The Role of Protein Kinase C During the Differentiation of Stem and Precursor Cells into Tissue Cells
Abstract
1. Introduction
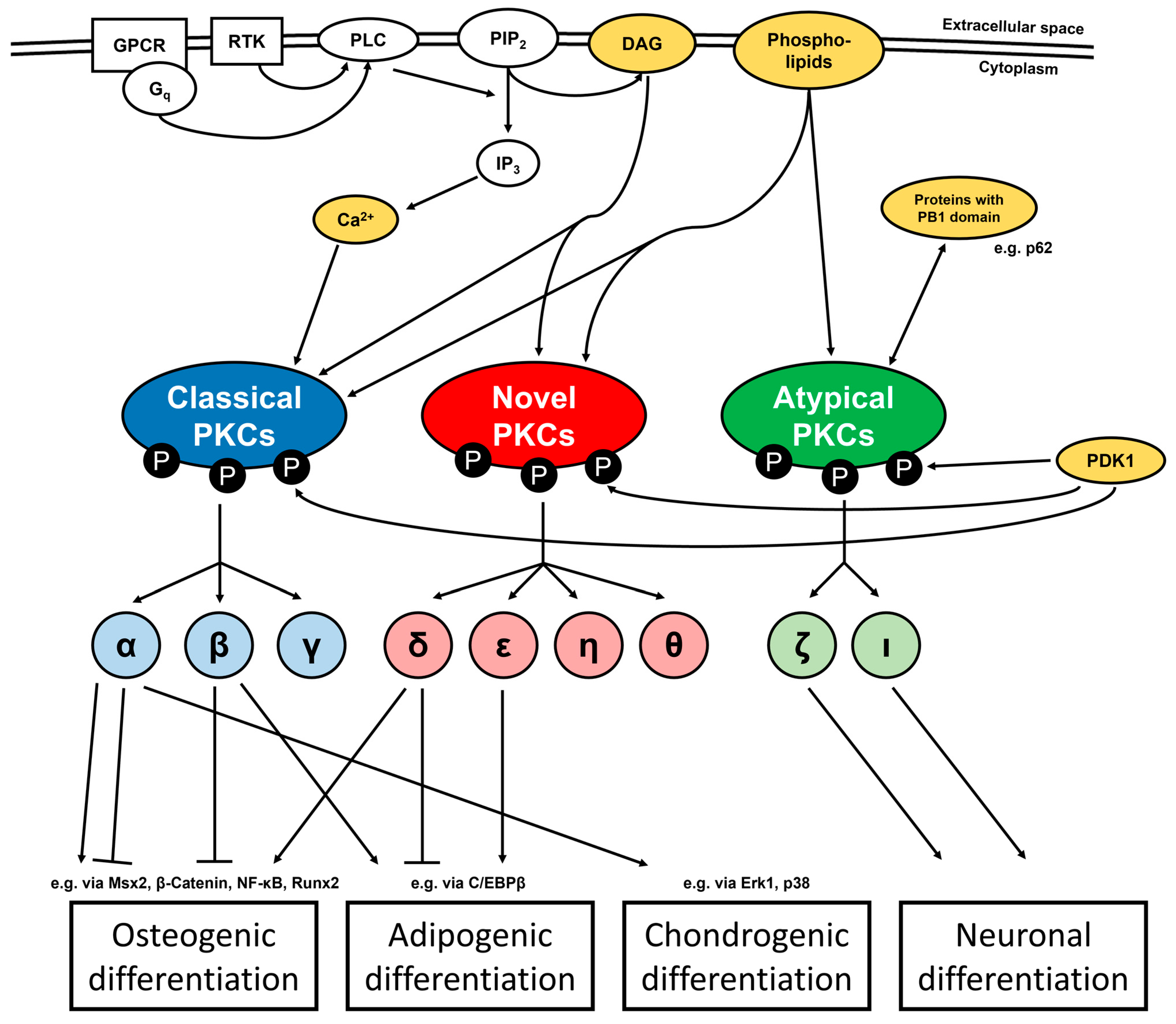
2. Regulation of Biological Processes by PKC
3. Impact of PKC on General Stem Cell Properties
4. Role of PKC During Osteogenic Differentiation
4.1. General Influence of PKC on Osteogenesis
4.2. Impact of Particular PKC Isoforms on Osteogenesis
4.3. Downstream Targets Mediating the Influence of PKC on Osteogenesis
4.4. Endogenous Regulation of PKC During Osteogenesis
4.5. Impact of PKC on Bone Resorption
5. Role of PKC During Adipogenic Differentiation
5.1. General Influence of PKC or Certain Isoforms During Adipogenesis
5.2. Endogenous Regulation of PKC During Adipogenesis
5.3. Downstream Targets Mediating the Influence of PKC on Adipogenesis
6. Role of PKC During Chondrogenic Differentiation
6.1. General Influence of PKC or Certain Isoforms During Chondrogenesis
6.2. Endogenous Regulation of PKC During Chondrogenesis
6.3. Downstream Targets Mediating the Influence of PKC on Chondrogenesis
7. Role of PKC During Differentiation into Other Tissue Cells
7.1. Role of PKC During Neuronal Differentiation
7.2. Role of PKC During Differentiation into Keratinocytes and Cardiomyocytes
8. Conclusions and Outlook
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| ADSCs | adipose-derived stem cells |
| AMPK | AMP-activated protein kinase |
| BMMSCs | bone marrow-derived mesenchymal stem cells |
| BMP2 | bone morphogenetic protein 2 |
| C/EBPβ | CCAAT/enhancer-binding protein β |
| DAG | diacylglycerol |
| DFCs | dental follicle cells |
| GPCRs | G protein-coupled receptors |
| IGF-1 | insulin-like growth factor 1 |
| IGF-2 | insulin-like growth factor 2 |
| IKK | IκB kinase |
| IP3 | inositol 1,4,5-trisphosphate |
| MAPK | mitogen-activated protein kinase |
| MSCs | mesenchymal stem cells |
| Msx2 | Msh homeobox 2 |
| NF-κB | nuclear factor “kappa-light-chain-enhancer” of activated B cells |
| PB1 | Phox and Bem1 |
| PDK1 | 3-phosphoinositide-dependent protein kinase-1 |
| PKA | protein kinase A |
| PKC | protein kinase C |
| PLC | phospholipase C |
| PTH | parathyroid hormone |
| RANKL | receptor activator of nuclear factor kappa B ligand |
| RTKs | receptor tyrosine kinases |
References
- Inoue, M.; Kishimoto, A.; Takai, Y.; Nishizuka, Y. Studies on a cyclic nucleotide-independent protein kinase and its proenzyme in mammalian tissues. II. Proenzyme and its activation by calcium-dependent protease from rat brain. J. Biol. Chem. 1977, 252, 7610–7616. [Google Scholar] [CrossRef] [PubMed]
- Kalive, M.; Faust, J.J.; Koeneman, B.A.; Capco, D.G. Involvement of the PKC family in regulation of early development. Mol. Reprod. Dev. 2010, 77, 95–104. [Google Scholar] [CrossRef] [PubMed]
- Rieger, M.E.; Zhou, B.; Solomon, N.; Sunohara, M.; Li, C.; Nguyen, C.; Liu, Y.; Pan, J.; Minoo, P.; Crandall, E.D.; et al. p300/β-Catenin Interactions Regulate Adult Progenitor Cell Differentiation Downstream of WNT5a/Protein Kinase C (PKC). J. Biol. Chem. 2016, 291, 6569–6582. [Google Scholar] [CrossRef] [PubMed]
- Mellor, H.; Parker, P.J. The extended protein kinase C superfamily. Biochem. J. 1998, 332 Pt 2, 281–292. [Google Scholar] [CrossRef] [PubMed]
- Goetzl, E.J.; Alpert, J.S.; Chen, Q.M. Human Stem Cells in Regenerative Medicine. Am. J. Med. 2024, 137, 805–809. [Google Scholar] [CrossRef]
- Dekker, L.V.; Palmer, R.H.; Parker, P.J. The protein kinase C and protein kinase C related gene families. Curr. Opin. Struct. Biol. 1995, 5, 396–402. [Google Scholar] [CrossRef]
- Nishizuka, Y. Discovery and prospect of protein kinase C research: Epilogue. J. Biochem. 2003, 133, 155–158. [Google Scholar] [CrossRef]
- Velnati, S.; Centonze, S.; Girivetto, F.; Capello, D.; Biondi, R.M.; Bertoni, A.; Cantello, R.; Ragnoli, B.; Malerba, M.; Graziani, A.; et al. Identification of Key Phospholipids That Bind and Activate Atypical PKCs. Biomedicines 2021, 9, 45. [Google Scholar] [CrossRef]
- Battaini, F.; Pascale, A. Protein kinase C signal transduction regulation in physiological and pathological aging. Ann. N. Y. Acad. Sci. 2005, 1057, 177–192. [Google Scholar] [CrossRef]
- Nishizuka, Y. The role of protein kinase C in cell surface signal transduction and tumour promotion. Nature 1984, 308, 693–698. [Google Scholar] [CrossRef]
- Singer, W.D.; Brown, H.A.; Sternweis, P.C. Regulation of eukaryotic phosphatidylinositol-specific phospholipase C and phospholipase D. Annu. Rev. Biochem. 1997, 66, 475–509. [Google Scholar] [CrossRef] [PubMed]
- Keranen, L.M.; Dutil, E.M.; Newton, A.C. Protein kinase C is regulated in vivo by three functionally distinct phosphorylations. Curr. Biol. 1995, 5, 1394–1403. [Google Scholar] [CrossRef] [PubMed]
- Le Good, J.A.; Ziegler, W.H.; Parekh, D.B.; Alessi, D.R.; Cohen, P.; Parker, P.J. Protein kinase C isotypes controlled by phosphoinositide 3-kinase through the protein kinase PDK1. Science 1998, 281, 2042–2045. [Google Scholar] [CrossRef] [PubMed]
- Balendran, A.; Hare, G.R.; Kieloch, A.; Williams, M.R.; Alessi, D.R. Further evidence that 3-phosphoinositide-dependent protein kinase-1 (PDK1) is required for the stability and phosphorylation of protein kinase C (PKC) isoforms. FEBS Lett. 2000, 484, 217–223. [Google Scholar] [CrossRef] [PubMed]
- Zaffagnini, G.; Savova, A.; Danieli, A.; Romanov, J.; Tremel, S.; Ebner, M.; Peterbauer, T.; Sztacho, M.; Trapannone, R.; Tarafder, A.K.; et al. p62 filaments capture and present ubiquitinated cargos for autophagy. EMBO J. 2018, 37, e98308. [Google Scholar] [CrossRef]
- Ren, J.; Wang, J.; Wang, Z.; Wu, J. Structural and biochemical insights into the homotypic PB1-PB1 complex between PKCζ and p62. Sci. China Life Sci. 2014, 57, 69–80. [Google Scholar] [CrossRef]
- Newton, A.C. Protein kinase C: Poised to signal. Am. J. Physiol. Endocrinol. Metab. 2010, 298, E395–E402. [Google Scholar] [CrossRef]
- Pu, Y.; Garfield, S.H.; Kedei, N.; Blumberg, P.M. Characterization of the differential roles of the twin C1a and C1b domains of protein kinase Cδ. J. Biol. Chem. 2009, 284, 1302–1312. [Google Scholar] [CrossRef]
- House, C.; Kemp, B.E. Protein kinase C contains a pseudosubstrate prototope in its regulatory domain. Science 1987, 238, 1726–1728. [Google Scholar] [CrossRef]
- Wu-Zhang, A.X.; Newton, A.C. Protein kinase C pharmacology: Refining the toolbox. Biochem. J. 2013, 452, 195–209. [Google Scholar] [CrossRef]
- Al-Alem, L.F.; McCord, L.A.; Southard, R.C.; Kilgore, M.W.; Curry, T.E. Activation of the PKC pathway stimulates ovarian cancer cell proliferation, migration, and expression of MMP7 and MMP10. Biol. Reprod. 2013, 89, 73. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.S.; Krebs, C.E.; Liu, S.J. Proliferation of human breast cancer cells and anti-cancer action of doxorubicin and vinblastine are independent of PKC-α. J. Cell. Biochem. 2007, 101, 517–528. [Google Scholar] [CrossRef] [PubMed]
- Galron, D.; Tamir, A.; Altman, A.; Isakov, N. Inhibition of PMA-induced human T cell proliferation by bryostatin is associated with enhanced degradation of conventional protein kinase C (cPKC): Ca2+ signals restore mitogenic activity without abrogating enhanced cPKC degradation. Cell. Immunol. 1994, 158, 195–207. [Google Scholar] [CrossRef] [PubMed]
- Caverzasio, J.; Biver, E.; Thouverey, C. Predominant role of PDGF receptor transactivation in Wnt3a-induced osteoblastic cell proliferation. J. Bone Miner. Res. 2013, 28, 260–270. [Google Scholar] [CrossRef]
- Russell, C.; Acevedo-Duncan, M. Effects of the PKC inhibitor PD 406976 on cell cycle progression, proliferation, PKC isozymes and apoptosis in glioma and SVG-transformed glial cells. Cell Prolif. 2005, 38, 87–106. [Google Scholar] [CrossRef]
- Chang, G.; Zheng, J.; Xiao, W.; Chang, S.; Wei, Q.; Wu, H.; Tao, Y.; Yang, G.; Xie, B.; Lan, X.; et al. PKC inhibition of sotrastaurin has antitumor activity in diffuse large B-cell lymphoma via regulating the expression of MCT-1. Acta Biochim. Biophys. Sin. 2018, 50, 399–407. [Google Scholar] [CrossRef]
- Liu, S.; Zhang, Y.; Yang, Q.; Zhang, Y.; Liu, H.; Huang, M.-H.; Wang, R.; Lu, F. PKC signal amplification suppresses non-small cell lung cancer growth by promoting p21 expression and phosphorylation. Heliyon 2022, 8, e10657. [Google Scholar] [CrossRef]
- Karhu, S.T.; Ruskoaho, H.; Talman, V. Distinct Regulation of Cardiac Fibroblast Proliferation and Transdifferentiation by Classical and Novel Protein Kinase C Isoforms: Possible Implications for New Antifibrotic Therapies. Mol. Pharmacol. 2021, 99, 104–113. [Google Scholar] [CrossRef]
- Tanaka, Y.; Gavrielides, M.V.; Mitsuuchi, Y.; Fujii, T.; Kazanietz, M.G. Protein kinase C promotes apoptosis in LNCaP prostate cancer cells through activation of p38 MAPK and inhibition of the Akt survival pathway. J. Biol. Chem. 2003, 278, 33753–33762. [Google Scholar] [CrossRef]
- Barboule, N.; Lafon, C.; Chadebech, P.; Vidal, S.; Valette, A. Involvement of p21 in the PKC-induced regulation of the G2/M cell cycle transition. FEBS Lett. 1999, 444, 32–37. [Google Scholar] [CrossRef]
- Wouters, M.M.; Roeder, J.L.; Tharayil, V.S.; Stanich, J.E.; Strege, P.R.; Lei, S.; Bardsley, M.R.; Ordog, T.; Gibbons, S.J.; Farrugia, G. Protein kinase Cγ mediates regulation of proliferation by the serotonin 5-hydroxytryptamine receptor 2B. J. Biol. Chem. 2009, 284, 21177–21184. [Google Scholar] [CrossRef] [PubMed]
- Murillo-Carretero, M.; Geribaldi-Doldán, N.; Flores-Giubi, E.; García-Bernal, F.; Navarro-Quiroz, E.A.; Carrasco, M.; Macías-Sánchez, A.J.; Herrero-Foncubierta, P.; Delgado-Ariza, A.; Verástegui, C.; et al. ELAC (3,12-di-O-acetyl-8-O-tigloilingol), a plant-derived lathyrane diterpene, induces subventricular zone neural progenitor cell proliferation through PKCβ activation. Br. J. Pharmacol. 2017, 174, 2373–2392. [Google Scholar] [CrossRef] [PubMed]
- Aaltonen, V.; Koivunen, J.; Laato, M.; Peltonen, J. PKC inhibitor Go6976 induces mitosis and enhances doxorubicin-paclitaxel cytotoxicity in urinary bladder carcinoma cells. Cancer Lett. 2007, 253, 97–107. [Google Scholar] [CrossRef] [PubMed]
- Poli, A.; Ramazzotti, G.; Matteucci, A.; Manzoli, L.; Lonetti, A.; Suh, P.-G.; McCubrey, J.A.; Cocco, L. A novel DAG-dependent mechanism links PKCa and Cyclin B1 regulating cell cycle progression. Oncotarget 2014, 5, 11526–11540. [Google Scholar] [CrossRef] [PubMed]
- Qiao, L.; Xu, Y.; Liu, X.; Xie, J.; Wang, J.; Du, C.; Zhang, J.; Ni, W.; Chen, S. PKC promotes proliferation of airway smooth muscle cells by regulating cyclinD1 expression in asthmatic rats. Acta Pharmacol. Sin. 2008, 29, 677–686. [Google Scholar] [CrossRef]
- Galea, G.L.; Meakin, L.B.; Williams, C.M.; Hulin-Curtis, S.L.; Lanyon, L.E.; Poole, A.W.; Price, J.S. Protein kinase Cα (PKCα) regulates bone architecture and osteoblast activity. J. Biol. Chem. 2014, 289, 25509–25522. [Google Scholar] [CrossRef]
- Poli, A.; Ratti, S.; Finelli, C.; Mongiorgi, S.; Clissa, C.; Lonetti, A.; Cappellini, A.; Catozzi, A.; Barraco, M.; Suh, P.-G.; et al. Nuclear translocation of PKC-α is associated with cell cycle arrest and erythroid differentiation in myelodysplastic syndromes (MDSs). FASEB J. 2018, 32, 681–692. [Google Scholar] [CrossRef]
- Oliva, J.L.; Caino, M.C.; Senderowicz, A.M.; Kazanietz, M.G. S-Phase-specific activation of PKCα induces senescence in non-small cell lung cancer cells. J. Biol. Chem. 2008, 283, 5466–5476. [Google Scholar] [CrossRef]
- Luan, Y.; Zhang, J.; Wang, M.; Fu, G.; Zhang, W. Advanced glycation end products facilitate the proliferation and reduce early apoptosis of cardiac microvascular endothelial cells via PKCβ signaling pathway: Insight from diabetic cardiomyopathy. Anatol. J. Cardiol. 2020, 23, 141–150. [Google Scholar] [CrossRef]
- Pysz, M.A.; Hao, F.; Hizli, A.A.; Lum, M.A.; Swetzig, W.M.; Black, A.R.; Black, J.D. Differential regulation of cyclin D1 expression by protein kinase C α and ϵ signaling in intestinal epithelial cells. J. Biol. Chem. 2014, 289, 22268–22283. [Google Scholar] [CrossRef]
- Youmell, M.; Park, S.J.; Basu, S.; Price, B.D. Regulation of the p53 protein by protein kinase Cα and protein kinase Cζ. Biochem. Biophys. Res. Commun. 1998, 245, 514–518. [Google Scholar] [CrossRef] [PubMed]
- Deeds, L.; Teodorescu, S.; Chu, M.; Yu, Q.; Chen, C.-Y. A p53-independent G1 cell cycle checkpoint induced by the suppression of protein kinase C α and θ isoforms. J. Biol. Chem. 2003, 278, 39782–39793. [Google Scholar] [CrossRef] [PubMed]
- Gao, Q.; Tan, J.; Ma, P.; Ge, J.; Liu, Y.; Sun, X.; Zhou, L. PKC alpha affects cell cycle progression and proliferation in human RPE cells through the downregulation of p27kip1. Mol. Vis. 2009, 15, 2683–2695. [Google Scholar] [PubMed]
- Skaletz-Rorowski, A.; Eschert, H.; Leng, J.; Stallmeyer, B.; Sindermann, J.R.; Pulawski, E.; Breithardt, G. PKC δ-induced activation of MAPK pathway is required for bFGF-stimulated proliferation of coronary smooth muscle cells. Cardiovasc. Res. 2005, 67, 142–150. [Google Scholar] [CrossRef]
- Okuwa, H.; Kanno, T.; Fujita, Y.; Gotoh, A.; Tabata, C.; Fukuoka, K.; Nakano, T.; Nishizaki, T. Sphingosine suppresses mesothelioma cell proliferation by inhibiting PKC-δ and inducing cell cycle arrest at the G0/G1 phase. Cell. Physiol. Biochem. 2012, 30, 995–1004. [Google Scholar] [CrossRef]
- BommaReddy, R.R.; Patel, R.; Smalley, T.; Acevedo-Duncan, M. Effects of Atypical Protein Kinase C Inhibitor (DNDA) on Lung Cancer Proliferation and Migration by PKC-ι/FAK Ubiquitination Through the Cbl-b Pathway. OncoTargets Ther. 2020, 13, 1661–1676. [Google Scholar] [CrossRef]
- Pillai, P.; Desai, S.; Patel, R.; Sajan, M.; Farese, R.; Ostrov, D.; Acevedo-Duncan, M. A novel PKC-ι inhibitor abrogates cell proliferation and induces apoptosis in neuroblastoma. Int. J. Biochem. Cell Biol. 2011, 43, 784–794. [Google Scholar] [CrossRef]
- Carlin, S.; Yang, K.X.; Donnelly, R.; Black, J.L. Protein kinase C isoforms in human airway smooth muscle cells: Activation of PKC-ζ during proliferation. Am. J. Physiol. 1999, 276, L506–L512. [Google Scholar] [CrossRef]
- Emoto, Y.; Manome, Y.; Meinhardt, G.; Kisaki, H.; Kharbanda, S.; Robertson, M.; Ghayur, T.; Wong, W.W.; Kamen, R.; Weichselbaum, R. Proteolytic activation of protein kinase C delta by an ICE-like protease in apoptotic cells. EMBO J. 1995, 14, 6148–6156. [Google Scholar] [CrossRef]
- Basu, A.; Lu, D.; Sun, B.; Moor, A.N.; Akkaraju, G.R.; Huang, J. Proteolytic activation of protein kinase C-epsilon by caspase-mediated processing and transduction of antiapoptotic signals. J. Biol. Chem. 2002, 277, 41850–41856. [Google Scholar] [CrossRef]
- Datta, R.; Kojima, H.; Yoshida, K.; Kufe, D. Caspase-3-mediated cleavage of protein kinase C θ in induction of apoptosis. J. Biol. Chem. 1997, 272, 20317–20320. [Google Scholar] [CrossRef]
- Heathcote, H.R.; Mancini, S.J.; Strembitska, A.; Jamal, K.; Reihill, J.A.; Palmer, T.M.; Gould, G.W.; Salt, I.P. Protein kinase C phosphorylates AMP-activated protein kinase α1 Ser487. Biochem. J. 2016, 473, 4681–4697. [Google Scholar] [CrossRef] [PubMed]
- Blair, D.; Dufort, F.J.; Chiles, T.C. Protein kinase Cβ is critical for the metabolic switch to glycolysis following B-cell antigen receptor engagement. Biochem. J. 2012, 448, 165–169. [Google Scholar] [CrossRef] [PubMed]
- Basu, A. Regulation of Autophagy by Protein Kinase C-ε in Breast Cancer Cells. Int. J. Mol. Sci. 2020, 21, 4247. [Google Scholar] [CrossRef] [PubMed]
- Herzig, S.; Shaw, R.J. AMPK: Guardian of metabolism and mitochondrial homeostasis. Nat. Rev. Mol. Cell Biol. 2018, 19, 121–135. [Google Scholar] [CrossRef]
- Nowak, G.; Bakajsova, D.; Samarel, A.M. Protein kinase C-epsilon activation induces mitochondrial dysfunction and fragmentation in renal proximal tubules. Am. J. Physiol. Ren. Physiol. 2011, 301, F197–F208. [Google Scholar] [CrossRef]
- Gong, J.; Hoyos, B.; Acin-Perez, R.; Vinogradov, V.; Shabrova, E.; Zhao, F.; Leitges, M.; Fischman, D.; Manfredi, G.; Hammerling, U. Two protein kinase C isoforms, δ and ε, regulate energy homeostasis in mitochondria by transmitting opposing signals to the pyruvate dehydrogenase complex. FASEB J. 2012, 26, 3537–3549. [Google Scholar] [CrossRef]
- Nowak, G. Protein kinase C-α and ERK1/2 mediate mitochondrial dysfunction, decreases in active Na+ transport, and cisplatin-induced apoptosis in renal cells. J. Biol. Chem. 2002, 277, 43377–43388. [Google Scholar] [CrossRef]
- Mahato, B.; Home, P.; Rajendran, G.; Paul, A.; Saha, B.; Ganguly, A.; Ray, S.; Roy, N.; Swerdlow, R.H.; Paul, S. Regulation of mitochondrial function and cellular energy metabolism by protein kinase C-λ/ι: A novel mode of balancing pluripotency. Stem Cells 2014, 32, 2880–2892. [Google Scholar] [CrossRef]
- Baker, C.L.; Pera, M.F. Capturing Totipotent Stem Cells. Cell Stem Cell 2018, 22, 25–34. [Google Scholar] [CrossRef]
- Ding, D.-C.; Shyu, W.-C.; Lin, S.-Z. Mesenchymal stem cells. Cell Transplant. 2011, 20, 5–14. [Google Scholar] [CrossRef] [PubMed]
- Shostak, S. (Re)defining stem cells. Bioessays 2006, 28, 301–308. [Google Scholar] [CrossRef] [PubMed]
- Digirolamo, C.M.; Stokes, D.; Colter, D.; Phinney, D.G.; Class, R.; Prockop, D.J. Propagation and senescence of human marrow stromal cells in culture: A simple colony-forming assay identifies samples with the greatest potential to propagate and differentiate. Br. J. Haematol. 1999, 107, 275–281. [Google Scholar] [CrossRef] [PubMed]
- Dominici, M.; Le Blanc, K.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F.; Krause, D.; Deans, R.; Keating, A.; Prockop, D.; Horwitz, E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 2006, 8, 315–317. [Google Scholar] [CrossRef]
- Bi, F.; Xiong, J.; Han, X.; Yang, C.; Li, X.; Chen, G.; Guo, W.; Tian, W. Dental follicle cells show potential for treating Parkinson’s disease through dopaminergic-neuronogenic differentiation. Hum. Cell 2022, 35, 1708–1721. [Google Scholar] [CrossRef]
- Jeong, H.M.; Jin, Y.-H.; Choi, Y.H.; Yum, J.; Choi, J.-K.; Yeo, C.-Y.; Lee, K.-Y. PKC signaling inhibits osteogenic differentiation through the regulation of Msx2 function. Biochim. Biophys. Acta BBA Mol. Cell Res. 2012, 1823, 1225–1232. [Google Scholar] [CrossRef]
- Hoang, D.M.; Pham, P.T.; Bach, T.Q.; Ngo, A.T.L.; Nguyen, Q.T.; Phan, T.T.K.; Nguyen, G.H.; Le, P.T.T.; van Hoang, T.; Forsyth, N.R.; et al. Stem cell-based therapy for human diseases. Signal Transduct. Target. Ther. 2022, 7, 272. [Google Scholar] [CrossRef]
- Feng, X.; Zhang, J.; Smuga-Otto, K.; Tian, S.; Yu, J.; Stewart, R.; Thomson, J.A. Protein kinase C mediated extraembryonic endoderm differentiation of human embryonic stem cells. Stem Cells 2012, 30, 461–470. [Google Scholar] [CrossRef]
- Ranieri, D.; Nanni, M.; Persechino, F.; Torrisi, M.R.; Belleudi, F. Role of PKCε in the epithelial-mesenchymal transition induced by FGFR2 isoform switch. Cell Commun. Signal. 2020, 18, 76. [Google Scholar] [CrossRef]
- Zafar, A.; Wu, F.; Hardy, K.; Li, J.; Tu, W.J.; McCuaig, R.; Harris, J.; Khanna, K.K.; Attema, J.; Gregory, P.A.; et al. Chromatinized protein kinase C-θ directly regulates inducible genes in epithelial to mesenchymal transition and breast cancer stem cells. Mol. Cell. Biol. 2014, 34, 2961–2980. [Google Scholar] [CrossRef]
- Varum, S.; Momcilović, O.; Castro, C.; Ben-Yehudah, A.; Ramalho-Santos, J.; Navara, C.S. Enhancement of human embryonic stem cell pluripotency through inhibition of the mitochondrial respiratory chain. Stem Cell Res. 2009, 3, 142–156. [Google Scholar] [CrossRef] [PubMed]
- Cho, Y.M.; Kwon, S.; Pak, Y.K.; Seol, H.W.; Choi, Y.M.; Park, D.J.; Park, K.S.; Lee, H.K. Dynamic changes in mitochondrial biogenesis and antioxidant enzymes during the spontaneous differentiation of human embryonic stem cells. Biochem. Biophys. Res. Commun. 2006, 348, 1472–1478. [Google Scholar] [CrossRef] [PubMed]
- Fu, X.; Liu, G.; Halim, A.; Ju, Y.; Luo, Q.; Song, A.G. Mesenchymal Stem Cell Migration and Tissue Repair. Cells 2019, 8, 784. [Google Scholar] [CrossRef] [PubMed]
- He, H.; Zhao, Z.-H.; Han, F.-S.; Wang, X.-F.; Zeng, Y.-J. Activation of protein kinase C ε enhanced movement ability and paracrine function of rat bone marrow mesenchymal stem cells partly at least independent of SDF-1/CXCR4 axis and PI3K/AKT pathway. Int. J. Clin. Exp. Med. 2015, 8, 188–202. [Google Scholar]
- Lin, C.-Y.; Zu, C.-H.; Yang, C.-C.; Tsai, P.-J.; Shyu, J.-F.; Chen, C.-P.; Weng, Z.-C.; Chen, T.-H.; Wang, H.-S. IL-1β-Induced Mesenchymal Stem Cell Migration Involves MLCK Activation via PKC Signaling. Cell Transplant. 2015, 24, 2011–2028. [Google Scholar] [CrossRef]
- Siddhanti, S.R.; Quarles, L.D. Molecular to pharmacologic control of osteoblast proliferation and differentiation. J. Cell. Biochem. 1994, 55, 310–320. [Google Scholar] [CrossRef]
- Filipak, M.; Estervig, D.N.; Tzen, C.Y.; Minoo, P.; Hoerl, B.J.; Maercklein, P.B.; Zschunke, M.A.; Edens, M.; Scott, R.E. Integrated control of proliferation and differentiation of mesenchymal stem cells. Environ. Health Perspect. 1989, 80, 117–125. [Google Scholar] [CrossRef]
- Santos, A.; Bakker, A.D.; de Blieck-Hogervorst, J.M.A.; Klein-Nulend, J. WNT5A induces osteogenic differentiation of human adipose stem cells via rho-associated kinase ROCK. Cytotherapy 2010, 12, 924–932. [Google Scholar] [CrossRef]
- Olivares-Navarrete, R.; Vesper, K.; Hyzy, S.L.; Almaguer-Flores, A.; Boyan, B.D.; Schwartz, Z. Role of the N-terminal peptide of amelogenin on osteoblastic differentiation of human mesenchymal stem cells. Eur. Cells Mater. 2014, 28, 1–10. [Google Scholar] [CrossRef]
- Li, S.; Hu, C.; Li, J.; Liu, L.; Jing, W.; Tang, W.; Tian, W.; Long, J. Effect of miR-26a-5p on the Wnt/Ca2+ Pathway and Osteogenic Differentiation of Mouse Adipose-Derived Mesenchymal Stem Cells. Calcif. Tissue Int. 2016, 99, 174–186. [Google Scholar] [CrossRef]
- Liu, Y.; Yang, R.; Liu, X.; Zhou, Y.; Qu, C.; Kikuiri, T.; Wang, S.; Zandi, E.; Du, J.; Ambudkar, I.S.; et al. Hydrogen sulfide maintains mesenchymal stem cell function and bone homeostasis via regulation of Ca2+ channel sulfhydration. Cell Stem Cell 2014, 15, 66–78. [Google Scholar] [CrossRef] [PubMed]
- Richardson, J.A.; Amantea, C.M.; Kianmahd, B.; Tetradis, S.; Lieberman, J.R.; Hahn, T.J.; Parhami, F. Oxysterol-induced osteoblastic differentiation of pluripotent mesenchymal cells is mediated through a PKC- and PKA-dependent pathway. J. Cell. Biochem. 2007, 100, 1131–1145. [Google Scholar] [CrossRef] [PubMed]
- Song, J.K.; Lee, C.H.; Hwang, S.-M.; Joo, B.S.; Lee, S.Y.; Jung, J.S. Effect of phorbol 12-myristate 13-acetate on the differentiation of adipose-derived stromal cells from different subcutaneous adipose tissue depots. Korean J. Physiol. Pharmacol. 2014, 18, 289–296. [Google Scholar] [CrossRef] [PubMed]
- Ogura, H.; Nakamura, T.; Ishii, T.; Saito, A.; Onodera, S.; Yamaguchi, A.; Nishii, Y.; Azuma, T. Mechanical stress-induced FGF-2 promotes proliferation and consequently induces osteoblast differentiation in mesenchymal stem cells. Biochem. Biophys. Res. Commun. 2023, 684, 149145. [Google Scholar] [CrossRef]
- Xia, S.-L.; Ma, Z.-Y.; Wang, B.; Gao, F.; Guo, S.-Y.; Chen, X.-H. Icariin promotes the proliferation and osteogenic differentiation of bone-derived mesenchymal stem cells in patients with osteoporosis and T2DM by upregulating GLI-1. J. Orthop. Surg. Res. 2023, 18, 500. [Google Scholar] [CrossRef]
- He, X.; Wang, H.; Jin, T.; Xu, Y.; Mei, L.; Yang, J. TLR4 Activation Promotes Bone Marrow MSC Proliferation and Osteogenic Differentiation via Wnt3a and Wnt5a Signaling. PLoS ONE 2016, 11, e0149876. [Google Scholar] [CrossRef]
- Chen, G.; Huang, G.; Lin, H.; Wu, X.; Tan, X.; Chen, Z. MicroRNA-425-5p modulates osteoporosis by targeting annexin A2. Immun. Ageing 2021, 18, 45. [Google Scholar] [CrossRef]
- Lotz, E.M.; Berger, M.B.; Boyan, B.D.; Schwartz, Z. Regulation of mesenchymal stem cell differentiation on microstructured titanium surfaces by semaphorin 3A. Bone 2020, 134, 115260. [Google Scholar] [CrossRef]
- Liu, J.; Someren, E.; Mentink, A.; Licht, R.; Dechering, K.; van Blitterswijk, C.; de Boer, J. The effect of PKC activation and inhibition on osteogenic differentiation of human mesenchymal stem cells. J. Tissue Eng. Regen. Med. 2010, 4, 329–339. [Google Scholar] [CrossRef]
- Nakura, A.; Higuchi, C.; Yoshida, K.; Yoshikawa, H. PKCα suppresses osteoblastic differentiation. Bone 2011, 48, 476–484. [Google Scholar] [CrossRef]
- Liu, Z.; Liu, H.; He, J.; Lin, P.; Tong, Q.; Yang, J. Myeloma cells shift osteoblastogenesis to adipogenesis by inhibiting the ubiquitin ligase MURF1 in mesenchymal stem cells. Sci. Signal. 2020, 13, eaay8203. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Wang, X.; Zhang, L.; Wang, B.; Xu, B.; Zhang, J. GLP-1 inhibits PKCβ2 phosphorylation to improve the osteogenic differentiation potential of hPDLSCs in the AGE microenvironment. J. Diabetes Complicat. 2020, 34, 107495. [Google Scholar] [CrossRef] [PubMed]
- Hua, W.-K.; Shiau, Y.-H.; Lee, O.K.; Lin, W.-J. Elevation of protein kinase Cα stimulates osteogenic differentiation of mesenchymal stem cells through the TAT-mediated protein transduction system. Biochem. Cell Biol. 2013, 91, 443–448. [Google Scholar] [CrossRef] [PubMed]
- Miraoui, H.; Oudina, K.; Petite, H.; Tanimoto, Y.; Moriyama, K.; Marie, P.J. Fibroblast growth factor receptor 2 promotes osteogenic differentiation in mesenchymal cells via ERK1/2 and protein kinase C signaling. J. Biol. Chem. 2009, 284, 4897–4904. [Google Scholar] [CrossRef]
- Kuo, S.-W.; Rimando, M.G.; Liu, Y.-S.; Lee, O.K. Intermittent Administration of Parathyroid Hormone 1-34 Enhances Osteogenesis of Human Mesenchymal Stem Cells by Regulating Protein Kinase Cδ. Int. J. Mol. Sci. 2017, 18, 2221. [Google Scholar] [CrossRef]
- Zhu, F.; Sweetwyne, M.T.; Hankenson, K.D. PKCδ is required for Jagged-1 induction of human mesenchymal stem cell osteogenic differentiation. Stem Cells 2013, 31, 1181–1192. [Google Scholar] [CrossRef]
- Lee, S.; Cho, H.-Y.; Bui, H.T.T.; Kang, D. The osteogenic or adipogenic lineage commitment of human mesenchymal stem cells is determined by protein kinase C delta. BMC Cell Biol. 2014, 15, 42. [Google Scholar] [CrossRef]
- Smyth, D.C.; Takenaka, S.; Yeung, C.; Richards, C.D. Oncostatin M regulates osteogenic differentiation of murine adipose-derived mesenchymal progenitor cells through a PKCdelta-dependent mechanism. Cell Tissue Res. 2015, 360, 309–319. [Google Scholar] [CrossRef]
- Tu, X.; Joeng, K.S.; Nakayama, K.I.; Nakayama, K.; Rajagopal, J.; Carroll, T.J.; McMahon, A.P.; Long, F. Noncanonical Wnt signaling through G protein-linked PKCδ activation promotes bone formation. Dev. Cell 2007, 12, 113–127. [Google Scholar] [CrossRef]
- Xi, G.; Shen, X.; Rosen, C.J.; Clemmons, D.R. IRS-1 Functions as a Molecular Scaffold to Coordinate IGF-I/IGFBP-2 Signaling During Osteoblast Differentiation. J. Bone Miner. Res. 2016, 31, 1300–1314. [Google Scholar] [CrossRef]
- Pieles, O.; Reichert, T.E.; Morsczeck, C. Classical isoforms of protein kinase C (PKC) and Akt regulate the osteogenic differentiation of human dental follicle cells via both β-catenin and NF-κB. Stem Cell Res. Ther. 2021, 12, 242. [Google Scholar] [CrossRef] [PubMed]
- De Pellegrin, M.; Reck, A.; Morsczeck, C. Sclerostin inhibits Protein kinase C inhibitor GÖ6976 induced osteogenic differentiation of dental follicle cells. Tissue Cell 2024, 90, 102522. [Google Scholar] [CrossRef] [PubMed]
- Lampasso, J.; Chen, W.; Marzec, N. The expression profile of PKC isoforms during MC3T3-E1 differentiation. Int. J. Mol. Med. 2006, 17, 1125–1131. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.-G.; Kim, H.-J.; Park, H.-J.; Kim, Y.-J.; Yoon, W.-J.; Lee, S.-J.; Ryoo, H.-M.; Cho, J.-Y. Runx2 phosphorylation induced by fibroblast growth factor-2/protein kinase C pathways. Proteomics 2006, 6, 1166–1174. [Google Scholar] [CrossRef] [PubMed]
- Cho, M.; Park, S.; Gwak, J.; Kim, D.-E.; Yea, S.S.; Shin, J.-G.; Oh, S. Bisindoylmaleimide I suppresses adipocyte differentiation through stabilization of intracellular β-catenin protein. Biochem. Biophys. Res. Commun. 2008, 367, 195–200. [Google Scholar] [CrossRef]
- Duong, M.; Yu, X.; Teng, B.; Schroder, P.; Haller, H.; Eschenburg, S.; Schiffer, M. Protein kinase C ϵ stabilizes β-catenin and regulates its subcellular localization in podocytes. J. Biol. Chem. 2017, 292, 12100–12110. [Google Scholar] [CrossRef]
- Choi, S.-W.; Song, J.-K.; Yim, Y.-S.; Yun, H.-G.; Chun, K.-H. Glucose deprivation triggers protein kinase C-dependent β-catenin proteasomal degradation. J. Biol. Chem. 2015, 290, 9863–9873. [Google Scholar] [CrossRef]
- Gwak, J.; Cho, M.; Gong, S.-J.; Won, J.; Kim, D.-E.; Kim, E.-Y.; Lee, S.S.; Kim, M.; Kim, T.K.; Shin, J.-G.; et al. Protein-kinase-C-mediated β-catenin phosphorylation negatively regulates the Wnt/β-catenin pathway. J. Cell Sci. 2006, 119, 4702–4709. [Google Scholar] [CrossRef]
- Shirakawa, F.; Mizel, S.B. In vitro activation and nuclear translocation of NF-κB catalyzed by cyclic AMP-dependent protein kinase and protein kinase C. Mol. Cell. Biol. 1989, 9, 2424–2430. [Google Scholar] [CrossRef]
- Lallena, M.J.; Diaz-Meco, M.T.; Bren, G.; Payá, C.V.; Moscat, J. Activation of IκB kinase β by protein kinase C isoforms. Mol. Cell. Biol. 1999, 19, 2180–2188. [Google Scholar] [CrossRef]
- Basson, M.D.; Zeng, B.; Downey, C.; Sirivelu, M.P.; Tepe, J.J. Increased extracellular pressure stimulates tumor proliferation by a mechanosensitive calcium channel and PKC-β. Mol. Oncol. 2015, 9, 513–526. [Google Scholar] [CrossRef] [PubMed]
- Xue, N.; Qi, L.; Zhang, G.; Zhang, Y. miRNA-125b Regulates Osteogenic Differentiation of Periodontal Ligament Cells Through NKIRAS2/NF-κB Pathway. Cell. Physiol. Biochem. 2018, 48, 1771–1781. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Zhang, X.; Ge, W.; Zhou, Y. Knockdown of LAP2α inhibits osteogenic differentiation of human adipose-derived stem cells by activating NF-κB. Stem Cell Res. Ther. 2020, 11, 263. [Google Scholar] [CrossRef] [PubMed]
- Ning, T.; Guo, H.; Ma, M.; Zha, Z. BRD4 facilitates osteogenic differentiation of human bone marrow mesenchymal stem cells through WNT4/NF-κB pathway. J. Orthop. Surg. Res. 2023, 18, 876. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Wang, Y.; Xue, J.; Xu, Q.; Zhang, Y.; Liu, J.; Xu, H.; Guan, Z.; Bian, C.; Zhang, G.; et al. Baicalin can enhance odonto/osteogenic differentiation of inflammatory dental pulp stem cells by inhibiting the NF-κB and β-catenin/Wnt signaling pathways. Mol. Biol. Rep. 2023, 50, 4435–4446. [Google Scholar] [CrossRef]
- Morsczeck, C.; Götz, W.; Schierholz, J.; Zeilhofer, F.; Kühn, U.; Möhl, C.; Sippel, C.; Hoffmann, K.H. Isolation of precursor cells (PCs) from human dental follicle of wisdom teeth. Matrix Biol. 2005, 24, 155–165. [Google Scholar] [CrossRef]
- Pan, K.; Sun, Q.; Zhang, J.; Ge, S.; Li, S.; Zhao, Y.; Yang, P. Multilineage differentiation of dental follicle cells and the roles of Runx2 over-expression in enhancing osteoblast/cementoblast-related gene expression in dental follicle cells. Cell Prolif. 2010, 43, 219–228. [Google Scholar] [CrossRef]
- Viale-Bouroncle, S.; Klingelhöffer, C.; Ettl, T.; Reichert, T.E.; Morsczeck, C. A protein kinase A (PKA)/β-catenin pathway sustains the BMP2/DLX3-induced osteogenic differentiation in dental follicle cells (DFCs). Cell. Signal. 2015, 27, 598–605. [Google Scholar] [CrossRef]
- Du, Y.; Ling, J.; Wei, X.; Ning, Y.; Xie, N.; Gu, H.; Yang, F. Wnt/β-catenin signaling participates in cementoblast/osteoblast differentiation of dental follicle cells. Connect. Tissue Res. 2012, 53, 390–397. [Google Scholar] [CrossRef]
- MacDonald, B.T.; Tamai, K.; He, X. Wnt/β-catenin signaling: Components, mechanisms, and diseases. Dev. Cell 2009, 17, 9–26. [Google Scholar] [CrossRef]
- Jouishomme, H.; Whitfield, J.F.; Chakravarthy, B.; Durkin, J.P.; Gagnon, L.; Isaacs, R.J.; MacLean, S.; Neugebauer, W.; Willick, G.; Rixon, R.H. The protein kinase-C activation domain of the parathyroid hormone. Endocrinology 1992, 130, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; Guo, J.; Divieti, P.; Bringhurst, F.R. Parathyroid hormone activates PKC-δ and regulates osteoblastic differentiation via a PLC-independent pathway. Bone 2006, 38, 485–496. [Google Scholar] [CrossRef] [PubMed]
- McCarty, M.F. Elevated sympathetic activity may promote insulin resistance syndrome by activating alpha-1 adrenergic receptors on adipocytes. Med. Hypotheses 2004, 62, 830–838. [Google Scholar] [CrossRef] [PubMed]
- Dossing, D.; Radeff, J.; Sanders, J.; Lee, S.-K.; Hsieh, M.-R.; Stern, P. Parathyroid hormone stimulates translocation of protein kinase C isozymes in UMR-106 osteoblastic osteosarcoma cells. Bone 2001, 29, 223–230. [Google Scholar] [CrossRef]
- Kulebyakin, K.; Tyurin-Kuzmin, P.; Sozaeva, L.; Voloshin, N.; Nikolaev, M.; Chechekhin, V.; Vigovskiy, M.; Sysoeva, V.; Korchagina, E.; Naida, D.; et al. Dynamic Balance between PTH1R-Dependent Signal Cascades Determines Its Pro- or Anti-Osteogenic Effects on MSC. Cells 2022, 11, 3519. [Google Scholar] [CrossRef]
- Kohn, A.D.; Moon, R.T. Wnt and calcium signaling: β-Catenin-independent pathways. Cell Calcium 2005, 38, 439–446. [Google Scholar] [CrossRef]
- Keller, K.C.; Ding, H.; Tieu, R.; Sparks, N.R.L.; Ehnes, D.D.; zur Nieden, N.I. Wnt5a Supports Osteogenic Lineage Decisions in Embryonic Stem Cells. Stem Cells Dev. 2016, 25, 1020–1032. [Google Scholar] [CrossRef]
- Koyanagi, M.; Iwasaki, M.; Haendeler, J.; Leitges, M.; Zeiher, A.M.; Dimmeler, S. Wnt5a increases cardiac gene expressions of cultured human circulating progenitor cells via a PKC delta activation. PLoS ONE 2009, 4, e5765. [Google Scholar] [CrossRef]
- Yao, J.; Li, J.; Zhou, L.; Cheng, J.; Chim, S.M.; Zhang, G.; Quinn, J.M.W.; Tickner, J.; Zhao, J.; Xu, J. Protein kinase C inhibitor, GF109203X attenuates osteoclastogenesis, bone resorption and RANKL-induced NF-κB and NFAT activity. J. Cell. Physiol. 2015, 230, 1235–1242. [Google Scholar] [CrossRef]
- Pang, C.; Wen, L.; Qin, H.; Zhu, B.; Lu, X.; Luo, S. Sotrastaurin, a PKC inhibitor, attenuates RANKL-induced bone resorption and attenuates osteochondral pathologies associated with the development of OA. J. Cell. Mol. Med. 2020, 24, 8452–8465. [Google Scholar] [CrossRef]
- Lee, S.; Kwak, H.; Chung, W.; Cheong, H.; Kim, H.-H.; Lee, Z. Participation of protein kinase c β in osteoclast differentiation and function. Bone 2003, 32, 217–227. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.Y.; Lee, K.; Shin, H.-I.; Jeong, D. Specific targeting of PKCδ suppresses osteoclast differentiation by accelerating proteolysis of membrane-bound macrophage colony-stimulating factor receptor. Sci. Rep. 2019, 9, 7044. [Google Scholar] [CrossRef] [PubMed]
- Durán, A.; Serrano, M.; Leitges, M.; Flores, J.M.; Picard, S.; Brown, J.P.; Moscat, J.; Diaz-Meco, M.T. The Atypical PKC-Interacting Protein p62 Is an Important Mediator of RANK-Activated Osteoclastogenesis. Dev. Cell 2004, 6, 303–309. [Google Scholar] [CrossRef] [PubMed]
- Wada, T.; Nakashima, T.; Hiroshi, N.; Penninger, J.M. RANKL-RANK signaling in osteoclastogenesis and bone disease. Trends Mol. Med. 2006, 12, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Valverde, A.M.; Lorenzo, M.; Navarro, P.; Benito, M. Phosphatidylinositol 3-kinase is a requirement for insulin-like growth factor I-induced differentiation, but not for mitogenesis, in fetal brown adipocytes. Mol. Endocrinol. 1997, 11, 595–607. [Google Scholar] [CrossRef]
- Zhang, K.; Wang, F.; Huang, J.; Lou, Y.; Xie, J.; Li, H.; Cao, D.; Huang, X. Insulin-like growth factor 2 promotes the adipogenesis of hemangioma-derived stem cells. Exp. Ther. Med. 2019, 17, 1663–1669. [Google Scholar] [CrossRef]
- Zhang, X.-X.; Wang, Y.-M.; Su, Y.-D.; Zuo, F.; Wu, B.; Nian, X. MiR-26a regulated adipogenic differentiation of ADSCs induced by insulin through CDK5/FOXC2 pathway. Mol. Cell. Biochem. 2021, 476, 1705–1716. [Google Scholar] [CrossRef]
- Morsczeck, C.; Moehl, C.; Götz, W.; Heredia, A.; Schäffer, T.E.; Eckstein, N.; Sippel, C.; Hoffmann, K.H. In vitro differentiation of human dental follicle cells with dexamethasone and insulin. Cell Biol. Int. 2005, 29, 567–575. [Google Scholar] [CrossRef]
- Saugspier, M.; Felthaus, O.; Viale-Bouroncle, S.; Driemel, O.; Reichert, T.E.; Schmalz, G.; Morsczeck, C. The differentiation and gene expression profile of human dental follicle cells. Stem Cells Dev. 2010, 19, 707–717. [Google Scholar] [CrossRef]
- Keats, E.C.; Dominguez, J.M.; Grant, M.B.; Khan, Z.A. Switch from canonical to noncanonical Wnt signaling mediates high glucose-induced adipogenesis. Stem Cells 2014, 32, 1649–1660. [Google Scholar] [CrossRef]
- Zhou, Y.; Wang, D.; Li, F.; Shi, J.; Song, J. Different roles of protein kinase C-βI and -δ in the regulation of adipocyte differentiation. Int. J. Biochem. Cell Biol. 2006, 38, 2151–2163. [Google Scholar] [CrossRef] [PubMed]
- Gaillard, D.; Négrel, R.; Lagarde, M.; Ailhaud, G. Requirement and role of arachidonic acid in the differentiation of pre-adipose cells. Biochem. J. 1989, 257, 389–397. [Google Scholar] [CrossRef] [PubMed]
- Artemenko, Y.; Gagnon, A.; Aubin, D.; Sorisky, A. Anti-adipogenic effect of PDGF is reversed by PKC inhibition. J. Cell. Physiol. 2005, 204, 646–653. [Google Scholar] [CrossRef] [PubMed]
- Shinohara, O.; Murata, Y.; Shimizu, M. Endothelin-1 suppression of rat adipocyte precursor cell differentiation in serum-free culture. Endocrinology 1992, 130, 2031–2036. [Google Scholar] [CrossRef]
- Wang, T.; Wang, Y.; Yamashita, H. Evodiamine inhibits adipogenesis via the EGFR-PKCα-ERK signaling pathway. FEBS Lett. 2009, 583, 3655–3659. [Google Scholar] [CrossRef]
- Pavan, C.; Vindigni, V.; Michelotto, L.; Rimessi, A.; Abatangelo, G.; Cortivo, R.; Pinton, P.; Zavan, B. Weight gain related to treatment with atypical antipsychotics is due to activation of PKC-β. Pharmacogenom. J. 2010, 10, 408–417. [Google Scholar] [CrossRef]
- Fleming, I.; MacKenzie, S.J.; Vernon, R.G.; Anderson, N.G.; Houslay, M.D.; Kilgour, E. Protein kinase C isoforms play differential roles in the regulation of adipocyte differentiation. Biochem. J. 1998, 333 Pt 3, 719–727. [Google Scholar] [CrossRef]
- McGowan, K.; DeVente, J.; Carey, J.O.; Ways, D.K.; Pekala, P.H. Protein kinase C isoform expression during the differentiation of 3T3-L1 preadipocytes: Loss of protein kinase C-α isoform correlates with loss of phorbol 12-myristate 13-acetate activation of nuclear factor κB and acquisition of the adipocyte phenotype. J. Cell. Physiol. 1996, 167, 113–120. [Google Scholar] [CrossRef]
- Carter, G.; Apostolatos, A.; Patel, R.; Mathur, A.; Cooper, D.; Murr, M.; Patel, N.A. Dysregulated Alternative Splicing Pattern of PKCδ during Differentiation of Human Preadipocytes Represents Distinct Differences between Lean and Obese Adipocytes. ISRN Obes. 2013, 2013, 161345. [Google Scholar] [CrossRef]
- Webb, P.R.; Doyle, C.; Anderson, N.G. Protein kinase C-epsilon promotes adipogenic commitment and is essential for terminal differentiation of 3T3-F442A preadipocytes. Cell. Mol. Life Sci. 2003, 60, 1504–1512. [Google Scholar] [CrossRef]
- Yu, Y.-H.; Liao, P.-R.; Guo, C.-J.; Chen, C.-H.; Mochly-Rosen, D.; Chuang, L.-M. PKC-ALDH2 Pathway Plays a Novel Role in Adipocyte Differentiation. PLoS ONE 2016, 11, e0161993. [Google Scholar] [CrossRef] [PubMed]
- Lacasa, D.; Agli, B.; Giudicelli, Y. ζPKC in rat preadipocytes: Modulation by insulin and serum mitogenic factors and possible role in adipogenesis. Biochem. Biophys. Res. Commun. 1995, 217, 123–130. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Li, X.; Tang, Q.-Q. Transcriptional regulation of adipocyte differentiation: A central role for CCAAT/enhancer-binding protein (C/EBP) β. J. Biol. Chem. 2015, 290, 755–761. [Google Scholar] [CrossRef] [PubMed]
- Kulyk, W.M.; Reichert, C. Staurosporine, a protein kinase inhibitor, stimulates cartilage differentiation by embryonic facial mesenchyme. J. Craniofac. Genet. Dev. Biol. 1992, 12, 90–97. [Google Scholar]
- Choi, B.; Chun, J.S.; Lee, Y.S.; Sonn, J.K.; Kang, S.S. Expression of protein kinase C isozymes that are required for chondrogenesis of chick limb bud mesenchymal cells. Biochem. Biophys. Res. Commun. 1995, 216, 1034–1040. [Google Scholar] [CrossRef]
- Tsai, T.-L.; Manner, P.A.; Li, W.-J. Regulation of mesenchymal stem cell chondrogenesis by glucose through protein kinase C/transforming growth factor signaling. Osteoarthr. Cartil. 2013, 21, 368–376. [Google Scholar] [CrossRef]
- Long, F.; Ornitz, D.M. Development of the endochondral skeleton. Cold Spring Harb. Perspect. Biol. 2013, 5, a008334. [Google Scholar] [CrossRef]
- Yoon, Y.M.; Oh, C.D.; Kim, D.Y.; Lee, Y.S.; Park, J.W.; Huh, T.L.; Kang, S.S.; Chun, J.S. Epidermal growth factor negatively regulates chondrogenesis of mesenchymal cells by modulating the protein kinase C-α, Erk-1, and p38 MAPK signaling pathways. J. Biol. Chem. 2000, 275, 12353–12359. [Google Scholar] [CrossRef]
- Yang, M.S.; Chang, S.H.; Sonn, J.K.; Lee, Y.S.; Kang, S.S.; Park, T.K.; Chun, J.S. Regulation of chondrogenic differentiation of mesenchymes by protein kinase Cα. Mol. Cells 1998, 8, 266–271. [Google Scholar] [CrossRef]
- Lim, Y.B.; Kang, S.S.; Park, T.K.; Lee, Y.S.; Chun, J.S.; Sonn, J.K. Disruption of actin cytoskeleton induces chondrogenesis of mesenchymal cells by activating protein kinase C-α signaling. Biochem. Biophys. Res. Commun. 2000, 273, 609–613. [Google Scholar] [CrossRef]
- Matta, C.; Juhász, T.; Szíjgyártó, Z.; Kolozsvári, B.; Somogyi, C.; Nagy, G.; Gergely, P.; Zákány, R. PKCδ is a positive regulator of chondrogenesis in chicken high density micromass cell cultures. Biochimie 2011, 93, 149–159. [Google Scholar] [CrossRef] [PubMed]
- Oh, C.-D.; Chun, J.-S. Signaling mechanisms leading to the regulation of differentiation and apoptosis of articular chondrocytes by insulin-like growth factor-1. J. Biol. Chem. 2003, 278, 36563–36571. [Google Scholar] [CrossRef] [PubMed]
- Jin, E.-J.; Park, J.-H.; Lee, S.-Y.; Chun, J.-S.; Bang, O.-S.; Kang, S.-S. Wnt-5a is involved in TGF-β3-stimulated chondrogenic differentiation of chick wing bud mesenchymal cells. Int. J. Biochem. Cell Biol. 2006, 38, 183–195. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.-K.; Chang, L.-H.; Hung, S.-H.; Wu, S.-C.; Lee, H.-Y.; Lin, Y.-S.; Chen, C.-H.; Fu, Y.-C.; Wang, G.-J.; Ho, M.-L. Parathyroid hormone 1-34 inhibits terminal differentiation of human articular chondrocytes and osteoarthritis progression in rats. Arthritis Rheum. 2009, 60, 3049–3060. [Google Scholar] [CrossRef]
- Zhen, X.; Wei, L.; Wu, Q.; Zhang, Y.; Chen, Q. Mitogen-activated protein kinase p38 mediates regulation of chondrocyte differentiation by parathyroid hormone. J. Biol. Chem. 2001, 276, 4879–4885. [Google Scholar] [CrossRef]
- Zhang, Y.; Pizzute, T.; Pei, M. A review of crosstalk between MAPK and Wnt signals and its impact on cartilage regeneration. Cell Tissue Res. 2014, 358, 633–649. [Google Scholar] [CrossRef]
- Chang, S.H.; Oh, C.D.; Yang, M.S.; Kang, S.S.; Lee, Y.S.; Sonn, J.K.; Chun, J.S. Protein kinase C regulates chondrogenesis of mesenchymes via mitogen-activated protein kinase signaling. J. Biol. Chem. 1998, 273, 19213–19219. [Google Scholar] [CrossRef]
- Yoon, Y.-M.; Kim, S.-J.; Oh, C.-D.; Ju, J.-W.; Song, W.K.; Yoo, Y.J.; Huh, T.-L.; Chun, J.-S. Maintenance of differentiated phenotype of articular chondrocytes by protein kinase C and extracellular signal-regulated protein kinase. J. Biol. Chem. 2002, 277, 8412–8420. [Google Scholar] [CrossRef]
- Oh, C.D.; Chang, S.H.; Yoon, Y.M.; Lee, S.J.; Lee, Y.S.; Kang, S.S.; Chun, J.S. Opposing role of mitogen-activated protein kinase subtypes, erk-1/2 and p38, in the regulation of chondrogenesis of mesenchymes. J. Biol. Chem. 2000, 275, 5613–5619. [Google Scholar] [CrossRef]
- Lim, Y.-B.; Kang, S.-S.; An, W.G.; Lee, Y.-S.; Chun, J.-S.; Sonn, J.K. Chondrogenesis induced by actin cytoskeleton disruption is regulated via protein kinase C-dependent p38 mitogen-activated protein kinase signaling. J. Cell. Biochem. 2003, 88, 713–718. [Google Scholar] [CrossRef]
- Castagna, M.; Takai, Y.; Kaibuchi, K.; Sano, K.; Kikkawa, U.; Nishizuka, Y. Direct activation of calcium-activated, phospholipid-dependent protein kinase by tumor-promoting phorbol esters. J. Biol. Chem. 1982, 257, 7847–7851. [Google Scholar] [CrossRef] [PubMed]
- Király, M.; Porcsalmy, B.; Pataki, A.; Kádár, K.; Jelitai, M.; Molnár, B.; Hermann, P.; Gera, I.; Grimm, W.-D.; Ganss, B.; et al. Simultaneous PKC and cAMP activation induces differentiation of human dental pulp stem cells into functionally active neurons. Neurochem. Int. 2009, 55, 323–332. [Google Scholar] [CrossRef] [PubMed]
- Lim, H.-S.; Joe, Y.A. A ROCK Inhibitor Blocks the Inhibitory Effect of Chondroitin Sulfate Proteoglycan on Morphological Changes of Mesenchymal Stromal/Stem Cells into Neuron-Like Cells. Biomol. Ther. 2013, 21, 447–453. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Navarro, S.; Marantz, Y.; Eyal, R.; Kalina, M.; Disatnik, M.H.; Mochly-Rosen, D.; Ben-Menahem, D.; Reiss, N.; Naor, Z. Developmental expression of protein kinase C subspecies in rat brain-pituitary axis. Mol. Cell. Endocrinol. 1994, 103, 133–138. [Google Scholar] [CrossRef]
- Tzeng, H.-H.; Hsu, C.-H.; Chung, T.-H.; Lee, W.-C.; Lin, C.-H.; Wang, W.-C.; Hsiao, C.-Y.; Leu, Y.-W.; Wang, T.-H. Cell Signaling and Differential Protein Expression in Neuronal Differentiation of Bone Marrow Mesenchymal Stem Cells with Hypermethylated Salvador/Warts/Hippo (SWH) Pathway Genes. PLoS ONE 2015, 10, e0145542. [Google Scholar] [CrossRef]
- Mardani, M.; Tiraihi, T.; Bathaie, S.Z.; Mirnajafi-Zadeh, J. Comparison of the proteome patterns of adipose-derived stem cells with those treated with selegiline using a two dimensional gel electrophoresis analysis. Biotech. Histochem. 2020, 95, 176–185. [Google Scholar] [CrossRef]
- Tsao, H.-K.; Chiu, P.-H.; Sun, S.H. PKC-dependent ERK phosphorylation is essential for P2X7 receptor-mediated neuronal differentiation of neural progenitor cells. Cell Death Dis. 2013, 4, e751. [Google Scholar] [CrossRef]
- Doonachar, A.; Schoenfeld, A.R. Expression of PKC iota affects neuronal differentiation of PC12 cells at least partly independent of kinase function. CellBio 2014, 3, 1–13. [Google Scholar] [CrossRef]
- Wooten, M.W.; Zhou, G.; Seibenhener, M.L.; Coleman, E.S. A role for zeta protein kinase C in nerve growth factor-induced differentiation of PC12 cells. Cell Growth Differ. 1994, 5, 395–403. [Google Scholar]
- Palazzo, E.; Kellett, M.D.; Cataisson, C.; Bible, P.W.; Bhattacharya, S.; Sun, H.-W.; Gormley, A.C.; Yuspa, S.H.; Morasso, M.I. A novel DLX3-PKC integrated signaling network drives keratinocyte differentiation. Cell Death Differ. 2017, 24, 717–730. [Google Scholar] [CrossRef]
- Jerome-Morais, A.; Rahn, H.R.; Tibudan, S.S.; Denning, M.F. Role for protein kinase C-α in keratinocyte growth arrest. J. Investig. Dermatol. 2009, 129, 2365–2375. [Google Scholar] [CrossRef] [PubMed]
- Kashiwagi, M.; Ohba, M.; Chida, K.; Kuroki, T. Protein kinase C eta (PKC eta): Its involvement in keratinocyte differentiation. J. Biochem. 2002, 132, 853–857. [Google Scholar] [CrossRef] [PubMed]
- Park, E.; Patel, A.N. PKC-delta induces cardiomyogenic gene expression in human adipose-derived stem cells. Biochem. Biophys. Res. Commun. 2010, 393, 582–586. [Google Scholar] [CrossRef] [PubMed]
- He, H.; Zhao, Z.-H.; Han, F.-S.; Liu, X.-H.; Wang, R.; Zeng, Y.-J. Overexpression of protein kinase C ε improves retention and survival of transplanted mesenchymal stem cells in rat acute myocardial infarction. Cell Death Dis. 2016, 7, e2056. [Google Scholar] [CrossRef] [PubMed]
- Rondeau, E.; Guidet, B.; Lacave, R.; Bens, M.; Sraer, J.; Nagamine, Y.; Ardaillou, R.; Sraer, J.D. Nordihydroguaiaretic acid inhibits urokinase synthesis by phorbol myristate acetate-stimulated LLC-PK1 cells. Biochim. Biophys. Acta (BBA) Mol. Cell Res. 1990, 1055, 165–172. [Google Scholar] [CrossRef]
- Song, H.; Hwang, H.J.; Chang, W.; Song, B.-W.; Cha, M.-J.; Kim, I.-K.; Lim, S.; Choi, E.J.; Ham, O.; Lee, C.Y.; et al. Cardiomyocytes from phorbol myristate acetate-activated mesenchymal stem cells restore electromechanical function in infarcted rat hearts. Proc. Natl. Acad. Sci. USA 2011, 108, 296–301. [Google Scholar] [CrossRef]
- Chang, W.; Lim, S.; Song, B.-W.; Lee, C.Y.; Park, M.-S.; Chung, Y.-A.; Yoon, C.; Lee, S.-Y.; Ham, O.; Park, J.-H.; et al. Phorbol myristate acetate differentiates human adipose-derived mesenchymal stem cells into functional cardiogenic cells. Biochem. Biophys. Res. Commun. 2012, 424, 740–746. [Google Scholar] [CrossRef]
- Pieles, O.P. Molekulare Untersuchung des Einflusses der Proteinkinase C auf die Osteogene Differenzierung von Dentalen Follikelzellen. Ph.D. Thesis, University of Regensburg, Regensburg, Germany, 2023. [Google Scholar] [CrossRef]


| Cells | Isoforms | Impact on Osteogenic Differentiation | Ref. | |
|---|---|---|---|---|
| ADSCs (human) | General | + | WNT5A induced PKC activity as well as osteogenic markers and mineralization | [78] |
| ADSCs (human) | General | + | PKC overactivation supported differentiation unless cells were pretreated with a PKC inhibitor | [83] |
| BMMSCs (human) | General | + | N-terminal amelogenin peptide induced both PKC activity and osteogenic differentiation | [79] |
| ADSCs (mouse) | General | + | Overexpression of miR-26a-5p inhibited both osteogenic differentiation and phosphorylation of PKC | [80] |
| BMMSCs (mouse) | General | + | Inhibition of cystathionine-β-synthase inhibited both the osteogenic differentiation and expression of phosphorylated PKC | [81] |
| Bone marrow stroma cells M2-10B4 (mouse) | General | + | Osteogenic markers were inhibited by PKC inhibitors | [82] |
| Myoblasts C2C12 (mouse) | General, | − | Inhibition of classical PKCs and general PKC inhibition induced osteogenic markers, while PKC overactivation inhibited osteogenesis | [66] |
| classical PKCs | − | |||
| BMMSCs (human) | General | +/− | Inhibition of PKC inhibited osteocalcin expression but increased BMP2 expression | [88] |
| DFCs (human) | Classical PKCs | − | Classical PKCs were downregulated during osteogenic differentiation; inhibition of classical PKCs stimulated mineralization | [101] |
| DFCs (human) | Classical PKCs | − | Inhibition of classical PKCs stimulated mineralization, which was impaired by treating cells with the protein sclerostin; expression of sclerostin was downregulated after inhibition of classical PKCs | [102] |
| BMMSCs (human) | Classical PKCs, | − | Inhibition of classical PKCs stimulated osteogenic markers and mineralization; inhibition of PKCδ hampered activity of alkaline phosphatase | [89] |
| PKCδ | + | |||
| BMMSCs (human and mouse) | PKCα | + | Overexpression of PKCα induced osteogenic markers (human and murine BMMSCs) and mineralization (murine BMMSCs) | [93] |
| Embryonic fibroblasts C3H10T1/2 (mouse) | PKCα | + | Inhibition of PKCα hampered osteogenic markers and mineralization | [94] |
| Osteogenic precursor cells MC3T3-E1 (mouse) | PKCα | − | Downregulation of PKCα supported osteogenic differentiation | [90] |
| BMMSCs (human) | PKCα, | +/− | Co-cultivation of BMMSCs and myeloma cells inhibited both mineralization and expression of phosphorylated PKCα und PKCδ, but increased expression of phosphorylated PKCβ1; inhibition of classical PKCssupported mineralization | [91] |
| PKCβ1, | − | |||
| PKCδ | + | |||
| Periodontal ligament stem cells (human) | PKCβ2 | − | Decreased expression of phosphorylated PKCβ2 was associated with increased expression of osteogenic markers | [92] |
| ADSCs (human) | PKCδ | + | Inhibition of PKCδ hampered osteogenic markers | [95] |
| BMMSCs (human) | PKCδ | + | Inhibition of PKCδ hampered Jagged-1-induced osteogenic differentiation | [96] |
| BMMSCs (human) | PKCδ | + | PKCδ was induced during osteogenic differentiation; inhibition of PKCδ hampered osteogenesis | [97] |
| ADSCs (mouse) | PKCδ | + | Oncostatin M stimulated both osteogenic differentiation and activity of PKCδ; downregulation of PKCδ inhibited osteogenesis | [98] |
| Bone marrow stroma cells ST2 (mouse) | PKCδ | + | Downregulation of PKCδ inhibited WNT3A-induced osteogenic differentiation | [99] |
| Osteogenic precursor cells MC3T3-E1 (mouse) | PKCη, | + | Expression of PKCη was associated with expression of osteogenic markers; expression of PKCθ was downregulated following osteogenic induction | [103] |
| PKCθ | − | |||
| Osteogenic precursor cells MC3T3-E1 (mouse) | PKCζ | + | PKCζ was activated after osteogenic induction and supported osteogenesis by phosphorylating vimentin | [100] |
| Cells | Isoforms | Impact on Adipogenic Differentiation | Ref. | |
|---|---|---|---|---|
| BMMSCs (human) | General, | + | PKC activity was induced during adipogenic differentiation; general PKC inhibition and specific inhibition of PKCε hampered adipogenic markers | [140] |
| PKCε | + | |||
| Embryonic fibroblasts 3T3-L1 (mouse) | General, | + | General PKC inhibition and specific inhibition of classical PKCs hampered adipogenic differentiation; inhibition of PKCδ supported adipogenesis | [141] |
| classical PKCs, | + | |||
| PKCδ | − | |||
| Embryonic fibroblasts 3T3-L1 (mouse) | General | + | PKC inhibition hampered adipogenic differentiation | [105] |
| Adipogenic precursor cells Ob1771 (mouse) | General | + | PKC overactivation supported adipogenesis when cells were simultaneously treated with substances that enhance cAMP concentration | [142] |
| ADSCs (human) | General | − | PKC overactivation inhibited adipogenic differentiation unless cells were pretreated with a PKC inhibitor | [83] |
| Embryonic fibroblasts 3T3-L1 (mouse) | General | − | PKC inhibition supported adipogenic differentiation | [143] |
| Adipogenic precursor cells (rat) | General | − | PKC inhibition supported adipogenic differentiation | [144] |
| Embryonic fibroblasts 3T3-F442A (mouse) | PKCα, | − | Expression of PKCα und PKCδ were reduced during adipogenic differentiation; downregulation of PKCγ and PKCε inhibited adipogenesis | [147] |
| PKCδ, | − | |||
| PKCγ, | + | |||
| PKCε | + | |||
| Embryonic fibroblasts 3T3-L1 (mouse) | PKCα | − | Expression of PKCα was downregulated during adipogenesis; expression of PKCβ was temporarily induced during differentiation, but declined at later periods; PKCθ was detected only in differentiated adipocytes | [148] |
| PKCβ | +/− | |||
| PKCθ | + | |||
| Embryonic fibroblasts 3T3-L1 (mouse) | PKCα | − | Phosphorylation of PKCα was associated with inhibition of adipogenesis after treatment with evodiamine | [145] |
| BMMSCs (human) | PKCα, | +/− | Co-cultivation of BMMSCs and myeloma cells supported adipogenesis and enhanced the expression of phosphorylated PKCβ1 while inhibiting the expression of phosphorylated PKCα und PKCδ; the inhibition of classical PKCs hampered adipogenic differentiation | [91] |
| PKCβ1, | + | |||
| PKCδ | − | |||
| ADSCs (human) | PKCβ | + | Activation of PKCβ was associated with induction of adipogenic differentiation by atypical antipsychotics; inhibition of PKCβ hampered adipogenesis | [146] |
| BMMSCs (human) | PKCδ | − | Inhibition of PKCδ induced adipogenic differentiation | [97] |
| Embryonic fibroblasts 3T3-F442A (mouse) | PKCε | + | Adipogenic differentiation stimulated expression of PKCε; overexpression of PKCε supported adipogenesis | [150] |
| Embryonic fibroblasts 3T3-L1 (mouse) | PKCε | + | Adipogenic differentiation was supported by PKCε stimulation and inhibited by PKCε downregulation | [151] |
| Adipogenic precursor cells (rat) | PKCζ | + | Expression of PKCζ in the cytoplasm was enhanced during adipogenic differentiation; insulin treatment increased expression of PKCζ in the cytoplasm, plasma membrane and nucleus | [152] |
| Fetal brown adipocytes (rat) | PKCζ | + | Activation of PKCζ was associated with IGF-1-induced adipogenic differentiation | [135] |
| Group | Isoform | Impact on… | |||
|---|---|---|---|---|---|
| Osteogenic Differentiation | Adipogenic Differentiation | Chondrogenic Differentiation | Neuronal Differentiation | ||
| Classical PKCs | α | +/− | − | + + | + |
| β | − | + + | ? | ? | |
| γ | ? | + | + | + | |
| Novel PKCs | δ | + + | − − | + | + |
| ε | + | + | + | ? | |
| η | + | ? | ? | + | |
| θ | − | + | ? | + | |
| Atypical PKCs | ζ | + | + | ? | + |
| ι | ? | ? | ? | + | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pieles, O.; Morsczeck, C. The Role of Protein Kinase C During the Differentiation of Stem and Precursor Cells into Tissue Cells. Biomedicines 2024, 12, 2735. https://doi.org/10.3390/biomedicines12122735
Pieles O, Morsczeck C. The Role of Protein Kinase C During the Differentiation of Stem and Precursor Cells into Tissue Cells. Biomedicines. 2024; 12(12):2735. https://doi.org/10.3390/biomedicines12122735
Chicago/Turabian StylePieles, Oliver, and Christian Morsczeck. 2024. "The Role of Protein Kinase C During the Differentiation of Stem and Precursor Cells into Tissue Cells" Biomedicines 12, no. 12: 2735. https://doi.org/10.3390/biomedicines12122735
APA StylePieles, O., & Morsczeck, C. (2024). The Role of Protein Kinase C During the Differentiation of Stem and Precursor Cells into Tissue Cells. Biomedicines, 12(12), 2735. https://doi.org/10.3390/biomedicines12122735






