Bone Marrow Stem Cell Population in Single- and Multiple-Level Aspiration
Abstract
1. Introduction
2. Materials and Methods
2.1. Antibodies and Reagents
2.2. Human Subjects
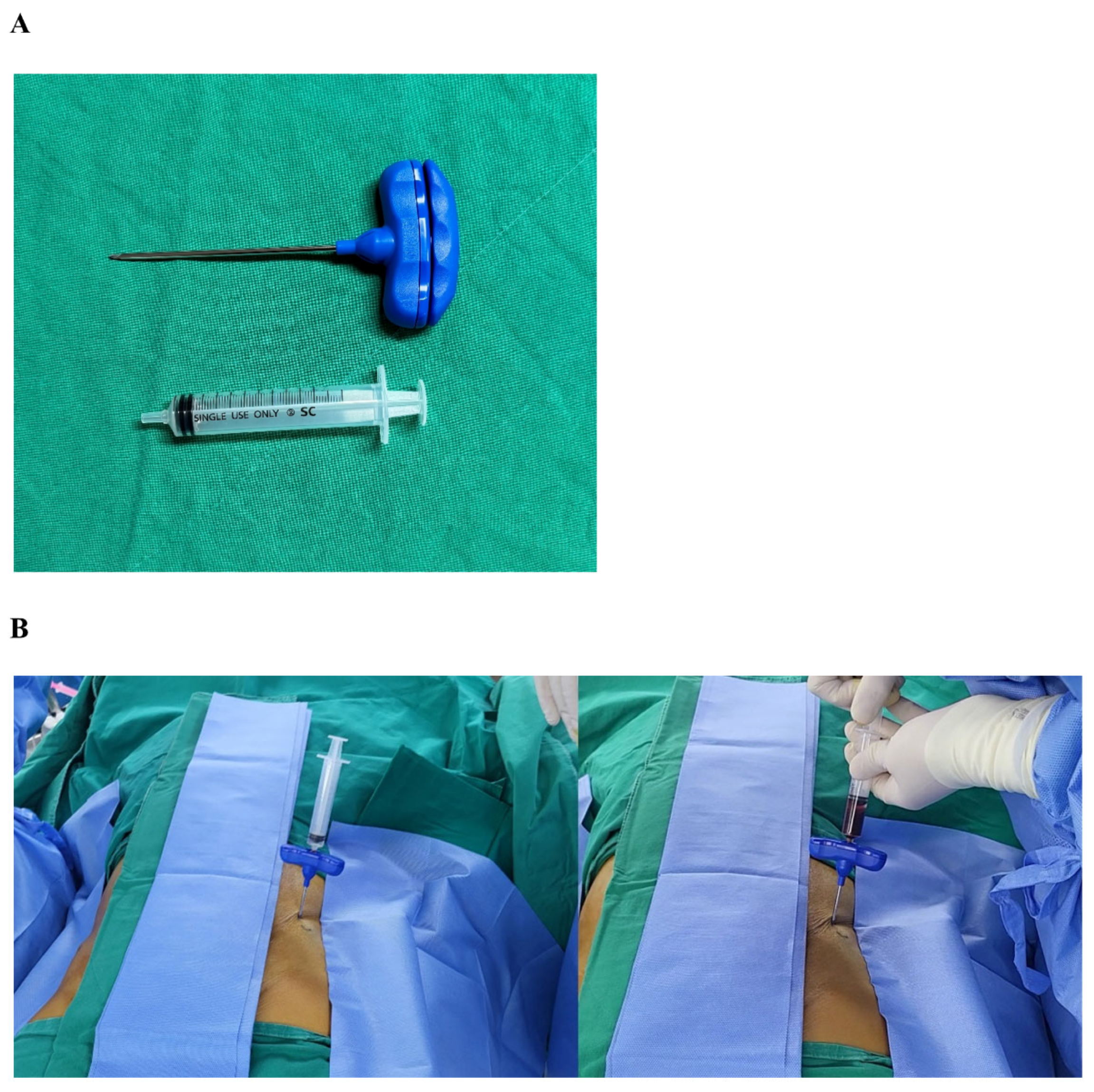
2.3. Bone Marrow Aspiration
2.4. Fluorescence-Activated Cell Sorting
2.5. Cell Seeding and Osteogenic Differentiation
2.6. Alizarin Red Staining
2.7. Statistical Analysis
3. Results
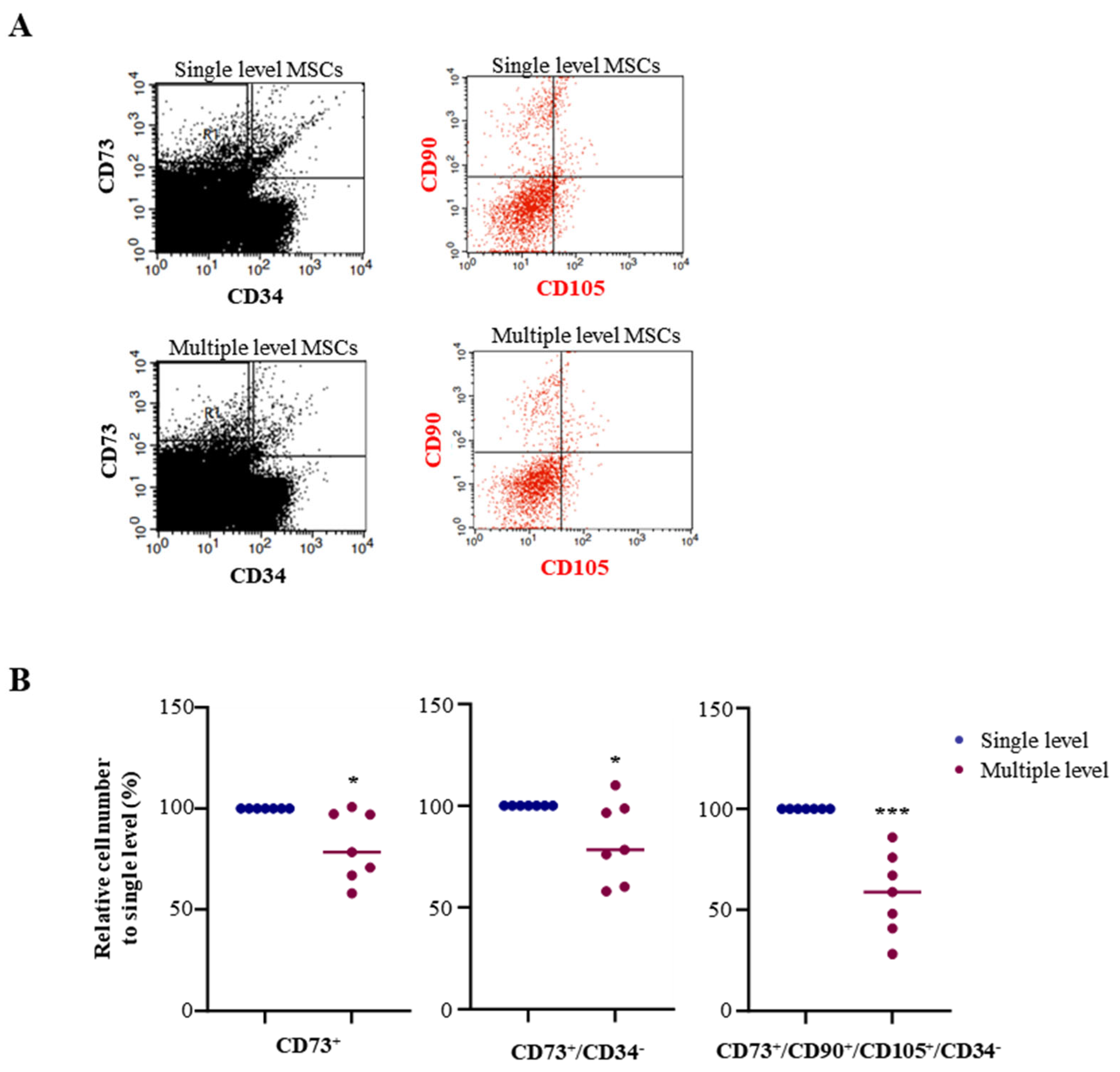
3.1. Advanced MSC Population in a Single-Level Bone Marrow Aspiration
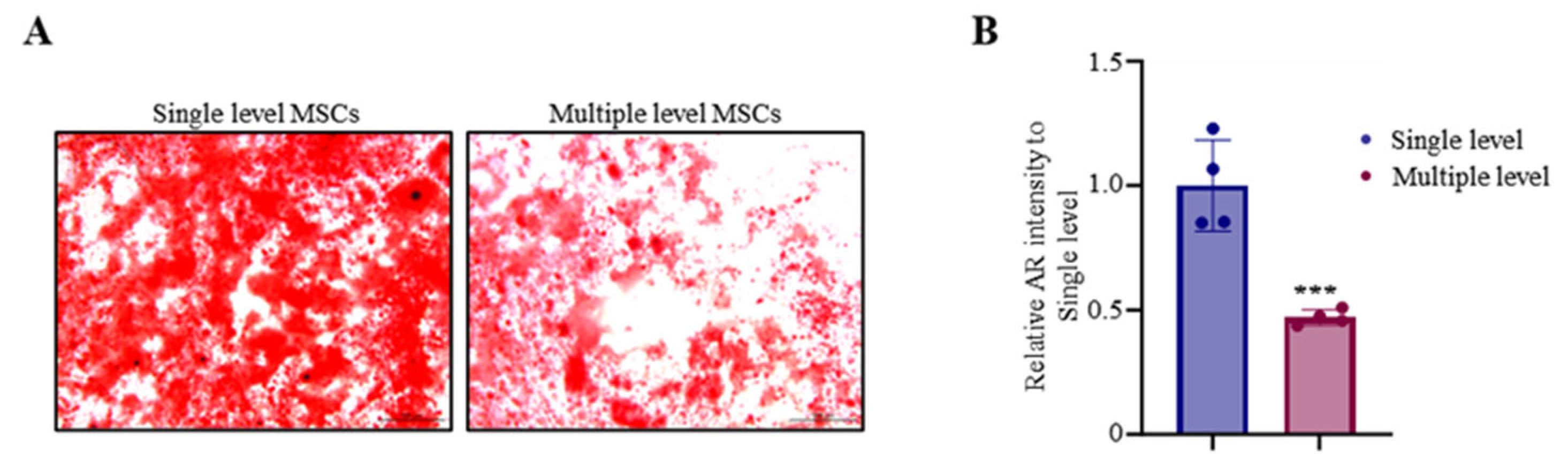
3.2. Advanced Osteoblast Mineralization in Single-Level MSCs
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Reznikov, N.; Shahar, R.; Weiner, S. Bone hierarchical structure in three dimensions. Acta Biomater. 2014, 10, 3815–3826. [Google Scholar] [CrossRef] [PubMed]
- Maestroni, L.; Read, P.; Bishop, C.; Papadopoulos, K.; Suchomel, T.J.; Comfort, P.; Turner, A. The benefits of strength training on musculoskeletal system health: Practical applications for interdisciplinary care. Sports Med. 2020, 50, 1431–1450. [Google Scholar] [CrossRef] [PubMed]
- Briggs, A.M.; Woolf, A.D.; Dreinhofer, K.; Homb, N.; Hoy, D.G.; Kopansky-Giles, D.; Akesson, K.; March, L. Reducing the global burden of musculoskeletal conditions. Bull. World Health Organ. 2018, 96, 366–368. [Google Scholar] [CrossRef] [PubMed]
- Dieleman, J.L.; Cao, J.; Chapin, A.; Chen, C.; Li, Z.; Liu, A.; Horst, C.; Kaldjian, A.; Matyasz, T.; Scott, K.W.; et al. Us health care spending by payer and health condition, 1996–2016. JAMA 2020, 323, 863–884. [Google Scholar] [CrossRef] [PubMed]
- Dieleman, J.L.; Baral, R.; Birger, M.; Bui, A.L.; Bulchis, A.; Chapin, A.; Hamavid, H.; Horst, C.; Johnson, E.K.; Joseph, J.; et al. Us spending on personal health care and public health, 1996–2013. JAMA 2016, 316, 2627–2646. [Google Scholar] [CrossRef]
- Mizoguchi, T.; Ono, N. The diverse origin of bone-forming osteoblasts. J. Bone Miner. Res. 2021, 36, 1432–1447. [Google Scholar] [CrossRef]
- Trapana, J.; Weinerman, J.; Lee, D.; Sedani, A.; Constantinescu, D.; Best, T.M.; Hornicek, F.J., Jr.; Hare, J.M. Cell-based therapy in the treatment of musculoskeletal diseases. Stem Cells Transl. Med. 2024, 13, 959–978. [Google Scholar] [CrossRef]
- Lana, J.F.; de Brito, G.C.; Kruel, A.; Brito, B.; Santos, G.S.; Caliari, C.; Salamanna, F.; Sartori, M.; Barbanti Brodano, G.; Costa, F.R.; et al. Evolution and Innovations in Bone Marrow Cellular Therapy for Musculoskeletal Disorders: Tracing the Historical Trajectory and Contemporary Advances. Bioengineering 2024, 11, 979. [Google Scholar] [CrossRef]
- Jin, Q.H.; Kim, H.K.; Na, J.Y.; Jin, C.; Seon, J.K. Anti-inflammatory effects of mesenchymal stem cell-conditioned media inhibited macrophages activation in vitro. Sci. Rep. 2022, 12, 4754–4764. [Google Scholar] [CrossRef]
- Salari, V.; Mengoni, F.; Del Gallo, F.; Bertini, G.; Fabene, P.F. The Anti-Inflammatory Properties of Mesenchymal Stem Cells in Epilepsy: Possible Treatments and Future Perspectives. Int. J. Mol. Sci. 2020, 21, 9683. [Google Scholar] [CrossRef]
- Piuzzi, N.S.; Chahla, J.; Schrock, J.B.; LaPrade, R.F.; Pascual-Garrido, C.; Mont, M.A.; Muschler, G.F. Evidence for the use of cell-based therapy for the treatment of osteonecrosis of the femoral head: A systematic review of the literature. J. Arthroplast. 2017, 32, 1698–1708. [Google Scholar] [CrossRef] [PubMed]
- Impieri, L.; Pezzi, A.; Hadad, H.; Peretti, G.M.; Mangiavini, L.; Rossi, N. Orthobiologics in delayed union and non-union of adult long bones fractures: A systematic review. Bone Rep. 2024, 21, 101760–101775. [Google Scholar] [CrossRef] [PubMed]
- Arthur, A.; Gronthos, S. Clinical Application of Bone Marrow Mesenchymal Stem/Stromal Cells to Repair Skeletal Tissue. Int. J. Mol. Sci. 2020, 21, 9759. [Google Scholar] [CrossRef] [PubMed]
- Jones, I.A.; Togashi, R.; Wilson, M.L.; Heckmann, N.; Vangsness, C.T., Jr. Intra-articular treatment options for knee osteoarthritis. Nat. Rev. Rheumatol. 2019, 15, 77–90. [Google Scholar] [CrossRef] [PubMed]
- Schafer, R.; DeBaun, M.R.; Fleck, E.; Centeno, C.J.; Kraft, D.; Leibacher, J.; Bieback, K.; Seifried, E.; Dragoo, J.L. Quantitation of progenitor cell populations and growth factors after bone marrow aspirate concentration. J. Transl. Med. 2019, 17, 115. [Google Scholar] [CrossRef]
- Chahla, J.; Mannava, S.; Cinque, M.E.; Geeslin, A.G.; Codina, D.; LaPrade, R.F. Bone Marrow Aspirate Concentrate Harvesting and Processing Technique. Arthrosc. Tech. 2017, 6, e441–e445. [Google Scholar] [CrossRef]
- Hernigou, P.; Poignard, A.; Beaujean, F.; Rouard, H. Percutaneous autologous bone-marrow grafting for nonunions. Influence of the number and concentration of progenitor cells. J. Bone Jt. Surg. Am. 2005, 87, 1430–1437. [Google Scholar]
- Choi, J.Y.; Lee, B.H.; Song, K.B.; Park, R.W.; Kim, I.S.; Sohn, K.Y.; Jo, J.S.; Ryoo, H.M. Expression patterns of bone-related proteins during osteoblastic differentiation in mc3t3-e1 cells. J. Cell. Biochem. 1996, 61, 609–618. [Google Scholar] [CrossRef]
- Caplan, A.I. Why are mscs therapeutic? New data: New insight. J. Pathol. 2009, 217, 318–324. [Google Scholar] [CrossRef] [PubMed]
- Harrell, C.; Markovic, B.; Fellabaum, C.; Arsenijevic, A.; Volarevic, V. Mesenchymal stem cell-based therapy of osteoarthritis: Current knowledge and future perspectives. Biomed. Pharmacother. 2019, 109, 2318–2326. [Google Scholar] [CrossRef]
- Yusuf, E. Pharmacologic and Non-Pharmacologic Treatment of Osteoarthritis. Curr. Treat. Options Rheum. 2016, 2, 111–125. [Google Scholar] [CrossRef]
- Ekegren, C.L.; Edwards, E.R.; de Steiger, R.; Gabbe, B.J. Incidence, costs and predictors of non-union, delayed union and mal-union following long bone fracture. Int. J. Environ. Res. Public Health 2018, 15, 2845. [Google Scholar] [CrossRef] [PubMed]
- Marolt Presen, D.; Traweger, A.; Gimona, M.; Redl, H. Mesenchymal stromal cell-based bone regeneration therapies: From cell transplantation and tissue engineering to therapeutic secretomes and extracellular vesicles. Front. Bioeng. Biotechnol. 2019, 7, 352. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Merchan, E.C. A review of recent developments in the molecular mechanisms of bone healing. Int. J. Mol. Sci. 2021, 22, 767. [Google Scholar] [CrossRef] [PubMed]
- Beresford, J.N. Osteogenic stem cells and the stromal system of bone and marrow. Clin. Orthop. Relat. Res. 1989, 240, 270–280. [Google Scholar] [CrossRef]
- Burwell, R.G. The function of bone marrow in the incorporation of a bone graft. Clin. Orthop. Relat. Res. 1985, 200, 125–141. [Google Scholar] [CrossRef]
- Connolly, J.F.; Guse, R.; Tiedeman, J.; Dehne, R. Autologous marrow injection as a substitute for operative grafting of tibial nonunions. Clin. Orthop. Relat. Res. 1991, 266, 259–270. [Google Scholar] [CrossRef]
- Healey, J.H.; Zimmerman, P.A.; McDonnell, J.M.; Lane, J.M. Percutaneous bone marrow grafting of delayed union and nonunion in cancer patients. Clin. Orthop. Relat. Res. 1990, 256, 280–285. [Google Scholar] [CrossRef]
- Connolly, J.; Guse, R.; Lippiello, L.; Dehne, R. Development of an osteogenic bone-marrow preparation. J. Bone Jt. Surg. Am. 1989, 71, 684–691. [Google Scholar] [CrossRef]
- Oliver, K.; Awan, T.; Bayes, M. Single-versus multiple-site harvesting techniques for bone marrow concentrate: Evaluation of aspirate quality and pain. Orthop. J. Sports Med. 2017, 5, 2325967117724398. [Google Scholar] [CrossRef]
- Piuzzi, N.S.; Mantripragada, V.P.; Sumski, A.; Selvam, S.; Boehm, C.; Muschler, G.F. Bone marrow-derived cellular therapies in orthopaedics: Part i: Recommendations for bone marrow aspiration technique and safety. JBJS Rev. 2018, 6, e4. [Google Scholar] [CrossRef] [PubMed]
- Dominici, M.; Le Blanc, K.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F.; Krause, D.; Deans, R.; Keating, A.; Prockop, D.; Horwitz, E. Minimal criteria for defining multipotent mesenchymal stromal cells. The international society for cellular therapy position statement. Cytotherapy 2006, 8, 315–317. [Google Scholar] [CrossRef] [PubMed]

| Patients | Age | Race | Location of Aspiration |
|---|---|---|---|
| 1 | 31 | Asian | Iliac crest |
| 2 | 31 | Asian | Iliac crest |
| 3 | 58 | Asian | Iliac crest |
| 4 | 58 | Asian | Iliac crest |
| 5 | 41 | Asian | Iliac crest |
| 6 | 58 | Asian | Iliac crest |
| 7 | 45 | Asian | Iliac crest |
| Markers | Relative MSC Number to Single Site (%) | p-Value |
|---|---|---|
| CD73+ | 81.3 ± 17.04 | <0.05 |
| CD73+/CD34− | 82.6 ± 19.92 | <0.05 |
| CD73+/CD90+/CD105+/CD34− | 57.8 ± 20.29 | <0.001 |
| Relative AR Intensity to a Single Site (%) | p-Value | |
|---|---|---|
| Compared with multiple sites | 47 ± 17.04 | <0.05 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Che, X.; Kim, H.-J.; Jin, X.; Kim, J.-W.; Park, K.-H.; Lim, J.-O.; Kyung, H.-S.; Oh, C.-W.; Choi, J.-Y. Bone Marrow Stem Cell Population in Single- and Multiple-Level Aspiration. Biomedicines 2024, 12, 2731. https://doi.org/10.3390/biomedicines12122731
Che X, Kim H-J, Jin X, Kim J-W, Park K-H, Lim J-O, Kyung H-S, Oh C-W, Choi J-Y. Bone Marrow Stem Cell Population in Single- and Multiple-Level Aspiration. Biomedicines. 2024; 12(12):2731. https://doi.org/10.3390/biomedicines12122731
Chicago/Turabian StyleChe, Xiangguo, Hee-June Kim, Xian Jin, Joon-Woo Kim, Kyeong-Hyeon Park, Jeong-Ok Lim, Hee-Soo Kyung, Chang-Wug Oh, and Je-Yong Choi. 2024. "Bone Marrow Stem Cell Population in Single- and Multiple-Level Aspiration" Biomedicines 12, no. 12: 2731. https://doi.org/10.3390/biomedicines12122731
APA StyleChe, X., Kim, H.-J., Jin, X., Kim, J.-W., Park, K.-H., Lim, J.-O., Kyung, H.-S., Oh, C.-W., & Choi, J.-Y. (2024). Bone Marrow Stem Cell Population in Single- and Multiple-Level Aspiration. Biomedicines, 12(12), 2731. https://doi.org/10.3390/biomedicines12122731










