Overview of the Trending Enteric Viruses and Their Pathogenesis in Intestinal Epithelial Cell Infection
Abstract
1. Introduction
| Potential Routes of Enteric Viruses That Causes Systemic Infection | ||||
|---|---|---|---|---|
| Enteric Virus Types | Infections and Mortality | Cellular and Molecular Mechanism of Infection | Types of Immune Cells Involved in the Infection | Refs. |
| Rotavirus (RV) |
|
| Monocyte, B cell, lymphocyte, dendritic cell | [8,9,10,11] |
| Human Norovirus (HNoV) | Low-grade fever, muscle aches: usually mild. | Virus infection induces proinflammatory cytokine release and triggers innate and adaptive immune system activation. | Neutrophils, monocytes, T cell, macrophages | [12,13,14] |
| Enterovirus A71 (EV-A71) | Neurological disease, cardiopulmonary disease: range from acute to long-term sequelae. | Virus infection induces the production of cytokines, which may increase endothelial permeability, causing systemic inflammation, | Neutrophils, monocytes, T cell, macrophages, dendritic cell | [15,16,17] |
| Coxsackieviruses A6 (CV-A6) | CNS disease, myocardial damage: high mortality in CNS disease patients. | Virus infection triggers inflammation, apoptosis, and astrocyte activation, and blocks innate immune response, causing neural damage and systemic inflammation. | Neutrophils, monocytes, T cell | [18,19,20] |
| Echovirus 11 (Echo11) | Hepatitis, respiratory disease, meningitis, myocardial injury: usually asymptomatic or mild, but can be severe in infected neonates. | Virus infection induces inflammation and reduces Type 1 IFN production. Some reports show that virus infection may induce pyroptosis in cells. | Neutrophils, monocytes, T cell, macrophages | [21,22,23,24] |
| Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) |
|
| Neutrophils, macrophages, dendritic cells, B cell, T cell | [25,26,27,28] |
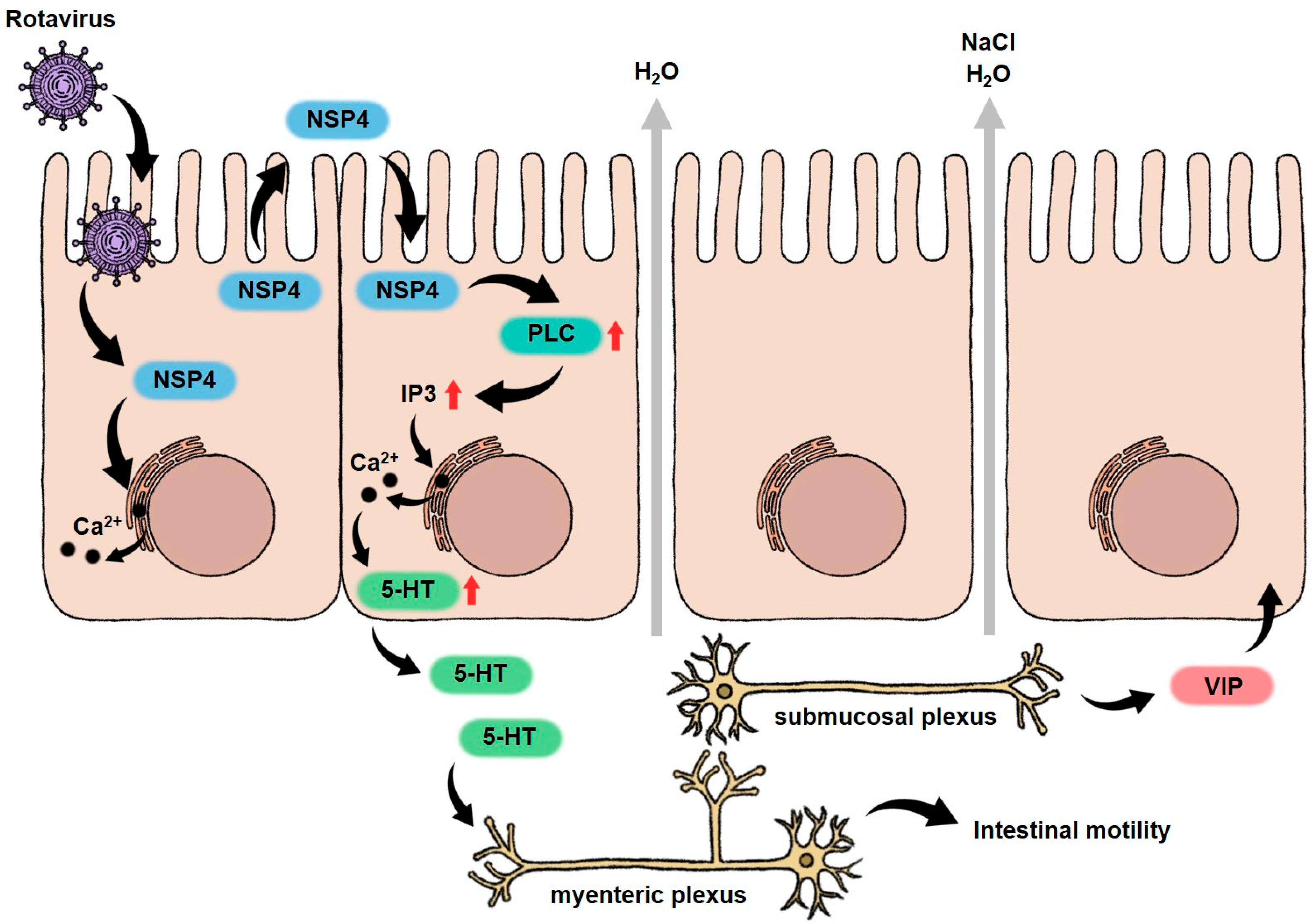
1.1. Rotavirus (RV)
| Enteric Viruses Types | In Vivo Model/In Vitro Model | Mechanism of Pathogenesis | Results | Clinical Interpretations | Refs. |
|---|---|---|---|---|---|
| Rotavirus (RV) | In vivo: mice, pig, rabbit In vitro: human intestinal epithelium cell lines, human intestinal enteroids (HIEs) |
| Villous atrophy, malabsorption, enteric nervous system activated | Watery diarrhea, vomiting, gastroenteritis, and fever | [8,10,33,45,46] |
| Human Norovirus (HNoV) | In vivo: zebrafish, gnotobiotic pigs, gnotobiotic calves. In vitro: B cell, intestinal enteroids. |
| Blunting of the villi, enlarged and pale mitochondria, intercellular edema, malabsorption | Watery diarrhea, nausea, vomiting, malaise, abdominal pain, gastroenteritis | [47,48,49,50] |
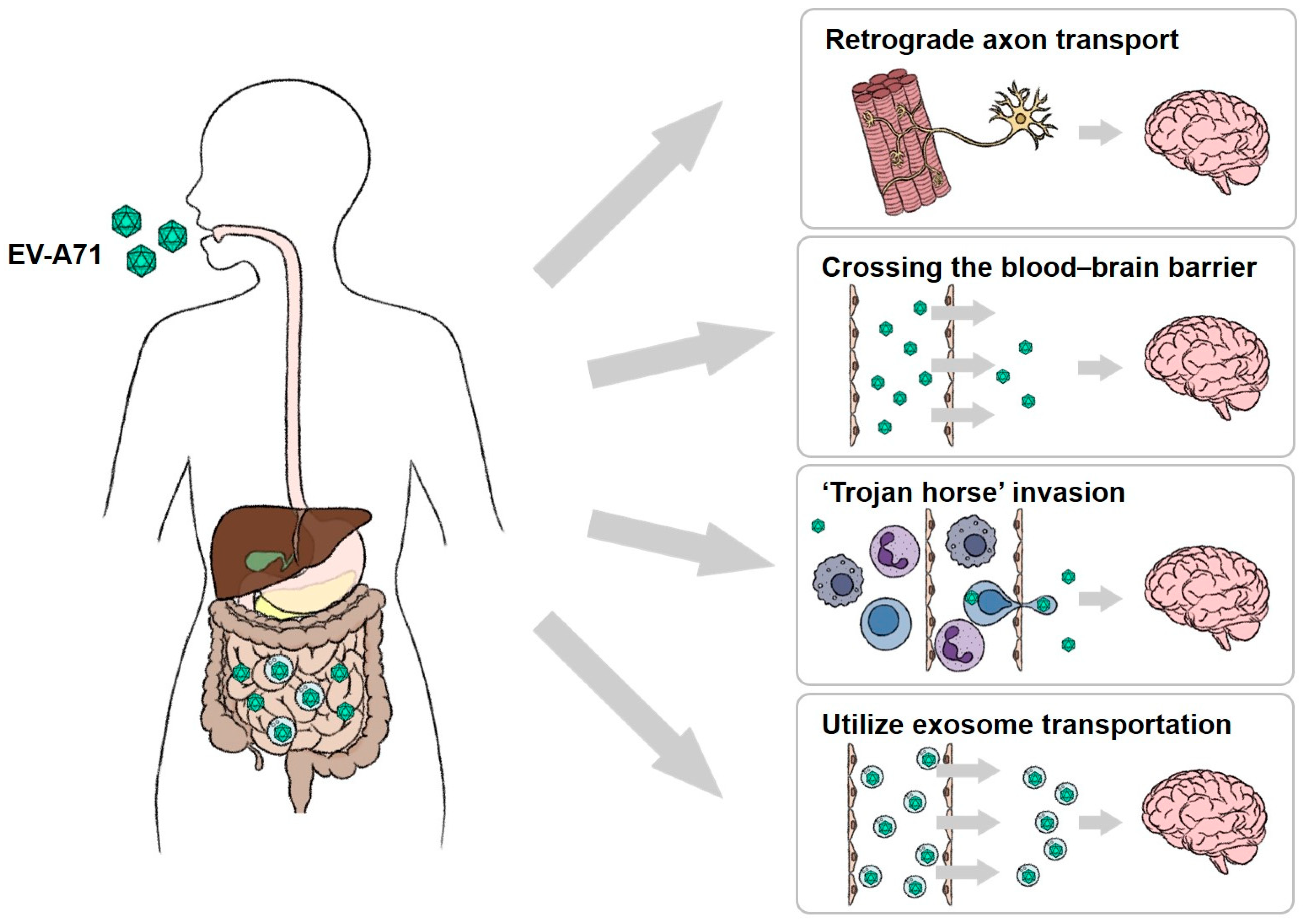
| Enterovirus A71 (EV-A71) | In vivo: mouse, non-human primates. In vitro: RD cell, Human intestinal epithelium cell lines, organoids, enteroids. | Virus infection triggers cell death or cell lysis to exit the cells. | Virus invades other tissues via circulatory system after breaking the intestinal epithelial barrier. | Hand, foot, and mouth disease (HFMD), neurologic complications, encephalitis, and paralysis | [1,51,52,53] |
| Coxsackieviruses A6 (CV-A6) | In vivo: mouse. In vitro: RD cell, Vero cell, KMB17 cell, HEK293A cell. | The KREMEN1 is the only known receptor for CV-A6. Virus infection may suppress interferon production and trigger cell death. | Altering host immune responses, Necroptosis | Herpangina, skin rash, vesiculobullous eruptions, desquamation, onychomadesis, and epididymitis | [20,54,55,56] |
| Echovirus 11 (Echo11) | In vivo: mouse. In vitro: RD cell, Vero cell, hepatocellular carcinoma cell lines, blood cells. | Virus utilizes CD55 and FcRn entering the cells and then activates the assembly of inflammasomes and triggers cell death. | Destroy epithelial barrier, Inflammation | Cough, rhinitis, hand, foot, and mouth disease (HFMD), myocarditis, aseptic meningitis, and rashes | [23,57,58] |
| Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) | In vivo: mice, rabbits, hamster, bats, cats, dogs, and non-human primates. In vitro: 2D or 3D cultures of primary or immortalized cells and tissues. | Virus infects intestinal epithelial cells, causing cytokine and chemokine release and the downregulation of ACE2, which leads to disorder of the RAS system. | Acute intestinal inflammation, Declining the anti-inflammatory ability, Epithelial damage | Diarrhea, anorexia, vomiting, nausea, encephalitis, hepatitis, and inflammatory bowel disease | [59,60,61,62] |
1.2. Norovirus (NoV)
| Enteric Virus Types | Family | Genome | Structure Protein | Receptor on Intestinal Epithelial Cells | Refs. |
|---|---|---|---|---|---|
| Rotavirus (RV) | Reoviridae | 11 segments of dsRNA | VP4 | sialic acid, integrins, Hsc70 | [38,84] |
| Human Norovirus (HNoV) | Caliciviridae | ssRNA (+), 7.5–7.7 kb | VP1 | HBGA | [85] |
| Human adenovirus (HAdV) | Adenoviridae | dsDNA, 36 kb | fiber | CAR | [86] |
| penton base | integrin | ||||
| Enterovirus A71 (EV-A71) | Picornaviridae | ssRNA, 7.4 kb | VP1 | PSGL-1, SCARB2 | [87] |
| Coxsackievirus A6 (CV-A6) | KREMEN1 | [54,88] | |||
| Echovirus 11 (Echo11) | VP2 and VP3 | CD55 | [89,90] | ||
| VP1 | FcRn | ||||
| Coronavirus (CoV) | Coronaviridae | ssRNA, 27–32 kb | spike | ACE2 | [91] |
1.3. Adenovirus (AdV)
1.4. Enteroviruses (EVs)
1.4.1. Enterovirus A71 (EV-A71)
1.4.2. Coxsackievirus A6 (CV-A6)
1.4.3. Echovirus 11 (Echo11)
1.5. Coronavirus (CoV)
2. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wells, A.I.; Coyne, C.B. Enteroviruses: A Gut-Wrenching Game of Entry, Detection, and Evasion. Viruses 2019, 11, 460. [Google Scholar] [CrossRef] [PubMed]
- Burgueño, J.F.; Abreu, M.T. Epithelial Toll-like receptors and their role in gut homeostasis and disease. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 263–278. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Shang, W.; Bao, L.; Peng, Z.; Wu, C. Epithelial-immune cell crosstalk for intestinal barrier homeostasis. Eur. J. Immunol. 2024, 54, e2350631. [Google Scholar] [CrossRef] [PubMed]
- Bishop, R.F.; Kirkwood, C.D. Enteric Viruses. In Encyclopedia of Virology, 3rd ed.; Mahy, B.W.J., Van Regenmortel, M.H.V., Eds.; Academic Press: Oxford, UK, 2008; pp. 116–123. [Google Scholar] [CrossRef]
- Rao, C.D. Enteroviruses in gastrointestinal diseases. Rev. Med. Virol. 2021, 31, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Manouana, G.P.; Nguema-Moure, P.A.; Mbong Ngwese, M.; Bock, C.-T.; Kremsner, P.G.; Borrmann, S.; Eibach, D.; Mordmüller, B.; Velavan, T.P.; Niendorf, S.; et al. Genetic Diversity of Enteric Viruses in Children under Five Years Old in Gabon. Viruses 2021, 13, 545. [Google Scholar] [CrossRef]
- Lockhart, A.; Mucida, D.; Parsa, R. Immunity to enteric viruses. Immunity 2022, 55, 800–818. [Google Scholar] [CrossRef]
- Dian, Z.; Sun, Y.; Zhang, G.; Xu, Y.; Fan, X.; Yang, X.; Pan, Q.; Peppelenbosch, M.; Miao, Z. Rotavirus-related systemic diseases: Clinical manifestation, evidence and pathogenesis. Crit. Rev. Microbiol. 2021, 47, 580–595. [Google Scholar] [CrossRef]
- Bednarek, J.; Traxinger, B.; Brigham, D.; Roach, J.; Orlicky, D.; Wang, D.; Pelanda, R.; Mack, C.L. Cytokine-Producing B Cells Promote Immune-Mediated Bile Duct Injury in Murine Biliary Atresia. Hepatology 2018, 68, 1890–1904. [Google Scholar] [CrossRef]
- Ramig Robert, F. Pathogenesis of Intestinal and Systemic Rotavirus Infection. J. Virol. 2004, 78, 10213–10220. [Google Scholar] [CrossRef]
- Rivero-Calle, I.; Gómez-Rial, J.; Martinón-Torres, F. Systemic features of rotavirus infection. J. Infect. 2016, 72, S98–S105. [Google Scholar] [CrossRef]
- Cutler, A.J.; Oliveira, J.; Ferreira, R.C.; Challis, B.; Walker, N.M.; Caddy, S.; Lu, J.; Stevens, H.E.; Smyth, D.J.; Pekalski, M.L.; et al. Capturing the systemic immune signature of a norovirus infection: An n-of-1 case study within a clinical trial. Wellcome Open Res. 2017, 2, 28. [Google Scholar] [CrossRef] [PubMed]
- Bouziat, R.; Biering, S.B.; Kouame, E.; Sangani, K.A.; Kang, S.; Ernest, J.D.; Varma, M.; Brown, J.J.; Urbanek, K.; Dermody, T.S.; et al. Murine Norovirus Infection Induces T(H)1 Inflammatory Responses to Dietary Antigens. Cell Host Microbe 2018, 24, 677–688.e5. [Google Scholar] [CrossRef] [PubMed]
- Wobus, C.E.; Karst, S.M.; Thackray, L.B.; Chang, K.O.; Sosnovtsev, S.V.; Belliot, G.; Krug, A.; Mackenzie, J.M.; Green, K.Y.; Virgin, H.W. Replication of Norovirus in cell culture reveals a tropism for dendritic cells and macrophages. PLoS Biol. 2004, 2, e432. [Google Scholar] [CrossRef] [PubMed]
- Hsia, S.-H.; Lin, J.-J.; Chan, O.-W.; Lin, T.-Y. Cardiopulmonary failure in children infected with Enterovirus A71. J. Biomed. Sci. 2020, 27, 53. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.Y.; Weng, K.F.; Chang, C.K.; Gong, Y.N.; Huang, G.J.; Lee, H.L.; Chen, Y.C.; Huang, C.C.; Lu, J.Y.; Huang, P.N.; et al. Enterovirus A71 Induces Neurological Diseases and Dynamic Variants in Oral Infection of Human SCARB2-Transgenic Weaned Mice. J. Virol. 2021, 95, e0089721. [Google Scholar] [CrossRef]
- Chang, L.Y.; Lin, H.Y.; Gau, S.S.; Lu, C.Y.; Hsia, S.H.; Huang, Y.C.; Huang, L.M.; Lin, T.Y. Enterovirus A71 neurologic complications and long-term sequelae. J. Biomed. Sci. 2019, 26, 57. [Google Scholar] [CrossRef]
- Sun, Q.; Li, J.; Wang, R.; Sun, T.; Zong, Y.; Wang, C.; Liu, Y.; Li, X.; Song, Y.; Zhang, Y. Coxsackievirus A6 Infection Causes Neurogenic Pathogenesis in a Neonatal Murine Model. Viruses 2023, 15, 511. [Google Scholar] [CrossRef]
- Jin, Y.; Zhang, R.; Wu, W.; Duan, G. Innate Immunity Evasion by Enteroviruses Linked to Epidemic Hand-Foot-Mouth Disease. Front. Microbiol. 2018, 9, 2422. [Google Scholar] [CrossRef]
- Lott, J.P.; Liu, K.; Landry, M.L.; Nix, W.A.; Oberste, M.S.; Bolognia, J.; King, B. Atypical hand-foot-and-mouth disease associated with coxsackievirus A6 infection. J. Am. Acad. Dermatol. 2013, 69, 736–741. [Google Scholar] [CrossRef]
- Zhang, G.; Li, J.; Sun, Q.; Zhang, K.; Xu, W.; Zhang, Y.; Wu, G. Pathological Features of Echovirus-11-Associated Brain Damage in Mice Based on RNA-Seq Analysis. Viruses 2021, 13, 2477. [Google Scholar] [CrossRef]
- Wang, P.; Xu, Y.; Liu, M.; Li, H.; Wang, H.; Liu, Y.; Wang, B.; Xia, S.; Su, H.; Wei, M.; et al. Risk factors and early markers for echovirus type 11 associated haemorrhage-hepatitis syndrome in neonates, a retrospective cohort study. Front. Pediatr. 2023, 11, 1063558. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Yang, R.; Yang, F.; Han, Y.; Ren, Y.; Xiong, X.; Wang, X.; Bi, Y.; Li, L.; Qiu, Y.; et al. Echovirus 11 infection induces pyroptotic cell death by facilitating NLRP3 inflammasome activation. PLoS Pathog. 2022, 18, e1010787. [Google Scholar] [CrossRef] [PubMed]
- Grapin, M.; Mirand, A.; Pinquier, D.; Basset, A.; Bendavid, M.; Bisseux, M.; Jeannoël, M.; Kireche, B.; Kossorotoff, M.; L’Honneur, A.S.; et al. Severe and fatal neonatal infections linked to a new variant of echovirus 11, France, July 2022 to April 2023. Eurosurveillance 2023, 28, 2300253. [Google Scholar] [CrossRef] [PubMed]
- Synowiec, A.; Szczepański, A.; Barreto-Duran, E.; Lie, L.K.; Pyrc, K. Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2): A Systemic Infection. Clin. Microbiol. Rev. 2021, 34, e00133-20. [Google Scholar] [CrossRef] [PubMed]
- Mahajan, R.K.; Paul, G.; Mahajan, R.; Gautam, P.L.; Paul, B. Systemic manifestations of COVID-19. J. Anaesthesiol. Clin. Pharmacol. 2020, 36, 435–442. [Google Scholar] [CrossRef]
- Munjal, M.; Das, S.; Chatterjee, N.; Setra, A.E.; Govil, D. Systemic Involvement of Novel Coronavirus (COVID-19): A Review of Literature. Indian J. Crit. Care Med. 2020, 24, 565–569. [Google Scholar] [CrossRef]
- El-Kassas, M.; Alboraie, M.; Elbadry, M.; El Sheemy, R.; Abdellah, M.; Afify, S.; Madkour, A.; Zaghloul, M.; Awad, A.; Wifi, M.N.; et al. Non-pulmonary involvement in COVID-19: A systemic disease rather than a pure respiratory infection. World J. Clin. Cases 2023, 11, 493–505. [Google Scholar] [CrossRef]
- Bergman, H.; Henschke, N.; Hungerford, D.; Pitan, F.; Ndwandwe, D.; Cunliffe, N.; Soares-Weiser, K. Vaccines for preventing rotavirus diarrhoea: Vaccines in use. Cochrane Database Syst. Rev. 2021, 11, Cd008521. [Google Scholar] [CrossRef]
- Bergelson, J.M.; Chan, M.; Solomon, K.R.; St John, N.F.; Lin, H.; Finberg, R.W. Decay-accelerating factor (CD55), a glycosylphosphatidylinositol-anchored complement regulatory protein, is a receptor for several echoviruses. Proc. Natl. Acad. Sci. USA 1994, 91, 6245–6248. [Google Scholar] [CrossRef]
- Guerrero, C.A.; Santana, A.Y.; Acosta, O. Mouse intestinal villi as a model system for studies of rotavirus infection. J. Virol. Methods 2010, 168, 22–30. [Google Scholar] [CrossRef]
- Gbebangi-Manzemu, D.; Kampunzu, V.M.; Vanzwa, H.M.; Mumbere, M.; Bukaka, G.M.; Likele, B.B.; Kasai, E.T.; Mukinayi, B.M.; Tonen-Wolyec, S.; Dauly, N.N.; et al. Clinical profile of children under 5 years of age with rotavirus diarrhoea in a hospital setting in Kisangani, DRC, after the introduction of the rotavirus vaccine, a cross-sectional study. BMC Pediatr. 2023, 23, 193. [Google Scholar] [CrossRef] [PubMed]
- Omatola, C.A.; Olaniran, A.O. Rotaviruses: From Pathogenesis to Disease Control—A Critical Review. Viruses 2022, 14, 875. [Google Scholar] [CrossRef] [PubMed]
- Shilpi Srivastava, A.J. Rotavirus Nonstructural Protein 4 (NSP4)-Viral Enterotoxin with Multiple roles in Pathogenesis of Diarrhoea in Children. J. Appl. Pharm. Sci. 2015, 5, 146–153. [Google Scholar] [CrossRef]
- Hyser, J.M.; Collinson-Pautz, M.R.; Utama, B.; Estes, M.K. Rotavirus disrupts calcium homeostasis by NSP4 viroporin activity. mBio 2010, 1, e00265-10. [Google Scholar] [CrossRef]
- Dong, Y.; Zeng, C.Q.; Ball, J.M.; Estes, M.K.; Morris, A.P. The rotavirus enterotoxin NSP4 mobilizes intracellular calcium in human intestinal cells by stimulating phospholipase C-mediated inositol 1,4,5-trisphosphate production. Proc. Natl. Acad. Sci. USA 1997, 94, 3960–3965. [Google Scholar] [CrossRef]
- Hagbom, M.; Sharma, S.; Lundgren, O.; Svensson, L. Towards a human rotavirus disease model. Curr. Opin. Virol. 2012, 2, 408–418. [Google Scholar] [CrossRef]
- Crawford, S.E.; Ramani, S.; Tate, J.E.; Parashar, U.D.; Svensson, L.; Hagbom, M.; Franco, M.A.; Greenberg, H.B.; O’Ryan, M.; Kang, G.; et al. Rotavirus infection. Nat. Rev. Dis. Primers 2017, 3, 17083. [Google Scholar] [CrossRef]
- Blutt, S.E.; Conner, M.E. Rotavirus: To the gut and beyond! Curr. Opin. Gastroenterol. 2007, 23, 39–43. [Google Scholar] [CrossRef]
- Laizane, G.; Smane, L.; Nokalna, I.; Gardovska, D.; Feemster, K.A. Rotavirus-associated seizures and reversible corpus callosum lesion. Acta Med. Litu. 2019, 26, 113–117. [Google Scholar] [CrossRef][Green Version]
- Borghan, M.A.; Mori, Y.; El-Mahmoudy, A.B.; Ito, N.; Sugiyama, M.; Takewaki, T.; Minamoto, N. Induction of nitric oxide synthase by rotavirus enterotoxin NSP4: Implication for rotavirus pathogenicity. J. Gen. Virol. 2007, 88 Pt 7, 2064–2072. [Google Scholar] [CrossRef]
- DiFazio, M.P.; Braun, L.; Freedman, S.; Hickey, P. Rotavirus-induced seizures in childhood. J. Child. Neurol. 2007, 22, 1367–1370. [Google Scholar] [CrossRef] [PubMed]
- Yeom, J.S.; Jo, J.Y.; Park, J.S.; Kim, Y.S.; Chung, J.Y.; Han, T.H.; Seo, J.H.; Park, E.S.; Lim, J.Y.; Woo, H.O.; et al. Monocyte Chemoattractant Protein (MCP)-1 in Rotavirus-Associated White Matter Injury in Newborns. Neuropediatrics 2019, 50, 228–234. [Google Scholar] [CrossRef] [PubMed]
- Karampatsas, K.; Spyridou, C.; Morrison, I.R.; Tong, C.Y.; Prendergast, A.J. Rotavirus-associated mild encephalopathy with a reversible splenial lesion (MERS)-case report and review of the literature. BMC Infect. Dis. 2015, 15, 446. [Google Scholar] [CrossRef] [PubMed]
- Conner, M.E.; Estes, M.K.; Graham, D.Y. Rabbit model of rotavirus infection. J. Virol. 1988, 62, 1625–1633. [Google Scholar] [CrossRef] [PubMed]
- Peña-Gil, N.; Randazzo, W.; Carmona-Vicente, N.; Santiso-Bellón, C.; Cárcamo-Cálvo, R.; Navarro-Lleó, N.; Monedero, V.; Yebra, M.J.; Buesa, J.; Gozalbo-Rovira, R.; et al. Culture of Human Rotaviruses in Relevant Models Shows Differences in Culture-Adapted and Nonculture-Adapted Strains. Int. J. Mol. Sci. 2023, 24, 17362. [Google Scholar] [CrossRef]
- Karst, S.M. Pathogenesis of noroviruses, emerging RNA viruses. Viruses 2010, 2, 748–781. [Google Scholar] [CrossRef]
- Lucero, Y.; Matson, D.O.; Ashkenazi, S.; George, S.; O’Ryan, M. Norovirus: Facts and Reflections from Past, Present, and Future. Viruses 2021, 13, 2399. [Google Scholar] [CrossRef]
- Netzler, N.E.; Enosi Tuipulotu, D.; White, P.A. Norovirus antivirals: Where are we now? Med. Res. Rev. 2019, 39, 860–886. [Google Scholar] [CrossRef]
- Omatola, C.A.; Mshelbwala, P.P.; Okolo, M.-L.O.; Onoja, A.B.; Abraham, J.O.; Adaji, D.M.; Samson, S.O.; Okeme, T.O.; Aminu, R.F.; Akor, M.E.; et al. Noroviruses: Evolutionary Dynamics, Epidemiology, Pathogenesis, and Vaccine Advances—A Comprehensive Review. Vaccines 2024, 12, 590. [Google Scholar] [CrossRef]
- Wei, Y.; Liu, H.; Hu, D.; He, Q.; Yao, C.; Li, H.; Hu, K.; Wang, J. Recent Advances in Enterovirus A71 Infection and Antiviral Agents. Lab. Investig. 2024, 104, 100298. [Google Scholar] [CrossRef]
- Wang, S.M.; Liu, C.C. Update of enterovirus 71 infection: Epidemiology, pathogenesis and vaccine. Expert Rev. Anti Infect. Ther. 2014, 12, 447–456. [Google Scholar] [CrossRef] [PubMed]
- Huang, P.N.; Shih, S.R. Update on enterovirus 71 infection. Curr. Opin. Virol. 2014, 5, 98–104. [Google Scholar] [CrossRef] [PubMed]
- Staring, J.; van den Hengel, L.G.; Raaben, M.; Blomen, V.A.; Carette, J.E.; Brummelkamp, T.R. KREMEN1 Is a Host Entry Receptor for a Major Group of Enteroviruses. Cell Host Microbe 2018, 23, 636–643.e5. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.H.; Du, J.; Yu, J.; Zhao, Y.; Wang, Y.; Hua, S.; Zhao, K. Coxsackievirus A6 2C protein antagonizes IFN-β production through MDA5 and RIG-I depletion. J. Virol. 2023, 97, e0107523. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Yu, X.; Meng, X.; Huo, W.; Su, Y.; Liu, J.; Liu, Y.; Zhang, J.; Wang, S.; Yu, J. Coxsackievirus A6 Induces Necroptosis for Viral Production. Front. Microbiol. 2020, 11, 42. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.W.; Bendig, J.W.A.; Ossuetta, I. Vertical transmission of human echovirus 11 at the time of bornholm disease in late pregnancy. Pediatr. Infect. Dis. J. 2005, 24, 88–89. [Google Scholar] [CrossRef] [PubMed]
- Wells, A.I.; Grimes, K.A.; Kim, K.; Branche, E.; Bakkenist, C.J.; DePas, W.H.; Shresta, S.; Coyne, C.B. Human FcRn expression and Type I Interferon signaling control Echovirus 11 pathogenesis in mice. PLoS Pathog. 2021, 17, e1009252. [Google Scholar] [CrossRef]
- Zhang, J.; Garrett, S.; Sun, J. Gastrointestinal symptoms, pathophysiology, and treatment in COVID-19. Genes Dis. 2021, 8, 385–400. [Google Scholar] [CrossRef]
- Zhang, H.; Shao, B.; Dang, Q.; Chen, Z.; Zhou, Q.; Luo, H.; Yuan, W.; Sun, Z. Pathogenesis and Mechanism of Gastrointestinal Infection with COVID-19. Front. Immunol. 2021, 12, 674074. [Google Scholar] [CrossRef]
- Vernia, F.; Ashktorab, H.; Cesaro, N.; Monaco, S.; Faenza, S.; Sgamma, E.; Viscido, A.; Latella, G. COVID-19 and Gastrointestinal Tract: From Pathophysiology to Clinical Manifestations. Medicina 2023, 59, 1709. [Google Scholar] [CrossRef]
- Rosa, R.B.; Dantas, W.M.; do Nascimento, J.C.F.; da Silva, M.V.; de Oliveira, R.N.; Pena, L.J. In Vitro and In Vivo Models for Studying SARS-CoV-2, the Etiological Agent Responsible for COVID-19 Pandemic. Viruses 2021, 13, 379. [Google Scholar] [CrossRef] [PubMed]
- Baciewicz, A.M.; Chrisman, C.R.; Finch, C.K.; Self, T.H. Update on rifampin, rifabutin, and rifapentine drug interactions. Curr. Med. Res. Opin. 2013, 29, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Cao, L.; Greenblatt, D.J.; Kwara, A. Inhibitory Effects of Selected Antituberculosis Drugs on Common Human Hepatic Cytochrome P450 and UDP-glucuronosyltransferase Enzymes. Drug Metab. Dispos. 2017, 45, 1035–1043. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.I.; Lin, J.Y.; Chen, S.H. EV71 Infection Induces IFNβ Expression in Neural Cells. Viruses 2019, 11, 1121. [Google Scholar] [CrossRef]
- Arnold, M.M.; Patton, J.T. Diversity of interferon antagonist activities mediated by NSP1 proteins of different rotavirus strains. J. Virol. 2011, 85, 1970–1979. [Google Scholar] [CrossRef]
- Khan, R.R.; Lawson, A.D.; Minnich, L.L.; Martin, K.; Nasir, A.; Emmett, M.K.; Welch, C.A.; Udall, J.N., Jr. Gastrointestinal norovirus infection associated with exacerbation of inflammatory bowel disease. J. Pediatr. Gastroenterol. Nutr. 2009, 48, 328–333. [Google Scholar] [CrossRef]
- Peiper, A.M.; Helm, E.W.; Nguyen, Q.; Phillips, M.; Williams, C.G.; Shah, D.; Tatum, S.; Iyer, N.; Grodzki, M.; Eurell, L.B.; et al. Infection of neonatal mice with the murine norovirus strain WU23 is a robust model to study norovirus pathogenesis. Lab. Anim. 2023, 52, 119–129. [Google Scholar] [CrossRef]
- Costantini, V.; Morantz, E.K.; Browne, H.; Ettayebi, K.; Zeng, X.L.; Atmar, R.L.; Estes, M.K.; Vinjé, J. Human norovirus targets enteroendocrine epithelial Intestinal Enteroids as Model to Evaluate Virus Inactivation. Emerg. Infect. Dis. 2018, 24, 1453–1464. [Google Scholar] [CrossRef]
- Zhang, D.; Tan, M.; Zhong, W.; Xia, M.; Huang, P.; Jiang, X. Human intestinal organoids express histo-blood group antigens, bind norovirus VLPs, and support limited norovirus replication. Sci. Rep. 2017, 7, 12621. [Google Scholar] [CrossRef]
- Ghosh, S.; Kumar, M.; Santiana, M.; Mishra, A.; Zhang, M.; Labayo, H.; Chibly, A.M.; Nakamura, H.; Tanaka, T.; Henderson, W.; et al. Enteric viruses replicate in salivary glands and infect through saliva. Nature 2022, 607, 345–350. [Google Scholar] [CrossRef]
- Lin, S.-C.; Bai, G.-H.; Lin, P.-C.; Chen, C.-Y.; Hsu, Y.-H.; Lee, Y.-C.; Chen, S.-Y. Molecular and Genetics-Based Systems for Tracing the Evolution and Exploring the Mechanisms of Human Norovirus Infections. Int. J. Mol. Sci. 2023, 24, 9093. [Google Scholar] [CrossRef] [PubMed]
- Kroneman, A.; Vega, E.; Vennema, H.; Vinjé, J.; White, P.A.; Hansman, G.; Green, K.; Martella, V.; Katayama, K.; Koopmans, M. Proposal for a unified norovirus nomenclature and genotyping. Arch. Virol. 2013, 158, 2059–2068. [Google Scholar] [CrossRef] [PubMed]
- Zang, R.; Gomez Castro, M.F.; McCune, B.T.; Zeng, Q.; Rothlauf, P.W.; Sonnek, N.M.; Liu, Z.; Brulois, K.F.; Wang, X.; Greenberg, H.B.; et al. TMPRSS2 and TMPRSS4 promote SARS-CoV-2 infection of human small intestinal enterocytes. Sci. Immunol. 2020, 5, eabc3582. [Google Scholar] [CrossRef] [PubMed]
- Graziano, V.R.; Walker, F.C.; Kennedy, E.A.; Wei, J.; Ettayebi, K.; Strine, M.S.; Filler, R.B.; Hassan, E.; Hsieh, L.L.; Kim, A.S.; et al. CD300lf is the primary physiologic receptor of murine norovirus but not human norovirus. PLoS Pathog. 2020, 16, e1008242. [Google Scholar] [CrossRef]
- Ettayebi, K.; Hardy, M.E. Norwalk virus nonstructural protein p48 forms a complex with the SNARE regulator VAP-A and prevents cell surface expression of vesicular stomatitis virus G protein. J. Virol. 2003, 77, 11790–11797. [Google Scholar] [CrossRef]
- Sharp, T.M.; Guix, S.; Katayama, K.; Crawford, S.E.; Estes, M.K. Inhibition of cellular protein secretion by norwalk virus nonstructural protein p22 requires a mimic of an endoplasmic reticulum export signal. PLoS ONE 2010, 5, e13130. [Google Scholar] [CrossRef]
- Roth, A.N.; Karst, S.M. Norovirus mechanisms of immune antagonism. Curr. Opin. Virol. 2016, 16, 24–30. [Google Scholar] [CrossRef]
- Sharp, T.M.; Estes, M.K. An inside job: Subversion of the host secretory pathway by intestinal pathogens. Curr. Opin. Infect. Dis. 2010, 23, 464–469. [Google Scholar] [CrossRef]
- Zhang, M.; Zhang, B.; Chen, R.; Li, M.; Zheng, Z.; Xu, W.; Zhang, Y.; Gong, S.; Hu, Q. Human Norovirus Induces Aquaporin 1 Production by Activating NF-κB Signaling Pathway. Viruses 2022, 14, 842. [Google Scholar] [CrossRef]
- Mboko, W.P.; Chhabra, P.; Valcarce, M.D.; Costantini, V.; Vinjé, J. Advances in understanding of the innate immune response to human norovirus infection using organoid models. J. Gen. Virol. 2022, 103, 001720. [Google Scholar] [CrossRef]
- Hassan, E.; Baldridge, M.T. Norovirus encounters in the gut: Multifaceted interactions and disease outcomes. Mucosal Immunol. 2019, 12, 1259–1267. [Google Scholar] [CrossRef] [PubMed]
- Ponterio, E.; Mariotti, S.; Tabolacci, C.; Ruggeri, F.M.; Nisini, R. Virus like particles of GII.4 norovirus bind Toll Like Receptors 2 and 5. Immunol. Lett. 2019, 215, 40–44. [Google Scholar] [CrossRef] [PubMed]
- Baker, M.; Prasad, B.V. Rotavirus cell entry. Curr. Top. Microbiol. Immunol. 2010, 343, 121–148. [Google Scholar] [CrossRef] [PubMed]
- Cotten, M.; Petrova, V.; Phan, M.V.; Rabaa, M.A.; Watson, S.J.; Ong, S.H.; Kellam, P.; Baker, S. Deep sequencing of norovirus genomes defines evolutionary patterns in an urban tropical setting. J. Virol. 2014, 88, 11056–11069. [Google Scholar] [CrossRef] [PubMed]
- Lecollinet, S.; Gavard, F.; Havenga, M.J.; Spiller, O.B.; Lemckert, A.; Goudsmit, J.; Eloit, M.; Richardson, J. Improved gene delivery to intestinal mucosa by adenoviral vectors bearing subgroup B and d fibers. J. Virol. 2006, 80, 2747–2759. [Google Scholar] [CrossRef]
- de Crom, S.C.M.; Rossen, J.W.A.; van Furth, A.M.; Obihara, C.C. Enterovirus and parechovirus infection in children: A brief overview. Eur. J. Pediatr. 2016, 175, 1023–1029. [Google Scholar] [CrossRef]
- Xu, L.; Zheng, Q.; Li, S.; He, M.; Wu, Y.; Li, Y.; Zhu, R.; Yu, H.; Hong, Q.; Jiang, J.; et al. Atomic structures of Coxsackievirus A6 and its complex with a neutralizing antibody. Nat. Commun. 2017, 8, 505. [Google Scholar] [CrossRef]
- Zhao, X.; Zhang, G.; Liu, S.; Chen, X.; Peng, R.; Dai, L.; Qu, X.; Li, S.; Song, H.; Gao, Z.; et al. Human Neonatal Fc Receptor Is the Cellular Uncoating Receptor for Enterovirus B. Cell 2019, 177, 1553–1565.e16. [Google Scholar] [CrossRef]
- Niu, S.; Liu, C.; Liu, C.; Liu, S.; Song, Y.; Zhang, Y.; Tian, W.; Zhao, X.; Wang, P.; Gao, G. Molecular and structural basis of Echovirus 11 infection by using the dual-receptor system of CD55 and FcRn. Chin. Sci. Bull. 2020, 65, 67–79. [Google Scholar] [CrossRef]
- Rajpal, V.R.; Sharma, S.; Sehgal, D.; Singh, A.; Kumar, A.; Vaishnavi, S.; Tiwari, M.; Bhalla, H.; Goel, S.; Raina, S.N. A comprehensive account of SARS-CoV-2 genome structure, incurred mutations, lineages and COVID-19 vaccination program. Future Virol. 2022, 17, 687–706. [Google Scholar] [CrossRef]
- Mennechet, F.J.D.; Paris, O.; Ouoba, A.R.; Salazar Arenas, S.; Sirima, S.B.; Takoudjou Dzomo, G.R.; Diarra, A.; Traore, I.T.; Kania, D.; Eichholz, K.; et al. A review of 65 years of human adenovirus seroprevalence. Expert Rev. Vaccines 2019, 18, 597–613. [Google Scholar] [CrossRef] [PubMed]
- Zaki, M.E.S.; Eid, A.-R.; Faried, O.A. Clinico-Pathological Study of Adenovirus Associated with Respiratory Infections in Children. Open Microbiol. J. 2020, 14, 48–52. [Google Scholar] [CrossRef]
- Shieh, W.-J. Human adenovirus infections in pediatric population—An update on clinico–pathologic correlation. Biomed. J. 2022, 45, 38–49. [Google Scholar] [CrossRef] [PubMed]
- Pan, Q.; Wang, J.; Gao, Y.; Wang, Q.; Cui, H.; Liu, C.; Qi, X.; Zhang, Y.; Wang, Y.; Li, K.; et al. Identification of chicken CAR homology as a cellular receptor for the emerging highly pathogenic fowl adenovirus 4 via unique binding mechanism. Emerg. Microbes Infect. 2020, 9, 586–596. [Google Scholar] [CrossRef] [PubMed]
- Lion, T. Adenovirus Infections in Immunocompetent and Immunocompromised Patients. Clin. Microbiol. Rev. 2014, 27, 441–462. [Google Scholar] [CrossRef]
- Anders, K.H.; Park, C.S.; Cornford, M.E.; Vinters, H.V. Adenovirus encephalitis and widespread ependymitis in a child with AIDS. Pediatr. Neurosurg. 1990, 16, 316–320. [Google Scholar] [CrossRef]
- Wasimuddin; Corman, V.M.; Ganzhorn, J.U.; Rakotondranary, J.; Ratovonamana, Y.R.; Drosten, C.; Sommer, S. Adenovirus infection is associated with altered gut microbial communities in a non-human primate. Sci. Rep. 2019, 9, 13410. [Google Scholar] [CrossRef]
- Schwartz, K.L.; Richardson, S.E.; MacGregor, D.; Mahant, S.; Raghuram, K.; Bitnun, A. Adenovirus-Associated Central Nervous System Disease in Children. J. Pediatr. 2019, 205, 130–137. [Google Scholar] [CrossRef]
- Corrier, D.E.; Montgomery, D.; Scutchfield, W.L. Adenovirus in the intestinal epithelium of a foal with prolonged diarrhea. Vet. Pathol. 1982, 19, 564–567. [Google Scholar] [CrossRef]
- Sanchez, L.H.G.; Shiau, H.; Baker, J.M.; Saaybi, S.; Buchfellner, M.; Britt, W.; Sanchez, V.; Potter, J.L.; Ingram, L.A.; Kelly, D.; et al. A Case Series of Children with Acute Hepatitis and Human Adenovirus Infection. N. Engl. J. Med. 2022, 387, 620–630. [Google Scholar] [CrossRef]
- Grand, R.J. Pathogenicity and virulence of human adenovirus F41: Possible links to severe hepatitis in children. Virulence 2023, 14, 2242544. [Google Scholar] [CrossRef] [PubMed]
- Holly, M.K.; Smith, J.G. Adenovirus Infection of Human Enteroids Reveals Interferon Sensitivity and Preferential Infection of Goblet Cells. J. Virol. 2018, 92, e00250-18. [Google Scholar] [CrossRef] [PubMed]
- Kosulin, K. Intestinal HAdV Infection: Tissue Specificity, Persistence, and Implications for Antiviral Therapy. Viruses 2019, 11, 804. [Google Scholar] [CrossRef] [PubMed]
- Reis, T.A.; Assis, A.S.; do Valle, D.A.; Barletta, V.H.; de Carvalho, I.P.; Rose, T.L.; Portes, S.A.; Leite, J.P.; da Rosa e Silva, M.L. The role of human adenoviruses type 41 in acute diarrheal disease in Minas Gerais after rotavirus vaccination. Braz. J. Microbiol. 2016, 47, 243–250. [Google Scholar] [CrossRef]
- Stasiak, A.C.; Stehle, T. Human adenovirus binding to host cell receptors: A structural view. Med. Microbiol. Immunol. 2020, 209, 325–333. [Google Scholar] [CrossRef]
- Kotha Lakshmi Narayan, P.; Readler, J.M.; Alghamri, M.S.; Brockman, T.L.; Yan, R.; Sharma, P.; Snitsarev, V.; Excoffon, K.J.D.A.; Kolawole, A.O. The Coxsackievirus and Adenovirus Receptor Has a Short Half-Life in Epithelial Cells. Pathogens 2022, 11, 173. [Google Scholar] [CrossRef]
- Seki, T.; Dmitriev, I.; Kashentseva, E.; Takayama, K.; Rots, M.; Suzuki, K.; Curiel, D.T. Artificial extension of the adenovirus fiber shaft inhibits infectivity in coxsackievirus and adenovirus receptor-positive cell lines. J. Virol. 2002, 76, 1100–1108. [Google Scholar] [CrossRef]
- Wang, H.; Li, Z.-Y.; Liu, Y.; Persson, J.; Beyer, I.; Möller, T.; Koyuncu, D.; Drescher, M.R.; Strauss, R.; Zhang, X.-B. Desmoglein 2 is a receptor for adenovirus serotypes 3, 7, 11 and 14. Nat. Med. 2011, 17, 96–104. [Google Scholar] [CrossRef]
- Nemerow, G.R.; Stewart, P.L. Role of alpha(v) integrins in adenovirus cell entry and gene delivery. Microbiol. Mol. Biol. Rev. 1999, 63, 725–734. [Google Scholar] [CrossRef]
- Rodríguez, E.; Romero, C.; Río, A.; Miralles, M.; Raventós, A.; Planells, L.; Burgueño, J.F.; Hamada, H.; Perales, J.C.; Bosch, A.; et al. Short-fiber protein of ad40 confers enteric tropism and protection against acidic gastrointestinal conditions. Hum. Gene Ther. Methods 2013, 24, 195–204. [Google Scholar] [CrossRef]
- Albinsson, B.; Kidd, A.H. Adenovirus type 41 lacks an RGD alpha(v)-integrin binding motif on the penton base and undergoes delayed uptake in A549 cells. Virus Res. 1999, 64, 125–136. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Illana, M.; Martínez, M.; Condezo, G.N.; Hernando-Pérez, M.; Mangroo, C.; Brown, M.; Marabini, R.; San Martín, C. Cryo-EM structure of enteric adenovirus HAdV-F41 highlights structural variations among human adenoviruses. Sci. Adv. 2021, 7, eabd9421. [Google Scholar] [CrossRef] [PubMed]
- Gaggar, A.; Shayakhmetov, D.M.; Lieber, A. CD46 is a cellular receptor for group B adenoviruses. Nat. Med. 2003, 9, 1408–1412. [Google Scholar] [CrossRef] [PubMed]
- Wu, E.; Trauger, S.A.; Pache, L.; Mullen, T.M.; von Seggern, D.J.; Siuzdak, G.; Nemerow, G.R. Membrane cofactor protein is a receptor for adenoviruses associated with epidemic keratoconjunctivitis. J. Virol. 2004, 78, 3897–3905. [Google Scholar] [CrossRef] [PubMed]
- Storm, R.J.; Persson, B.D.; Skalman, L.N.; Frängsmyr, L.; Lindström, M.; Rankin, G.; Lundmark, R.; Domellöf, F.P.; Arnberg, N. Human Adenovirus Type 37 Uses αVβ1 and α3β1 Integrins for Infection of Human Corneal Cells. J. Virol. 2017, 91. [Google Scholar] [CrossRef]
- Weitzman, M.D.; Ornelles, D.A. Inactivating intracellular antiviral responses during adenovirus infection. Oncogene 2005, 24, 7686–7696. [Google Scholar] [CrossRef]
- Greber, U.F.; Flatt, J.W. Adenovirus Entry: From Infection to Immunity. Annu. Rev. Virol. 2019, 6, 177–197. [Google Scholar] [CrossRef]
- Berk, A.J. Adenovirus promoters and E1A transactivation. Annu. Rev. Genet. 1986, 20, 45–79. [Google Scholar] [CrossRef]
- Prusinkiewicz, M.A.; Mymryk, J.S. Metabolic Reprogramming of the Host Cell by Human Adenovirus Infection. Viruses 2019, 11, 141. [Google Scholar] [CrossRef]
- Cook, J.L.; Walker, T.A.; Worthen, G.S.; Radke, J.R. Role of the E1A Rb-binding domain in repression of the NF-kappa B-dependent defense against tumor necrosis factor-alpha. Proc. Natl. Acad. Sci. USA 2002, 99, 9966–9971. [Google Scholar] [CrossRef]
- Ackrill, A.M.; Foster, G.R.; Laxton, C.D.; Flavell, D.M.; Stark, G.R.; Kerr, I.M. Inhibition of the cellular response to interferons by products of the adenovirus type 5 E1A oncogene. Nucleic Acids Res. 1991, 19, 4387–4393. [Google Scholar] [CrossRef] [PubMed]
- Lowe, S.W.; Ruley, H.E. Stabilization of the p53 tumor suppressor is induced by adenovirus 5 E1A and accompanies apoptosis. Genes Dev. 1993, 7, 535–545. [Google Scholar] [CrossRef] [PubMed]
- Teodoro, J.G.; Branton, P.E. Regulation of p53-dependent apoptosis, transcriptional repression, and cell transformation by phosphorylation of the 55-kilodalton E1B protein of human adenovirus type 5. J. Virol. 1997, 71, 3620–3627. [Google Scholar] [CrossRef]
- Chahal, J.S.; Qi, J.; Flint, S.J. The human adenovirus type 5 E1B 55 kDa protein obstructs inhibition of viral replication by type I interferon in normal human cells. PLoS Pathog. 2012, 8, e1002853. [Google Scholar] [CrossRef] [PubMed]
- Lecuit, M.; Eloit, M. Chapter 21—The Viruses of the Gut Microbiota. In The Microbiota in Gastrointestinal Pathophysiology; Floch, M.H., Ringel, Y., Allan Walker, W., Eds.; Academic Press: Boston, MA, USA, 2017; pp. 179–183. [Google Scholar] [CrossRef]
- Tarris, G.; de Rougemont, A.; Charkaoui, M.; Michiels, C.; Martin, L.; Belliot, G. Enteric Viruses and Inflammatory Bowel Disease. Viruses 2021, 13, 104. [Google Scholar] [CrossRef] [PubMed]
- Vidal, L.R.; de Almeida, S.M.; Cavalli, B.M.; Dieckmann, T.G.; Raboni, S.M.; Salvador, G.L.O.; Pereira, L.A.; Rotta, I.; Nogueira, M.B. Human adenovirus meningoencephalitis: A 3-years’ overview. J. Neurovirol 2019, 25, 589–596. [Google Scholar] [CrossRef]
- Itani, T.; Chalapa, V.; Semenov, A.; Sergeev, A. Laboratory diagnosis of nonpolio enteroviruses: A review of the current literature. Biosaf. Health 2023, 5, 112–119. [Google Scholar] [CrossRef]
- Pons-Salort, M.; Parker, E.P.; Grassly, N.C. The epidemiology of non-polio enteroviruses: Recent advances and outstanding questions. Curr. Opin. Infect. Dis. 2015, 28, 479–487. [Google Scholar] [CrossRef]
- Kuo, R.-L.; Shih, S.-R. Strategies to develop antivirals against enterovirus 71. Virol. J. 2013, 10, 28. [Google Scholar] [CrossRef]
- Fujii, K.; Nagata, N.; Sato, Y.; Ong, K.C.; Wong, K.T.; Yamayoshi, S.; Shimanuki, M.; Shitara, H.; Taya, C.; Koike, S. Transgenic mouse model for the study of enterovirus 71 neuropathogenesis. Proc. Natl. Acad. Sci. USA 2013, 110, 14753–14758. [Google Scholar] [CrossRef]
- Good, C.; Wells, A.I.; Coyne, C.B. Type III interferon signaling restricts enterovirus 71 infection of goblet cells. Sci. Adv. 2019, 5, eaau4255. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Tijsma, A.; Mirabelli, C.; Baggen, J.; Wahedi, M.; Franco, D.; De Palma, A.; Leyssen, P.; Verbeken, E.; van Kuppeveld, F.J.M.; et al. Intra-host emergence of an enterovirus A71 variant with enhanced PSGL1 usage and neurovirulence. Emerg. Microbes Infect. 2019, 8, 1076–1085. [Google Scholar] [CrossRef] [PubMed]
- Tee, H.K.; Tan, C.W.; Yogarajah, T.; Lee, M.H.P.; Chai, H.J.; Hanapi, N.A.; Yusof, S.R.; Ong, K.C.; Lee, V.S.; Sam, I.C.; et al. Electrostatic interactions at the five-fold axis alter heparin-binding phenotype and drive enterovirus A71 virulence in mice. PLoS Pathog. 2019, 15, e1007863. [Google Scholar] [CrossRef] [PubMed]
- Yamayoshi, S.; Fujii, K.; Koike, S. Receptors for enterovirus 71. Emerg. Microbes Infect. 2014, 3, e53. [Google Scholar] [CrossRef] [PubMed]
- Zhou, D.; Zhao, Y.; Kotecha, A.; Fry, E.E.; Kelly, J.T.; Wang, X.; Rao, Z.; Rowlands, D.J.; Ren, J.; Stuart, D.I. Unexpected mode of engagement between enterovirus 71 and its receptor SCARB2. Nat. Microbiol. 2019, 4, 414–419. [Google Scholar] [CrossRef]
- Kobayashi, K.; Koike, S. Adaptation and Virulence of Enterovirus-A71. Viruses 2021, 13, 1661. [Google Scholar] [CrossRef]
- Laszik, Z.; Jansen, P.J.; Cummings, R.D.; Tedder, T.F.; McEver, R.P.; Moore, K.L. P-selectin glycoprotein ligand-1 is broadly expressed in cells of myeloid, lymphoid, and dendritic lineage and in some nonhematopoietic cells. Blood 1996, 88, 3010–3021. [Google Scholar] [CrossRef]
- Lai, J.; Li, Z.; Pan, L.; Huang, Y.; Zhou, Z.; Ma, C.; Guo, J.; Xu, L. Research progress on pathogenic and therapeutic mechanisms of Enterovirus A71. Arch. Virol. 2023, 168, 260. [Google Scholar] [CrossRef]
- Chang, C.-S.; Liao, C.-C.; Liou, A.-T.; Chang, Y.-S.; Chang, Y.-T.; Tzeng, B.-H.; Chen, C.-C.; Shih, C. Enterovirus 71 targets the cardiopulmonary system in a robust oral infection mouse model. Sci. Rep. 2019, 9, 11108. [Google Scholar] [CrossRef]
- Huang, H.-I.; Chio, C.-C.; Lin, J.-Y.; Chou, C.-J.; Lin, C.-C.; Chen, S.-H.; Yu, L.-S. EV-A71 induced IL-1β production in THP-1 macrophages is dependent on NLRP3, RIG-I, and TLR3. Sci. Rep. 2022, 12, 21425. [Google Scholar] [CrossRef]
- Wang, H.; Lei, X.; Xiao, X.; Yang, C.; Lu, W.; Huang, Z.; Leng, Q.; Jin, Q.; He, B.; Meng, G.; et al. Reciprocal Regulation between Enterovirus 71 and the NLRP3 Inflammasome. Cell Rep. 2015, 12, 42–48. [Google Scholar] [CrossRef] [PubMed]
- Pan, P.; Zhang, Q.; Liu, W.; Wang, W.; Yu, Z.; Lao, Z.; Zhang, W.; Shen, M.; Wan, P.; Xiao, F.; et al. Dengue Virus Infection Activates Interleukin-1β to Induce Tissue Injury and Vascular Leakage. Front. Microbiol. 2019, 10, 2637. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.Y. Enterovirus 71 infection and neurological complications. Korean J. Pediatr. 2016, 59, 395–401. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Sun, J.; Wang, N.; Sun, Z.; Ma, Q.; Li, J.; Zhang, M.; Xu, J. Enterovirus A71 capsid protein VP1 increases blood-brain barrier permeability and virus receptor vimentin on the brain endothelial cells. J. Neurovirol 2020, 26, 84–94. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.S.; Yao, Y.C.; Lin, S.C.; Lee, Y.P.; Wang, Y.F.; Wang, J.R.; Liu, C.C.; Lei, H.Y.; Yu, C.K. Retrograde axonal transport: A major transmission route of enterovirus 71 in mice. J. Virol. 2007, 81, 8996–9003. [Google Scholar] [CrossRef]
- Gu, J.; Wu, J.; Fang, D.; Qiu, Y.; Zou, X.; Jia, X.; Yin, Y.; Shen, L.; Mao, L. Exosomes cloak the virion to transmit Enterovirus 71 non-lytically. Virulence 2020, 11, 32–38. [Google Scholar] [CrossRef]
- Huang, H.I.; Lin, J.Y.; Chiang, H.C.; Huang, P.N.; Lin, Q.D.; Shih, S.R. Exosomes Facilitate Transmission of Enterovirus A71 from Human Intestinal Epithelial Cells. J. Infect. Dis. 2020, 222, 456–469. [Google Scholar] [CrossRef]
- Moshiri, J.; Craven, A.R.; Mixon, S.B.; Amieva, M.R.; Kirkegaard, K. Mechanosensitive extrusion of Enterovirus A71-infected cells from colonic organoids. Nat. Microbiol. 2023, 8, 629–639. [Google Scholar] [CrossRef]
- Gu, J.; Zhao, Y.; Wu, J.; Chen, Y.; Yin, Y.; Jia, X.; Mao, L. Enterovirus-71 utilizes small extracellular vesicles to cross the blood-brain barrier for infecting the central nervous system via transcytosis. J. Med. Virol. 2023, 95, 28120. [Google Scholar] [CrossRef]
- Osterback, R.; Vuorinen, T.; Linna, M.; Susi, P.; Hyypiä, T.; Waris, M. Coxsackievirus A6 and hand, foot, and mouth disease, Finland. Emerg. Infect. Dis. 2009, 15, 1485–1488. [Google Scholar] [CrossRef]
- Zhao, T.S.; Du, J.; Sun, D.P.; Zhu, Q.R.; Chen, L.Y.; Ye, C.; Wang, S.; Liu, Y.Q.; Cui, F.; Lu, Q.B. A review and meta-analysis of the epidemiology and clinical presentation of coxsackievirus A6 causing hand-foot-mouth disease in China and global implications. Rev. Med. Virol. 2020, 30, e2087. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.; Maimaiti, H.; Zhou, L.; Gao, J.; Lu, Y. Changing serotypes of hand, foot and mouth disease in Shanghai, 2017–2019. Gut Pathog 2022, 14, 12. [Google Scholar] [CrossRef] [PubMed]
- Zhu, F.; Xu, W.; Xia, J.; Liang, Z.; Liu, Y.; Zhang, X.; Tan, X.; Wang, L.; Mao, Q.; Wu, J.; et al. Efficacy, safety, and immunogenicity of an enterovirus 71 vaccine in China. N. Engl. J. Med. 2014, 370, 818–828. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.T.; Chiu, C.H.; Lin, C.Y.; Chiu, N.C.; Chen, P.Y.; Le, T.T.V.; Le, D.N.; Duong, A.H.; Nguyen, V.L.; Huynh, T.N.; et al. Efficacy, safety, and immunogenicity of an inactivated, adjuvanted enterovirus 71 vaccine in infants and children: A multiregion, double-blind, randomised, placebo-controlled, phase 3 trial. Lancet 2022, 399, 1708–1717. [Google Scholar] [CrossRef]
- Kimmis, B.D.; Downing, C.; Tyring, S. Hand-foot-and-mouth disease caused by coxsackievirus A6 on the rise. Cutis 2018, 102, 353–356. [Google Scholar]
- Laga, A.C.; Shroba, S.M.; Hanna, J. Atypical hand, foot and mouth disease in adults associated with coxsackievirus A6: A clinico-pathologic study. J. Cutan. Pathol. 2016, 43, 940–945. [Google Scholar] [CrossRef]
- Li, D.; Sun, T.; Tao, L.; Ji, W.; Zhu, P.; Liang, R.; Zhang, Y.; Chen, S.; Yang, H.; Jin, Y.; et al. A mouse-adapted CVA6 strain exhibits neurotropism and triggers systemic manifestations in a novel murine model. Emerg. Microbes Infect. 2022, 11, 2248–2263. [Google Scholar] [CrossRef]
- Li, J.; Yan, D.; Chen, L.; Zhang, Y.; Song, Y.; Zhu, S.; Ji, T.; Zhou, W.; Gan, F.; Wang, X.; et al. Multiple genotypes of Echovirus 11 circulated in mainland China between 1994 and 2017. Sci. Rep. 2019, 9, 10583. [Google Scholar] [CrossRef]
- Zhou, D.; Qin, L.; Duyvesteyn, H.M.E.; Zhao, Y.; Lin, T.-Y.; Fry, E.E.; Ren, J.; Huang, K.-Y.A.; Stuart, D.I. Switching of Receptor Binding Poses between Closely Related Enteroviruses. Viruses 2022, 14, 2625. [Google Scholar] [CrossRef]
- Chevaliez, S.; Szendröi, A.; Caro, V.; Balanant, J.; Guillot, S.; Berencsi, G.; Delpeyroux, F. Molecular comparison of echovirus 11 strains circulating in Europe during an epidemic of multisystem hemorrhagic disease of infants indicates that evolution generally occurs by recombination. Virology 2004, 325, 56–70. [Google Scholar] [CrossRef]
- Ho, S.-Y.; Chiu, C.-H.; Huang, Y.-C.; Chen, C.-J.; Lien, R.; Chu, S.-M.; Huang, C.-G.; Tsao, K.-C.; Shih, S.-R.; Hsu, J.-F. Investigation and successful control of an echovirus 11 outbreak in neonatal intensive care units. Pediatr. Neonatol. 2020, 61, 180–187. [Google Scholar] [CrossRef]
- Gong, Y.N.; Yang, S.L.; Chen, Y.C.; Liu, Y.C.; Huang, Y.C.; Tsao, K.C. Novel intertypic recombination of Echovirus 11 in the Enterovirus species B. J. Med. Virol. 2024, 96, e29323. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Kang, M.; Zeng, H.; Zhong, Y.; Fang, L.; Zheng, X.; Liu, L.; Yi, L.; Lin, H.; Peng, J. Tracking echovirus eleven outbreaks in Guangdong, China: A metatranscriptomic, phylogenetic, and epidemiological study. Virus Evol. 2020, 6, veaa029. [Google Scholar] [CrossRef] [PubMed]
- Pellegrinelli, L.; Galli, C.; Giardina, F.; Ferrari, G.; Uceda Renteria, S.C.; Ceriotti, F.; Seiti, A.; Binda, S.; Pitrolo, A.M.G.; Schiavo, R.; et al. Increased circulation of echovirus 11 in the general population and hospital patients as elicited by the non-polio enterovirus laboratory-based sentinel surveillance in northern Italy, 2023. Int. J. Infect. Dis. 2024, 142, 106998. [Google Scholar] [CrossRef] [PubMed]
- Wells, A.I.; Coyne, C.B. An In Vivo Model of Echovirus-Induced Meningitis Defines the Differential Roles of Type I and Type III Interferon Signaling in Central Nervous System Infection. J. Virol. 2022, 96, e00330-22. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-C.; Yang, S.-L.; Yang, H.; Lin, T.-Y.; Hsieh, Y.-C.; Huang, K.-Y.A.; Kuo, C.-Y.; Chiu, C.-H.; Huang, Y.-C.; Chu, S.-M.; et al. Clinical characteristics of echovirus 11 and coxsackievirus B5 infections in Taiwanese children requiring hospitalization. J. Microbiol. Immunol. Infect. 2021, 54, 581–587. [Google Scholar] [CrossRef]
- Williams, P.; Chaudhry, Y.; Goodfellow, I.G.; Billington, J.; Powell, R.; Spiller, O.B.; Evans, D.J.; Lea, S. Mapping CD55 function. The structure of two pathogen-binding domains at 1.7 A. J. Biol. Chem. 2003, 278, 10691–10696. [Google Scholar] [CrossRef]
- Nasu, J.; Mizuno, M.; Uesu, T.; Takeuchi, K.; Inaba, T.; Ohya, S.; Kawada, M.; Shimo, K.; Okada, H.; Fujita, T.; et al. Cytokine-stimulated release of decay-accelerating factor (DAF;CD55) from HT-29 human intestinal epithelial cells. Clin. Exp. Immunol. 1998, 113, 379–385. [Google Scholar] [CrossRef]
- Morosky, S.; Wells, A.I.; Lemon, K.; Evans, A.S.; Schamus, S.; Bakkenist, C.J.; Coyne, C.B. The neonatal Fc receptor is a pan-echovirus receptor. Proc. Natl. Acad. Sci. 2019, 116, 3758–3763. [Google Scholar] [CrossRef]
- Chen, X.; Qu, X.; Liu, C.; Zhang, Y.; Zhang, G.; Han, P.; Duan, Y.; Li, Q.; Wang, L.; Ruan, W.; et al. Human FcRn Is a Two-in-One Attachment-Uncoating Receptor for Echovirus 18. mBio 2022, 13, e0116622. [Google Scholar] [CrossRef]
- Lin, C.N.; Chan, K.R.; Ooi, E.E.; Chiou, M.T.; Hoang, M.; Hsueh, P.R.; Ooi, P.T. Animal Coronavirus Diseases: Parallels with COVID-19 in Humans. Viruses 2021, 13, 1507. [Google Scholar] [CrossRef] [PubMed]
- Weiss, S.R.; Navas-Martin, S. Coronavirus pathogenesis and the emerging pathogen severe acute respiratory syndrome coronavirus. Microbiol. Mol. Biol. Rev. 2005, 69, 635–664. [Google Scholar] [CrossRef] [PubMed]
- Cui, J.; Li, F.; Shi, Z.L. Origin and evolution of pathogenic coronaviruses. Nat. Rev. Microbiol. 2019, 17, 181–192. [Google Scholar] [CrossRef]
- Wong, S.H.; Lui, R.N.; Sung, J.J. COVID-19 and the digestive system. J. Gastroenterol. Hepatol. 2020, 35, 744–748. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Lau, R.I.; Liu, Q.; Su, Q.; Chan, F.K.L.; Ng, S.C. Gut microbiota in COVID-19: Key microbial changes, potential mechanisms and clinical applications. Nat. Rev. Gastroenterol. Hepatol. 2023, 20, 323–337. [Google Scholar] [CrossRef] [PubMed]
- Lai, C.C.; Shih, T.P.; Ko, W.C.; Tang, H.J.; Hsueh, P.R. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and coronavirus disease-2019 (COVID-19): The epidemic and the challenges. Int. J. Antimicrob. Agents 2020, 55, 105924. [Google Scholar] [CrossRef]
- Muralidar, S.; Ambi, S.V.; Sekaran, S.; Krishnan, U.M. The emergence of COVID-19 as a global pandemic: Understanding the epidemiology, immune response and potential therapeutic targets of SARS-CoV-2. Biochimie 2020, 179, 85–100. [Google Scholar] [CrossRef]
- Meyerowitz, E.A.; Richterman, A.; Gandhi, R.T.; Sax, P.E. Transmission of SARS-CoV-2: A Review of Viral, Host, and Environmental Factors. Ann. Intern. Med. 2021, 174, 69–79. [Google Scholar] [CrossRef]
- Essalmani, R.; Jain, J.; Susan-Resiga, D.; Andréo, U.; Evagelidis, A.; Derbali, R.M.; Huynh, D.N.; Dallaire, F.; Laporte, M.; Delpal, A.; et al. Distinctive Roles of Furin and TMPRSS2 in SARS-CoV-2 Infectivity. J. Virol. 2022, 96, e0012822. [Google Scholar] [CrossRef]
- Jackson, C.B.; Farzan, M.; Chen, B.; Choe, H. Mechanisms of SARS-CoV-2 entry into cells. Nat. Rev. Mol. Cell Biol. 2022, 23, 3–20. [Google Scholar] [CrossRef]
- Xiao, F.; Tang, M.; Zheng, X.; Liu, Y.; Li, X.; Shan, H. Evidence for Gastrointestinal Infection of SARS-CoV-2. Gastroenterology 2020, 158, 1831–1833.e1833. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Chen, C.; Zhu, S.; Shu, C.; Wang, D.; Song, J.; Song, Y.; Zhen, W.; Feng, Z.; Wu, G.; et al. Isolation of 2019-nCoV from a Stool Specimen of a Laboratory-Confirmed Case of the Coronavirus Disease 2019 (COVID-19). China CDC Wkly 2020, 2, 123–124. [Google Scholar] [CrossRef] [PubMed]
- Harrison, A.G.; Lin, T.; Wang, P. Mechanisms of SARS-CoV-2 Transmission and Pathogenesis. Trends Immunol. 2020, 41, 1100–1115. [Google Scholar] [CrossRef] [PubMed]
- Lamers, M.M.; Beumer, J.; van der Vaart, J.; Knoops, K.; Puschhof, J.; Breugem, T.I.; Ravelli, R.B.G.; Paul van Schayck, J.; Mykytyn, A.Z.; Duimel, H.Q.; et al. SARS-CoV-2 productively infects human gut enterocytes. Science 2020, 369, 50–54. [Google Scholar] [CrossRef]
- Chen, T.H.; Hsu, M.T.; Lee, M.Y.; Chou, C.K. Gastrointestinal Involvement in SARS-CoV-2 Infection. Viruses 2022, 14, 1188. [Google Scholar] [CrossRef]
- Zeng, F.M.; Li, Y.w.; Deng, Z.h.; He, J.z.; Li, W.; Wang, L.; Lyu, T.; Li, Z.; Mei, C.; Yang, M.; et al. SARS-CoV-2 spike spurs intestinal inflammation via VEGF production in enterocytes. EMBO Mol. Med. 2022, 14, e14844. [Google Scholar] [CrossRef]
- Liu, H.; Wang, Z.; Sun, H.; Teng, T.; Li, Y.; Zhou, X.; Yang, Q. Thrombosis and Coagulopathy in COVID-19: Current Understanding and Implications for Antithrombotic Treatment in Patients Treated with Percutaneous Coronary Intervention. Front. Cardiovasc. Med. 2021, 7, 599334. [Google Scholar] [CrossRef]
- Wu, X.; Jing, H.; Wang, C.; Wang, Y.; Zuo, N.; Jiang, T.; Novakovic, V.A.; Shi, J. Intestinal Damage in COVID-19: SARS-CoV-2 Infection and Intestinal Thrombosis. Front. Microbiol. 2022, 13, 860931. [Google Scholar] [CrossRef]
- King, C.K.; Glass, R.; Bresee, J.S.; Duggan, C. Managing acute gastroenteritis among children: Oral rehydration, maintenance, and nutritional therapy. MMWR Recomm. Rep. 2003, 52, 1–16. [Google Scholar]
- Zhang, Y.; Cui, J.; Liu, F.; Song, Y.; Wang, Q.; Liu, Y.; Zhang, Y.; Li, Z.; Chang, Z. Effectiveness of Enterovirus 71 inactivated vaccines against hand, foot, and mouth disease: A test-negative case-control study. Hum. Vaccin. Immunother. 2024, 20, 2330163. [Google Scholar] [CrossRef]
- Varghese, T.; Kang, G.; Steele, A.D. Understanding Rotavirus Vaccine Efficacy and Effectiveness in Countries with High Child Mortality. Vaccines 2022, 10, 346. [Google Scholar] [CrossRef] [PubMed]
- Green, K.Y.; Kaufman, S.S.; Nagata, B.M.; Chaimongkol, N.; Kim, D.Y.; Levenson, E.A.; Tin, C.M.; Yardley, A.B.; Johnson, J.A.; Barletta, A.B.F.; et al. Human norovirus targets enteroendocrine epithelial cells in the small intestine. Nat. Commun. 2020, 11, 2759. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chio, C.-C.; Chien, J.-C.; Chan, H.-W.; Huang, H.-I. Overview of the Trending Enteric Viruses and Their Pathogenesis in Intestinal Epithelial Cell Infection. Biomedicines 2024, 12, 2773. https://doi.org/10.3390/biomedicines12122773
Chio C-C, Chien J-C, Chan H-W, Huang H-I. Overview of the Trending Enteric Viruses and Their Pathogenesis in Intestinal Epithelial Cell Infection. Biomedicines. 2024; 12(12):2773. https://doi.org/10.3390/biomedicines12122773
Chicago/Turabian StyleChio, Chi-Chong, Jou-Chun Chien, Hio-Wai Chan, and Hsing-I Huang. 2024. "Overview of the Trending Enteric Viruses and Their Pathogenesis in Intestinal Epithelial Cell Infection" Biomedicines 12, no. 12: 2773. https://doi.org/10.3390/biomedicines12122773
APA StyleChio, C.-C., Chien, J.-C., Chan, H.-W., & Huang, H.-I. (2024). Overview of the Trending Enteric Viruses and Their Pathogenesis in Intestinal Epithelial Cell Infection. Biomedicines, 12(12), 2773. https://doi.org/10.3390/biomedicines12122773






