Coley’s Toxin to First Approved Therapeutic Vaccine—A Brief Historical Account in the Progression of Immunobiology-Based Cancer Treatment
Abstract
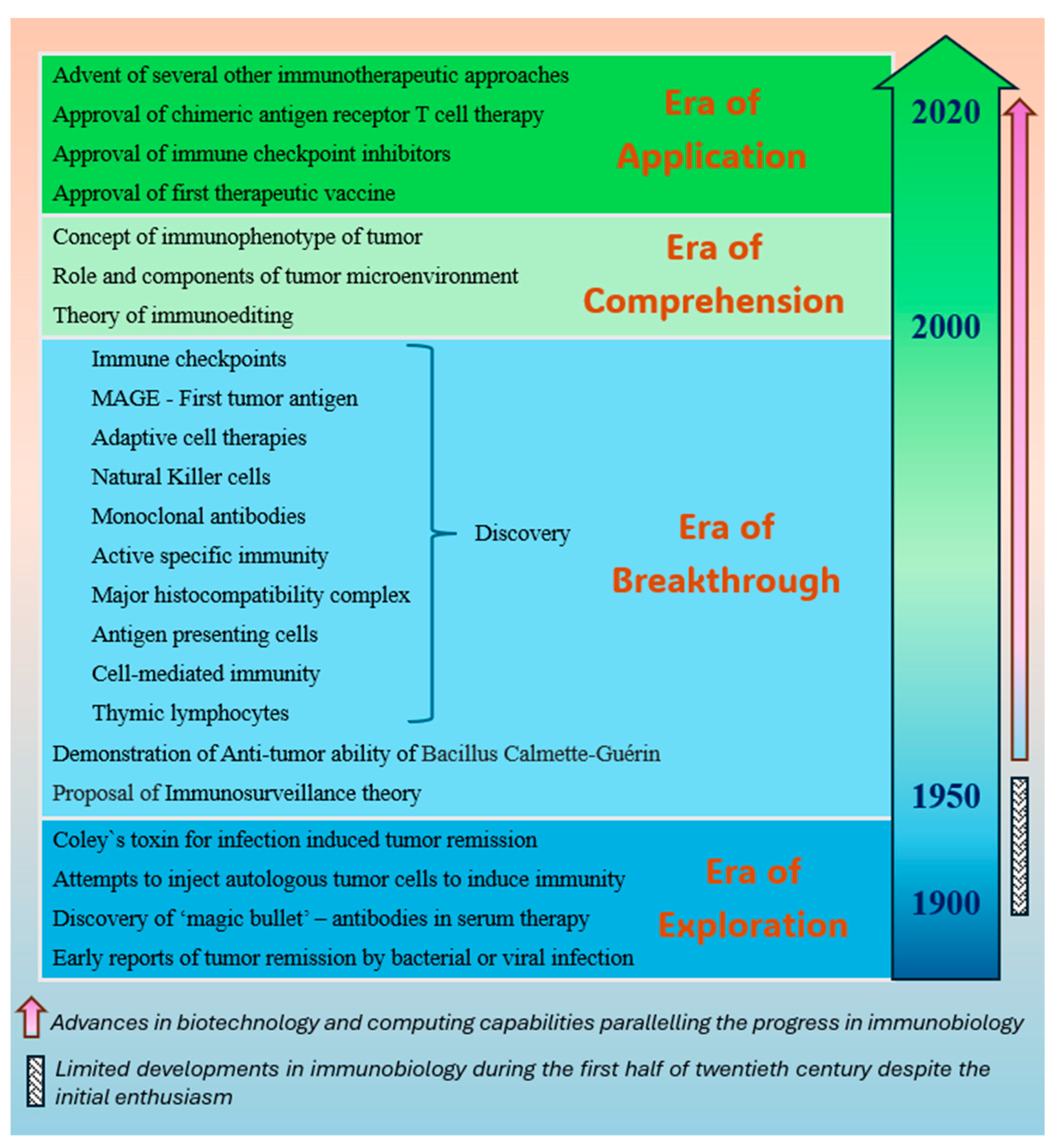
1. Introduction
2. Early Developments in Application of Immunology in Cancer Treatment at the Turn of the Twentieth century
2.1. First Reports of Infection-Induced Cancer Remission
2.2. Coley’s Toxin—The First Immunotherapeutic
2.3. Discovery of the “Magic Bullet”
2.4. Other Attempts to Induce Anti-Tumor Immunity
3. Institutional Support and Theoretical Advancements in Cancer Immunology During the Second Half of the Twentieth Century
3.1. Establishment of a Dedicated Institute for Research on Cancer Immunology
3.2. Genesis of the Theory of Immune Surveillance
4. Evolution of Cellular Immunology in Cancer Research
4.1. Discovery of T Lymphocytes—The Dawn of the Era of Immunotherapy
4.2. Discovery of Other Key Apparatus of Antigen-Mediated Immune Mechanisms
4.3. Introduction of Alternate Apporaches in Cancer Immunotherapeuctics
5. Therapeutic Vaccines as Prototype of Immunotherapeutic Approaches
5.1. BCG—The First Therapeutic Vaccine with Definitive Indication
5.2. Central Mechanism of Therapeutic Vaccines
5.3. Melacine—The First Therapeutic Vaccine to Be Engineered
5.4. Enhanced Ability to Measure the Elicited ASIR
5.5. Isolation of DCs
5.6. MAGE—The First Tumor Antigen to Be Recognized
5.7. Provenge—The First FDA-Approved Therapeutic Vaccine
6. Explosion of Immunological Concepts—At the Turn of the Twenty-First Century
6.1. Discovery of Immune Tolerance Mechanisms
6.2. Theory of Immunoediting
6.3. Role of the TME in the Process of Immunoediting
6.4. Immunophenotyping of Cancer
7. Other Prominent Immunotherapeutic Modalities
7.1. Adoptive Cell Therapies (ACTs)
7.2. MABs
7.3. NK Cell-Based Therapies
7.4. Nuclear Factor-κB (NF-κB)
8. Prospects of Immunotherapy
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Waldman, A.D.; Fritz, J.M.; Lenardo, M.J. A Guide to Cancer Immunotherapy: From T Cell Basic Science to Clinical Practice. Nat. Rev. Immunol. 2020, 20, 651–668. [Google Scholar] [CrossRef] [PubMed]
- Blass, E.; Ott, P.A. Advances in the Development of Personalized Neoantigen-Based Therapeutic Cancer Vaccines. Nat. Rev. Clin. Oncol. 2021, 18, 215–229. [Google Scholar] [CrossRef] [PubMed]
- Devaraja, K. Current Prospects of Molecular Therapeutics in Head and Neck Squamous Cell Carcinoma. Pharm. Med. 2019, 33, 269–289. [Google Scholar] [CrossRef] [PubMed]
- Devaraja, K.; Aggarwal, S.; Singh, M. Therapeutic Vaccination in Head and Neck Squamous Cell Carcinoma—A Review. Vaccines 2023, 11, 634. [Google Scholar] [CrossRef]
- Levine, D.B. The Hospital for the Ruptured and Crippled: William Bradley Coley, Third Surgeon-in-Chief 1925–1933. HSS J. 2008, 4, 1–9. [Google Scholar] [CrossRef]
- Hobohm, U. Fever and Cancer in Perspective. Cancer Immunol. Immunother. 2001, 50, 391–396. [Google Scholar] [CrossRef]
- Oiseth, S.J.; Aziz, M.S. Cancer Immunotherapy: A Brief Review of the History, Possibilities, and Challenges Ahead. J. Cancer Metastasis Treat. 2017, 3, 250–261. [Google Scholar] [CrossRef]
- Suzuki, A.; Abe, S.; Koyama, K.; Suzuki, S.; Nagao, M.; Kobayashi, M.; Nomura, J.; Tsutsumi, T.; Takeda, T.; Oka, Y.; et al. Spontaneous Regression of Blastic Plasmacytoid Dendritic Cell Neoplasm Following Sepsis by Serratia Marcescens: A Case Report and Literature Review. Intern. Med. 2021, 60, 927–933. [Google Scholar] [CrossRef]
- Burnet, M. Cancer; a Biological Approach. I. The Processes of Control. Br. Med. J. 1957, 1, 779–786. [Google Scholar] [CrossRef]
- Thomas, L.; Lawrence, H. Cellular and Humoral Aspects of the Hypersensitive States; Hoeber-Harper: New York, NY, USA, 1959; pp. 529–532. [Google Scholar]
- McCarthy, E.F. The Toxins of William B. Coley and the Treatment of Bone and Soft-Tissue Sarcomas. Iowa Orthop. J. 2006, 26, 154–158. [Google Scholar]
- Busch, W. Aus Der Sitzung Der Medicinischen Section Vom 13 November 1867. Berl. Klin. Wochenschr. 1868, 5, 137. [Google Scholar]
- Fehleisen, N. Ueber die Züchtung der Erysipelkokken auf künstlichem Nährboden und ihre Uebertragbarkeit auf den Menschen. Dtsch. Med. Wochenschr. 1882, 8, 553–554. [Google Scholar] [CrossRef]
- Bruns, P. von Die Heilwirkung Des Erysipels Auf Geschwulste. Beitr. Klin. Chir. 1887, 3, 443–466. [Google Scholar]
- Coley, W.B. The Treatment of Inoperable Sarcoma by Bacterial Toxins (The Mixed Toxins of the Streptococcus erysipelas and the Bacillus prodigiosus). Proc. R. Soc. Med. 1910, 3, 1–48. [Google Scholar] [CrossRef] [PubMed]
- Coley, W.B., II. Contribution to the Knowledge of Sarcoma. Ann. Surg. 1891, 14, 199–220. [Google Scholar] [CrossRef] [PubMed]
- Ehrlich, P. Ueber Den Jetzigen Stand Der Karzinomforschung. Weekblad Jaargang Eerst Helft 1909, 5, 273. [Google Scholar]
- Miller, J.F. Immunological Function of the Thymus. Lancet 1961, 2, 748–749. [Google Scholar] [CrossRef]
- Miller, J.F.; Mitchell, G.F.; Weiss, N.S. Cellular Basis of the Immunological Defects in Thymectomized Mice. Nature 1967, 214, 992–997. [Google Scholar] [CrossRef]
- Burnet, M. Role of the Thymus and Related Organs in Immunity. Br. Med. J. 1962, 2, 807–811. [Google Scholar] [CrossRef][Green Version]
- Claman, H.N.; Chaperon, E.A.; Triplett, R.F. Thymus-Marrow Cell Combinations. Synergism in Antibody Production. Proc. Soc. Exp. Biol. Med. 1966, 122, 1167–1171. [Google Scholar] [CrossRef]
- Wagner, H.; Röllinghoff, M.; Nossal, G.J. T-Cell-Mediated Immune Responses Induced in Vitro: A Probe for Allograft and Tumor Immunity. Transplant. Rev. 1973, 17, 3–36. [Google Scholar] [CrossRef] [PubMed]
- Carlson, R.D.; Flickinger, J.C.; Snook, A.E. Talkin’ Toxins: From Coley’s to Modern Cancer Immunotherapy. Toxins 2020, 12, 241. [Google Scholar] [CrossRef] [PubMed]
- Blevins, S.M.; Bronze, M.S. Robert Koch and the “golden Age” of Bacteriology. Int. J. Infect. Dis. 2010, 14, e744–e751. [Google Scholar] [CrossRef]
- Loeffler, F. Untersuchungen Über Die Bedeutung Der Mikroorganismen Für Die Entstehung Der Diphtherie Beim Menschen, Bei Der Taube Und Beim Kalbe; Mitt Klein Gesundheitsamte: Berlin, Germany, 1884; Volume 2, pp. 421–499. [Google Scholar]
- Winau, F.; Winau, R. Emil von Behring and Serum Therapy. Microbes Infect. 2002, 4, 185–188. [Google Scholar] [CrossRef] [PubMed]
- Emil von, B. Über Die Ursache Der Immunität von Ratten Gegen Milzbrand.: Centralblatt für Klinische Medicin 38. 1888; pp. 681–690.
- Von Behring, E.; Kitasato, S. Uber Das Zustandekommen Der Diphtherie-Immunitat und Der Tetanus-Immunitat Bei Thieren. Deut. Med. Wochenschr. 1890, 16, 1113–1114. [Google Scholar]
- Winau, F.; Westphal, O.; Winau, R. Paul Ehrlich—In Search of the Magic Bullet. Microbes Infect. 2004, 6, 786–789. [Google Scholar] [CrossRef]
- Ehrlich, P. Die Seitenkettentheorie Und Ihre Gegner. Münchner. Med. Wochenschr. 1901, 52, 2123–2124. [Google Scholar]
- Currie, G.A. Eighty Years of Immunotherapy: A Review of Immunological Methods Used for the Treatment of Human Cancer. Br. J. Cancer 1972, 26, 141–153. [Google Scholar] [CrossRef][Green Version]
- Von Leyden, V.; Blumenthal, F. Vorläufige Mittheilungen Über Einige Ergebnisse Der Krebsforshung Auf Der 1. Medizinschen Klinik. Dtsch. Med. Wschr. 1902, 28, 637–638. [Google Scholar] [CrossRef][Green Version]
- Risley, E.H. The Gilman–Coca Vaccine Emulsion Treatment of Cancer. Boston Med. Surg. J. 1911, 165, 784–788. [Google Scholar] [CrossRef]
- Vaughan, J.W. Cancer Vaccine and Anticancer Globulins as an Aid in the Surgical Treatment of Malignancy. J. Am. Med. Assoc. 1914, 63, 1258–1265. [Google Scholar] [CrossRef]
- Dobosz, P.; Dzieciątkowski, T. The Intriguing History of Cancer Immunotherapy. Front. Immunol. 2019, 10, 2965. [Google Scholar] [CrossRef] [PubMed]
- Dock, G. The Influence of Complicating Diseases upon Leukaemia. Am. J. Med. Sci. (1827–1924) 1904, 127, 563. [Google Scholar] [CrossRef]
- Larson, C.; Oronsky, B.; Scicinski, J.; Fanger, G.R.; Stirn, M.; Oronsky, A.; Reid, T.R. Going Viral: A Review of Replication-Selective Oncolytic Adenoviruses. Oncotarget 2015, 6, 19976–19989. [Google Scholar] [CrossRef]
- Jensen, C. Experimental Studies on Cancer in Mice (from German). Zentralblatt Bacteriol. Parasitenkd. Infekt. 1903, 34, 28–34. [Google Scholar]
- Little, C.C. A possible mendelian explanation for a type of inheritance apparently non-mendelian in nature. Science 1914, 40, 904–906. [Google Scholar] [CrossRef][Green Version]
- Murphy, J.B.; Morton, J.J. The Lymphocyte as a Factor in Natural and Induced Resistance to Transplanted Cancer. Proc. Natl. Acad. Sci. USA 1915, 1, 435–437. [Google Scholar] [CrossRef]
- The CRI Timeline. Available online: https://www.cancerresearch.org/timeline (accessed on 26 July 2023).
- Everson, T.C.; Cole, W.H. Spontaneous Regression of Malignant Disease. J. Am. Med. Assoc. 1959, 169, 1758–1759. [Google Scholar] [CrossRef]
- Old, L.J.; Clarke, D.A.; Benacerraf, B. Effect of Bacillus Calmette-Guerin Infection on Transplanted Tumours in the Mouse. Nature 1959, 184 (Suppl. S5), 291–292. [Google Scholar] [CrossRef]
- Silverstein, A.M. The Curious Case of the 1960 Nobel Prize to Burnet and Medawar. Immunology 2016, 147, 269–274. [Google Scholar] [CrossRef]
- Liston, A. Immunological Tolerance 50 Years after the Burnet Nobel Prize. Immunol. Cell Biol. 2011, 89, 14–15. [Google Scholar] [CrossRef] [PubMed]
- Gowans, J.L.; McGregor, D.D.; Cowen, D.M. Initiation of Immune Responses by Small Lymphocytes. Nature 1962, 196, 651–655. [Google Scholar] [CrossRef] [PubMed]
- Miller, J.F.; Grant, G.A.; Roe, F.J. Effect of thymectomy on the induction of skin tumours by 3,4-benzopyrene. Nature 1963, 199, 920–922. [Google Scholar] [CrossRef] [PubMed]
- Miller, J.F. Influence of thymectomy on tumor induction by polyoma virus in C57BL mice. Proc. Soc. Exp. Biol. Med. 1964, 116, 323–327. [Google Scholar] [CrossRef] [PubMed]
- Miller, J.F. Events That Led to the Discovery of T-Cell Development and Function—A Personal Recollection. Tissue Antigens 2004, 63, 509–517. [Google Scholar] [CrossRef]
- Miller, J. The Early Work on the Discovery of the Function of the Thymus, an Interview with Jacques Miller. Cell Death Differ. 2020, 27, 396–401. [Google Scholar] [CrossRef]
- Cerottini, J.C.; Nordin, A.A.; Brunner, K.T. In Vitro Cytotoxic Activity of Thymus Cells Sensitized to Alloantigens. Nature 1970, 227, 72–73. [Google Scholar] [CrossRef]
- Cerottini, J.C.; Nordin, A.A.; Brunner, K.T. Specific in Vitro Cytotoxicity of Thymus-Derived Lymphocytes Sensitized to Alloantigens. Nature 1970, 228, 1308–1309. [Google Scholar] [CrossRef]
- Steinman, R.M.; Cohn, Z.A. Identification of a Novel Cell Type in Peripheral Lymphoid Organs of Mice. I. Morphology, Quantitation, Tissue Distribution. J. Exp. Med. 1973, 137, 1142–1162. [Google Scholar] [CrossRef]
- Banchereau, J.; Steinman, R.M. Dendritic Cells and the Control of Immunity. Nature 1998, 392, 245–252. [Google Scholar] [CrossRef]
- Geissmann, F.; Gordon, S.; Hume, D.A.; Mowat, A.M.; Randolph, G.J. Unravelling Mononuclear Phagocyte Heterogeneity. Nat. Rev. Immunol. 2010, 10, 453–460. [Google Scholar] [CrossRef] [PubMed]
- Zinkernagel, R.M.; Doherty, P.C. Restriction of in Vitro T Cell-Mediated Cytotoxicity in Lymphocytic Choriomeningitis within a Syngeneic or Semiallogeneic System. Nature 1974, 248, 701–702. [Google Scholar] [CrossRef] [PubMed]
- Zinkernagel, R.M.; Doherty, P.C. Immunological Surveillance against Altered Self Components by Sensitised T Lymphocytes in Lymphocytic Choriomeningitis. Nature 1974, 251, 547–548. [Google Scholar] [CrossRef] [PubMed]
- Doherty, P.C. Challenged by Complexity: My Twentieth Century in Immunology. Annu. Rev. Immunol. 2007, 25, 1–19. [Google Scholar] [CrossRef][Green Version]
- Raju, T.N. The Nobel Chronicles. 1996: Peter Charles Doherty (b 1940) and Rolf M Zinkernagel (b 1944). Lancet 2000, 356, 172. [Google Scholar] [CrossRef]
- Volchenkov, R.; Sprater, F.; Vogelsang, P.; Appel, S. The 2011 Nobel Prize in Physiology or Medicine. Scand. J. Immunol. 2012, 75, 1–4. [Google Scholar] [CrossRef]
- Scott, A.M.; Allison, J.P.; Wolchok, J.D. Monoclonal Antibodies in Cancer Therapy. Cancer Immun. 2012, 12, 14. [Google Scholar]
- Damián-Blanco, P.; Ahuexoteco-Sánchez, S.; Carbajal-Gallardo, A.A.; Coctecon-Chávelas, F.C.; Rodríguez-Nava, C.; Vences-Velázquez, A.; Medina-Flores, Y.; Mata-Ruíz, O.; Lloret-Sánchez, L.; Cortés-Sarabia, K. Use of Monoclonal Antibodies in Cancer Immunotherapy: Types and Mechanisms of Action. Bol. Med. Hosp. Infant. Mex. 2023, 80, 153–164. [Google Scholar] [CrossRef]
- Schwaber, J.; Cohen, E.P. Human x Mouse Somatic Cell Hybrid Clone Secreting Immunoglobulins of Both Parental Types. Nature 1973, 244, 444–447. [Google Scholar] [CrossRef]
- Köhler, G.; Milstein, C. Continuous Cultures of Fused Cells Secreting Antibody of Predefined Specificity. Nature 1975, 256, 495–497. [Google Scholar] [CrossRef]
- Ribatti, D. From the Discovery of Monoclonal Antibodies to Their Therapeutic Application: An Historical Reappraisal. Immunol. Lett. 2014, 161, 96–99. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.K.H. The History of Monoclonal Antibody Development—Progress, Remaining Challenges and Future Innovations. Ann. Med. Surg. 2014, 3, 113–116. [Google Scholar] [CrossRef] [PubMed]
- Kaunitz, J.D. Development of Monoclonal Antibodies: The Dawn of mAb Rule. Dig. Dis. Sci. 2017, 62, 831–832. [Google Scholar] [CrossRef] [PubMed]
- Herberman, R.B.; Nunn, M.E.; Lavrin, D.H.; Asofsky, R. Effect of Antibody to Theta Antigen on Cell-Mediated Immunity Induced in Syngeneic Mice by Murine Sarcoma Virus. J. Natl. Cancer Inst. 1973, 51, 1509–1512. [Google Scholar] [CrossRef]
- Herberman, R.B. In Vivo and in Vitro Assays of Cellular Immunity to Human Tumor Antigens. Fed. Proc. 1973, 32, 160–164. [Google Scholar]
- Kiessling, R.; Klein, E.; Wigzell, H. “Natural” Killer Cells in the Mouse. I. Cytotoxic Cells with Specificity for Mouse Moloney Leukemia Cells. Specificity and Distribution According to Genotype. Eur. J. Immunol. 1975, 5, 112–117. [Google Scholar] [CrossRef]
- Kiessling, R.; Klein, E.; Pross, H.; Wigzell, H. “Natural” Killer Cells in the Mouse. II. Cytotoxic Cells with Specificity for Mouse Moloney Leukemia Cells. Characteristics of the Killer Cell. Eur. J. Immunol. 1975, 5, 117–121. [Google Scholar] [CrossRef]
- Kiessling, R.; Petrányi, G.; Klein, G.; Wigzell, H. Non-T-Cell Resistance against a Mouse Moloney Lymphoma. Int. J. Cancer 1976, 17, 275–281. [Google Scholar] [CrossRef]
- Chen, J.; Gao, L.; Wu, X.; Fan, Y.; Liu, M.; Peng, L.; Song, J.; Li, B.; Liu, A.; Bao, F. BCG-Induced Trained Immunity: History, Mechanisms and Potential Applications. J. Transl. Med. 2023, 21, 106. [Google Scholar] [CrossRef]
- Pearl, R. On the Pathological Relations Between Cancer and Tuberculosis. Proc. Soc. Exp. Biol. Med. 1928, 26, 73–75. [Google Scholar] [CrossRef]
- Morales, A.; Eidinger, D. Bacillus Calmette-Guerin in the Treatment of Adenocarcinoma of the Kidney. J. Urol. 1976, 115, 377–380. [Google Scholar] [CrossRef] [PubMed]
- Morales, A.; Eidinger, D.; Bruce, A.W. Intracavitary Bacillus Calmette-Guerin in the Treatment of Superficial Bladder Tumors. J. Urol. 1976, 116, 180–183. [Google Scholar] [CrossRef] [PubMed]
- Hanna, M.G.; Peters, L.C. Specific Immunotherapy of Established Visceral Micrometastases by BCG-Tumor Cell Vaccine Alone or as an Adjunct to Surgery. Cancer 1978, 42, 2613–2625. [Google Scholar] [CrossRef] [PubMed]
- Grant, R.M.; Mackie, R.; Cochran, A.J.; Murray, E.L.; Hoyle, D.; Ross, C. Results of Administering B.C.G. to Patients with Melanoma. Lancet 1974, 2, 1096–1100. [Google Scholar] [CrossRef]
- Donaldson, R.C. Chemoimmunotherapy for Cancer of the Head and Neck. Am. J. Surg. 1973, 126, 507–512. [Google Scholar] [CrossRef]
- Carswell, E.A.; Old, L.J.; Kassel, R.L.; Green, S.; Fiore, N.; Williamson, B. An Endotoxin-Induced Serum Factor That Causes Necrosis of Tumors. Proc. Natl. Acad. Sci. USA 1975, 72, 3666–3670. [Google Scholar] [CrossRef]
- Old, L.J. Cancer Immunology. Sci. Am. 1977, 236, 62–70. [Google Scholar] [CrossRef]
- Ashley, M.P.; Zbar, B.; Hunter, J.T.; Rapp, H.J.; Sugimoto, T. Adjuvant-Antigen Requirements for Active Specific Immunotherapy of Microscopic Metastases Remaining after Surgery. Cancer Res. 1980, 40, 4197–4203. [Google Scholar]
- Sukumar, S.; Hunter, J.T.; Terata, N.; Rapp, H.J. Eradication of Microscopic Hepatic Metastases by Active Specific Immunization. Cancer Immunol. Immunother. 1983, 14, 151–154. [Google Scholar] [CrossRef]
- Bier, H.; Armonat, G.; Bier, J.; Schirrmacher, V.; Ganzer, U. Postoperative Active-Specific Immunotherapy of Lymph Node Micrometastasis in a Guinea Pig Tumor Model. Orl J. Otorhinolaryngol. Relat. Spec. 1989, 51, 197–205. [Google Scholar] [CrossRef]
- Morton, D.L.; Foshag, L.J.; Hoon, D.S.; Nizze, J.A.; Famatiga, E.; Wanek, L.A.; Chang, C.; Davtyan, D.G.; Gupta, R.K.; Elashoff, R. Prolongation of Survival in Metastatic Melanoma after Active Specific Immunotherapy with a New Polyvalent Melanoma Vaccine. Ann. Surg. 1992, 216, 463–482. [Google Scholar] [CrossRef] [PubMed]
- Böhle, A.; Brandau, S. Immune Mechanisms in Bacillus Calmette-Guerin Immunotherapy for Superficial Bladder Cancer. J. Urol. 2003, 170, 964–969. [Google Scholar] [CrossRef] [PubMed]
- de Jong, W.H.; Teppema, J.S.; Wagenaar, S.S.; Paques, M.; Steerenberg, P.A.; Ruitenberg, E.J. Histological Evaluation of Immunologically Mediated Tumor Regression of the Line 10 Guinea Pig Hepatocarcinoma. Virchows Arch. B Cell Pathol. Incl. Mol. Pathol. 1986, 50, 249–269. [Google Scholar] [CrossRef] [PubMed]
- Miyazaki, J.; Onozawa, M.; Takaoka, E.; Yano, I. Bacillus Calmette-Guérin Strain Differences as the Basis for Immunotherapies against Bladder Cancer. Int. J. Urol. 2018, 25, 405–413. [Google Scholar] [CrossRef]
- Klein, E. Lymphocyte-Mediated Lysis of Tumor Cells in Vitro. Antigen-Restricted Clonal and Unrestricted Polyclonal Effects. Springer Semin. Immunopathol. 1982, 5, 147–159. [Google Scholar] [CrossRef]
- Stauss, H.J.; Van Waes, C.; Fink, M.A.; Starr, B.; Schreiber, H. Identification of a Unique Tumor Antigen as Rejection Antigen by Molecular Cloning and Gene Transfer. J. Exp. Med. 1986, 164, 1516–1530. [Google Scholar] [CrossRef]
- Cole, W.H. Efforts to Explain Spontaneous Regression of Cancer. J. Surg. Oncol. 1981, 17, 201–209. [Google Scholar] [CrossRef]
- Thomas, L. On Immunosurveillance in Human Cancer. Yale J. Biol. Med. 1982, 55, 329–333. [Google Scholar]
- Burnet, M. Donn. Br. Med. Bull. 1964, 20, 154–158. [Google Scholar] [CrossRef]
- Ribatti, D. The Concept of Immune Surveillance against Tumors. The First Theories. Oncotarget 2017, 8, 7175–7180. [Google Scholar] [CrossRef]
- Mitchell, M.S.; Kan-Mitchell, J.; Kempf, R.A.; Harel, W.; Shau, H.Y.; Lind, S. Active Specific Immunotherapy for Melanoma: Phase I Trial of Allogeneic Lysates and a Novel Adjuvant. Cancer Res. 1988, 48, 5883–5893. [Google Scholar] [PubMed]
- Mitchell, M.S.; Harel, W.; Kempf, R.A.; Hu, E.; Kan-Mitchell, J.; Boswell, W.D.; Dean, G.; Stevenson, L. Active-Specific Immunotherapy for Melanoma. J. Clin. Oncol. 1990, 8, 856–869. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, M.S. Perspective on Allogeneic Melanoma Lysates in Active Specific Immunotherapy. Semin. Oncol. 1998, 25, 623–635. [Google Scholar] [PubMed]
- Sondak, V.K.; Sosman, J.A. Results of Clinical Trials with an Allogenic Melanoma Tumor Cell Lysate Vaccine: Melacine. Semin. Cancer Biol. 2003, 13, 409–415. [Google Scholar] [CrossRef]
- Sondak, V.K.; Liu, P.-Y.; Tuthill, R.J.; Kempf, R.A.; Unger, J.M.; Sosman, J.A.; Thompson, J.A.; Weiss, G.R.; Redman, B.G.; Jakowatz, J.G.; et al. Adjuvant Immunotherapy of Resected, Intermediate-Thickness, Node-Negative Melanoma with an Allogeneic Tumor Vaccine: Overall Results of a Randomized Trial of the Southwest Oncology Group. J. Clin. Oncol. 2002, 20, 2058–2066. [Google Scholar] [CrossRef]
- Morton, D.L.; Mozzillo, N.; Thompson, J.F.; Kelley, M.C.; Faries, M.; Wagner, J.; Schneebaum, S.; Schuchter, L.; Gammon, G.; Elashoff, R. An International, Randomized, Phase III Trial of Bacillus Calmette-Guerin (BCG) plus Allogeneic Melanoma Vaccine (MCV) or Placebo after Complete Resection of Melanoma Metastatic to Regional or Distant Sites. JCO 2007, 25, 8508. [Google Scholar] [CrossRef]
- Apostólico, J.d.S.; Lunardelli, V.A.S.; Coirada, F.C.; Boscardin, S.B.; Rosa, D.S. Adjuvants: Classification, Modus Operandi, and Licensing. J. Immunol. Res. 2016, 2016, 1459394. [Google Scholar] [CrossRef]
- Firdaus, F.Z.; Skwarczynski, M.; Toth, I. Developments in Vaccine Adjuvants. Methods Mol. Biol. 2022, 2412, 145–178. [Google Scholar] [CrossRef]
- Czerkinsky, C.C.; Nilsson, L.A.; Nygren, H.; Ouchterlony, O.; Tarkowski, A. A Solid-Phase Enzyme-Linked Immunospot (ELISPOT) Assay for Enumeration of Specific Antibody-Secreting Cells. J. Immunol. Methods 1983, 65, 109–121. [Google Scholar] [CrossRef]
- Slota, M.; Lim, J.-B.; Dang, Y.; Disis, M.L. ELISpot for Measuring Human Immune Responses to Vaccines. Expert. Rev. Vaccines 2011, 10, 299–306. [Google Scholar] [CrossRef]
- Ranieri, E.; Netti, G.S.; Gigante, M. CTL ELISPOT Assay and T Cell Detection. Methods Mol. Biol. 2021, 2325, 65–77. [Google Scholar] [CrossRef] [PubMed]
- Saxena, M.; Bhardwaj, N. Turbocharging Vaccines: Emerging Adjuvants for Dendritic Cell Based Therapeutic Cancer Vaccines. Curr. Opin. Immunol. 2017, 47, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Inaba, K.; Inaba, M.; Romani, N.; Aya, H.; Deguchi, M.; Ikehara, S.; Muramatsu, S.; Steinman, R.M. Generation of Large Numbers of Dendritic Cells from Mouse Bone Marrow Cultures Supplemented with Granulocyte/Macrophage Colony-Stimulating Factor. J. Exp. Med. 1992, 176, 1693–1702. [Google Scholar] [CrossRef] [PubMed]
- Young, J.W.; Szabolcs, P.; Moore, M.A. Identification of Dendritic Cell Colony-Forming Units among Normal Human CD34+ Bone Marrow Progenitors That Are Expanded by c-Kit-Ligand and Yield Pure Dendritic Cell Colonies in the Presence of Granulocyte/Macrophage Colony-Stimulating Factor and Tumor Necrosis Factor Alpha. J. Exp. Med. 1995, 182, 1111–1119. [Google Scholar] [CrossRef] [PubMed]
- De Plaen, E.; Lurquin, C.; Van Pel, A.; Mariamé, B.; Szikora, J.P.; Wölfel, T.; Sibille, C.; Chomez, P.; Boon, T. Immunogenic (Tum-) Variants of Mouse Tumor P815: Cloning of the Gene of Tum- Antigen P91A and Identification of the Tum- Mutation. Proc. Natl. Acad. Sci. USA 1988, 85, 2274–2278. [Google Scholar] [CrossRef]
- van der Bruggen, P.; Traversari, C.; Chomez, P.; Lurquin, C.; De Plaen, E.; Van den Eynde, B.; Knuth, A.; Boon, T. A Gene Encoding an Antigen Recognized by Cytolytic T Lymphocytes on a Human Melanoma. Science 1991, 254, 1643–1647. [Google Scholar] [CrossRef]
- Boon, T.; van der Bruggen, P. Human Tumor Antigens Recognized by T Lymphocytes. J. Exp. Med. 1996, 183, 725–729. [Google Scholar] [CrossRef]
- Wang, R.F.; Rosenberg, S.A. Human Tumor Antigens for Cancer Vaccine Development. Immunol. Rev. 1999, 170, 85–100. [Google Scholar] [CrossRef]
- Devaraja, K.; Aggarwal, S.; Verma, S.S.; Gupta, S.C. Clinico-Pathological Peculiarities of Human Papilloma Virus Driven Head and Neck Squamous Cell Carcinoma: A Comprehensive Update. Life Sci. 2020, 245, 117383. [Google Scholar] [CrossRef]
- Coulie, P.G.; Van den Eynde, B.J.; van der Bruggen, P.; Boon, T. Tumour Antigens Recognized by T Lymphocytes: At the Core of Cancer Immunotherapy. Nat. Rev. Cancer 2014, 14, 135–146. [Google Scholar] [CrossRef]
- Zarour, H.M.; DeLeo, A.; Finn, O.J.; Storkus, W.J. Categories of Tumor Antigens. In Holland-Frei Cancer Medicine, 6th ed.; BC Decker: Hamilton, ON, Canada, 2003. [Google Scholar]
- Kosaka, A.; Yajima, Y.; Hatayama, M.; Ikuta, K.; Sasaki, T.; Hirai, N.; Yasuda, S.; Nagata, M.; Hayashi, R.; Harabuchi, S.; et al. A Stealth Antigen SPESP1, Which Is Epigenetically Silenced in Tumors, Is a Suitable Target for Cancer Immunotherapy. Cancer Sci. 2021, 112, 2705–2713. [Google Scholar] [CrossRef] [PubMed]
- Yajima, Y.; Kosaka, A.; Ishibashi, K.; Yasuda, S.; Komatsuda, H.; Nagato, T.; Oikawa, K.; Kitada, M.; Takekawa, M.; Kumai, T.; et al. A Tumor Metastasis-Associated Molecule TWIST1 Is a Favorable Target for Cancer Immunotherapy Due to Its Immunogenicity. Cancer Sci. 2022, 113, 2526–2535. [Google Scholar] [CrossRef]
- Cheever, M.A.; Allison, J.P.; Ferris, A.S.; Finn, O.J.; Hastings, B.M.; Hecht, T.T.; Mellman, I.; Prindiville, S.A.; Viner, J.L.; Weiner, L.M.; et al. The Prioritization of Cancer Antigens: A National Cancer Institute Pilot Project for the Acceleration of Translational Research. Clin. Cancer Res. 2009, 15, 5323–5337. [Google Scholar] [CrossRef] [PubMed]
- Higano, C.S.; Schellhammer, P.F.; Small, E.J.; Burch, P.A.; Nemunaitis, J.; Yuh, L.; Provost, N.; Frohlich, M.W. Integrated Data from 2 Randomized, Double-Blind, Placebo-Controlled, Phase 3 Trials of Active Cellular Immunotherapy with Sipuleucel-T in Advanced Prostate Cancer. Cancer 2009, 115, 3670–3679. [Google Scholar] [CrossRef] [PubMed]
- Burch, P.A.; Croghan, G.A.; Gastineau, D.A.; Jones, L.A.; Kaur, J.S.; Kylstra, J.W.; Richardson, R.L.; Valone, F.H.; Vuk-Pavlović, S. Immunotherapy (APC8015, Provenge) Targeting Prostatic Acid Phosphatase Can Induce Durable Remission of Metastatic Androgen-Independent Prostate Cancer: A Phase 2 Trial. Prostate 2004, 60, 197–204. [Google Scholar] [CrossRef]
- Kantoff, P.W.; Higano, C.S.; Shore, N.D.; Berger, E.R.; Small, E.J.; Penson, D.F.; Redfern, C.H.; Ferrari, A.C.; Dreicer, R.; Sims, R.B.; et al. Sipuleucel-T Immunotherapy for Castration-Resistant Prostate Cancer. N. Engl. J. Med. 2010, 363, 411–422. [Google Scholar] [CrossRef]
- Cheever, M.A.; Higano, C.S. PROVENGE (Sipuleucel-T) in Prostate Cancer: The First FDA-Approved Therapeutic Cancer Vaccine. Clin. Cancer Res. 2011, 17, 3520–3526. [Google Scholar] [CrossRef]
- Brunet, J.F.; Denizot, F.; Luciani, M.F.; Roux-Dosseto, M.; Suzan, M.; Mattei, M.G.; Golstein, P. A New Member of the Immunoglobulin Superfamily—CTLA-4. Nature 1987, 328, 267–270. [Google Scholar] [CrossRef]
- Linsley, P.S.; Wallace, P.M.; Johnson, J.; Gibson, M.G.; Greene, J.L.; Ledbetter, J.A.; Singh, C.; Tepper, M.A. Immunosuppression in Vivo by a Soluble Form of the CTLA-4 T Cell Activation Molecule. Science 1992, 257, 792–795. [Google Scholar] [CrossRef]
- Iwai, Y.; Ishida, M.; Tanaka, Y.; Okazaki, T.; Honjo, T.; Minato, N. Involvement of PD-L1 on Tumor Cells in the Escape from Host Immune System and Tumor Immunotherapy by PD-L1 Blockade. Proc. Natl. Acad. Sci. USA 2002, 99, 12293–12297. [Google Scholar] [CrossRef]
- Dong, H.; Strome, S.E.; Salomao, D.R.; Tamura, H.; Hirano, F.; Flies, D.B.; Roche, P.C.; Lu, J.; Zhu, G.; Tamada, K.; et al. Tumor-Associated B7-H1 Promotes T-Cell Apoptosis: A Potential Mechanism of Immune Evasion. Nat. Med. 2002, 8, 793–800. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.S.; Mellman, I. Elements of Cancer Immunity and the Cancer-Immune Set Point. Nature 2017, 541, 321–330. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-S.; Shen, C.-R. Immune Checkpoint Blockade Therapy: The 2014 Tang Prize in Biopharmaceutical Science. Biomed. J. 2015, 38, 5–8. [Google Scholar] [CrossRef] [PubMed]
- Huang, P.-W.; Chang, J.W.-C. Immune Checkpoint Inhibitors Win the 2018 Nobel Prize. Biomed. J. 2019, 42, 299–306. [Google Scholar] [CrossRef] [PubMed]
- Dunn, G.P.; Bruce, A.T.; Ikeda, H.; Old, L.J.; Schreiber, R.D. Cancer Immunoediting: From Immunosurveillance to Tumor Escape. Nat. Immunol. 2002, 3, 991–998. [Google Scholar] [CrossRef]
- Dunn, G.P.; Old, L.J.; Schreiber, R.D. The Immunobiology of Cancer Immunosurveillance and Immunoediting. Immunity 2004, 21, 137–148. [Google Scholar] [CrossRef]
- Song, Q.; Zhang, C.-D.; Wu, X.-H. Therapeutic Cancer Vaccines: From Initial Findings to Prospects. Immunol. Lett. 2018, 196, 11–21. [Google Scholar] [CrossRef]
- Shibata, H.; Xu, N.; Saito, S.; Zhou, L.; Ozgenc, I.; Webb, J.; Fu, C.; Zolkind, P.; Egloff, A.M.; Uppaluri, R. Integrating CD4+ T Cell Help for Therapeutic Cancer Vaccination in a Preclinical Head and Neck Cancer Model. Oncoimmunology 2021, 10, 1958589. [Google Scholar] [CrossRef]
- Wei, C.; Ma, Y.; Wang, F.; Liao, Y.; Chen, Y.; Zhao, B.; Zhao, Q.; Wang, D.; Tang, D. Igniting Hope for Tumor Immunotherapy: Promoting the “Hot and Cold” Tumor Transition. Clin. Med. Insights Oncol. 2022, 16, 11795549221120708. [Google Scholar] [CrossRef]
- Smyth, M.J.; Godfrey, D.I.; Trapani, J.A. A Fresh Look at Tumor Immunosurveillance and Immunotherapy. Nat. Immunol. 2001, 2, 293–299. [Google Scholar] [CrossRef]
- Hanahan, D.; Weinberg, R.A. Hallmarks of Cancer: The next Generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef] [PubMed]
- Hegde, P.S.; Karanikas, V.; Evers, S. The Where, the When, and the How of Immune Monitoring for Cancer Immunotherapies in the Era of Checkpoint Inhibition. Clin. Cancer Res. 2016, 22, 1865–1874. [Google Scholar] [CrossRef]
- de Visser, K.E.; Eichten, A.; Coussens, L.M. Paradoxical Roles of the Immune System during Cancer Development. Nat. Rev. Cancer 2006, 6, 24–37. [Google Scholar] [CrossRef] [PubMed]
- Kather, J.N.; Suarez-Carmona, M.; Charoentong, P.; Weis, C.-A.; Hirsch, D.; Bankhead, P.; Horning, M.; Ferber, D.; Kel, I.; Herpel, E.; et al. Topography of Cancer-Associated Immune Cells in Human Solid Tumors. eLife 2018, 7, e36967. [Google Scholar] [CrossRef] [PubMed]
- Troiano, G.; Rubini, C.; Togni, L.; Caponio, V.C.A.; Zhurakivska, K.; Santarelli, A.; Cirillo, N.; Lo Muzio, L.; Mascitti, M. The Immune Phenotype of Tongue Squamous Cell Carcinoma Predicts Early Relapse and Poor Prognosis. Cancer Med. 2020, 9, 8333–8344. [Google Scholar] [CrossRef]
- Duan, Q.; Zhang, H.; Zheng, J.; Zhang, L. Turning Cold into Hot: Firing up the Tumor Microenvironment. Trends Cancer 2020, 6, 605–618. [Google Scholar] [CrossRef]
- Rosenberg, S.A.; Spiess, P.; Lafreniere, R. A New Approach to the Adoptive Immunotherapy of Cancer with Tumor-Infiltrating Lymphocytes. Science 1986, 233, 1318–1321. [Google Scholar] [CrossRef]
- Rosenberg, S.A.; Lotze, M.T.; Muul, L.M.; Chang, A.E.; Avis, F.P.; Leitman, S.; Linehan, W.M.; Robertson, C.N.; Lee, R.E.; Rubin, J.T. A Progress Report on the Treatment of 157 Patients with Advanced Cancer Using Lymphokine-Activated Killer Cells and Interleukin-2 or High-Dose Interleukin-2 Alone. N. Engl. J. Med. 1987, 316, 889–897. [Google Scholar] [CrossRef]
- Mitra, A.; Barua, A.; Huang, L.; Ganguly, S.; Feng, Q.; He, B. From Bench to Bedside: The History and Progress of CAR T Cell Therapy. Front. Immunol. 2023, 14, 1188049. [Google Scholar] [CrossRef]
- Pang, Z.; Wang, Z.; Li, F.; Feng, C.; Mu, X. Current Progress of CAR-NK Therapy in Cancer Treatment. Cancers 2022, 14, 4318. [Google Scholar] [CrossRef]
- Mellman, I.; Coukos, G.; Dranoff, G. Cancer Immunotherapy Comes of Age. Nature 2011, 480, 480–489. [Google Scholar] [CrossRef] [PubMed]
- Kuwana, Y.; Asakura, Y.; Utsunomiya, N.; Nakanishi, M.; Arata, Y.; Itoh, S.; Nagase, F.; Kurosawa, Y. Expression of Chimeric Receptor Composed of Immunoglobulin-Derived V Regions and T-Cell Receptor-Derived C Regions. Biochem. Biophys. Res. Commun. 1987, 149, 960–968. [Google Scholar] [CrossRef] [PubMed]
- Gross, G.; Waks, T.; Eshhar, Z. Expression of Immunoglobulin-T-Cell Receptor Chimeric Molecules as Functional Receptors with Antibody-Type Specificity. Proc. Natl. Acad. Sci. USA 1989, 86, 10024–10028. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Lin, Q.; Song, Y.; Liu, D. Universal CARs, Universal T Cells, and Universal CAR T Cells. J. Hematol. Oncol. 2018, 11, 132. [Google Scholar] [CrossRef]
- Demaria, O.; Gauthier, L.; Debroas, G.; Vivier, E. Natural Killer Cell Engagers in Cancer Immunotherapy: Next Generation of Immuno-Oncology Treatments. Eur. J. Immunol. 2021, 51, 1934–1942. [Google Scholar] [CrossRef]
- Tsao, L.-C.; Force, J.; Hartman, Z.C. Mechanisms of Therapeutic Antitumor Monoclonal Antibodies. Cancer Res. 2021, 81, 4641–4651. [Google Scholar] [CrossRef]
- Ramakrishnan, M.S.; Eswaraiah, A.; Crombet, T.; Piedra, P.; Saurez, G.; Iyer, H.; Arvind, A.S. Nimotuzumab, a Promising Therapeutic Monoclonal for Treatment of Tumors of Epithelial Origin. MAbs 2009, 1, 41–48. [Google Scholar] [CrossRef]
- Parakh, S.; King, D.; Gan, H.K.; Scott, A.M. Current Development of Monoclonal Antibodies in Cancer Therapy. Recent. Results Cancer Res. 2020, 214, 1–70. [Google Scholar] [CrossRef]
- Thomas, A.; Teicher, B.A.; Hassan, R. Antibody-Drug Conjugates for Cancer Therapy. Lancet Oncol. 2016, 17, e254–e262. [Google Scholar] [CrossRef]
- Akbari, B.; Farajnia, S.; Ahdi Khosroshahi, S.; Safari, F.; Yousefi, M.; Dariushnejad, H.; Rahbarnia, L. Immunotoxins in Cancer Therapy: Review and Update. Int. Rev. Immunol. 2017, 36, 207–219. [Google Scholar] [CrossRef]
- Waldhauer, I.; Steinle, A. NK Cells and Cancer Immunosurveillance. Oncogene 2008, 27, 5932–5943. [Google Scholar] [CrossRef] [PubMed]
- Basílio-Queirós, D.; Mischak-Weissinger, E. Natural Killer Cells- from Innate Cells to the Discovery of Adaptability. Front. Immunol. 2023, 14, 1172437. [Google Scholar] [CrossRef] [PubMed]
- Nicholson, S.E.; Keating, N.; Belz, G.T. Natural Killer Cells and Anti-Tumor Immunity. Mol. Immunol. 2019, 110, 40–47. [Google Scholar] [CrossRef] [PubMed]
- Mizutani, Y.; Okada, Y.; Terachi, T.; Yoshida, O. Prognostic Significance of Circulating Cytotoxic Lymphocytes against Autologous Tumors in Patients with Bladder Cancer. J. Urol. 1996, 155, 888–892. [Google Scholar] [CrossRef]
- Imai, K.; Matsuyama, S.; Miyake, S.; Suga, K.; Nakachi, K. Natural Cytotoxic Activity of Peripheral-Blood Lymphocytes and Cancer Incidence: An 11-Year Follow-up Study of a General Population. Lancet 2000, 356, 1795–1799. [Google Scholar] [CrossRef]
- Zhang, M.; Lam, K.-P.; Xu, S. Natural Killer Cell Engagers (NKCEs): A New Frontier in Cancer Immunotherapy. Front. Immunol. 2023, 14, 1207276. [Google Scholar] [CrossRef]
- Gauthier, L.; Morel, A.; Anceriz, N.; Rossi, B.; Blanchard-Alvarez, A.; Grondin, G.; Trichard, S.; Cesari, C.; Sapet, M.; Bosco, F.; et al. Multifunctional Natural Killer Cell Engagers Targeting NKp46 Trigger Protective Tumor Immunity. Cell 2019, 177, 1701–1713.e16. [Google Scholar] [CrossRef]
- Lee, R.B.; Maddineni, S.; Landry, M.; Diaz, C.; Tashfeen, A.; Yamada-Hunter, S.A.; Mackall, C.L.; Beinat, C.; Sunwoo, J.B.; Cochran, J.R. An Engineered NKp46 Antibody for Construction of Multi-Specific NK Cell Engagers. Protein Eng. Des. Sel. 2024, 37, gzae013. [Google Scholar] [CrossRef]
- Zhu, A.; Bai, Y.; Nan, Y.; Ju, D. Natural Killer Cell Engagers: From Bi-Specific to Tri-Specific and Tetra-Specific Engagers for Enhanced Cancer Immunotherapy. Clin. Transl. Med. 2024, 14, e70046. [Google Scholar] [CrossRef]
- Sen, R.; Baltimore, D. Multiple Nuclear Factors Interact with the Immunoglobulin Enhancer Sequences. Cell 1986, 46, 705–716. [Google Scholar] [CrossRef]
- Ghosh, S.; Hayden, M.S. Celebrating 25 Years of NF-κB Research. Immunol. Rev. 2012, 246, 5–13. [Google Scholar] [CrossRef] [PubMed]
- Bassères, D.S.; Baldwin, A.S. Nuclear Factor-kappaB and Inhibitor of kappaB Kinase Pathways in Oncogenic Initiation and Progression. Oncogene 2006, 25, 6817–6830. [Google Scholar] [CrossRef] [PubMed]
- Zinatizadeh, M.R.; Schock, B.; Chalbatani, G.M.; Zarandi, P.K.; Jalali, S.A.; Miri, S.R. The Nuclear Factor Kappa B (NF-kB) Signaling in Cancer Development and Immune Diseases. Genes Dis. 2021, 8, 287–297. [Google Scholar] [CrossRef] [PubMed]
- Zou, J.-Y.; Chen, Q.-L.; Luo, X.-C.; Damdinjav, D.; Abdelmohsen, U.R.; Li, H.-Y.; Battulga, T.; Chen, H.-B.; Wang, Y.-Q.; Zhang, J.-Y. Natural Products Reverse Cancer Multidrug Resistance. Front. Pharmacol. 2024, 15, 1348076. [Google Scholar] [CrossRef] [PubMed]
- Vlahopoulos, S.A. Aberrant Control of NF-κB in Cancer Permits Transcriptional and Phenotypic Plasticity, to Curtail Dependence on Host Tissue: Molecular Mode. Cancer Biol. Med. 2017, 14, 254–270. [Google Scholar] [CrossRef]
- Yu, H.; Lin, L.; Zhang, Z.; Zhang, H.; Hu, H. Targeting NF-κB Pathway for the Therapy of Diseases: Mechanism and Clinical Study. Signal Transduct. Target. Ther. 2020, 5, 209. [Google Scholar] [CrossRef]
- Baud, V.; Karin, M. Is NF-kappaB a Good Target for Cancer Therapy? Hopes and Pitfalls. Nat. Rev. Drug Discov. 2009, 8, 33–40. [Google Scholar] [CrossRef]
- Li, Y.; Zhao, B.; Peng, J.; Tang, H.; Wang, S.; Peng, S.; Ye, F.; Wang, J.; Ouyang, K.; Li, J.; et al. Inhibition of NF-κB Signaling Unveils Novel Strategies to Overcome Drug Resistance in Cancers. Drug Resist. Updat. 2024, 73, 101042. [Google Scholar] [CrossRef]
- Betzler, A.C.; Theodoraki, M.-N.; Schuler, P.J.; Döscher, J.; Laban, S.; Hoffmann, T.K.; Brunner, C. NF-κB and Its Role in Checkpoint Control. Int. J. Mol. Sci. 2020, 21, 3949. [Google Scholar] [CrossRef]
- Grinberg-Bleyer, Y.; Oh, H.; Desrichard, A.; Bhatt, D.M.; Caron, R.; Chan, T.A.; Schmid, R.M.; Klein, U.; Hayden, M.S.; Ghosh, S. NF-κB c-Rel Is Crucial for the Regulatory T Cell Immune Checkpoint in Cancer. Cell 2017, 170, 1096–1108.e13. [Google Scholar] [CrossRef]
- Xia, L.; Tan, S.; Zhou, Y.; Lin, J.; Wang, H.; Oyang, L.; Tian, Y.; Liu, L.; Su, M.; Wang, H.; et al. Role of the NFκB-Signaling Pathway in Cancer. Onco Targets Ther. 2018, 11, 2063–2073. [Google Scholar] [CrossRef] [PubMed]
- Baldo, B.A. Monoclonal Antibodies Approved for Cancer Therapy. Saf. Biol. Ther. 2016, 57. [Google Scholar] [CrossRef]
- Kumar, M.; Jalota, A.; Sahu, S.K.; Haque, S. Therapeutic Antibodies for the Prevention and Treatment of Cancer. J. Biomed. Sci. 2024, 31, 6. [Google Scholar] [CrossRef] [PubMed]
- Shiravand, Y.; Khodadadi, F.; Kashani, S.M.A.; Hosseini-Fard, S.R.; Hosseini, S.; Sadeghirad, H.; Ladwa, R.; O’Byrne, K.; Kulasinghe, A. Immune Checkpoint Inhibitors in Cancer Therapy. Curr. Oncol. 2022, 29, 3044–3060. [Google Scholar] [CrossRef]
- Wada, S.; Kobayashi, S.; Tsunoda, T. Future Prospects for Cancer Immunotherapy—Strategies for Ineffective Cancers. Hum. Vaccines Immunother. 2022, 18, 2031699. [Google Scholar] [CrossRef]
- Schaft, N.; Dörrie, J.; Schuler, G.; Schuler-Thurner, B.; Sallam, H.; Klein, S.; Eisenberg, G.; Frankenburg, S.; Lotem, M.; Khatib, A. The Future of Affordable Cancer Immunotherapy. Front. Immunol. 2023, 14, 1248867. [Google Scholar] [CrossRef]
| Group | Agent | Mechanism of Action |
|---|---|---|
| Therapeutic vaccines | ISA 101 * | Peptide vaccine targeting HPV-16 E6/E7 |
| CIMAvax | Recombinant human EGF-rp64k | |
| IPI-549 | A specific PI3K inhibitor | |
| UCPVax | Universal cancer peptides derived from hTERT | |
| UV1 * | Three synthetic long peptides derived from hTERT | |
| VTX-2337 | Toll-like receptor 8 agonist | |
| PDS0101 * | Liposomal-based HPV-16 E6/E7 multipeptide vaccine | |
| CMP-001 | Virus-like particle containing Toll-like receptor 9 agonist | |
| N 803 | Interleukin-15 superagonist complex | |
| NT-I7 | Recombinant human interleukin-17 | |
| Immune checkpoints | Pembrolizumab ** | Anti-programmed cell death 1 antibody |
| Nivolumab ** | Anti-programmed cell death 1 antibody | |
| Durvalumab ** | Anti-programmed cell death ligand 1 antibody | |
| Tremelimumab | Anti-cytotoxic T lymphocyte-associated protein 4 | |
| Ipilimumab ** | Anti-cytotoxic T lymphocyte-associated protein 4 | |
| Atezolizumab ** | Anti-programmed cell death-ligand 1 antibody | |
| Avelumab ** | Anti-programmed cell death-ligand 1 antibody | |
| Cemiplimab ** | Anti-programmed cell death 1 antibody | |
| Monoclonal antibodies | Rituximab ** | Anti-CD20 |
| Cetuximab ** | Anti-epidermal growth factor receptor antibody | |
| Palbociclib | Anti-cyclin-dependent kinase 4/6 | |
| Monalizumab | Anti-NKG2A antibody | |
| Ramucirumab ** | Anti-vascular endothelial growth factor receptor 2 | |
| Bevacizumab ** | Anti-vascular endothelial growth factor A | |
| Panitumumab ** | Anti-epidermal growth factor receptor antibody | |
| Trastuzumab ** | Anti-HER 2 | |
| Denosumab ** | Anti-RANKL | |
| NK cell-based therapies | NK Cell ADCC | NK cell antibody-dependent cellular cytotoxicity |
| CAR-NK cell therapy | Adoptive transfer of genetically engineered CAR-NK cells | |
| BiKE | Bispecific killer cell engager | |
| TriKE | Trispecific killer cell engager | |
| Adaptive cell therapy | Infusion of CTL | Adoptive transfer of autologous or allogenic CTLs |
| TCR-T cell therapy | Adoptive transfer of clone TCR containing T cells | |
| CAR-T cell therapy ** | Adoptive transfer of genetically modified CAR-T cells | |
| Other Personalized therapy | AlloVax | Personalized cancer vaccine from the patient’s tumor |
| PNeoVCA | Personalized peptide-based vaccine with 20 neoantigens | |
| PANDA-VAC | Personalized and adjusted neoantigen peptide vaccine |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Devaraja, K.; Singh, M.; Sharan, K.; Aggarwal, S. Coley’s Toxin to First Approved Therapeutic Vaccine—A Brief Historical Account in the Progression of Immunobiology-Based Cancer Treatment. Biomedicines 2024, 12, 2746. https://doi.org/10.3390/biomedicines12122746
Devaraja K, Singh M, Sharan K, Aggarwal S. Coley’s Toxin to First Approved Therapeutic Vaccine—A Brief Historical Account in the Progression of Immunobiology-Based Cancer Treatment. Biomedicines. 2024; 12(12):2746. https://doi.org/10.3390/biomedicines12122746
Chicago/Turabian StyleDevaraja, K., Manisha Singh, Krishna Sharan, and Sadhna Aggarwal. 2024. "Coley’s Toxin to First Approved Therapeutic Vaccine—A Brief Historical Account in the Progression of Immunobiology-Based Cancer Treatment" Biomedicines 12, no. 12: 2746. https://doi.org/10.3390/biomedicines12122746
APA StyleDevaraja, K., Singh, M., Sharan, K., & Aggarwal, S. (2024). Coley’s Toxin to First Approved Therapeutic Vaccine—A Brief Historical Account in the Progression of Immunobiology-Based Cancer Treatment. Biomedicines, 12(12), 2746. https://doi.org/10.3390/biomedicines12122746






