Genetic Variation in Targets of Antidiabetic Drugs and Amyotrophic Lateral Sclerosis Risk
Abstract
1. Introduction
2. Materials and Methods
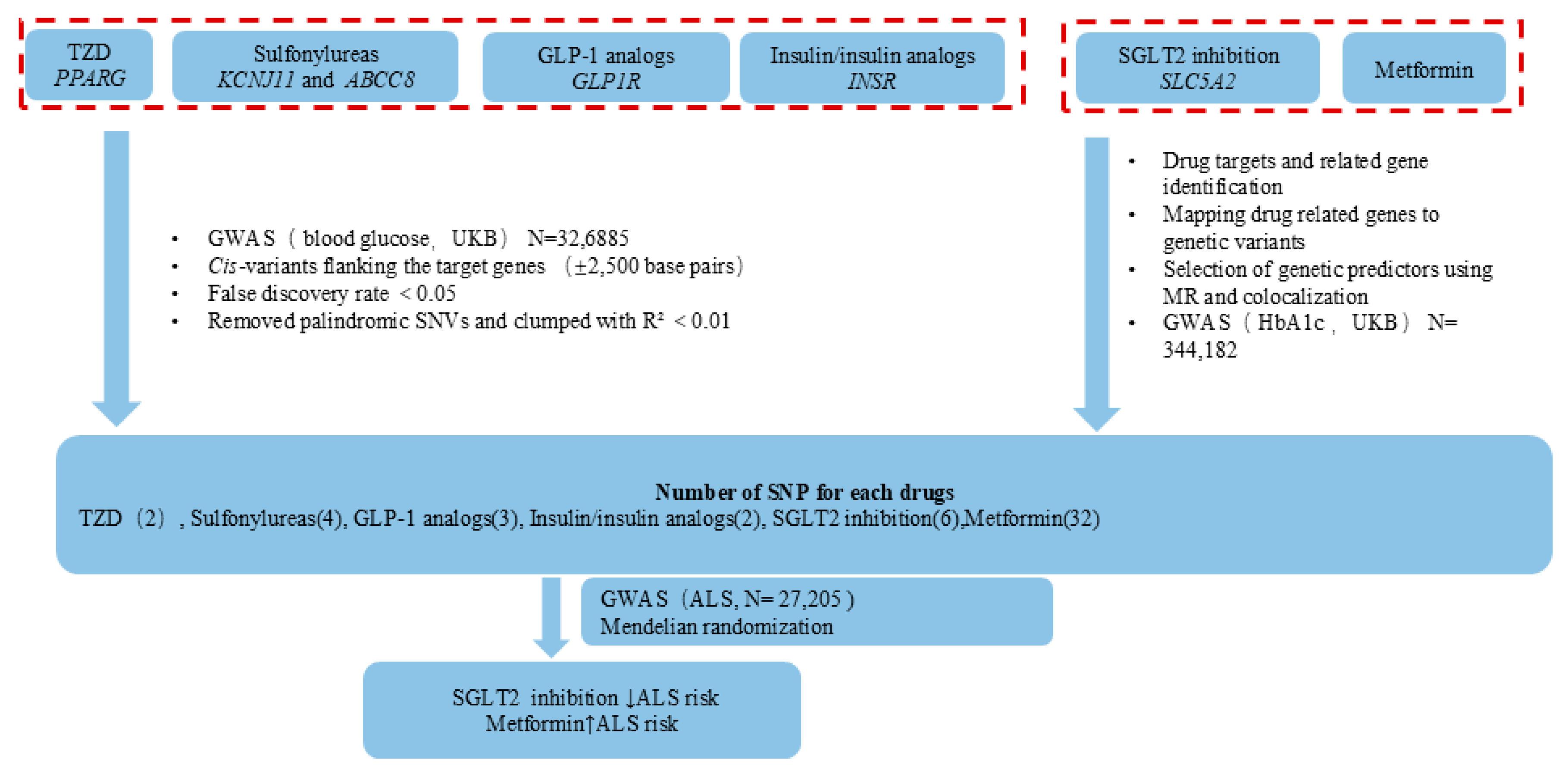
2.1. Identification of Antidiabetic Drugs
2.2. Instrument Selection
2.3. ALS GWAS Data
2.4. Mendelian Randomization
2.5. Ethical Issues
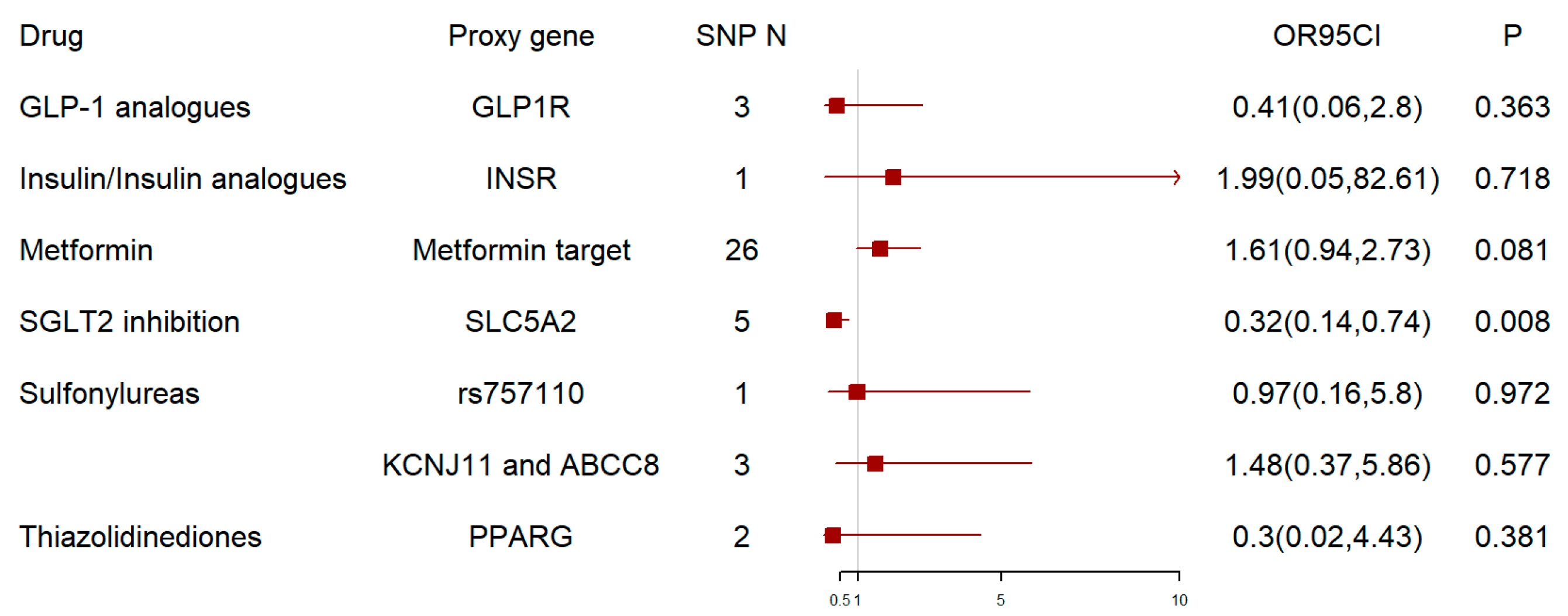
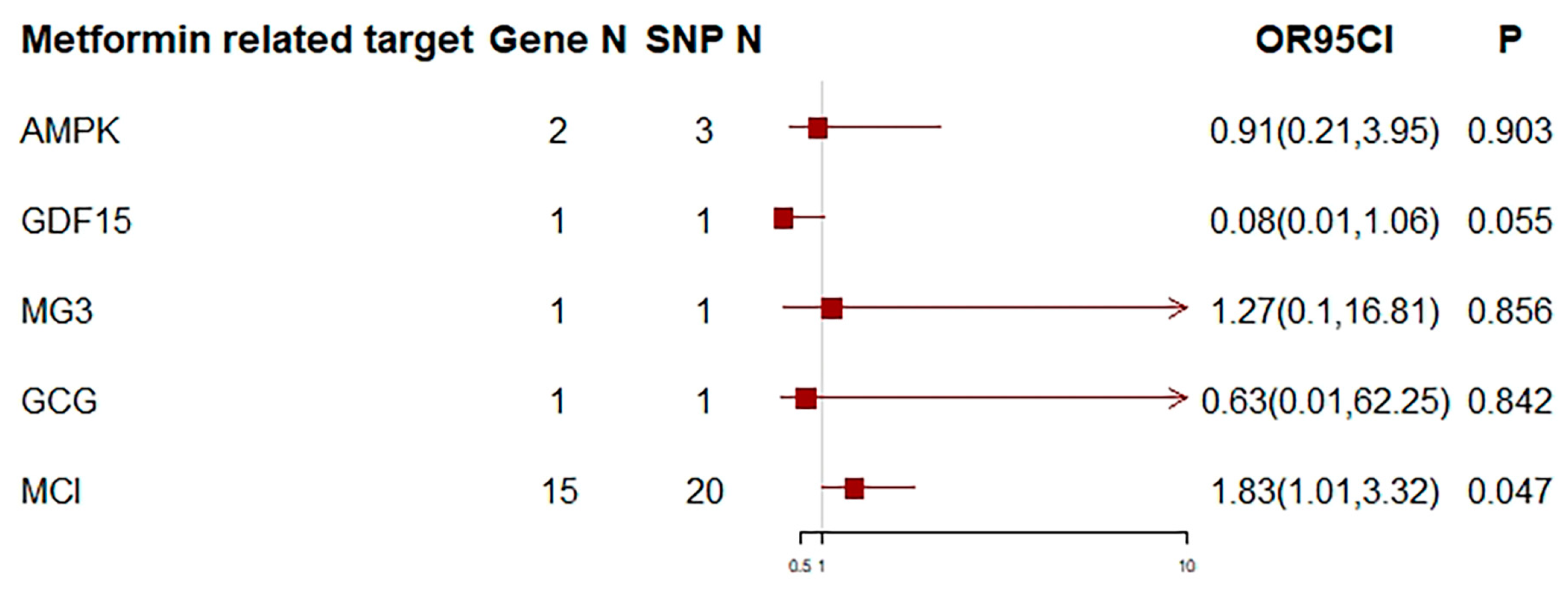
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Brooks, B.R.; Miller, R.G.; Swash, M.; Munsat, T.L.; World Federation of Neurology Research Group on Motor Neuron, Diseases. El Escorial revisited: Revised criteria for the diagnosis of amyotrophic lateral sclerosis. Amyotroph Lateral Scler Other Motor Neuron Disord. 2000, 1, 293–299. [Google Scholar] [CrossRef]
- Feldman, E.L.; Goutman, S.A.; Petri, S.; Mazzini, L.; Savelieff, M.G.; Shaw, P.J.; Sobue, G. Amyotrophic lateral sclerosis. Lancet 2022, 400, 1363–1380. [Google Scholar] [CrossRef]
- Kioumourtzoglou, M.A.; Rotem, R.S.; Seals, R.M.; Gredal, O.; Hansen, J.; Weisskopf, M.G. Diabetes Mellitus, Obesity, and Diagnosis of Amyotrophic Lateral Sclerosis: A Population-Based Study. JAMA Neurol. 2015, 72, 905–911. [Google Scholar] [CrossRef]
- D’Ovidio, F.; d’Errico, A.; Carna, P.; Calvo, A.; Costa, G.; Chio, A. The role of pre-morbid diabetes on developing amyotrophic lateral sclerosis. Eur. J. Neurol. 2018, 25, 164–170. [Google Scholar] [CrossRef]
- Mariosa, D.; Kamel, F.; Bellocco, R.; Ye, W.; Fang, F. Association between diabetes and amyotrophic lateral sclerosis in Sweden. Eur. J. Neurol. 2015, 22, 1436–1442. [Google Scholar] [CrossRef]
- Zhang, L.; Tang, L.; Huang, T.; Fan, D. Association between type 2 diabetes and amyotrophic lateral sclerosis. Sci. Rep. 2022, 12, 2544. [Google Scholar] [CrossRef]
- Zeng, P.; Wang, T.; Zheng, J.; Zhou, X. Causal association of type 2 diabetes with amyotrophic lateral sclerosis: New evidence from Mendelian randomization using GWAS summary statistics. BMC Med. 2019, 17, 225. [Google Scholar] [CrossRef]
- Jawaid, A.; Paganoni, S.; Hauser, C.; Schulz, P.E. Trials of antidiabetic drugs in amyotrophic lateral sclerosis: Proceed with caution? Neurodegener Dis. 2014, 13, 205–208. [Google Scholar] [CrossRef]
- Cardoso, S.; Correia, S.; Santos, R.X.; Carvalho, C.; Santos, M.S.; Oliveira, C.R.; Perry, G.; Smith, M.A.; Zhu, X.; Moreira, P.I. Insulin is a two-edged knife on the brain. J. Alzheimer’s Dis. 2009, 18, 483–507. [Google Scholar] [CrossRef]
- Sun, H.; Knippenberg, S.; Thau, N.; Ragancokova, D.; Korner, S.; Huang, D.; Dengler, R.; Dohler, K.; Petri, S. Therapeutic potential of N-acetyl-glucagon-like peptide-1 in primary motor neuron cultures derived from non-transgenic and SOD1-G93A ALS mice. Cell Mol. Neurobiol. 2013, 33, 347–357. [Google Scholar] [CrossRef]
- Diekmann, K.; Kuzma-Kozakiewicz, M.; Piotrkiewicz, M.; Gromicho, M.; Grosskreutz, J.; Andersen, P.M.; de Carvalho, M.; Uysal, H.; Osmanovic, A.; Schreiber-Katz, O.; et al. Impact of comorbidities and co-medication on disease onset and progression in a large German ALS patient group. J. Neurol. 2020, 267, 2130–2141. [Google Scholar] [CrossRef] [PubMed]
- Sutedja, N.A.; van der Schouw, Y.T.; Fischer, K.; Sizoo, E.M.; Huisman, M.H.; Veldink, J.H.; Van den Berg, L.H. Beneficial vascular risk profile is associated with amyotrophic lateral sclerosis. J. Neurol. Neurosurg. Psychiatry 2011, 82, 638–642. [Google Scholar] [CrossRef] [PubMed]
- Mariosa, D.; Kamel, F.; Bellocco, R.; Ronnevi, L.O.; Almqvist, C.; Larsson, H.; Ye, W.; Fang, F. Antidiabetics, statins and the risk of amyotrophic lateral sclerosis. Eur. J. Neurol. 2020, 27, 1010–1016. [Google Scholar] [CrossRef] [PubMed]
- Pfeiffer, R.M.; Mayer, B.; Kuncl, R.W.; Check, D.P.; Cahoon, E.K.; Rivera, D.R.; Freedman, D.M. Identifying potential targets for prevention and treatment of amyotrophic lateral sclerosis based on a screen of medicare prescription drugs. Amyotroph Lateral Scler Front. Degener 2020, 21, 235–245. [Google Scholar] [CrossRef]
- Zu, T.; Guo, S.; Bardhi, O.; Ryskamp, D.A.; Li, J.; Khoramian Tusi, S.; Engelbrecht, A.; Klippel, K.; Chakrabarty, P.; Nguyen, L.; et al. Metformin inhibits RAN translation through PKR pathway and mitigates disease in C9orf72 ALS/FTD mice. Proc. Natl. Acad. Sci. USA 2020, 117, 18591–18599. [Google Scholar] [CrossRef]
- Kaneb, H.M.; Sharp, P.S.; Rahmani-Kondori, N.; Wells, D.J. Metformin treatment has no beneficial effect in a dose-response survival study in the SOD1(G93A) mouse model of ALS and is harmful in female mice. PLoS ONE 2011, 6, e24189. [Google Scholar] [CrossRef]
- Schutz, B.; Reimann, J.; Dumitrescu-Ozimek, L.; Kappes-Horn, K.; Landreth, G.E.; Schurmann, B.; Zimmer, A.; Heneka, M.T. The oral antidiabetic pioglitazone protects from neurodegeneration and amyotrophic lateral sclerosis-like symptoms in superoxide dismutase-G93A transgenic mice. J. Neurosci. 2005, 25, 7805–7812. [Google Scholar] [CrossRef]
- Dupuis, L.; Dengler, R.; Heneka, M.T.; Meyer, T.; Zierz, S.; Kassubek, J.; Fischer, W.; Steiner, F.; Lindauer, E.; Otto, M.; et al. A randomized, double blind, placebo-controlled trial of pioglitazone in combination with riluzole in amyotrophic lateral sclerosis. PLoS ONE 2012, 7, e37885. [Google Scholar] [CrossRef]
- Emdin, C.A.; Khera, A.V.; Kathiresan, S. Mendelian Randomization. JAMA 2017, 318, 1925–1926. [Google Scholar] [CrossRef]
- Schmidt, A.F.; Finan, C.; Gordillo-Maranon, M.; Asselbergs, F.W.; Freitag, D.F.; Patel, R.S.; Tyl, B.; Chopade, S.; Faraway, R.; Zwierzyna, M.; et al. Genetic drug target validation using Mendelian randomisation. Nat. Commun. 2020, 11, 3255. [Google Scholar] [CrossRef]
- Tang, B.; Wang, Y.; Jiang, X.; Thambisetty, M.; Ferrucci, L.; Johnell, K.; Hagg, S. Genetic Variation in Targets of Antidiabetic Drugs and Alzheimer Disease Risk: A Mendelian Randomization Study. Neurology 2022, 99, e650–e659. [Google Scholar] [CrossRef] [PubMed]
- Allen, N.E.; Sudlow, C.; Peakman, T.; Collins, R.; Biobank, U.K. UK biobank data: Come and get it. Sci. Transl. Med. 2014, 6, 224ed4. [Google Scholar] [CrossRef] [PubMed]
- Wishart, D.S.; Knox, C.; Guo, A.C.; Shrivastava, S.; Hassanali, M.; Stothard, P.; Chang, Z.; Woolsey, J. DrugBank: A comprehensive resource for in silico drug discovery and exploration. Nucleic Acids Res. 2006, 34, D668–D672. [Google Scholar] [CrossRef] [PubMed]
- Mendez, D.; Gaulton, A.; Bento, A.P.; Chambers, J.; De Veij, M.; Felix, E.; Magarinos, M.P.; Mosquera, J.F.; Mutowo, P.; Nowotka, M.; et al. ChEMBL: Towards direct deposition of bioassay data. Nucleic Acids Res. 2019, 47, D930–D940. [Google Scholar] [CrossRef]
- Zheng, J.; Xu, M.; Walker, V.; Yuan, J.; Korologou-Linden, R.; Robinson, J.; Huang, P.; Burgess, S.; Au Yeung, S.L.; Luo, S.; et al. Evaluating the efficacy and mechanism of metformin targets on reducing Alzheimer’s disease risk in the general population: A Mendelian randomisation study. Diabetologia 2022, 65, 1664–1675. [Google Scholar] [CrossRef]
- Xu, M.; Zheng, J.; Hou, T.; Lin, H.; Wang, T.; Wang, S.; Lu, J.; Zhao, Z.; Li, M.; Xu, Y.; et al. SGLT2 Inhibition, Choline Metabolites, and Cardiometabolic Diseases: A Mediation Mendelian Randomization Study. Diabetes Care 2022, 45, 2718–2728. [Google Scholar] [CrossRef]
- van Rheenen, W.; van der Spek, R.A.A.; Bakker, M.K.; van Vugt, J.J.F.A.; Hop, P.J.; Zwamborn, R.A.J.; de Klein, N.; Westra, H.-J.; Bakker, O.B.; Deelen, P.; et al. Common and rare variant association analyses in amyotrophic lateral sclerosis identify 15 risk loci with distinct genetic architectures and neuron-specific biology. Nat. Genet. 2021, 53, 1636–1648. [Google Scholar] [CrossRef]
- Burgess, S.; Thompson, S.G. Interpreting findings from Mendelian randomization using the MR-Egger method. Eur. J. Epidemiol. 2017, 32, 377–389. [Google Scholar] [CrossRef]
- Bowden, J.; Davey Smith, G.; Haycock, P.C.; Burgess, S. Consistent Estimation in Mendelian Randomization with Some Invalid Instruments Using a Weighted Median Estimator. Genet. Epidemiol. 2016, 40, 304–314. [Google Scholar] [CrossRef]
- Hemani, G.; Bowden, J.; Davey Smith, G. Evaluating the potential role of pleiotropy in Mendelian randomization studies. Hum. Mol. Genet. 2018, 27, R195–R208. [Google Scholar] [CrossRef]
- Verbanck, M.; Chen, C.Y.; Neale, B.; Do, R. Detection of widespread horizontal pleiotropy in causal relationships inferred from Mendelian randomization between complex traits and diseases. Nat. Genet. 2018, 50, 693–698. [Google Scholar] [CrossRef] [PubMed]
- Lingli, X.; Wenfang, X. Characteristics and molecular mechanisms through which SGLT2 inhibitors improve metabolic diseases: A mechanism review. Life Sci. 2022, 300, 120543. [Google Scholar] [CrossRef] [PubMed]
- Gyimesi, G.; Pujol-Gimenez, J.; Kanai, Y.; Hediger, M.A. Sodium-coupled glucose transport, the SLC5 family, and therapeutically relevant inhibitors: From molecular discovery to clinical application. Pflug. Arch. 2020, 472, 1177–1206. [Google Scholar] [CrossRef] [PubMed]
- Koekkoek, L.L.; Mul, J.D.; la Fleur, S.E. Glucose-Sensing in the Reward System. Front. Neurosci. 2017, 11, 716. [Google Scholar] [CrossRef]
- Arab, H.H.; Safar, M.M.; Shahin, N.N. Targeting ROS-Dependent AKT/GSK-3beta/NF-kappaB and DJ-1/Nrf2 Pathways by Dapagliflozin Attenuates Neuronal Injury and Motor Dysfunction in Rotenone-Induced Parkinson’s Disease Rat Model. ACS Chem. Neurosci. 2021, 12, 689–703. [Google Scholar] [CrossRef]
- Erdogan, M.A.; Yusuf, D.; Christy, J.; Solmaz, V.; Erdogan, A.; Taskiran, E.; Erbas, O. Highly selective SGLT2 inhibitor dapagliflozin reduces seizure activity in pentylenetetrazol-induced murine model of epilepsy. BMC Neurol. 2018, 18, 81. [Google Scholar] [CrossRef]
- Rizzo, M.R.; Di Meo, I.; Polito, R.; Auriemma, M.C.; Gambardella, A.; di Mauro, G.; Capuano, A.; Paolisso, G. Cognitive impairment and type 2 diabetes mellitus: Focus of SGLT2 inhibitors treatment. Pharmacol. Res. 2022, 176, 106062. [Google Scholar] [CrossRef]
- Hu, N.; Ji, H. Medications on hypertension, hyperlipidemia, diabetes, and risk of amyotrophic lateral sclerosis: A systematic review and meta-analysis. Neurol. Sci. 2022, 43, 5189–5199. [Google Scholar] [CrossRef]
- Sai Swaroop, R.; Akhil, P.S.; Sai Sanwid, P.; Bandana, P.; Raksha, R.K.; Meghana, M.; Bibha, C.; Sivaramakrishnan, V. Integrated multi-omic data analysis and validation with yeast model show oxidative phosphorylation modulates protein aggregation in amyotrophic lateral sclerosis. J. Biomol. Struct. Dyn. 2022, 41, 5548–5567. [Google Scholar] [CrossRef]
- Rena, G.; Hardie, D.G.; Pearson, E.R. The mechanisms of action of metformin. Diabetologia 2017, 60, 1577–1585. [Google Scholar] [CrossRef]
| Drug Class | Proxy Gene | IVW-Q Test | MR-Egger | |||
|---|---|---|---|---|---|---|
| Q-Statistic | Q-p | Intercept | SE | p-Value | ||
| GLP-1 analogues | GLP1R | 0.084 | 0.959 | −0.003 | 0.019 | 0.890 |
| Insulin/Insulin Analogues | INSR | NA | NA | NA | NA | NA |
| Metformin | Metformin target | 49.321 | 0.003 | −0.003 | 0.008 | 0.755 |
| SGLT2 Inhibition | SLC5A2 | 2.212 | 0.697 | 0.002 | 0.024 | 0.935 |
| Sulfonylureas | rs757110 | NA | NA | NA | NA | NA |
| KCNJ11 and ABCC8 | 1.581 | 0.454 | −0.012 | 0.026 | 0.719 | |
| Thiazolidinediones | PPARG | 0.599 | 0.439 | NA | NA | NA |
| Metformin-Related Drug Target | IVW-Q Test | MR-Egger | |||
|---|---|---|---|---|---|
| Q-Statistic | Q-p | Intercept | SE | p-Value | |
| AMPK | 1.35 | 0.51 | 0 | 0.04 | 0.931 |
| GDF15 | NA | NA | NA | NA | NA |
| Mitochondrial-glycerol-3 | NA | NA | NA | NA | NA |
| GCG | NA | NA | NA | NA | NA |
| Mitochondrial-complex-I | 41.6 | 0.002 | 0.01 | 0.01 | 0.486 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wan, M.; Zhang, L.; Huo, J.; Fu, Y.; Huang, T.; Fan, D. Genetic Variation in Targets of Antidiabetic Drugs and Amyotrophic Lateral Sclerosis Risk. Biomedicines 2024, 12, 2733. https://doi.org/10.3390/biomedicines12122733
Wan M, Zhang L, Huo J, Fu Y, Huang T, Fan D. Genetic Variation in Targets of Antidiabetic Drugs and Amyotrophic Lateral Sclerosis Risk. Biomedicines. 2024; 12(12):2733. https://doi.org/10.3390/biomedicines12122733
Chicago/Turabian StyleWan, Mengxia, Linjing Zhang, Junyan Huo, Yu Fu, Tao Huang, and Dongsheng Fan. 2024. "Genetic Variation in Targets of Antidiabetic Drugs and Amyotrophic Lateral Sclerosis Risk" Biomedicines 12, no. 12: 2733. https://doi.org/10.3390/biomedicines12122733
APA StyleWan, M., Zhang, L., Huo, J., Fu, Y., Huang, T., & Fan, D. (2024). Genetic Variation in Targets of Antidiabetic Drugs and Amyotrophic Lateral Sclerosis Risk. Biomedicines, 12(12), 2733. https://doi.org/10.3390/biomedicines12122733







