Microsatellite Instability in Urine: Breakthrough Method for Bladder Cancer Identification
Abstract
1. Introduction
2. Traditional Techniques for Bladder Cancer Detection
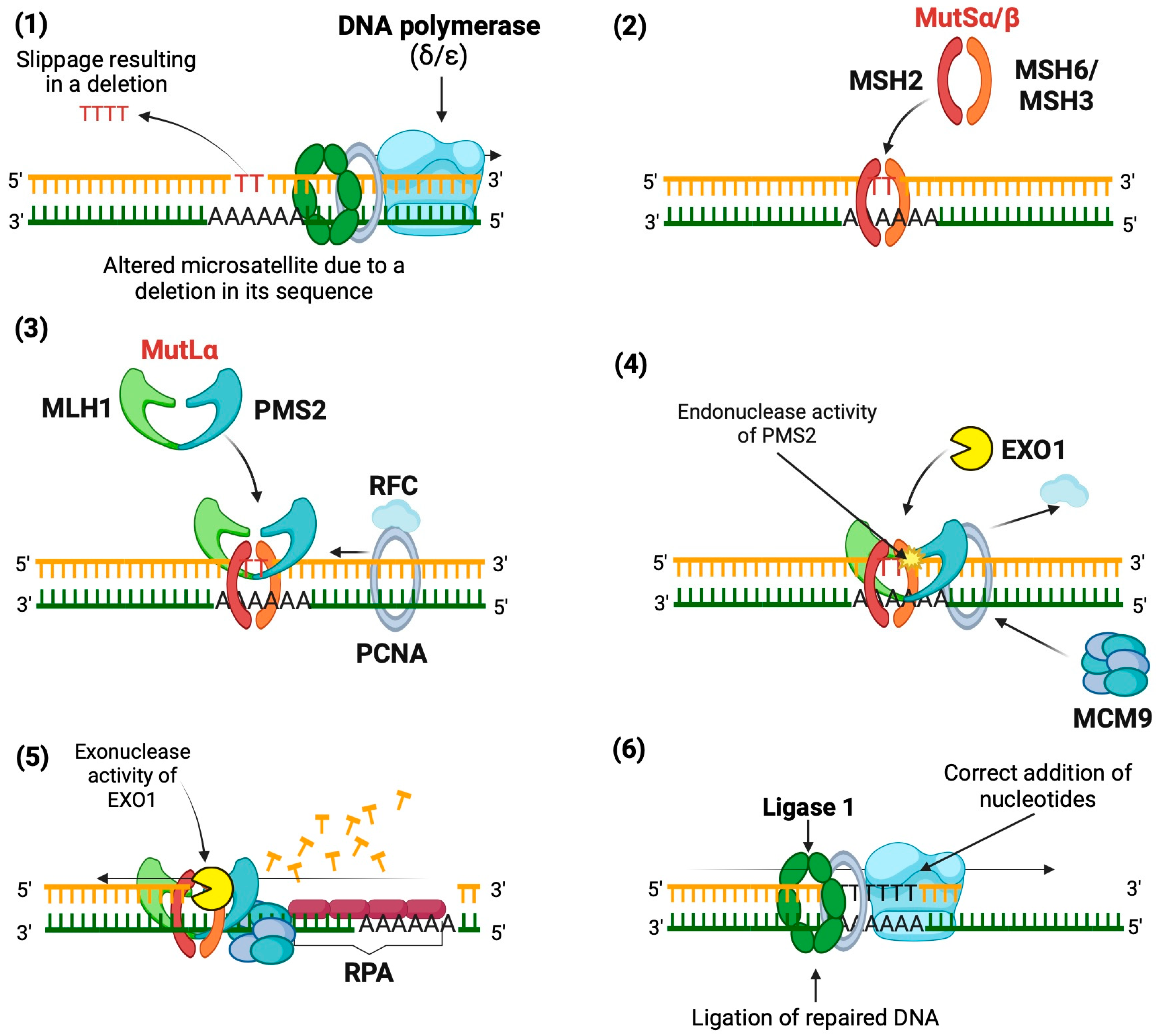
3. Microsatellite Instability Development
- Base mismatch: The DNA polymerase (δ or ε, depending on the DNA strand being polymerized) causes a slippage error in the microsatellite sequence, resulting in a deletion.
- Mismatch recognition: The MutSα heterodimer (MSH2 and MSH6) or MutSβ heterodimer (MSH2 and MSH3) binds to mismatches in the DNA, depending on the size of the mismatch. MutSα recognizes SNVs and indels of 1 to 2 bp, while MutSβ identifies larger indels. This binding induces a conformational change that allows MutSα/β to move along the DNA.
- Recruitment of MutLα: MutSα/β facilitates the recruitment of MutLα (MLH1 and PMS2), forming a tetrameric complex along with proliferating cell nuclear antigen (PCNA) and replication factor C (RFC), which binds to the DNA strand.
- Activation of PMS2: PCNA activates MutLα, enabling PMS2 to exert its endonuclease activity specifically on the nascent strand, creating entry sites for exonuclease 1 (EXO1).
- DNA excision: EXO1 excises the nascent strand from the mismatch to a gap in the single-stranded DNA, with the help of helicase MCM9, forming a protein complex (MutSα/MutLα/EXO1/MCM9).
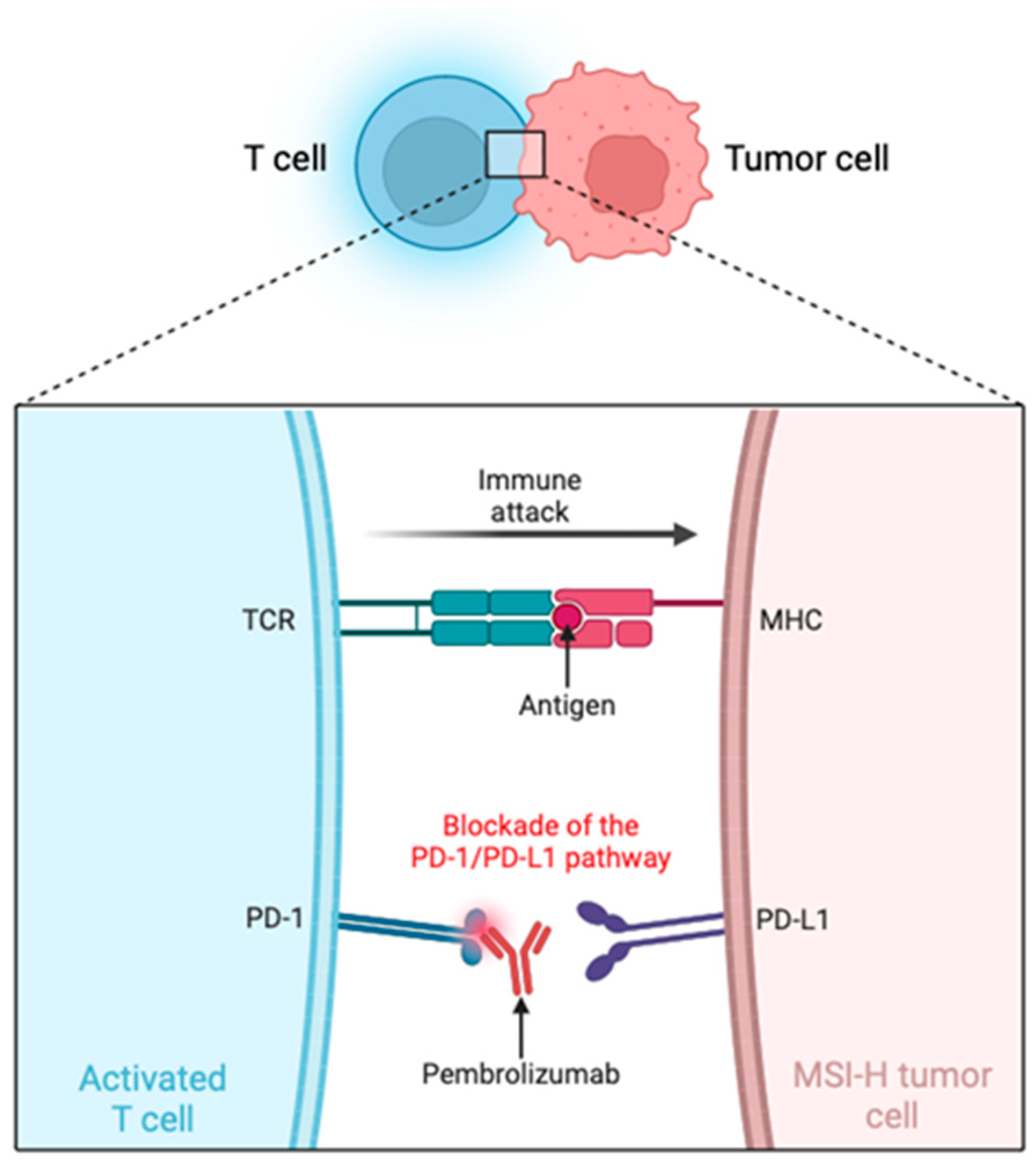
4. Role of Microsatellite Instability in Pembrolizumab Response for MSI-H Tumors
5. Role of Microsatellite Instability in Bladder Cancer Development
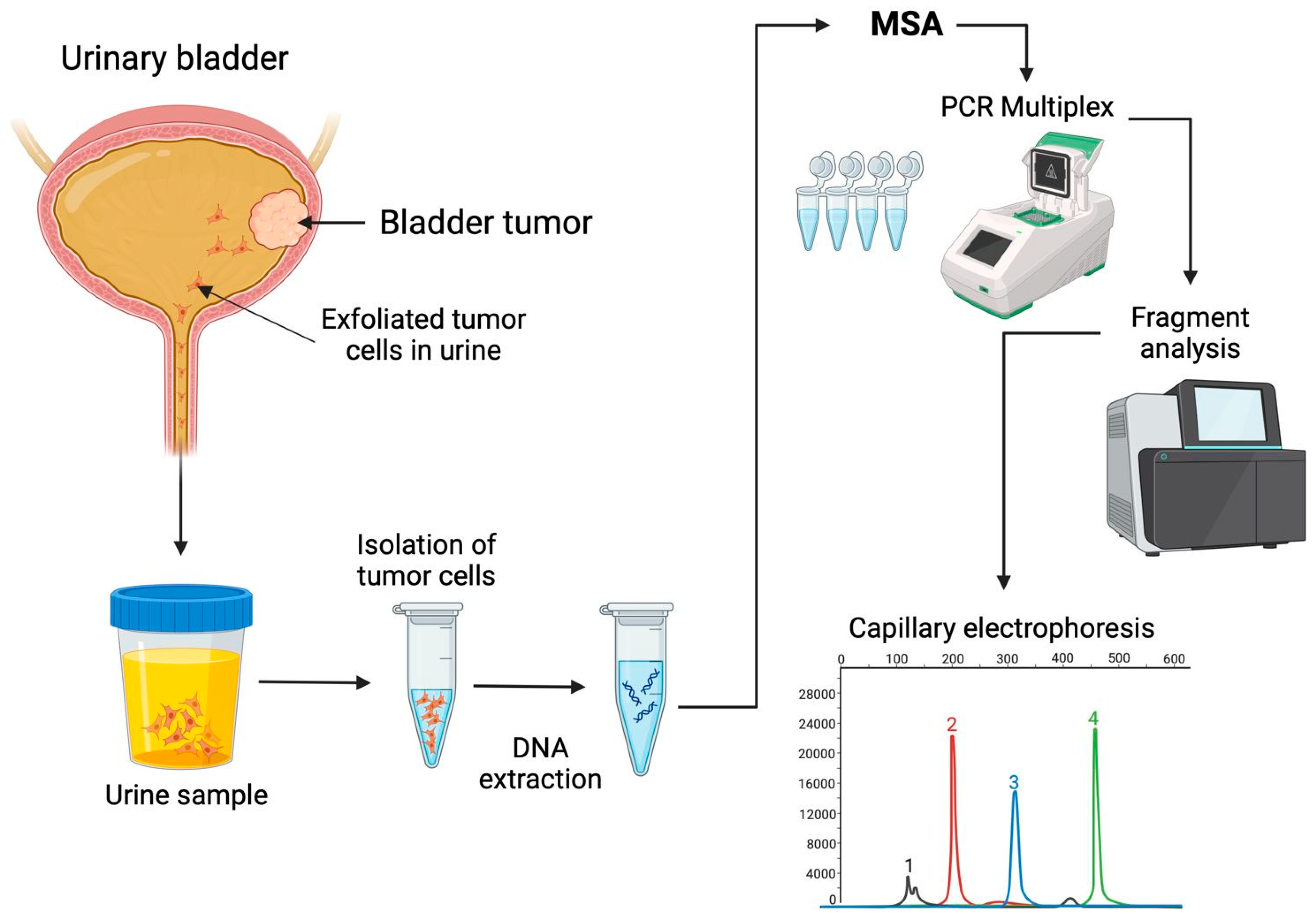
6. Microsatellite Instability in Urine-Derived Bladder Tumor Cells
7. Discussion and Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Rizzo, A.; Mollica, V.; Cimadamore, A.; Santoni, M.; Scarpelli, M.; Schiavina, R.; Cheng, L.; Lopez-Beltran, A.; Brunocilla, E.; Montironi, R.; et al. TNM staging towards a personalized approach in metastatic urothelial carcinoma: What will the future be like?-a narrative review. Transl. Androl. Urol. 2021, 10, 1541–1552. [Google Scholar] [CrossRef] [PubMed]
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef] [PubMed]
- Ferlay, J.; Ervik, M.; Lam, F.; Laversanne, M.; Colombet, M.; Mery, L.; Piñeros, M.; Znaor, A.; Soerjomataram, I.; Bray, F. Global Cancer Observatory: Cancer Today. Lyon, France: International Agency for Research on Cancer. Available online: https://gco.iarc.who.int/today (accessed on 14 September 2024).
- Fantini, D.; Meeks, J.J. Genomic classification and risk stratification of bladder cancer. World J. Urol. 2019, 37, 1751–1757. [Google Scholar] [CrossRef] [PubMed]
- Dobruch, J.; Oszczudłowski, M. Bladder cancer: Current challenges and future directions. Medicina 2021, 57, 749. [Google Scholar] [CrossRef] [PubMed]
- Soria, F.; Droller, M.J.; Lotan, Y.; Gontero, P.; D’Andrea, D.; Gust, K.M.; Rouprêt, M.; Babjuk, M.; Palou, J.; Shariat, S.F. An up-to-date catalog of available urinary biomarkers for the surveillance of non-muscle invasive bladder cancer. World J. Urol. 2018, 36, 1981–1995. [Google Scholar] [CrossRef] [PubMed]
- Moon, C.; Gordon, M.; Moon, D.; Reynolds, T. Microsatellite instability analysis (MSA) for bladder cancer: Past history and future directions. Int. J. Mol. Sci. 2021, 22, 12864. [Google Scholar] [CrossRef]
- Marcus, L.; Lemery, S.J.; Keegan, P.; Pazdur, R. FDA approval summary: Pembrolizumab for the treatment of microsatellite instability-high solid tumors. Clin. Cancer Res. 2019, 25, 3753–3758. [Google Scholar] [CrossRef]
- Sepulveda, A.R.; Hamilton, S.R.; Allegra, C.J.; Grody, W.; Cushman-Vokoun, A.M.; Funkhouser, W.K.; Kopetz, S.E.; Lieu, C.; Lindor, N.M.; Minsky, B.D.; et al. Molecular biomarkers for the evaluation of colorectal cancer: Guideline from the American Society for Clinical Pathology, College of American Pathologists, Association for Molecular Pathology, and American Society of Clinical Oncology. J. Mol. Diagn. 2017, 19, 187–225. [Google Scholar] [CrossRef]
- Colombo, N.; Creutzberg, C.; Amant, F.; Bosse, T.; González-Martín, A.; Ledermann, J.; Marth, C.; Nout, R.; Querleu, D.; Mirza, M.R.; et al. ESMO-ESGO-ESTRO Endometrial Consensus Conference Working Group. ESMO-ESGO-ESTRO consensus conference on endometrial cancer: Diagnosis, treatment and follow-up. Ann. Oncol. 2016, 27, 16–41. [Google Scholar] [CrossRef]
- Smyth, E.C.; Verheij, M.; Allum, W.; Cunningham, D.; Cervantes, A.; Arnold, D.; ESMO Guidelines Committee. Gastric cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2016, 27 (Suppl. S5), v38–v49. [Google Scholar] [CrossRef] [PubMed]
- van Vugt, M.J.H.; Stone, J.A.; De Greef, R.H.J.M.M.; Snyder, E.S.; Lipka, L.; Turner, D.C.; Chain, A.; Lala, M.; Li, M.; Robey, S.H.; et al. Immunogenicity of pembrolizumab in patients with advanced tumors. J. Immunother. Cancer 2019, 7, 212. [Google Scholar] [CrossRef] [PubMed]
- Kwok, G.; Yau, T.C.; Chiu, J.W.; Tse, E.; Kwong, Y.L. Pembrolizumab (Keytruda). Hum. Vaccines Immunother. 2016, 12, 2777–2789. [Google Scholar] [CrossRef] [PubMed]
- Busetto, G.M.; Del Giudice, F.; de Cobelli, O.; Carrieri, G.; Porreca, A.; Cimmino, A.; Terracciano, D. Liquid Biopsy Biomarkers in Urine: A Route towards Molecular Diagnosis and Personalized Medicine of Bladder Cancer. J. Pers. Med. 2021, 11, 237. [Google Scholar] [CrossRef] [PubMed]
- Lenis, A.T.; Lec, P.M.; Chamie, K. Bladder cancer: A review. JAMA 2020, 324, 1980–1991. [Google Scholar] [CrossRef] [PubMed]
- Stav, K.; Leibovici, D.; Goren, E.; Livshitz, A.; Siegel, Y.I.; Lindner, A.; Zisman, A. Adverse Effects of Cystoscopy and Its Impact on Patients’ Quality of Life and Sexual Performance. Isr. Med. Assoc. J. 2004, 6, 474–478. [Google Scholar] [PubMed]
- Ahmadi, H.; Duddalwar, V.; Daneshmand, S. Diagnosis and Staging of Bladder Cancer. Hematol. Oncol. Clin. N. Am. 2021, 35, 531–541. [Google Scholar] [CrossRef]
- Lee, C.S.; Yoon, C.Y.; Witjes, J.A. The Past, Present and Future of Cystoscopy: The Fusion of Cystoscopy and Novel Imaging Technology. BJU Int. 2008, 102, 1228–1233. [Google Scholar] [CrossRef]
- Chen, D.; Xu, T.; Wang, S.; Chang, H.; Yu, T.; Zhu, Y.; Chen, J. Liquid biopsy applications in the clinic. Mol. Diagn. Ther. 2020, 24, 125–132. [Google Scholar] [CrossRef]
- Crocetto, F.; Barone, B.; Ferro, M.; Busetto, G.M.; La Civita, E.; Buonerba, C.; Di Lorenzo, G.; Terracciano, D.; Schalken, J.A. Liquid biopsy in bladder cancer: State of the art and future perspectives. Crit. Rev. Oncol. Hematol. 2022, 170, 103577. [Google Scholar] [CrossRef]
- Jeong, S.H.; Ku, J.H. Urinary markers for bladder cancer diagnosis and monitoring. Front. Cell Dev. Biol. 2022, 10, 892067. [Google Scholar] [CrossRef]
- Nagai, T.; Naiki, T.; Etani, T.; Iida, K.; Noda, Y.; Shimizu, N.; Isobe, T.; Nozaki, S.; Okamura, T.; Ando, R.; et al. UroVysion fluorescence in situ hybridization in urothelial carcinoma: A narrative review and future perspectives. Transl. Androl. Urol. 2021, 10, 1908–1917. [Google Scholar] [CrossRef] [PubMed]
- Ng, K.; Stenzl, A.; Sharma, A.; Vasdev, N. Urinary biomarkers in bladder cancer: A review of the current landscape and future directions. Urol. Oncol. 2021, 39, 41–51. [Google Scholar] [CrossRef] [PubMed]
- Vasdev, N.; Hampson, A.; Agarwal, S.; Swamy, R.; Chilvers, M.; Hampson, A.; Jahanfard, S.; Kim, N. The role of URO17™ biomarker to enhance diagnosis of urothelial cancer in new hematuria patients—First European data. BJUI Compass 2020, 2, 46–52. [Google Scholar] [CrossRef] [PubMed]
- Shang, D.; Liu, Y.; Xu, X.; Chen, Z.; Wang, D. Diagnostic value comparison of CellDetect, fluorescent in situ hybridization (FISH), and cytology in urothelial carcinoma. Cancer Cell Int. 2021, 21, 465. [Google Scholar] [CrossRef]
- Ecke, T.H.; Meisl, C.J.; Schlomm, T.; Rabien, A.; Labonté, F.; Rong, D.; Hofbauer, S.; Friedersdorff, F.; Sommerfeldt, L.; Gagel, N.; et al. BTA stat®, NMP22® BladderChek®, UBC® Rapid Test, and CancerCheck® UBC® rapid VISUAL as urinary marker for bladder cancer: Final results of a German multicenter study. Urol. Oncol. 2023, 41, 484.e17–484.e26. [Google Scholar] [CrossRef]
- Guo, A.; Wang, X.; Gao, L.; Shi, J.; Sun, C.; Wan, Z. Bladder tumour antigen (BTA stat) test compared to the urine cytology in the diagnosis of bladder cancer: A meta-analysis. Can. Urol. Assoc. J. 2014, 8, E347–E352. [Google Scholar] [CrossRef]
- Malkowicz, S.B. The application of human complement factor H-related protein (BTA TRAK) in monitoring patients with bladder cancer. Urol. Clin. N. Am. 2000, 27, 63–73. [Google Scholar] [CrossRef]
- Wang, J.; Zhao, X.; Jiang, X.L.; Lu, D.; Yuan, Q.; Li, J. Diagnostic performance of nuclear matrix protein 22 and urine cytology for bladder cancer: A meta-analysis. Diagn. Cytopathol. 2022, 50, 300–312. [Google Scholar] [CrossRef]
- Ecke, T.H.; Weiß, S.; Stephan, C.; Hallmann, S.; Barski, D.; Otto, T.; Gerullis, H. UBC® Rapid Test for detection of carcinoma in situ for bladder cancer. Tumour Biol. 2017, 39, 1010428317701624. [Google Scholar] [CrossRef]
- Roupret, M.; Gontero, P.; McCracken, S.R.C.; Dudderidge, T.; Stockley, J.; Kennedy, A.; Rodriguez, O.; Sieverink, C.; Vanié, F.; Allasia, M.; et al. Diagnostic accuracy of MCM5 for the detection of recurrence in nonmuscle invasive bladder cancer follow-up: A blinded, prospective cohort, multicenter European study. J. Urol. 2020, 204, 685–690. [Google Scholar] [CrossRef]
- Cai, Q.; Wu, Y.; Guo, Z.; Gong, R.; Tang, Y.; Yang, K.; Li, X.; Guo, X.; Niu, Y.; Zhao, Y. Urine BLCA-4 exerts potential role in detecting patients with bladder cancers: A pooled analysis of individual studies. Oncotarget 2015, 6, 37500–37510. [Google Scholar] [CrossRef] [PubMed]
- Hirasawa, Y.; Pagano, I.; Chen, R.; Sun, Y.; Dai, Y.; Gupta, A.; Tikhonenkov, S.; Goodison, S.; Rosser, C.J.; Furuya, H. Diagnostic performance of Oncuria™, a urinalysis test for bladder cancer. J. Transl. Med. 2021, 19, 141. [Google Scholar] [CrossRef] [PubMed]
- Sharma, G.; Sharma, A.; Krishna, M.; Devana, S.K.; Singh, S.K. Xpert bladder cancer monitor in surveillance of bladder cancer: Systematic review and meta-analysis. Urol. Oncol. 2022, 40, 163.e1–163.e9. [Google Scholar] [CrossRef] [PubMed]
- Chai, C.A.; Yeoh, W.S.; Rajandram, R.; Aung, K.P.; Ong, T.A.; Kuppusamy, S.; Nazran, A.; Kumaran, K.; Razack, A.H.A.; Teoh, J.Y. Comparing CxBladder to urine cytology as adjunct to cystoscopy in surveillance of non-muscle invasive bladder cancer—A pilot study. Front. Surg. 2021, 8, 659292. [Google Scholar] [CrossRef]
- Avogbe, P.H.; Manel, A.; Vian, E.; Durand, G.; Forey, N.; Voegele, C.; Zvereva, M.; Hosen, M.I.; Meziani, S.; De Tilly, B.; et al. Urinary TERT promoter mutations as non-invasive biomarkers for the comprehensive detection of urothelial cancer. EBioMedicine 2019, 44, 431–438. [Google Scholar] [CrossRef]
- Sieverink, C.A.; Batista, R.P.M.; Prazeres, H.J.M.; Vinagre, J.; Sampaio, C.; Leão, R.R.; Máximo, V.; Witjes, J.A.; Soares, P. Clinical validation of a urine test (Uromonitor-V2®) for the surveillance of non-muscle-invasive bladder cancer patients. Diagnostics 2020, 10, 745. [Google Scholar] [CrossRef] [PubMed]
- Dudley, J.C.; Schroers-Martin, J.; Lazzareschi, D.V.; Shi, W.Y.; Chen, S.B.; Esfahani, M.S.; Trivedi, D.; Chabon, J.J.; Chaudhuri, A.A.; Stehr, H.; et al. Detection and surveillance of bladder cancer using urine tumor DNA. Cancer Discov. 2019, 9, 500–509. [Google Scholar] [CrossRef]
- Fang, Q.; Zhang, X.; Nie, Q.; Hu, J.; Zhou, S.; Wang, C. Improved urine DNA methylation panel for early bladder cancer detection. BMC Cancer 2022, 22, 237. [Google Scholar] [CrossRef]
- Piatti, P.; Chew, Y.C.; Suwoto, M.; Yamada, T.; Jara, B.; Jia, X.Y.; Guo, W.; Ghodoussipour, S.; Daneshmand, S.; Ahmadi, H.; et al. Clinical evaluation of Bladder CARE, a new epigenetic test for bladder cancer detection in urine samples. Clin. Epigenetics 2021, 13, 84. [Google Scholar] [CrossRef]
- Witjes, J.A.; Morote, J.; Cornel, E.B.; Gakis, G.; van Valenberg, F.J.P.; Lozano, F.; Sternberg, I.A.; Willemsen, E.; Hegemann, M.L.; Paitan, Y.; et al. Performance of the Bladder EpiCheck™ methylation test for patients under surveillance for non-muscle-invasive bladder cancer: Results of a multicenter, prospective, blinded clinical trial. Eur. Urol. Oncol. 2018, 1, 307–313. [Google Scholar] [CrossRef] [PubMed]
- Feber, A.; Dhami, P.; Dong, L.; de Winter, P.; Tan, W.S.; Martínez-Fernández, M.; Paul, D.S.; Hynes-Allen, A.; Rezaee, S.; Gurung, P.; et al. UroMark—A urinary biomarker assay for the detection of bladder cancer. Clin. Epigenetics 2017, 9, 8. [Google Scholar] [CrossRef]
- Bagshaw, A.T.M. Functional mechanisms of microsatellite DNA in eukaryotic genomes. Genome Biol. Evol. 2017, 9, 2428–2443. [Google Scholar] [CrossRef] [PubMed]
- Kristmundsdottir, S.; Jonsson, H.; Hardarson, M.T.; Palsson, G.; Beyter, D.; Eggertsson, H.P.; Gylfason, A.; Sveinbjornsson, G.; Holley, G.; Stefansson, O.A.; et al. Sequence variants affecting the genome-wide rate of germline microsatellite mutations. Nat. Commun. 2023, 14, 3855. [Google Scholar] [CrossRef] [PubMed]
- Söreide, K.; Janssen, E.A.M.; Söiland, H.; Körner, H.; Baak, J.P.A. Microsatellite instability in colorectal cancer. Br. J. Surg. 2006, 93, 395–406. [Google Scholar] [CrossRef] [PubMed]
- Tamura, K.; Kaneda, M.; Futagawa, M.; Takeshita, M.; Kim, S.; Nakama, M.; Kawashita, N.; Tatsumi-Miyajima, J. Genetic and genomic basis of the mismatch repair system involved in Lynch syndrome. Int. J. Clin. Oncol. 2019, 24, 999–1011. [Google Scholar] [CrossRef] [PubMed]
- Gupta, D.; Heinen, C.D. The mismatch repair-dependent DNA damage response: Mechanisms and implications. DNA Repair 2019, 78, 60–69. [Google Scholar] [CrossRef] [PubMed]
- Kadyrova, L.Y.; Kadyrov, F.A. Endonuclease activities of MutLα and its homologs in DNA mismatch repair. DNA Repair 2016, 38, 42–49. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Martin, A. Mismatch repair and colon cancer: Mechanisms and therapies explored. Trends Mol. Med. 2016, 22, 274–289. [Google Scholar] [CrossRef]
- Kunkel, T.A.; Erie, D.A. Eukaryotic mismatch repair in relation to DNA replication. Annu. Rev. Genet. 2015, 49, 291–313. [Google Scholar] [CrossRef]
- Traver, S.; Coulombe, P.; Peiffer, I.; Hutchins, J.R.; Kitzmann, M.; Latreille, D.; Méchali, M. MCM9 is required for mammalian DNA mismatch repair. Mol. Cell 2015, 59, 831–839. [Google Scholar] [CrossRef]
- Goel, A.; Nagasaka, T.; Hamelin, R.; Boland, C.R. An optimized pentaplex PCR for detecting DNA mismatch repair-deficient colorectal cancers. PLoS ONE 2010, 5, e9393. [Google Scholar] [CrossRef]
- Yang, G.; Zheng, R.Y.; Jin, Z.S. Correlations between microsatellite instability and the biological behaviour of tumours. J. Cancer Res. Clin. Oncol. 2019, 145, 2891–2899. [Google Scholar] [CrossRef] [PubMed]
- Le, D.T.; Kim, T.W.; Van Cutsem, E.; Geva, R.; Jäger, D.; Hara, H.; Burge, M.; O’Neil, B.; Kavan, P.; Yoshino, T.; et al. Phase II Open-Label Study of Pembrolizumab in Treatment-Refractory, Microsatellite Instability-High/Mismatch Repair-Deficient Metastatic Colorectal Cancer: KEYNOTE-164. J. Clin. Oncol. 2020, 38, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Diaz, L.A.; Shiu, K.K.; Kim, T.W.; Jensen, B.V.; Jensen, L.H.; Punt, C.; Smith, D.; Garcia-Carbonero, R.; Benavides, M.; Gibbs, P.; et al. KEYNOTE-177 Investigators. Pembrolizumab versus chemotherap for microsatellite instability-high or mismatch repair-deficient metastatic colorectal cancer (KEYNOTE-177): Final analysis of a randomised, open-label, phase 3 study. Lancet Oncol. 2022, 23, 659–670. [Google Scholar] [CrossRef] [PubMed]
- Marabelle, A.; Le, D.T.; Ascierto, P.A.; Di Giacomo, A.M.; De Jesus-Acosta, A.; Delord, J.P.; Geva, R.; Gottfried, M.; Penel, N.; Hansen, A.R.; et al. Efficacy of Pembrolizumab in Patients With Noncolorectal High Microsatellite Instability/Mismatch Repair-Deficient Cancer: Results From the Phase II KEYNOTE-158 Study. J. Clin. Oncol. 2020, 38, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Luchini, C.; Bibeau, F.; Ligtenberg, M.J.L. ESMO recommendations on microsatellite instability testing for immunotherapy in cancer, and its relationship with PD-1/PD-L1 expression and tumour mutational burden: A systematic review-based approach. Ann. Oncol. 2019, 30, 1232–1243. [Google Scholar] [CrossRef]
- Liu, J.; Chen, Z.; Li, Y.; Zhao, W.; Wu, J.; Zhang, Z. PD-1/PD-L1 checkpoint inhibitors in tumor immunotherapy. Front. Pharmacol. 2021, 12, 731798. [Google Scholar] [CrossRef]
- Selvajaran, G. Pembrolizumab: The Nut Cracker. Indian J. Med. Paediatr. Oncol. 2020, 41, 393–396. [Google Scholar]
- André, T.; Cohen, R.; Salem, M.E. Immune checkpoint blockade therapy in patients with colorectal cancer harboring microsatellite instability/mismatch repair deficiency in 2022. Am. Soc. Clin. Oncol. Educ. Book 2022, 42, 233–241. [Google Scholar] [CrossRef]
- De’ Angelis, G.L.; Bottarelli, L.; Azzoni, C.; De’ Angelis, N.; Leandro, G.; Di Mario, F.; Gaiani, F.; Negri, F. Microsatellite instability in colorectal cancer. Acta Biomed. 2018, 89, 97–101. [Google Scholar]
- Hause, R.J.; Pritchard, C.C.; Shendure, J.; Salipante, S.J. Classification and characterization of microsatellite instability across 18 cancer types. Nat. Med. 2016, 22, 1342–1350. [Google Scholar] [CrossRef] [PubMed]
- Huang, R.; Zhou, P.K. DNA damage repair: Historical perspectives, mechanistic pathways, and clinical translation for targeted cancer therapy. Signal Transduct. Target. Ther. 2021, 6, 254. [Google Scholar] [CrossRef] [PubMed]
- Mouw, K.W. DNA repair pathway alterations in bladder cancer. Cancers 2017, 9, 28. [Google Scholar] [CrossRef]
- Fraune, C.; Simon, R.; Hube-Magg, C.; Makrypidi-Fraune, G.; Kähler, C.; Kluth, M.; Höflmayer, D.; Büscheck, F.; Dum, D.; Luebke, A.M.; et al. MMR deficiency in urothelial carcinoma of the bladder presents with temporal and spatial homogeneity throughout the tumor mass. Urol. Oncol. Semin. Orig. Investig. 2020, 38, 488–495. [Google Scholar] [CrossRef] [PubMed]
- Mohamedali, R.; Adhya, A.K.; Mandal, S.; Mitra, S. Expression of mismatch repair proteins in urothelial carcinoma of the urinary bladder. Indian J. Cancer 2022, 59, 279–281. [Google Scholar] [CrossRef]
- Catto, J.W.F.; Xinarianos, G.; Burton, J.L.; Meuth, M.; Hamdy, F.C. Differential expression of hMLH1 and hMSH2 is related to bladder cancer grade, stage and prognosis but not microsatellite instability. Int. J. Cancer 2003, 105, 484–490. [Google Scholar] [CrossRef]
- Burger, M.; Burger, S.J.; Denzinger, S.; Wild, P.J.; Wieland, W.F.; Blaszyk, H.; Obermann, E.C.; Stoehr, R.; Hartmann, A. Elevated microsatellite instability at selected tetranucleotide repeats does not correlate with clinicopathologic features of bladder cancer. Eur. Urol. 2006, 50, 770–776. [Google Scholar] [CrossRef] [PubMed]
- Vageli, D.P.; Giannopoulos, S.; Doukas, S.G.; Kalaitzis, C.; Giannakopoulos, S.; Giatromanolaki, A.; Koukouli, G.K.; Touloupidis, S. Mismatch repair hMSH2, hMLH1, hMSH6 and hPMS2 mRNA expression profiles in precancerous and cancerous urothelium. Oncol. Lett. 2013, 5, 283–294. [Google Scholar] [CrossRef][Green Version]
- Vaish, M.; Mandhani, A.; Mittal, R.; Mittal, B. Microsatellite instability as a prognostic marker in bladder tumors: Clinical significance. BMC Urol. 2005, 5, 2. [Google Scholar] [CrossRef]
- Awadalla, A.; Harraz, A.M.; Abol-Enein, H.; Laymon, M.; Ahmed, A.E.; Abdel-Rahim, M.; Zekri, A.-R.N.; Shokeir, A.A. Prognostic influence of microsatellite alterations of muscle-invasive bladder cancer treated with radical cystectomy. Urol. Oncol. Semin. Orig. Investig. 2022, 40, e9–e64. [Google Scholar] [CrossRef]
- Saidi, S.; Popov, Z.; Stavridis, S.; Panov, S. Alterations of microsatellite loci GSN and D18S51 in urinary bladder cancer. Hippokratia 2015, 19, 200–204. [Google Scholar] [PubMed]
- Walz, S.; Pollehne, P.; Geng, R.; Schneider, J.; Maas, M.; Aicher, W.K.; Stenzl, A.; Amend, B.; Harland, N. A protocol for organoids from the urine of bladder cancer patients. Cells 2023, 12, 2188. [Google Scholar] [CrossRef] [PubMed]
- Andersson, E.; Dahmcke, C.M.; Steven, K.; Larsen, L.K.; Guldberg, P. Filtration Device for On-Site Collection, Storage and Shipment of Cells from Urine and Its Application to DNA-Based Detection of Bladder Cancer. PLoS ONE 2015, 10, e0131889. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Jordaens, S.; Arora, A.; MacDonald, K.W.; Wood, C.; Hendrickx, J.O.; Zwaenepoel, K.; Deben, C.; Tjalma, W.; Pauwels, P.; Beyers, K.; et al. UAS™-A Urine Preservative for Oncology Applications. Cancers 2023, 15, 3119. [Google Scholar] [CrossRef]
- Linn, J.F.; Lango, M.; Halachmi, S.; Schoenberg, M.P.; Sidransky, D. Microsatellite Analysis and Telomerase Activity in Archived Tissue and Urine Samples of Bladder Cancer Patients. Int. J. Cancer 1997, 74, 625–629. [Google Scholar] [CrossRef]
- Mao, L.; Schoenberg, M.P.; Scicchitano, M.; Erozan, Y.S.; Merlo, A.; Schwab, D.; Sidransky, D. Molecular detection of primary bladder cancer by microsatellite analysis. Science 1996, 271, 659–662. [Google Scholar] [CrossRef]
- Mourah, S.; Cussenot, O.; Vimont, V.; Desgrandchamps, F.; Teillac, P.; Cochant-Priollet, B.; Le Duc, A.; Fiet, J.; Soliman, H. Assessment of microsatellite instability in urine in the detection of transitional-cell carcinoma of the bladder. Int. J. Cancer 1998, 79, 629–633. [Google Scholar] [CrossRef]
- Seripa, D.; Parrella, P.; Gallucci, M.; Gravina, C.; Papa, S.; Fortunato, P.; Alcini, A.; Flammia, G.; Lazzari, M.; Fazio, V.M. Sensitive detection of transitional cell carcinoma of the bladder by microsatellite analysis of cells exfoliated in urine. Int. J. Cancer 2001, 95, 364–369. [Google Scholar]
- Amira, N.; Mourah, S.; Rozet, F.; Teillac, P.; Fiet, J.; Aubin, P.; Cortesse, A.; Desgrandchamps, F.; Le Duc, A.; Cussenot, O.; et al. Non-invasive molecular detection of bladder cancer recurrence. Int. J. Cancer 2002, 101, 293–297. [Google Scholar] [CrossRef]
- Molina Burgos, R.; Millán Salvador, J.M.; Oltra Soler, J.S.; Jiménez Cruz, J.F. Microsatellite analysis in exfoliated cells from urine sediment. Utility for bladder cancer detection. Comparative study with urinary cytology. Actas Urol. Esp. 2003, 27, 618–628. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wadhwa, N.; Mathew, B.B.; Tandon, S.; Biju, V.G.; Tiwari, A. Assessment of microsatellite instability for screening bladder cancer in a high-risk population. J. Cancer Res. Ther. 2018, 14, 916–920. [Google Scholar] [CrossRef] [PubMed]
- Andersson, E.; Steven, K.; Guldberg, P. Size-based enrichment of exfoliated tumor cells in urine increases the sensitivity for DNA-based detection of bladder cancer. PLoS ONE 2014, 9, e94023. [Google Scholar] [CrossRef]
- Brennetot, C.; Buhard, O.; Jourdan, F.; Flejou, J.F.; Duval, A.; Hamelin, R. Mononucleotide repeats BAT-26 and BAT-25 accurately detect MSI-H tumors and predict tumor content: Implications for population screening. Int. J. Cancer 2005, 113, 446–450. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, T.; Bertsche, K.; Moon, D.; Moon, C. Qualification of the microsatellite instability analysis (MSA) for bladder cancer detection: The technical challenges of concordance analysis. Int. J. Mol. Sci. 2023, 25, 209. [Google Scholar] [CrossRef] [PubMed]
- Haghighi, M.M.; Javadi, G.R.; Parivar, K. Frequent MSI mononucleotide markers for diagnosis of hereditary nonpolyposis colorectal cancer. Asian Pac. J. Cancer Prev. 2010, 11, 1033–1035. [Google Scholar] [PubMed]
- Suraweera, N.; Duval, A.; Reperant, M.; Vaury, C.; Furlan, D.; Leroy, K.; Seruca, R.; Iacopetta, B.; Hamelin, R. Evaluation of tumor microsatellite instability using five quasimonomorphic mononucleotide repeats and pentaplex PCR. Gastroenterology 2002, 123, 1804–1811. [Google Scholar] [CrossRef]
- Buhard, O.; Cattaneo, F.; Wong, Y.F.; Yim, S.F.; Friedman, E.; Flejou, J.F.; Duval, A.; Hamelin, R. Multipopulation analysis of polymorphisms in five mononucleotide repeats used to determine the microsatellite instability status of human tumors. J. Clin. Oncol. 2006, 24, 241–251. [Google Scholar] [CrossRef]
- Chung, Y.; Nam, S.K.; Chang, H.E.; Lee, C.; Kang, G.H.; Lee, H.S.; Park, K.U. Evaluation of an eight marker-panel including long mononucleotide repeat markers to detect microsatellite instability in colorectal, gastric, and endometrial cancers. BMC Cancer 2023, 23, 1100. [Google Scholar] [CrossRef]
- Kang, S.Y.; Kim, K.M. Highly sensitive duplex MSI test and BAT40 germline polymorphism. APMIS 2021, 129, 607–615. [Google Scholar] [CrossRef]
- Balar, A.V.; Kamat, A.M.; Kulkarni, G.S.; Uchio, E.M.; Boormans, J.L.; Roumiguié, M.; Krieger, L.E.M.; Singer, E.A.; Bajorin, D.F.; Grivas, P.; et al. Pembrolizumab monotherapy for the treatment of high-risk non-muscle-invasive bladder cancer unresponsive to BCG (KEYNOTE-057): An open-label, single-arm, multicentre, phase 2 study. Lancet Oncol. 2021, 22, 919–930. [Google Scholar] [CrossRef]
| Panel | Principle | Detection Unit | Sensitivity | Specificity | References |
|---|---|---|---|---|---|
| Exfoliated bladder tumor cells | |||||
| UroVysion * | FISH | Aneuploidies in chromosomes 3, 7, and 17 and deletions in 9p21 | 73% | 83% | [21,22] |
| Immunocyt * | F-LMab | Carcinoembryonic antigen and sulfated mucin glycoproteins | 60–100% | 75–84% | [23] |
| URO17 | IHC | Cytokeratin 17 | 100% | 92.6% | [24] |
| Cell detect | Immunostaining | Malignant cells | 82.8% | 88.2% | [25] |
| Proteins | |||||
| BTA-STAT * | ICA | Proteins associated with human complement factor H | 76.7% | 67.9% | [26,27] |
| BTA TRAK * | ELISA | Proteins associated with human complement factor H | 58% | 73% | [21,28] |
| NMP22 * | ELISA | NMP22 protein | 50–70% | 60–80% | [21,29] |
| UBC Rapid test | ELISA | Cytokeratins 8 and 18 | 72.2% | 79.4% | [24,30] |
| ADXBLADDER | ELISA | MCM5 protein | 75.6% | 71.1% | [31] |
| BLCA-4 | ELISA | BLCA-4 protein | 93% | 97% | [32] |
| OncuriaTM | Multiplex Immunoassay | A1AT, APOE, ANG, CA9, IL8, MMP9, MMP10, PAI1, SDC1, and VEGFA proteins | 93% | 93% | [33] |
| mRNA | |||||
| Xpert Bladder Cancer Monitor | RT-PCR | mRNA levels of CRH, IGF2, UPK1B, ANXA10, and ABL1 genes. | 75% | 77% | [34] |
| CxBladder | RT-PCR | mRNA levels of MDK, HOXA13, CDC2, IGFBP5, and CXCR2 genes | 100% | 75% | [35] |
| DNA alterations | |||||
| UroMuTERT | Sequencing | Mutations in TERT promoter | 87.1% | 94.7% | [36] |
| Uromonitor-V2® | qPCR | Mutations in TERT, FGFR3, and KRAS genes | 93.1% | 85.4% | [37] |
| uCAPP-Seq | Sequencing | DNA alterations in regions from 460 genes | 83% | 97% | [38] |
| DNA methylation | |||||
| P3 panel | qMSP | Methylation status of PCDH17, POU4F2, and PENK genes | 84% | 100% | [39] |
| Bladder Care | qMSP | Methylation status of TRNA-Cys, SIM2 and NKX1-1 | 93.5% | 92.6% | [40] |
| EpiCheck | qMSP | Methylation status of 15 loci | 68.2% | 88% | [41] |
| UroMark | Targeted bisulfite sequencing | Methylation status of 150 loci | 96% | 97% | [42] |
| n | MSI Frequency | Analyzed Markers | Reference |
|---|---|---|---|
| 44 | 72.7% | BAT-26, BAT-40, BAX, TGFβ, IGFIIR, MSH3, D2S123, D9S283, D9S1851, and D18S58 | [70] |
| 448 | 1.1% | BAT-25, BAT-26, D2S123, D5S346, D17S250, and MYCL1 | [65] |
| 220 | 39% | ACTBP2, D16S310, D16S476, D18S51, D4S243, D9S162, D9S171, D9S747, FGA, INF-a, MBP, and MJD | [71] |
| 70 | 65.7% | GSN and D18S51 | [72] |
| 84 | 8% | BAT25, BAT26, D17S250, D2S123, D5S346, BAT40, D10S197, MYC1L, D18S58, D18S69, TGFβR2, BAX, hMSH3, and hMSH6 | [67] |
| 117 | 8.5% | BAT25, BAT26, D2S123, APC, and D17S250 | [68] |
| n | MSI Frequency | Analyzed Markers | Reference |
|---|---|---|---|
| 20 | 95% | ACTBP2, D16S310, FGA, D21S1245, D4S243, D16S476, D9S747, D18S51, MBP, MJD, D9S162, IFNA, and D9S171 | [77] |
| 12 | 83% | D4S243, FGA, ACTBP2, D9S162, D9S171, D9S747, IFNA, MJD52, D16S310, D16S476, D18S51, MBP, and D21S1245 | [78] |
| 34 | 97% | D4S243, FGA, ACTBP2, D8S307, IFNA, D9S162, D9S171, D9S747, D11S488, THO, D13S802, MJD, D16S310, D16S476, D17S695, D17S654, D18S51, MBP, D20S48, and D21S1245 | [79] |
| 47 | 94% | D4S243, FGA, ACTBP2, D9S162, D9S171, D9S747, IFNA, MJD52, D16S310, D16S476, D18S51, MBP, and D21S1245 | [80] |
| 150 | 74% | D9S747, D9S171, D9S162, IFNA, and D4S243 | [81] |
| 50 | 76% | D9S63, D9S156, and D9S283 | [82] |
| 220 | 43.5% | ACTBP2, D16S310, D16S476, D18S51, D4S243, D9S162, D9S171, D9S747, FGA, INF-a, MBP, and MJD | [71] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rico-Méndez, M.A.; Ayala-Madrigal, M.d.l.L.; González-Mercado, A.; Gutiérrez-Angulo, M.; Ramírez de Arellano Sánchez, J.A.; Beltrán-Ontiveros, S.A.; Contreras-Haro, B.; Gutiérrez-Hurtado, I.A.; Moreno-Ortiz, J.M. Microsatellite Instability in Urine: Breakthrough Method for Bladder Cancer Identification. Biomedicines 2024, 12, 2726. https://doi.org/10.3390/biomedicines12122726
Rico-Méndez MA, Ayala-Madrigal MdlL, González-Mercado A, Gutiérrez-Angulo M, Ramírez de Arellano Sánchez JA, Beltrán-Ontiveros SA, Contreras-Haro B, Gutiérrez-Hurtado IA, Moreno-Ortiz JM. Microsatellite Instability in Urine: Breakthrough Method for Bladder Cancer Identification. Biomedicines. 2024; 12(12):2726. https://doi.org/10.3390/biomedicines12122726
Chicago/Turabian StyleRico-Méndez, Manuel Alejandro, María de la Luz Ayala-Madrigal, Anahí González-Mercado, Melva Gutiérrez-Angulo, Jorge Adrián Ramírez de Arellano Sánchez, Saul Armando Beltrán-Ontiveros, Betsabe Contreras-Haro, Itzae Adonai Gutiérrez-Hurtado, and José Miguel Moreno-Ortiz. 2024. "Microsatellite Instability in Urine: Breakthrough Method for Bladder Cancer Identification" Biomedicines 12, no. 12: 2726. https://doi.org/10.3390/biomedicines12122726
APA StyleRico-Méndez, M. A., Ayala-Madrigal, M. d. l. L., González-Mercado, A., Gutiérrez-Angulo, M., Ramírez de Arellano Sánchez, J. A., Beltrán-Ontiveros, S. A., Contreras-Haro, B., Gutiérrez-Hurtado, I. A., & Moreno-Ortiz, J. M. (2024). Microsatellite Instability in Urine: Breakthrough Method for Bladder Cancer Identification. Biomedicines, 12(12), 2726. https://doi.org/10.3390/biomedicines12122726










