Integrating Drug Target Information in Deep Learning Models to Predict the Risk of Adverse Events in Patients with Comorbid Post-Traumatic Stress Disorder and Alcohol Use Disorder
Abstract
1. Introduction
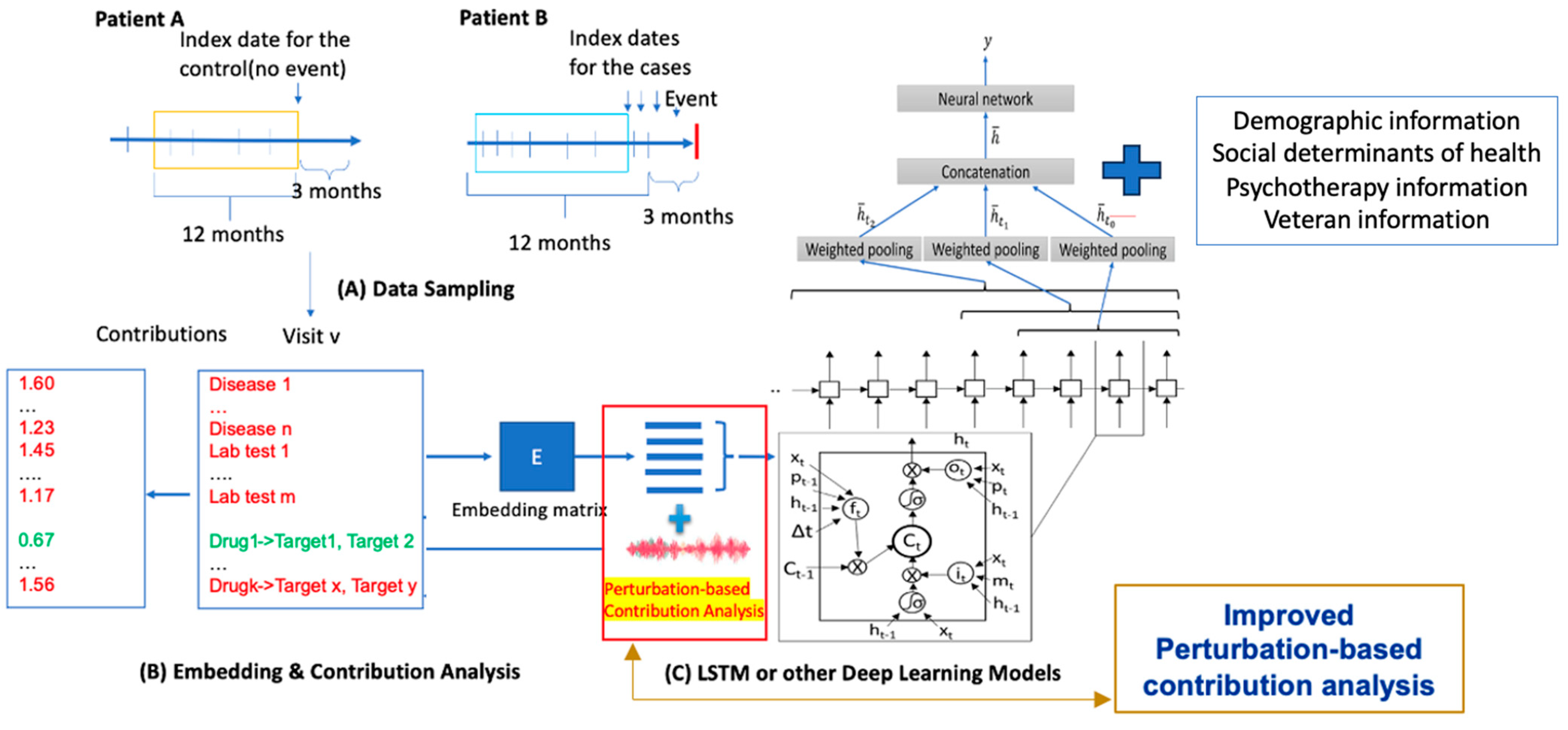
2. Materials and Methods
2.1. Source of Data
2.2. Data Preparation
2.2.1. Definition of PTSD and AUD
2.2.2. Definition of Adverse Events
2.2.3. Augmentation Techniques
2.3. Dataset Splitting
2.4. Drug Target Dataset Collection
2.5. T-DeepBiomarker
2.6. Statistical Analysis
Assessment of Biomarker Importance for Prediction of Adverse Events
3. Results
3.1. Strong Performance Demonstrated by T-DeepBiomarker
3.2. Identification of Beneficial Drugs and Their Associated Drug Targets for Prediction of Adverse Events
4. Discussion
4.1. Impact of Medications on Prediction of Adverse Events in PTSD + AUD Patients
Potential Beneficial Medication Options
4.2. Leveraging Advanced Predictive Models to Reduce Adverse Outcomes in PTSD and AUD Patients
4.3. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A. Diagnosis Codes (See References [15,16])
Appendix A.1. PTSD
Appendix A.2. ASUD
Appendix A.3. SRE
References
- Renaud, F.; Jakubiec, L.; Swendsen, J.; Fatseas, M. The Impact of Co-occurring Post-traumatic Stress Disorder and Substance Use Disorders on Craving: A Systematic Review of the Literature. Front. Psychiatry 2021, 12, 786664. [Google Scholar] [CrossRef] [PubMed]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 5th ed.; APA: Washington, DC, USA, 2013. [Google Scholar]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 4th ed.; APA: Washington, DC, USA, 1995. [Google Scholar]
- Kilpatrick, D.G.; Resnick, H.S.; Milanak, M.E.; Miller, M.W.; Keyes, K.M.; Friedman, M.J. National estimates of exposure to traumatic events and PTSD prevalence using DSM-IV and DSM-5 criteria. J. Trauma Stress 2013, 26, 537–547. [Google Scholar] [CrossRef] [PubMed]
- Kessler, R.C.; Sonnega, A.; Bromet, E.; Hughes, M.; Nelson, C.B. Posttraumatic stress disorder in the National Comorbidity Survey. Arch. Gen. Psychiatry 1995, 52, 1048–1060. [Google Scholar] [CrossRef] [PubMed]
- Sheerin, C.M.; Bountress, K.E.; Meyers, J.L.; Saenz de Viteri, S.S.; Shen, H.; Maihofer, A.X.; Duncan, L.E.; Amstadter, A.B. Shared molecular genetic risk of alcohol dependence and posttraumatic stress disorder (PTSD). Psychol. Addict. Behav. 2020, 34, 613–619. [Google Scholar] [CrossRef]
- Meyers, J.L.; Shmulewitz, D.; Elliott, J.C.; Thompson, R.G.; Aharonovich, E.; Spivak, B.; Weizman, A.; Frisch, A.; Grant, B.F.; Hasin, D.S. Parental alcohol history differentially predicts off-spring disorders in distinct subgroups in Israel. J. Stud. Alcohol Drugs 2014, 75, 859–869. [Google Scholar] [CrossRef][Green Version]
- Dube, S.R.; Anda, R.F.; Felitti, V.J.; Croft, J.B.; Edwards, V.J.; Giles, W.H. Growing up with parental alcohol abuse: Exposure to childhood abuse, neglect, and household dysfunction. Child Abus. Negl. 2001, 25, 1627–1640. [Google Scholar] [CrossRef]
- Brady, K.T.; Sonne, S.; Anton, R.F.; Randall, C.L.; Back, S.E.; Simpson, K. Sertraline in the treatment of co-occurring alcohol dependence and posttraumatic stress disorder. Alcohol. Clin. Exp. Res. 2005, 29, 395–401. [Google Scholar] [CrossRef]
- Petrakis, I.L.; Poling, J.; Levinson, C.; Nich, C.; Carroll, K.; Ralevski, E.; Rounsaville, B. Naltrexone and disulfiram in patients with alcohol dependence and comorbid post-traumatic stress disorder. Biol. Psychiatry 2006, 60, 777–783. [Google Scholar] [CrossRef]
- Watts, B.V.; Schnurr, P.P.; Mayo, L.; Young-Xu, Y.; Weeks, W.B.; Friedman, M.J. Meta-analysis of the efficacy of treatments for posttraumatic stress disorder. J. Clin. Psychiatry 2013, 74, e541–e550. [Google Scholar] [CrossRef]
- Petrakis, I.L.; Ralevski, E.; Desai, N.; Trevisan, L.; Gueorguieva, R.; Rounsaville, B.; Krystal, J.H. Noradrenergic vs. serotonergic antidepressant with or without naltrexone for veterans with PTSD and comorbid alcohol dependence. Neuropsychopharmacology 2012, 37, 996–1004. [Google Scholar] [CrossRef][Green Version]
- Fan, P.; Miranda, O.; Qi, X.; Kofler, J.; Sweet, R.A.; Wang, L. Unveiling the Enigma: Exploring Risk Factors and Mechanisms for Psychotic Symptoms in Alzheimer’s Disease through Electronic Medical Records with Deep Learning Models. Pharmaceuticals 2023, 16, 911. [Google Scholar] [CrossRef] [PubMed]
- Miranda, O.; Fan, P.; Qi, X.; Yu, Z.; Ying, J.; Wang, H.; Brent, D.A.; Silverstein, J.C.; Chen, Y.; Wang, L. DeepBiomarker: Identifying Important Lab Tests from Electronic Medical Records for the Prediction of Suicide-Related Events among PTSD Patients. J. Pers. Med. 2022, 12, 524. [Google Scholar] [CrossRef] [PubMed]
- Miranda, O.; Fan, P.; Qi, X.; Wang, H.; Brannock, M.D.; Kosten, T.; Ryan, N.D.; Kirisci, L.; Wang, L. Prediction of adverse events risk in patients with comorbid post-traumatic stress disorder and alcohol use disorder using electronic medical records by deep learning models. Drug Alcohol Depend. 2024, 255, 111066. [Google Scholar] [CrossRef] [PubMed]
- Miranda, O.; Fan, P.; Qi, X.; Wang, H.; Brannock, M.D.; Kosten, T.R.; Ryan, N.D.; Kirisci, L.; Wang, L. DeepBiomarker2, Prediction of Alcohol and Substance Use Disorder Risk in Post-Traumatic Stress Disorder Patients Using Electronic Medical Records and Multiple Social Determinants of Health. J. Pers. Med. 2024, 14, 94. [Google Scholar] [CrossRef]
- Heslin, K.C.; Steiner, C.A. Hospitalizations Involving Mental and Substance Use Disorders Among Adults, 2012: Statistical Brief #191—Table 4: ICD-9-CM diagnosis codes defining substance use disorders. In Healthcare Cost and Utilization Project (HCUP) Statistical Briefs [Internet]; Agency for Healthcare Research and Quality: Rockville, MD, USA, 2006. Available online: https://www.ncbi.nlm.nih.gov/books/NBK310986/table/sb191.t4/ (accessed on 25 March 2024).
- Rasmy, L.; Zhu, J.; Li, Z.; Hao, X.; Tran, H.T.; Zhou, Y.; Tiryaki, F.; Xiang, Y.; Xu, H.; Zhi, D. Simple Recurrent Neural Networks is all we need for clinical events predictions using EHR data. arXiv 2021, arXiv:2110.00998. [Google Scholar]
- Choi, E.; Bahadori, M.T.; Kulas, J.; Schuetz, A.; Stewart, W.; Sun, J. RETAIN: An Interpretable Predictive Model for Healthcare using Reverse Time Attention Mechanism. In Proceedings of the 30th International Conference on Neural Information Processing Systems (NIPS’16), Barcelona, Spain, 5–10 December 2016; pp. 3512–3520. [Google Scholar]
- Guan, C.; Wang, X.; Zhang, Q.; Chen, R.; He, D.; Xie, X. Towards a deep and unified understanding of deep neural models in nlp. Proc. Mach. Learn. Res. 2019, 97, 2454–2463. [Google Scholar]
- Arendt, J.; Aulinas, A. Physiology of the Pineal Gland and Melatonin. In Endotext [Internet]; Feingold, K.R., Anawalt, B., Blackman, M.R., Boyce, A., Chrousos, G., Corpas, E., de Herder, W.W., Dhatariya, K., Dungan, K., Hofland, J., et al., Eds.; MDText.com, Inc.: South Dartmouth, MA, USA, 2022. [Google Scholar] [PubMed]
- Romano, R.; Del Fiore, V.S.; Bucci, C. Role of the Intermediate Filament Protein Peripherin in Health and Disease. Int. J. Mol. Sci. 2022, 23, 15416. [Google Scholar] [CrossRef]
- Duez, H.; Staels, B. The nuclear receptors Reverbs and RORs integrate circadian rhythms and metabolism. Diabetes Vasc. Dis. Res. 2008, 5, 82–88. [Google Scholar] [CrossRef]
- Zou, S.; Kumar, U. Cannabinoid Receptors and the Endocannabinoid System: Signaling and Function in the Central Nervous System. Int. J. Mol. Sci. 2018, 19, 833. [Google Scholar] [CrossRef]
- Fuller-Bicer, G.A.; Varadi, G.; Koch, S.E.; Ishii, M.; Bodi, I.; Kadeer, N.; Muth, J.N.; Mikala, G.; Petrashevskaya, N.N.; Jordan, M.A.; et al. Targeted disruption of the voltage-dependent calcium channel α2/δ-1-subunit. Am. J. Physiol. Heart Circ. Physiol. 2009, 297, H117–H124. [Google Scholar] [CrossRef]
- Murad, R.; Mahboob, T.; Rehman, R.; Baig, R. Comparison of serum levels of vitamin D and vitamin D-binding protein in normal, osteopenic and osteoporotic postmenopausal women. Pak. J. Med. Sci. 2019, 35, 543–548. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.Y.; Jin, H.K.; Bae, J.S. Sphingolipids in neuroinflammation: A potential target for diagnosis and therapy. BMB Rep. 2020, 53, 28–34. [Google Scholar] [CrossRef] [PubMed]
- Antonucci, F.; Corradini, I.; Fossati, G.; Tomasoni, R.; Menna, E.; Matteoli, M. SNAP-25, a Known Presynaptic Protein with Emerging Postsynaptic Functions. Front. Synaptic Neurosci. 2016, 8, 7. [Google Scholar] [CrossRef] [PubMed]
- Ru, Q.; Wang, Y.; Zhou, E.; Chen, L.; Wu, Y. The potential therapeutic roles of Rho GTPases in substance dependence. Front. Mol. Neurosci. 2023, 16, 1125277. [Google Scholar] [CrossRef]
- Biose, I.J.; Ismael, S.; Ouvrier, B.; White, A.L.; Bix, G.J. The Potential Role of Integrin Signaling in Memory and Cognitive Impairment. Biomolecules 2023, 13, 108. [Google Scholar] [CrossRef]
- Frei, J.A.; Niescier, R.F.; Bridi, M.S.; Durens, M.; Nestor, J.E.; Kilander, M.B.C.; Yuan, X.; Dykxhoorn, D.M.; Nestor, M.W.; Huang, S.; et al. Regulation of Neural Circuit Development by Cadherin-11 Provides Implications for Autism. eNeuro 2021, 8, 8. [Google Scholar] [CrossRef]
- Niu, Y.; Zeng, X.; Zhao, L.; Zhou, Y.; Qin, G.; Zhang, D.; Fu, Q.; Zhou, J.; Chen, L. Metabotropic glutamate receptor 5 regulates synaptic plasticity in a chronic migraine rat model through the PKC/NR2B signal. J. Headache Pain 2020, 21, 139. [Google Scholar] [CrossRef]
- Idrizaj, E.; Biagioni, C.; Traini, C.; Vannucchi, M.G.; Baccari, M.C. Glucagon-like Peptide-2 Depresses Ileal Contractility in Preparations from Mice through Opposite Modulatory Effects on Nitrergic and Cholinergic Neurotransmission. Int. J. Mol. Sci. 2024, 25, 1855. [Google Scholar] [CrossRef]
- Parker, A.L.; Teo, W.S.; Brayford, S.; Moorthi, U.K.; Arumugam, S.; Ferguson, C.; Parton, R.G.; McCarroll, J.A.; Kavallaris, M. βIII-Tubulin Structural Domains Regulate Mitochondrial Network Architecture in an Isotype-Specific Manner. Cells 2022, 11, 776. [Google Scholar] [CrossRef]
- Kaplan, G.B.; Lakis, G.A.; Zhoba, H. Sleep-wake and arousal dysfunctions in post-traumatic stress disorder: Role of orexin systems. Brain Res. Bull. 2022, 186, 106–122. [Google Scholar] [CrossRef]
- Burnette, D.T.; Ji, L.; Schaefer, A.W.; Medeiros, N.A.; Danuser, G.; Forscher, P. Myosin II activity facilitates microtubule bundling in the neuronal growth cone neck. Dev. Cell 2008, 15, 163–169. [Google Scholar] [CrossRef] [PubMed]
- Lewerenz, J.; Hewett, S.; Huang, Y.; Lambros, M.; Gout, P.W.; Kalivas, P.W.; Massie, A.; Smolders, I.; Methner, A.; Pergande, M.; et al. The cystine/glutamate antiporter system xc− in health and disease: From molecular mechanisms to novel therapeutic opportunities. Antioxid. Redox Signal. 2013, 18, 522–555. [Google Scholar] [CrossRef] [PubMed]
- Grieco, M.; Giorgi, A.; Gentile, M.C.; D’erme, M.; Morano, S.; Maras, B.; Filardi, T. Glucagon-Like Peptide-1, A Focus on Neurodegenerative Diseases. Front. Neurosci. 2019, 13, 1112. [Google Scholar] [CrossRef] [PubMed]
- Xiao, L.; Mackey, S.; Hui, H.; Xong, D.; Zhang, Q.; Zhang, D. Subcutaneous Injection of Botulinum Toxin A Is Beneficial in Postherpetic Neuralgia. Pain Med. 2010, 11, 1827–1833. [Google Scholar] [CrossRef]
- Yuan, R.Y.; Sheu, J.J.; Yu, J.M.; Chen, W.T.; Tseng, I.J.; Chang, H.H.; Hu, C.J. Botulinum toxin for diabetic neuropathic pain: A randomized double-blind crossover trial. Neurology 2009, 72, 1473–1478. [Google Scholar] [CrossRef]
- Donoghue, K.; Hermann, L.; Brobbin, E.; Drummond, C. The rates and measurement of adherence to acamprosate in randomised controlled clinical trials: A systematic review. PLoS ONE 2022, 17, e0263350. [Google Scholar] [CrossRef]
- Jafari, S.; Khajehpour, S.; Razzaghi, E.M.; Heidari, K.; Soleimani, M.; Ghaeli, P. Craving and Drug Reward: A Comparison of Celecoxib and Ibuprofen in Detoxifying Opiate Addicts. Iran. J. Psychiatry 2017, 12, 229–235. [Google Scholar]
- Gendy, M.N.S.; Lagzdins, D.; Schaman, J.; Le Foll, B. Melatonin for Treatment-Seeking Alcohol Use Disorder patients with sleeping problems: A randomized clinical pilot trial. Sci. Rep. 2020, 10, 8739. [Google Scholar] [CrossRef]
- Suzuki, M.; Shiraishi, E.; Cronican, J.; Kimura, H. Effects of the orexin receptor 2 agonist danavorexton on emergence from general anaesthesia and opioid-induced sedation, respiratory depression, and analgesia in rats and monkeys. Br. J. Anaesth. 2024, 132, 541–552. [Google Scholar] [CrossRef]
- Kron, J.O.-Z.; Keenan, R.J.; Hoyer, D.; Jacobson, L.H. Orexin Receptor Antagonism: Normalizing Sleep Architecture in Old Age and Disease. Annu. Rev. Pharmacol. Toxicol. 2024, 64, 359–386. [Google Scholar] [CrossRef]
- Couvineau, A.; Dayot, S.; Nicole, P.; Gratio, V.; Rebours, V.; Couvelard, A.; Voisin, T. The Anti-tumoral Properties of Orexin/Hypocretin Hypothalamic Neuropeptides: An Unexpected Therapeutic Role. Front. Endocrinol. 2018, 9, 573. [Google Scholar] [CrossRef] [PubMed]
- Konofal, E. From past to future: 50 years of pharmacological interventions to treat narcolepsy. Pharmacol. Biochem. Behav. 2024, 241, 173804. [Google Scholar] [CrossRef] [PubMed]
- Dauvilliers, Y.; Mignot, E.; Villegas, R.d.R.; Du, Y.; Hanson, E.; Inoue, Y.; Kadali, H.; Koundourakis, E.; Meyer, S.; Rogers, R.; et al. Oral Orexin Receptor 2 Agonist in Narcolepsy Type 1. N. Engl. J. Med. 2023, 389, 309–321. [Google Scholar] [CrossRef] [PubMed]
- Rezvani, A.H.; Janowsky, D.S. Decreased alcohol consumption by verapamil in alcohol preferring rats. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 1990, 14, 623–631. [Google Scholar] [CrossRef]
- Wu, S.; White, R.; Wikman-Coffelt, J.; Sievers, R.; Wendland, M.; Garrett, J.; Higgins, C.B.; James, T.; Parmley, W.W. The preventive effect of verapamil on ethanol-induced cardiac depression: Phosphorus-31 nuclear magnetic resonance and high-pressure liquid chromatographic studies of hamsters. Circulation 1987, 75, 1058–1064. [Google Scholar] [CrossRef]
- Kähkönen, S.; Bondarenko, B.B.; Lipsanen, J.; Zvartau, E.E. Effects of verapamil, an antagonist of L-type calcium channels, on cardiovascular symptoms in alcohol withdrawal. Neuropsychobiology 2008, 58, 123–127. [Google Scholar] [CrossRef]
- Tardelli, V.S.; Lago, M.P.P.D.; da Silveira, D.X.; Fidalgo, T.M. Vitamin D and alcohol: A review of the current literature. Psychiatry Res. 2017, 248, 83–86. [Google Scholar] [CrossRef]
- Repova, K.; Aziriova, S.; Krajcirovicova, K.; Simko, F. Cardiovascular therapeutics: A new potential for anxiety treatment? Med. Res. Rev. 2022, 42, 1202–1245. [Google Scholar] [CrossRef]
- Uma, D.; Rabbani, R.; Lee, J.H.; Gavini, D.R.; Shah, P.H.; Hamid, P. Does Hormone Supplementation With Levothyroxine Improve Hypothyroid Impaired Cognitive Dysfunction? Cureus 2021, 13, e17885. [Google Scholar] [CrossRef]
- Liu, Q.; Ding, X.; Wang, Y.; Chu, H.; Guan, Y.; Li, M.; Sun, K. Artemisinin reduces PTSD-like symptoms, improves synaptic plasticity, and inhibits apoptosis in rats subjected to single prolonged stress. Front. Pharmacol. 2024, 15, 1303123. [Google Scholar] [CrossRef]
- Zhao, X.; Wang, L.; Zhang, H.; Zhang, D.; Zhang, Z.; Zhang, J. Protective effect of artemisinin on chronic alcohol induced-liver damage in mice. Environ. Toxicol. Pharmacol. 2017, 52, 221–226. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.; Patel, P.; Fenando, A. Sulfasalazine. [Updated 21 March 2024]. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2024. Available online: https://www.ncbi.nlm.nih.gov/books/NBK557809/ (accessed on 22 July 2024).
- Heilig, M.; Witkiewitz, K.; Ray, L.A.; Leggio, L. Novel medications for problematic alcohol use. J. Clin. Investig. 2024, 134, e172889. [Google Scholar] [CrossRef] [PubMed]
- Klausen, M.K.; Jensen, M.E.; Møller, M.; Le Dous, N.; Jensen, A.-M.; Zeeman, V.A.; Johannsen, C.-F.; Lee, A.; Thomsen, G.K.; Macoveanu, J.; et al. Exenatide once weekly for alcohol use disorder investigated in a randomized, placebo-controlled clinical trial. J. Clin. Investig. 2022, 7, e159863. [Google Scholar] [CrossRef]
- Richards, J.R.; Dorand, M.F.; Royal, K.; Mnajjed, L.; Paszkowiak, M.; Simmons, W.K. Significant Decrease in Alcohol Use Disorder Symptoms Secondary to Semaglutide Therapy for Weight Loss: A Case Series. J. Clin. Psychiatry 2023, 85, 50515. [Google Scholar] [CrossRef]
| RETAIN(+SDOH) | 1 | 2 | 3 | 4 | 5 | Average | Standard Deviation |
| Validation Data: AUC | 0.962 | 0.964 | 0.956 | 0.956 | 0.965 | 0.961 | 0.004 |
| Test Data: AUC | 0.954 | 0.952 | 0.953 | 0.951 | 0.96 | 0.954 | 0.004 |
| Test Data: Precision | 0.912 | 0.886 | 0.904 | 0.903 | 0.931 | 0.907 | 0.016 |
| Test Data: Recall | 0.889 | 0.907 | 0.896 | 0.899 | 0.881 | 0.894 | 0.010 |
| Test Data: F1 | 0.9 | 0.897 | 0.9 | 0.901 | 0.905 | 0.901 | 0.003 |
| LR(+SDoH) | 1 | 2 | 3 | 4 | 5 | Average | Standard Deviation |
| Validation Data: AUC | 0.878 | 0.874 | 0.875 | 0.866 | 0.862 | 0.871 | 0.007 |
| Test Data: AUC | 0.868 | 0.856 | 0.86 | 0.851 | 0.846 | 0.856 | 0.008 |
| Test Data: Precision | 0.75 | 0.7 | 0.696 | 0.671 | 0.696 | 0.703 | 0.029 |
| Test Data: Recall | 0.823 | 0.864 | 0.899 | 0.917 | 0.863 | 0.873 | 0.036 |
| Test Data: F1 | 0.785 | 0.774 | 0.784 | 0.775 | 0.771 | 0.778 | 0.006 |
| TLSTM(+SDoH) | 1 | 2 | 3 | 4 | 5 | Average | Standard Deviation |
| Validation Data: AUC | 0.96 | 0.963 | 0.966 | 0.964 | 0.962 | 0.963 | 0.002 |
| Test Data: AUC | 0.941 | 0.953 | 0.96 | 0.961 | 0.958 | 0.955 | 0.008 |
| Test Data: Precision | 0.849 | 0.896 | 0.866 | 0.887 | 0.884 | 0.876 | 0.019 |
| Test Data: Recall | 0.903 | 0.884 | 0.929 | 0.914 | 0.916 | 0.909 | 0.017 |
| Test Data: F1 | 0.875 | 0.89 | 0.896 | 0.9 | 0.899 | 0.892 | 0.010 |
| Feature Name | Protein Name | Relative Contribution | Wilcoxon p Value | FDR-Q Non-Parametric Test | Bonferroni p Value | DrugBank ID | Drug Name |
|---|---|---|---|---|---|---|---|
| ASMT_HUMAN | Acetylserotonin O-methyltransferase | 0.682 | 2.15 × 10−12 | 1.94 × 10−10 | 6.60 × 10−9 | DB01065 | Melatonin |
| PERE_HUMAN | Peripherin | 0.656 | 2.66 × 10−11 | 1.74 × 10−9 | 8.17 × 10−8 | DB01065 | Melatonin |
| RORB_HUMAN | Nuclear receptor ROR-beta | 0.680 | 8.03 × 10−10 | 3.85 × 10−8 | 2.47 × 10−6 | DB01065 | Melatonin |
| CNR1_HUMAN | Cannabinoid receptor 1 | 0.198 | 1.08 × 10−9 | 3.34 × 10−8 | 3.11 × 10−6 | DB00470; DB00486; DB02955; DB05077; DB05201; DB05750; DB06155; DB09061; DB09288; DB11745; DB11755; DB14009; DB14011 | Dronabinol, Nabilone, Ricinoleic acid, SLV319, V24343, Drinabant, Rimonabant, Canabidiol, Propacetamol, Otenabant, Tetrahydrocanabivarin, Medical Cannabis, Nabiximols |
| CNR2_HUMAN | Cannabinoid receptor 2 | 0.198 | 1.08 × 10−9 | 3.34 × 10−8 | 3.11 × 10−6 | DB00470; DB00486; DB02955; DB06202; DB09061; DB11755; DB14009; DB14011; DB16321 | Dronabinol, Nabilone, Ricinoleic acid, Lasofoxine, Tetrahydro-canabivarin, Medical Cannabis, SLV319, Canabidiol, Nabiximols |
| CA2D3_HUMAN | Voltage-dependent calcium channel subunit alpha-2 | 0.756 | 4.63 × 10−9 | 1.65 × 10−7 | 1.42 × 10−5 | DB00153; DB00228; DB00421; DB00622; DB00661; DB09235; DB11148; DB13746 | Ergocalciferol, Enflurane, Spironolactone, Nicardipine, Verapamil, Efonidipine, Butamben, Bioallethrin |
| CA2D3_HUMAN | Voltage-dependent calcium channel subunit delta-3 | 0.756 | 4.63 × 10−9 | 1.65 × 10−7 | 1.42 × 10−5 | DB00153; DB00228; DB00421; DB00622; DB00661; DB09235; DB11148; DB13746 | Ergocalciferol, Enflurane, Spironolactone, Nicardipine, Verapamil, Efonidipine, Butamben, Bioallethrin |
| VTDB_HUMAN | Vitamin D-binding protein | 0.721 | 2.44 × 10−7 | 5.77 × 10−6 | 0.00075 | DB00169 | Vitamin D |
| ASM_HUMAN | Sphingomyelin phosphodiesterase | 0.765 | 1.32 × 10−6 | 2.53 × 10−5 | 0.00405 | DB00381; DB00477; DB01151; DB14009 | Amlodipine, Chlorpromazine, Desipramine, Cannabis |
| SNP25_HUMAN | Synaptosomal-associated protein 25 | 0.203 | 2.12 × 10−6 | 2.42 × 10−5 | 0.00610 | DB00083; DB16820 | BotulinumtoxinA, LetibotulinumtoxinA |
| RHOB_HUMAN | Rho-related GTP-binding protein RhoB | 0.203 | 2.12 × 10−6 | 2.42 × 10−5 | 0.00610 | DB00083 | BotulinumtoxinA |
| ITAV_HUMAN | Integrin alpha-V | 0.783 | 3.94 × 10−6 | 6.40 × 10−5 | 0.0121 | DB00451; DB16515 | PLN-74809, Levothyroxine |
| H3BUU9_HUMAN | Cadherin 11 | 0.476 | 4.13 × 10−6 | 6.63 × 10−5 | 0.0127 | DB00482 | Celecoxib |
| GRM5_HUMAN | Metabotropic glutamate receptor 5 | 0.233 | 5.37 × 10−6 | 5.66 × 10−5 | 0.0154 | DB00659; DB05070; DB06201; DB12733 | Acamprosate, ADX10059, Rufinamide, Dipraglurant |
| GLP2R_HUMAN | Glucagon-like peptide 2 receptor | 0.374 | 2.86 × 10−5 | 0.000315 | 0.0877 | DB00040; DB08900 | Teduglutide, Glucagon |
| TBB5_HUMAN | Tubulin beta-5 chain | 0.182 | 0.000121 | 0.000760 | 0.347 | DB00361; DB00541; DB00570; DB01179; DB01394; DB01873; DB03010; DB05147; DB05284; DB06042; DB06137; DB09130; DB11638; DB11641; DB11731; DB12334; DB15534 | Vinorelbine, Vincristine, Vinblastine, Podofilox, Colchicine, Epothilone Ds, Patupilone, CYT997, CA4P, ZEN-012, Tirbanibulin, Copper, Artenimol, Vinflunine, Depatuxizumab, Milataxel, Colchicine |
| MAP4_HUMAN | Microtubule-associated protein 4 | 0.418 | 0.000183 | 0.00107 | 0.525 | DB01229; DB01248; DB11638 | Paclitaxel, Docetaxel, Artenimol |
| OX1R_HUMAN | Orexin receptor type 1 | 0.398 | 0.00799 | 0.0237 | 1 | DB09034; DB11951; DB15031 | Daridorexant, Lemborexant, Suvorexant |
| XCT_HUMAN | Myosin-2 | 0.284 | 0.00146 | 0.00565 | 1 | DB00138; DB00142; DB00740; DB00795; DB06151; DB11590 | Cystine, Glutamic Acid, Riluzole, Sulfasalazine, Acetyl Cysteine, Thimerosal |
| GLP1R_HUMAN | Cystine/glutamate transporter | 0.625 | 0.00616 | 0.0226 | 1 | DB00040; DB01276; DB06655; DB09043; DB09045; DB09265; DB13928; DB14027; DB15171; DB15650; DB16697 | Glucagon, Exenatide, Liraglutide, Albiglutide, Dulaglutide, Lixisenatide, Semaglutide, Taspoglutide, Tirzepatide, Efpeglenatide, Glutazumab |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miranda, O.; Qi, X.; Brannock, M.D.; Whitworth, R.; Kosten, T.R.; Ryan, N.D.; Haas, G.L.; Kirisci, L.; Wang, L. Integrating Drug Target Information in Deep Learning Models to Predict the Risk of Adverse Events in Patients with Comorbid Post-Traumatic Stress Disorder and Alcohol Use Disorder. Biomedicines 2024, 12, 2772. https://doi.org/10.3390/biomedicines12122772
Miranda O, Qi X, Brannock MD, Whitworth R, Kosten TR, Ryan ND, Haas GL, Kirisci L, Wang L. Integrating Drug Target Information in Deep Learning Models to Predict the Risk of Adverse Events in Patients with Comorbid Post-Traumatic Stress Disorder and Alcohol Use Disorder. Biomedicines. 2024; 12(12):2772. https://doi.org/10.3390/biomedicines12122772
Chicago/Turabian StyleMiranda, Oshin, Xiguang Qi, M. Daniel Brannock, Ryan Whitworth, Thomas R. Kosten, Neal David Ryan, Gretchen L. Haas, Levent Kirisci, and Lirong Wang. 2024. "Integrating Drug Target Information in Deep Learning Models to Predict the Risk of Adverse Events in Patients with Comorbid Post-Traumatic Stress Disorder and Alcohol Use Disorder" Biomedicines 12, no. 12: 2772. https://doi.org/10.3390/biomedicines12122772
APA StyleMiranda, O., Qi, X., Brannock, M. D., Whitworth, R., Kosten, T. R., Ryan, N. D., Haas, G. L., Kirisci, L., & Wang, L. (2024). Integrating Drug Target Information in Deep Learning Models to Predict the Risk of Adverse Events in Patients with Comorbid Post-Traumatic Stress Disorder and Alcohol Use Disorder. Biomedicines, 12(12), 2772. https://doi.org/10.3390/biomedicines12122772








