Choroidal Response to Intravitreal Bevacizumab Injections in Treatment-Naïve Macular Neovascularization Secondary to Chronic Central Serous Chorioretinopathy
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Setting, Duration, and Participant Recruitment
2.2. Data Collection
2.3. Image Acquisition and Analysis
2.4. Anti-VEGF Treatment
2.5. Outcome Measures
2.6. Statistical Analysis
3. Results
3.1. Baseline Characteristics
3.2. Anatomic and Visual Outcomes over Time
3.3. Anatomic and Visual Outcomes Relative to Number of Injections
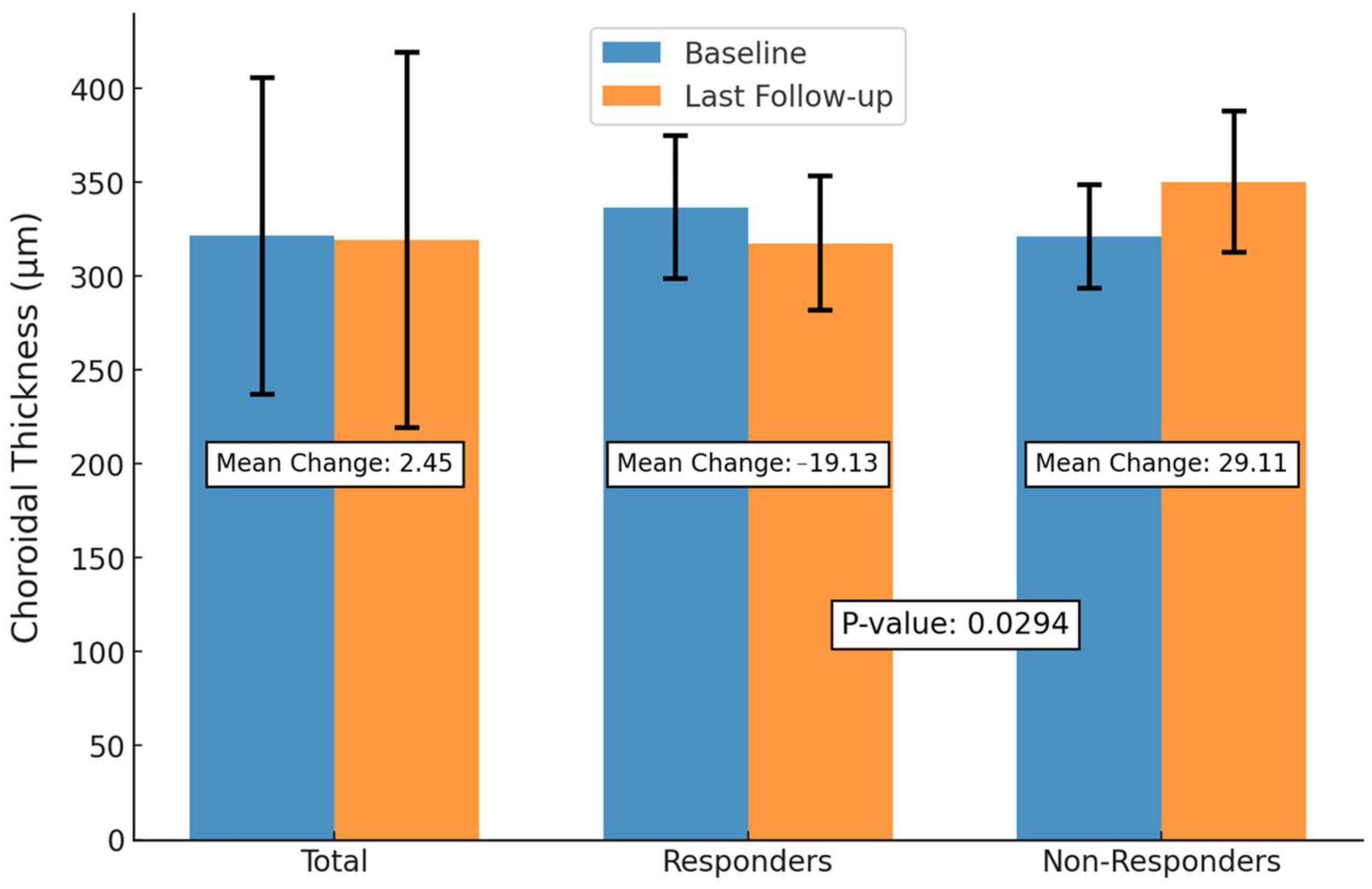
3.4. Anatomic and Visual Outcomes Between SRF Responders and Non-Responders
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AMD | Age-Related Macular Degeneration |
| BCVA | Best-Corrected Visual Acuity |
| CMT | Central Macular Thickness |
| CNV | Choroidal Neovascularization |
| CSC | Central Serous Chorioretinopathy |
| cCSC | Chronic Central Serous Chorioretinopathy |
| CT | Choroidal Thickness |
| EDI | Enhanced Depth Imaging |
| FA | Fluorescein Angiography |
| FAF | Fundus Autofluorescence |
| FIPED | Flat Irregular Pigment Epithelial Detachment |
| IRF | Intraretinal Fluid |
| IVB | Intravitreal Bevacizumab |
| MNV | Macular Neovascularization |
| OCT | Optical Coherence Tomography |
| OCTA | Optical Coherence Tomography Angiography |
| FA | Fluorescein Angiography |
| PED | Pigment Epithelial Detachment |
| RPE | Retinal Pigment Epithelium |
| SRF | Subretinal Fluid |
| SRD | Serous Retinal Detachment |
| SFCT | Subfoveal Choroidal Thickness |
| VEGF | Vascular Endothelial Growth Factor |
| Anti-VEGF | Anti-Vascular Endothelial Growth Factor |
References
- Nicholson, B.; Noble, J.; Forooghian, F.; Meyerle, C. Central Serous Chorioretinopathy: Update on Pathophysiology and Treatment. Surv. Ophthalmol. 2013, 58, 103–126. [Google Scholar] [CrossRef] [PubMed]
- Yannuzzi, L.A. Central Serous Chorioretinopathy: A Personal Perspective. Am. J. Ophthalmol. 2010, 149, 361–363. [Google Scholar] [CrossRef] [PubMed]
- van Rijssen, T.J.; van Dijk, E.H.C.; Yzer, S.; Ohno-Matsui, K.; Keunen, J.E.E.; Schlingemann, R.O.; Sivaprasad, S.; Querques, G.; Downes, S.M.; Fauser, S.; et al. Central serous chorioretinopathy: Towards an evidence-based treatment guideline. Prog. Retin. Eye Res. 2019, 73, 100770. [Google Scholar] [CrossRef] [PubMed]
- Lee, G.I.; Kim, A.Y.; Kang, S.W. Risk Factors and Outcomes of Choroidal Neovascularization Secondary to Central Serous Chorioretinopathy. Sci. Rep. 2019, 9, 3927. [Google Scholar] [CrossRef]
- Daruich, A.; Matet, A.; Dirani, A. Central Serous Chorioretinopathy: Recent Findings and New Physiopathology Hypothesis. Prog. Retin. Eye Res. 2015, 48, 82–118. [Google Scholar] [CrossRef]
- Cheung, C.M.G.; Lee, W.K.; Koizumi, H.; Dansingani, K.; Lai, T.Y.Y.; Freund, K.B. Pachychoroid Disease. Eye 2019, 33, 14–33. [Google Scholar] [CrossRef]
- Spaide, R.F.; Gemmy Cheung, C.M.; Matsumoto, H.; Kishi, S.; Boon, C.J.; van Dijk, E.H.; Mauget-Faysse, M.; Behar-Cohen, F.; Hartnett, M.E.; Sivaprasad, S.; et al. Venous Overload Choroidopathy: A Hypothetical Framework for Central Serous Chorioretinopathy and Allied Disorders. Prog. Retin. Eye Res. 2022, 86, 100973. [Google Scholar] [CrossRef]
- Imamura, Y.; Fujiwara, T.; Margolis, R.; Spaide, R.F. Enhanced Depth Imaging Optical Coherence Tomography of the Choroid in Central Serous Chorioretinopathy. Retina 2009, 29, 1469–1473. [Google Scholar] [CrossRef]
- Zarnegar, A.; Ong, J.; Matsyaraja, T.; Arora, S.; Chhablani, J. Pathomechanisms in Central Serous Chorioretinopathy: A Recent Update. Int. J. Retin. Vitr. 2023, 9, 3. [Google Scholar] [CrossRef]
- Lai, T.Y.Y.; Staurenghi, G.; Lanzetta, P.; Holz, F.G.; Melissa Liew, S.H.; Desset-Brethes, S.; Staines, H.; Hykin, P.G.; MINERVA Study Group. Efficacy and Safety of Ranibizumab for the Treatment of Choroidal Neovascularization Due to Uncommon Cause: Twelve-Month Results of the MINERVA Study. Retina 2018, 38, 1464–1477. [Google Scholar] [CrossRef]
- Razavi, S.; Souied, E.H.; Darvizeh, F.; Querques, G. Assessment of Choroidal Topographic Changes by Swept-Source Optical Coherence Tomography After Intravitreal Ranibizumab for Exudative Age-Related Macular Degeneration. Am. J. Ophthalmol. 2015, 160, 1006–1013. [Google Scholar] [CrossRef] [PubMed]
- Sacconi, R.; Tomasso, L.; Corbelli, E. Early Response to the Treatment of Choroidal Neovascularization Complicating Central Serous Chorioretinopathy: A OCT-Angiography Study. Eye 2019, 33, 1809–1817. [Google Scholar] [CrossRef] [PubMed]
- Schworm, B.; Luft, N.; Keidel, L.F. Response of Neovascular Central Serous Chorioretinopathy to an Extended Upload of Anti-VEGF Agents. Graefes Arch. Clin. Exp. Ophthalmol. 2020, 258, 1013–1021. [Google Scholar] [CrossRef] [PubMed]
- Kim, G.A.; Rim, T.H.; Lee, S.C.; Byeon, S.H.; Koh, H.J.; Kim, S.S.; Lee, C.S. Clinical Characteristics of Responders to Intravitreal Bevacizumab in Central Serous Chorioretinopathy Patients. Eye 2015, 29, 732–741. [Google Scholar] [CrossRef]
- Koizumi, H.; Kano, M.; Yamamoto, A.; Saito, M.; Maruko, I.; Sekiryu, T.; Okada, A.A.; Iida, T. Subfoveal Choroidal Thickness during Aflibercept Therapy for Neovascular Age-Related Macular Degeneration: Twelve-Month Results. Ophthalmology 2016, 123, 617–624. [Google Scholar] [CrossRef]
- Ting, D.S.W.; Ng, W.Y.; Ng, S.R.; Tan, S.P.; Yeo, I.Y.S.; Mathur, R.; Chan, C.M.; Tan, A.C.S.; Tan, G.S.W.; Wong, T.Y.; et al. Choroidal Thickness Changes in Age-Related Macular Degeneration and Polypoidal Choroidal Vasculopathy: A 12-Month Prospective Study. Am. J. Ophthalmol. 2016, 164, 128–136.e1. [Google Scholar] [CrossRef]
- Inan, S.; Baysal, Z.; Inan, U.U. Long-Term Changes in Submacular Choroidal Thickness after Intravitreal Ranibizumab Therapy for Neovascular Age-Related Macular Degeneration: 14-Mo Follow-Up. Curr. Eye Res. 2020, 45, 527–528. [Google Scholar] [CrossRef]
- Feenstra, H.M.A.; van Dijk, E.H.C.; Cheung, C.M.G.; Ohno-Matsui, K.; Lai, T.Y.Y.; Koizumi, H.; Larsen, M.; Querques, G.; Downes, S.M.; Yzer, S.; et al. Central serous chorioretinopathy: An evidence-based treatment guideline. Prog. Retin. Eye Res. 2024, 101, 101236. [Google Scholar] [CrossRef]
- Shiragami, C.; Takasago, Y.; Osaka, R. Clinical Features of Central Serous Chorioretinopathy with Type 1 Choroidal Neovascularization. Am. J. Ophthalmol. 2018, 193, 80–86. [Google Scholar] [CrossRef]
- Mrejen, S.; Balaratnasingam, C.; Kaden, T.R. Long-Term Visual Outcomes and Causes of Vision Loss in Chronic Central Serous Chorioretinopathy. Ophthalmology 2019, 126, 576–588. [Google Scholar] [CrossRef]
- Lejoyeux, R.; Behar-Cohen, F.; Mantel, I. Type One Macular Neovascularization in Central Serous Chorioretinopathy: Short-Term Response to Anti-Vascular Endothelial Growth Factor Therapy. Eye 2021, 36, 1945–1950. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.; Zhang, X.; Gan, Y.; Ji, Y.; Wen, F. Characteristics and Associated Factors of Flat Irregular Pigment Epithelial Detachment with Choroidal Neovascularization in Chronic Central Serous Chorioretinopathy. Front. Med. 2021, 8, 687023. [Google Scholar] [CrossRef] [PubMed]
- Margolis, R.; Spaide, R.F. A pilot study of enhanced depth imaging optical coherence tomography of the choroid in normal eyes. Am. J. Ophthalmol. 2009, 147, 811–815. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.J.; Roh, M.I.; Kwon, O.W. Intravitreal Bevacizumab Injection for Central Serous Chorioretinopathy. Retina 2010, 30, 100. [Google Scholar] [CrossRef]
- Cozzupoli, G.M.; Sacconi, R.; Tombolini, B.; Fantaguzzi, F.; Servillo, A.; Menean, M.; Ribarich, N.; Querques, L.; Zucchiatti, I.; Fedeli, R.; et al. Long-Term Predictors of Anti-VEGF Treatment Response in Patients with Neovascularization Secondary to CSCR: A Longitudinal Study. Graefes Arch. Clin. Exp. Ophthalmol. 2024, 262, 73–80. [Google Scholar] [CrossRef]
- Romdhane, K.; Zola, M.; Matet, A. Predictors of Treatment Response to Intravitreal Anti-Vascular Endothelial Growth Factor (Anti-VEGF) Therapy for Choroidal Neovascularization Secondary to Chronic Central Serous Chorioretinopathy. Br. J. Ophthalmol. 2020, 104, 910–916. [Google Scholar] [CrossRef]
- Padron-Perez, N.; Arias, L.; Rubio, M. Changes in Choroidal Thickness After Intravitreal Injection of Anti-Vascular Endothelial Growth Factor in Pachychoroid Neovasculopathy. Investig. Ophthalmol. Vis. Sci. 2018, 59, 1119–1124. [Google Scholar] [CrossRef]
- Carosielli, M.; Carnevali, A.; Fallico, M.; Pirozzi, E.; Chiosi, F.; Chronopoulos, A.; Cucciniello, P.; Affatato, M.; Rapino, G.; dell’Omo, R.; et al. Intravitreal Brolucizumab for Pachychoroid Neovasculopathy Associated with Chronic Central Serous Chorioretinopathy. Transl. Vis. Sci. Technol. 2023, 12, 17. [Google Scholar] [CrossRef]
- Song, Y.-Y.; Yu, H.-Y.; Baek, S.-K.; Lee, Y.-H.; Lee, M.-W. Short-Term Effect of Anti-VEGF for Chronic Central Serous Chorioretinopathy According to the Presence of Choroidal Neovascularization Using Optical Coherence Tomography Angiography. PLoS ONE 2021, 16, e0245342. [Google Scholar] [CrossRef]
- Hagag, A.M.; Chandra, S.; Khalid, H.; Lamin, A.; Keane, P.A.; Lotery, A.J.; Sivaprasad, S. Multimodal Imaging in the Management of Choroidal Neovascularization Secondary to Central Serous Chorioretinopathy. J. Clin. Med. 2020, 9, 1934. [Google Scholar] [CrossRef]
- Guo, J.; Tang, W.; Liu, W.; Chang, Q.; Xu, G. Clinical Features of Flat Irregular Pigment Epithelial Detachment Associated with Choroidal Neovascularization in Chronic Central Serous Chorioretinopathy. Retina 2021, 41, 199–207. [Google Scholar] [CrossRef] [PubMed]
- Mao, J.; Zhang, C.; Zhang, S.; Liu, C.; Chen, N.; Tao, J.; She, X.; Zheng, Z.; Lv, Z.; Shen, L. Predictors of Anti-VEGF Efficacy in Chronic Central Serous Chorioretinopathy Based on Intraocular Cytokine Levels and Pigment Epithelium Detachment Subtypes. Acta Ophthalmol. 2022, 100, E1385–E1394. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-C.; Chen, S.-N. Three-Year Follow-Up of Choroidal Neovascularization in Eyes of Chronic Central Serous Chorioretinopathy. Br. J. Ophthalmol. 2020, 104, 1561–1566. [Google Scholar] [CrossRef] [PubMed]
- Gołębiewska, J.; Brydak-Godowska, J.; Moneta-Wielgoś, J.; Turczyńska, M.; Kęcik, D.; Hautz, W. Correlation between Choroidal Neovascularization Shown by OCT Angiography and Choroidal Thickness in Patients with Chronic Central Serous Chorioretinopathy. J. Ophthalmol. 2017, 2017, 3048013. [Google Scholar] [CrossRef] [PubMed]
- Altinisik, M.; Kurt, E.; Kayikcioglu, O.; Yildirim, A. Quantitative Analysis of the Activity in Choroidal Neovascularizations after a Single Anti-VEGF Injection: OCT Versus OCT Angiography. Semin. Ophthalmol. 2021, 36, 573–581. [Google Scholar] [CrossRef]
- Chhablani, J.; Kozak, I.; Pichi, F.; Chenworth, M.; Berrocal, M.H.; Bedi, R.; Singh, R.P.; Wu, L.; Meyerle, C.; Casella, A.M.; et al. Outcomes of Treatment of Choroidal Neovascularization Associated with Central Serous Chorioretinopathy with Intravitreal Antiangiogenic Agents. Retina 2015, 35, 2489. [Google Scholar] [CrossRef]
- Chhablani, J.; Pichi, F.; Silva, R.; Casella, A.M.; Murthy, H.; Banker, A.; Nowilaty, S.R.; Carrai, P.; Nucci, P.; Arevalo, J.F. Antiangiogenics in Choroidal Neovascularization Associated with Laser in Central Serous Chorioretinopathy. Retina 2016, 36, 901. [Google Scholar] [CrossRef]
- Peiretti, E.; Caminiti, G.; Serra, R.; Querques, L.; Pertile, R.; Querques, G. Anti-Vascular Endothelial Growth Factor Therapy Versus Photodynamic Therapy in the Treatment of Choroidal Neovascularization Secondary to Central Serous Chorioretinopathy. Retina 2018, 38, 1526. [Google Scholar] [CrossRef]
- Lee, G.W.; Roh, H.C.; Kang, S.W.; Kim, A.Y.; Noh, H.; Choi, K.J. The Implications of Subretinal Fluid in Pachychoroid Neovasculopathy. Sci. Rep. 2021, 11, 4066. [Google Scholar] [CrossRef]
- Spaide, R.F. Optical Coherence Tomography Angiography Signs of Vascular Abnormalization with Antiangiogenic Therapy for Choroidal Neovascularization. Am. J. Ophthalmol. 2015, 160, 6–16. [Google Scholar] [CrossRef]
- Wu, J.; Zhang, J. Neovascular Remodeling and Subretinal Fibrosis as Biomarkers for Predicting Incomplete Response to Anti-VEGF Therapy in Neovascular Age-Related Macular Degeneration. Front. Biosci. (Landmark Ed.) 2022, 27, 135. [Google Scholar] [CrossRef] [PubMed]
- Demirel, S.; Yanık, Ö.; Nalcı, H.; Batıoğlu, F.; Özmert, E. The use of optical coherence tomography angiography in pachychoroid spectrum diseases: A concurrent comparison with dye angiography. Graefes Arch. Clin. Exp. Ophthalmol. 2017, 255, 2317–2324. [Google Scholar] [CrossRef] [PubMed]
- Carnevali, A.; Capuano, V.; Sacconi, R.; Querques, L.; Marchese, A.; Rabiolo, A.; Souied, E.; Scorcia, V.; Bandello, F.; Querques, G. OCT Angiography of Treatment-Naïve Quiescent Choroidal Neovascularization in Pachychoroid Neovasculopathy. Ophthalmol. Retin. 2017, 1, 328–332. [Google Scholar] [CrossRef] [PubMed]
- Costanzo, E.; Cohen, S.Y.; Miere, A.; Querques, G.; Capuano, V.; Semoun, O.; El Ameen, A.; Oubraham, H.; Souied, E.H. Optical Coherence Tomography Angiography in Central Serous Chorioretinopathy. J. Ophthalmol. 2015, 2015, 134783. [Google Scholar] [CrossRef]
- Bousquet, E.; Bonnin, S.; Mrejen, S.; Krivosic, V.; Tadayoni, R.; Gaudric, A. Optical Coherence Tomography Angiography of Flat Irregular Pigment Epithelium Detachment in Chronic Central Serous Chorioretinopathy. Retina 2018, 38, 629–638. [Google Scholar] [CrossRef]
- Chhablani, J.; Cohen, F.B.; Central Serous Chorioretinopathy International Group. Multimodal Imaging-Based Central Serous Chorioretinopathy Classification. Ophthalmol. Retin. 2020, 4, 1043–1046. [Google Scholar] [CrossRef]
- Weng, S.; Mao, L.; Yu, S.; Gong, Y.; Cheng, L.; Chen, X. Detection of Choroidal Neovascularization in Central Serous Chorioretinopathy Using Optical Coherence Tomographic Angiography. Ophthalmologica 2016, 236, 114–121. [Google Scholar] [CrossRef]
- Hage, R.; Mrejen, S.; Krivosic, V.; Quentel, G.; Tadayoni, R.; Gaudric, A. Flat Irregular Retinal Pigment Epithelium Detachments in Chronic Central Serous Chorioretinopathy and Choroidal Neovascularization. Am. J. Ophthalmol. 2015, 159, 890–903.e3. [Google Scholar] [CrossRef]
- Quaranta-El Maftouhi, M.; El Maftouhi, A.; Eandi, C.M. Chronic Central Serous Chorioretinopathy Imaged by Optical Coherence Tomographic Angiography. Am. J. Ophthalmol. 2015, 160, 581–587.e1. [Google Scholar] [CrossRef]
- Bonini Filho, M.A.; de Carlo, T.E.; Ferrara, D.; Adhi, M.; Baumal, C.R.; Witkin, A.J.; Reichel, E.; Duker, J.S.; Waheed, N.K. Association of Choroidal Neovascularization and Central Serous Chorioretinopathy with Optical Coherence Tomography Angiography. JAMA Ophthalmol. 2015, 133, 899–906. [Google Scholar] [CrossRef]
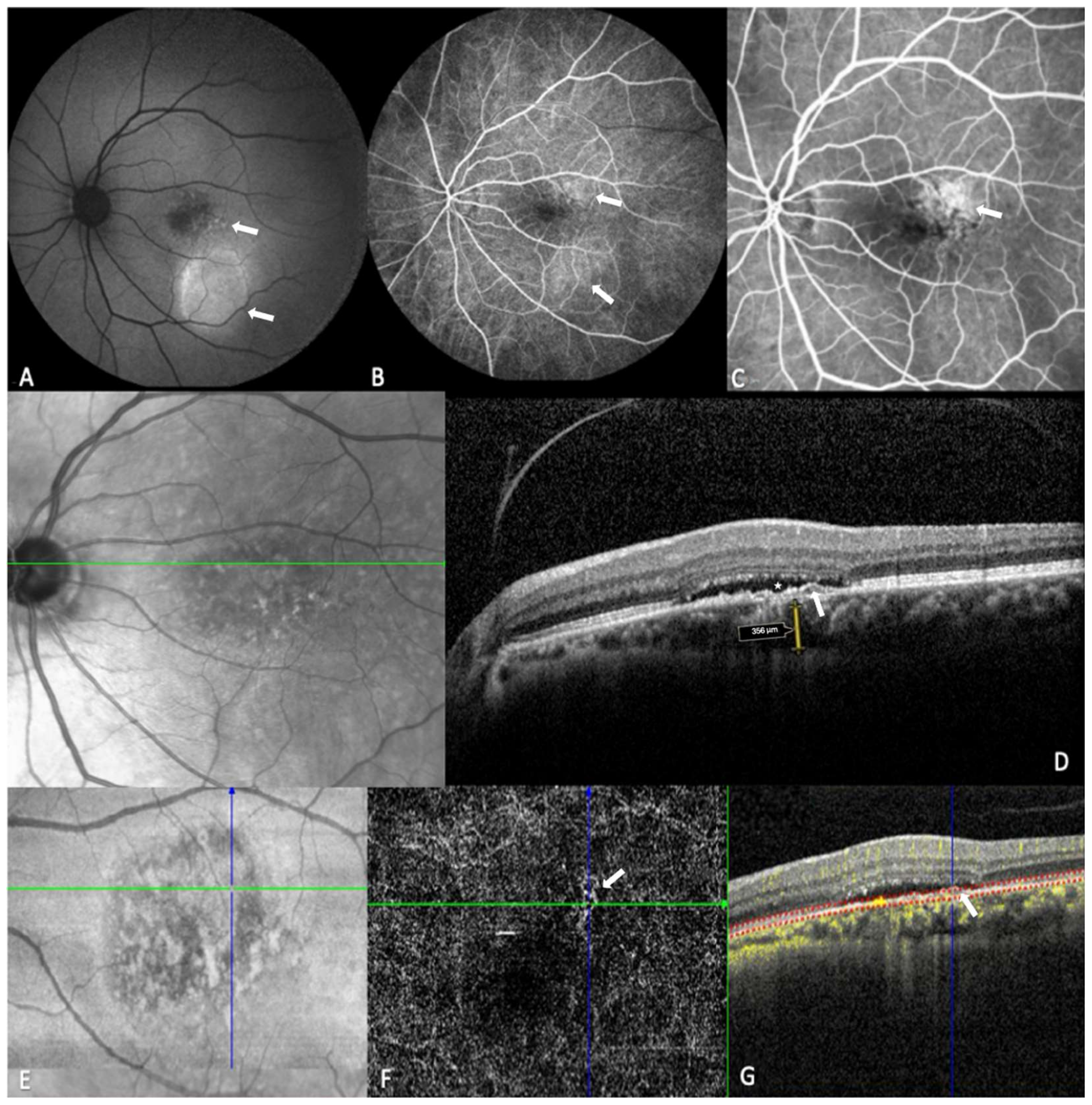


| Characteristics | |
|---|---|
| Total no. eyes (patients) | 22 |
| Age (years), mean ± SD | 67.61 ± 10.65 |
| Sex | |
| Female, no. (%) | 4 (18.00) |
| Male, no. (%) | 18 (82.00) |
| Eye | |
| OD, no. (%) | 14 (64.00) |
| OS, no. (%) | 8 (36.00) |
| MNV | |
| Type 1, no. (%) | 11 (50.00) |
| Mixed type 1 + 2, no. (%) | 11 (50.00) |
| Time from diagnosis to first injection (days), mean ± SD | 16.35 ± 13.80 |
| Total follow-up period (months), mean ± SD (IQR) | 21.0 ± 14.6 (3.5–51.0) |
| No. injections, mean ± SD (IQR) | 10 ± 8 (2.0–29.0) |
| BCVA (LogMAR), mean ± SD | 0.51 ± 0.47 |
| Anatomic parameters | |
| SFCT, mean ± SD | 321.59 ± 84.32 |
| SRF, mean ± SD | 176.86 ± 115.62 |
| CMT, mean ± SD | 331.32 ± 130.49 |
| PED, no. (%) | 12 (54.55) |
| FIPED, no. (%) | 13 (59.09) |
| IRF, no. (%) | 11 (50.00) |
| Baseline | Post Injection | Last Follow-Up | p Value | |
|---|---|---|---|---|
| SFCT, mean ± SD | 321.59 ± 84.32 | 317.45 ± 84.96 | 319.14 ± 99.96 | 0.850 |
| Nasal CT, mean ± SD | 318.23 ± 84.45 | 317.09 ± 83.57 | 319.68 ± 102.81 | 0.928 |
| Temporal CT, mean ± SD | 310.23 ± 80.04 | 304.50 ± 79.46 | 308.05 ± 79.07 | 0.779 |
| SRF, mean ± SD * | 176.86 ± 115.62 | 94.45 ± 83.24 | 80.95 ± 87.32 | 0.003 |
| CMT, mean ± SD ** | 331.32 ± 130.49 | 291.32 ± 106.70 | 284.77 ± 99.19 | 0.133 |
| BCVA (LogMAR), mean ± SD | 0.51 ± 0.47 | 0.56 ± 0.50 | 0.55 ± 0.54 | 0.336 |
| 1–6 (N = 9) | 7–12 (N = 6) | >12 (N = 7) | p-Value | ||
|---|---|---|---|---|---|
| Baseline | SFCT (1a) | 341.89 (79.59) | 350.83 (111.02) | 270.43 (40.49) | 0.148 |
| Nasal CT (1b) | 338.22 (76.43) | 344.67 (119.24) | 269.86 (35.98) | 0.188 | |
| Temporal CT (1c) | 324.22 (64.26) | 345.67 (115.44) | 261.86 (39.16) | 0.134 | |
| SRF (1d) | 159.44 (88.59) | 183.00 (151.06) | 194.00 (128.33) | 0.843 | |
| CMT (1e) | 366.89 (152.83) | 286.67 (111.36) | 323.86 (119.00) | 0.521 | |
| VA LogMAR (1f) | 0.58 (0.61) | 0.44 (0.17) | 0.47 (0.49) | 0.854 | |
| Post Injection | SFCT (2a) | 335.89 (62.73) | 347.17 (127.02) | 268.29 (48.54) | 0.177 |
| Nasal CT (2b) | 337.33 (62.39) | 342.00 (126.12) | 269.71 (46.30) | 0.196 | |
| Temporal CT (2c) | 316.22 (57.66) | 336.33 (122.05) | 262.14 (44.02) | 0.214 | |
| SRF (2d) | 108.89 (94.74) | 109.67 (87.85) | 62.86 (64.37) | 0.500 | |
| CMT (2e) | 315.11 (103.02) | 284.33 (87.06) | 266.71 (133.32) | 0.677 | |
| VA LogMAR (2f) | 0.59 (0.66) | 0.53 (0.30) | 0.54 (0.45) | 0.973 | |
| Last Follow-up | SFCT (3a) | 360.44 (110.28) | 324.50 (108.48) | 261.43 (49.77) | 0.143 |
| Nasal CT (3b) | 364.33 (112.93) | 321.17 (113.44) | 261.00 (47.51) | 0.136 | |
| Temporal CT (3c) | 340.11 (66.31) | 317.17 (108.58) | 259.00 (41.99) | 0.117 | |
| SRF (3d) | 134.78 (91.10) | 41.83 (70.16) | 45.29 (64.48) | 0.047 | |
| CMT (3e) | 334.00 (114.85) | 257.83 (71.62) | 244.57 (79.54) | 0.149 | |
| VA LogMAR (3f) | 0.62 (0.64) | 0.43 (0.33) | 0.57 (0.59) | 0.800 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rabinovitch, D.; Shulman, S.; Goldenberg, D.; Wang, L.; Iyer, P.; Loewenstein, A.; Igra, N.; Levine, O.; Herrera, G.; Trivizki, O. Choroidal Response to Intravitreal Bevacizumab Injections in Treatment-Naïve Macular Neovascularization Secondary to Chronic Central Serous Chorioretinopathy. Biomedicines 2024, 12, 2760. https://doi.org/10.3390/biomedicines12122760
Rabinovitch D, Shulman S, Goldenberg D, Wang L, Iyer P, Loewenstein A, Igra N, Levine O, Herrera G, Trivizki O. Choroidal Response to Intravitreal Bevacizumab Injections in Treatment-Naïve Macular Neovascularization Secondary to Chronic Central Serous Chorioretinopathy. Biomedicines. 2024; 12(12):2760. https://doi.org/10.3390/biomedicines12122760
Chicago/Turabian StyleRabinovitch, David, Shiri Shulman, Dafna Goldenberg, Liang Wang, Prashanth Iyer, Anat Loewenstein, Noah Igra, Olivia Levine, Gissel Herrera, and Omer Trivizki. 2024. "Choroidal Response to Intravitreal Bevacizumab Injections in Treatment-Naïve Macular Neovascularization Secondary to Chronic Central Serous Chorioretinopathy" Biomedicines 12, no. 12: 2760. https://doi.org/10.3390/biomedicines12122760
APA StyleRabinovitch, D., Shulman, S., Goldenberg, D., Wang, L., Iyer, P., Loewenstein, A., Igra, N., Levine, O., Herrera, G., & Trivizki, O. (2024). Choroidal Response to Intravitreal Bevacizumab Injections in Treatment-Naïve Macular Neovascularization Secondary to Chronic Central Serous Chorioretinopathy. Biomedicines, 12(12), 2760. https://doi.org/10.3390/biomedicines12122760






