Y.; Okada, Y.; Manabe, T. Effects of Helicobacter pylori infection on human pancreatic cancer cell line. Hepato-Gastroenterol. 2007, 54, 2387–2391. [Google Scholar]
- Hashemi Goradel, N.; Heidarzadeh, S.; Jahangiri, S.; Farhood, B.; Mortezaee, K.; Khanlarkhani, N.; Negahdari, B. Fusobacterium nucleatum and colorectal cancer: A mechanistic overview. J. Cell. Physiol. 2018, 234, 2337–2344. [Google Scholar] [CrossRef]
- Nejman, D.; Livyatan, I.; Fuks, G.; Gavert, N.; Zwang, Y.; Geller, L.; Rotter-Maskowitz, A.; Weiser, R.; Mallel, G.; Gigi, E.; et al. The human tumor microbiome is composed of tumor type-specific intracellular bacteria. Science 2020, 368, 973–980. [Google Scholar] [CrossRef]
- Pushalkar, S.; Hundeyin, M.; Daley, D.; Zambirinis, C.P.; Kurz, E.; Mishra, A.; Mohan, N.; Aykut, B.; Usyk, M.; Torres, L.E.; et al. The Pancreatic Cancer Microbiome Promotes Oncogenesis by Induction of Innate and Adaptive Immune Suppression. Cancer Discov. 2018, 8, 403–416. [Google Scholar] [CrossRef] [PubMed]
- Waldman, A.; Fritz, J.M.; Lenardo, M.X. A guide to cancer immunotherapy: From T cell basic science to clinical practice. Nat. Rev. Immunol. 2020, 20, 651–668. [Google Scholar] [CrossRef] [PubMed]
- Basudan, A. The Role of Immune Checkpoint Inhibitors in Cancer Therapy. Clin. Pr. 2022, 13, 22–40. [Google Scholar] [CrossRef] [PubMed]
- Keam, S.J. Tremelimumab: First Approval. Drugs 2022, 83, 93–102. [Google Scholar] [CrossRef] [PubMed]
- Meng, L.; Wu, H.; Wu, J.; Ding, P.; He, J.; Sang, M.; Liu, L. Mechanisms of immune checkpoint inhibitors: Insights into the regulation of circular RNAS involved in cancer hallmarks. Cell Death Dis. 2024, 15, 3. [Google Scholar]
- Abiko, K.; Matsumura, N.; Hamanishi, J.; Horikawa, N.; Murakami, R.; Yamaguchi, K.; Yoshioka, Y.; Baba, T.; Konishi, I.; Mandai, M. IFN-γ from lymphocytes induces PD-L1 expression and promotes progression of ovarian cancer. Br. J. Cancer 2015, 112, 1501–1509. [Google Scholar] [CrossRef]
- Zhao, L.; Yang, Y.; Gao, Q. Efficacy and safety of nivolumab plus apatinib in advanced liver carcinosarcoma: A case report. Immunotherapy 2019, 11, 651–656. [Google Scholar] [CrossRef]
- Wu, Z.; Sun, W.; Wang, C. Clinical characteristics, treatment, and outcomes of pembrolizumab-induced uveitis. Investig. New Drugs 2024. online ahead of print. [Google Scholar] [CrossRef]
- Ogata, D.; Tsuchida, T. Systemic Immunotherapy for Advanced Cutaneous Squamous Cell Carcinoma. Curr. Treat. Options Oncol. 2019, 20, 30. [Google Scholar] [CrossRef]
- Derosa, L.; Iebba, V.; Silva, C.A.C.; Piccinno, G.; Wu, G.; Lordello, L.; Routy, B.; Zhao, N.; Thelemaque, C.; Birebent, R.; et al. Custom scoring based on ecological topology of gut microbiota associated with cancer immunotherapy outcome. Cell 2024, 187, 3373–3389.e3316. [Google Scholar] [CrossRef]
- Smith, M.; Dai, A.; Ghilardi, G.; Amelsberg, K.V.; Devlin, S.M.; Pajarillo, R.; Slingerland, J.B.; Beghi, S.; Herrera, P.S.; Giardina, P.; et al. Gut microbiome correlates of response and toxicity following anti-CD19 CAR T cell therapy. Nat. Med. 2022, 28, 713–723. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Tan, Y.; Cheng, X.; Zhang, Z.; Huang, J.; Hui, S.; Zhu, L.; Liu, Y.; Zhao, D.; Liu, Z. Untargeted metabolomics analysis of the hippocampus and cerebral cortex identified the neuroprotective mechanisms of Bushen Tiansui formula in an aβ25-35-induced rat model of Alzheimer’s disease. Front. Pharmacol. 2022, 13, 990307. [Google Scholar] [CrossRef] [PubMed]
- Hajjar, R.; Gonzalez, E.; Fragoso, G.; Oliero, M.; Alaoui, A.A.; Calvé, A.; Rendos, H.V.; Djediai, S.; Cuisiniere, T.; Laplante, P. Gut microbiota influence anastomotic healing in colorectal cancer surgery through modulation of mucosal proinflammatory cytokines. Gut 2023, 72, 1143–1154. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Li, A.; Wang, Y.; Zhang, Y. Intratumoral microbiota: Roles in cancer initiation, development and therapeutic efficacy. Signal Transduct. Target. Ther. 2023, 8, 35. [Google Scholar] [CrossRef] [PubMed]
- Kareva, I. Metabolism and Gut Microbiota in Cancer Immunoediting, CD8/Treg Ratios, Immune Cell Homeostasis, and Cancer (Immuno)Therapy: Concise Review. Stem. Cells 2019, 37, 1273–1280. [Google Scholar] [CrossRef]
- Li, Z.; Xiong, W.; Liang, Z.; Wang, J.; Zeng, Z.; Kolat, D.; Li, X.; Zhou, D.; Xu, X.; Zhao, L. Critical role of the gut microbiota in immune responses and cancer immunotherapy. J. Hematol. Oncol. 2024, 17, 33. [Google Scholar] [CrossRef]
- Zhu, C.; Wang, Y.; Zhu, R.; Wang, S.; Xue, J.; Zhang, D.; Lan, Z.; Zhang, C.; Liang, Y.; Zhang, N.; et al. Gut microbiota and metabolites signatures of clinical response in anti-PD-1/PD-L1 based immunotherapy of biliary tract cancer. Biomark. Res. 2024, 12, 56. [Google Scholar] [CrossRef]
- Inamura, K. Roles of microbiota in response to cancer immunotherapy. Semin. Cancer Biol. 2020, 65, 164–175. [Google Scholar] [CrossRef]
- Gopalakrishnan, V.; Spencer, C.N.; Nezi, L.; Reuben, A.; Andrews, M.C.; Karpinets, T.V.; Prieto, P.A.; Vicente, D.; Hoffman, K.; Wei, S.C.; et al. Gut microbiome modulates response to anti-PD-1 immunotherapy in melanoma patients. Science 2018, 359, 97–103. [Google Scholar] [CrossRef]
| Bacterial Species | Anticancer Immunity | Mechanism of Action | Notes | References |
|---|---|---|---|---|
| Bacteroides fragilis | ↑ CRC | ↑ ROS in colon epithelial cells, activates NF-κB through IL-1 and TNF-α, driving chronic inflammation and carcinogenesis | Linked with higher polyp and dysplasia rates; key risk factor in CRC | [4,9] |
| Fusobacterium nucleatum | Inhibits anticancer immunity, ↑tumor progression | Blocks NK cell activity, inhibits adaptive immune responses to evade anticancer immunity | Causes chronic inflammation and DNA damage | [1,9] |
| Faecalibaculum rodentium | ↓ Tumor cell proliferation | Produces SCFAs that inhibit calcineurin/NFATc3, ↓ tumor cell growth | ↑Gut homeostasis and having tumor-suppressing effects | |
| Lactobacillus johnsonii | ↑Anticancer immunity, restores mucosal integrity | Translocates to lymph nodes, induces Th1/Th17 responses with IL-17 and IFN-γ during cyclophosphamide therapy | ↑ Anticancer drug response by supporting T-cell activity | [2,4] |
| Enterococcus hirae | ↑ Anticancer responses | Activates immune cells, especially Th1 cells | Important in chemotherapy, especially cyclophosphamide treatment | |
| Bacteroides fragilis | Essential for anti-CTLA-4 immunotherapy efficacy | Engages TLR2/TLR4 pathways to activate dendritic cells, stimulating Th1 responses with IFN-γ | Required for optimal immunotherapy response in anti-CTLA-4 treatments | |
| Clostridium spp. | Supports anticancer immunity | ↑ Differentiation of CD4+ Tregs via IL-10, aiding immune tolerance and ↓ inflammation | Plays a significant role in maintaining immune regulation | |
| Escherichia coli | ↓ Chemotherapy efficacy, ↑ infection risk | ↑ Pathogenic strains due to altered gut microbiota, leading to epithelial barrier disruption and systemic infections | ↓ Beneficial Lactobacillus and Bifidobacterium populations | |
| Bifidobacterium spp. | Protects against chemotherapy-induced toxicity, supports immune homeostasis | Contributes to barrier integrity, ↑ SCFA production which modulates IL-6 and IL-12 | ↓ By chemotherapy, which impacts treatment efficacy | |
| B. pseudolongum | ↑ Anticancer immunity and therapeutic response | Produces inosine; with IFN-γ, ↑ Th1 differentiation, ↑ responses to anti-PD-L1 therapy | Improved outcomes in immunotherapy | [2,3] |
| Microbial Metabolites | ||||
| Polyamines | ↓ Anticancer immunity | Inhibits lymphocyte proliferation, ↓anticancer immune responses, enables tumor protease activity | High levels in obese patients, supports tumor invasion | [9] |
| SCFAs | Inhibits metastasis, maintains barrier integrity, supports immune recruitment | Acts on G protein-coupled receptors, recruits/activates immune cells (neutrophils, macrophages, T-cells); modulates IL-6, IL-12 | ↓ Breast cancer cell metastasis, protects gut barrier integrity | |
| Imidazole propionate | ↑ Tumor growth, especially in type 2 diabetes patients | Activates mTOR signaling, ↑ insulin resistance and tumorigenesis | Abundant in diabetic microbiota, contributing to higher cancer risk | [1] |
| Vitamin B (various types) | Supports metabolic functions, potentially impacting cancer development | Influences the SGOC pathway; may modulate immune responses and cellular metabolism | Impact on cancer linked to specific vitamin B pathways in SGOC metabolic regulation | |
| Inosine | ↑ T-cell differentiation and anticancer immunity | Produced by B. pseudolongum, works with IFN-γ to drive Th1 cell differentiation and ↑ responses to checkpoint inhibitors like anti-PD-L1 therapy | ↑ Anti-tumor responses in checkpoint inhibitor treatments | [2,3] |
| Bacteria | Role of Signaling Pathway | Immunomodulatory Role in Cancer Defense | Refs. |
|---|---|---|---|
| Lactobacillus rhamnosus GG | Stimulates IFN-α and IFN-β via cGAS-STING pathway in DC | ↑ Anti-tumor effects of PD-1 immunotherapy by ↑ immune response to ICIs. | [1,27] |
| Lactobacillus reuteri | Alters CD4+ T cells into CD4+ CD8αα+ intraepithelial lymphocytes | ↓ IBD by modulating immune cells, contributing to overall cancer defense mechanisms. | |
| Bifidobacterium bifidum | Expresses surface polysaccharides (β-glucan/galactan) to induce Tregs | ↓ Colitis and ↑ tumor inhibition, with effects similar to PD-L1 antibody therapy, by inducing Tregs. | |
| Streptococcus thermophiles | Secretes β-galactosidase enzyme | Inhibits CRC cell proliferation, ↓ tumor growth, and ↑ probiotics by regulating lymphocytes and Tregs, creating a supportive environment for immune modulation. | [1] |
| Bifidobacterium spp. | Activates STING pathway and type I interferons in hypoxic tumor microenvironment | ↑ Anti-CD47 immunotherapy by ↑ macrophage-mediated phagocytosis of tumor cells, taking advantage of the tumor’s low-oxygen state. | [3] |
| Bifidobacterium Pseudolongum Olsenella Lactobacillus johnsonii | Activates Th1 cells via inosine metabolite | ↑ Immune response against colon cancer and melanoma; inosine acts as an adjuvant to ↑ effectiveness of ICIs, helping ↓ tumor growth. | [1,28] |
| Bacteroides fragilis | Induces T-cell-mediated immune response by activating Th1 cells | ↑ PD-1/PD-L1 and CTLA-4 immunotherapies by stimulating Th1 cells, thus ↑ anti-tumor immunity and facilitating an active immune response against cancer cells. | [2,9] |
| Bacteroides thetaiotaomicron, Burkholderia cepacia | Activates Th1 cells and facilitates DC maturation in lymph nodes | Boosts anti-CTLA-4 therapy efficacy by promoting maturation of DC in the tumor microenvironment and stimulating Th1 cells; ↑ immune activation against tumors. | [9] |
| Gut microbiota (General) | Upregulates Nox1 and Cybb genes for NADPH oxidase 2, aiding ROS production | Assists cytotoxic drugs (oxaliplatin) via ↑ ROS production, which induces cancer cell apoptosis, thereby ↑ chemotherapy’s anticancer effects. | [4] |
| Gut microbiota (General) | Activates TLRs to stimulate NF-κB and MAPK pathways, leading to cytokine production | Supports inflammation and ROS production in myeloid cells, ↑ immune response against cancer cells. Facilitates production of immunomodulatory cytokines to strengthen anticancer immunity alongside chemotherapy. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Farhadi Rad, H.; Tahmasebi, H.; Javani, S.; Hemati, M.; Zakerhamidi, D.; Hosseini, M.; Alibabaei, F.; Banihashemian, S.Z.; Oksenych, V.; Eslami, M. Microbiota and Cytokine Modulation: Innovations in Enhancing Anticancer Immunity and Personalized Cancer Therapies. Biomedicines 2024, 12, 2776. https://doi.org/10.3390/biomedicines12122776
Farhadi Rad H, Tahmasebi H, Javani S, Hemati M, Zakerhamidi D, Hosseini M, Alibabaei F, Banihashemian SZ, Oksenych V, Eslami M. Microbiota and Cytokine Modulation: Innovations in Enhancing Anticancer Immunity and Personalized Cancer Therapies. Biomedicines. 2024; 12(12):2776. https://doi.org/10.3390/biomedicines12122776
Chicago/Turabian StyleFarhadi Rad, Hamidreza, Hamed Tahmasebi, Samaneh Javani, Maral Hemati, Darya Zakerhamidi, Masoomeh Hosseini, Farnaz Alibabaei, Seyedeh Zahra Banihashemian, Valentyn Oksenych, and Majid Eslami. 2024. "Microbiota and Cytokine Modulation: Innovations in Enhancing Anticancer Immunity and Personalized Cancer Therapies" Biomedicines 12, no. 12: 2776. https://doi.org/10.3390/biomedicines12122776
APA StyleFarhadi Rad, H., Tahmasebi, H., Javani, S., Hemati, M., Zakerhamidi, D., Hosseini, M., Alibabaei, F., Banihashemian, S. Z., Oksenych, V., & Eslami, M. (2024). Microbiota and Cytokine Modulation: Innovations in Enhancing Anticancer Immunity and Personalized Cancer Therapies. Biomedicines, 12(12), 2776. https://doi.org/10.3390/biomedicines12122776







