Craniofacial Effects of Zoledronic Acid on the Osteogenesis Imperfecta Mouse (−/−) Model of Severe Osteogenesis Imperfecta
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Procedures
2.2. In Vivo Computed Tomography
2.3. Peripheral Quantitative Computed Tomography (pQCT)
2.4. Histology
2.5. Statistics
3. Results
3.1. In Vivo CT
3.2. pQCT
3.3. Correlation
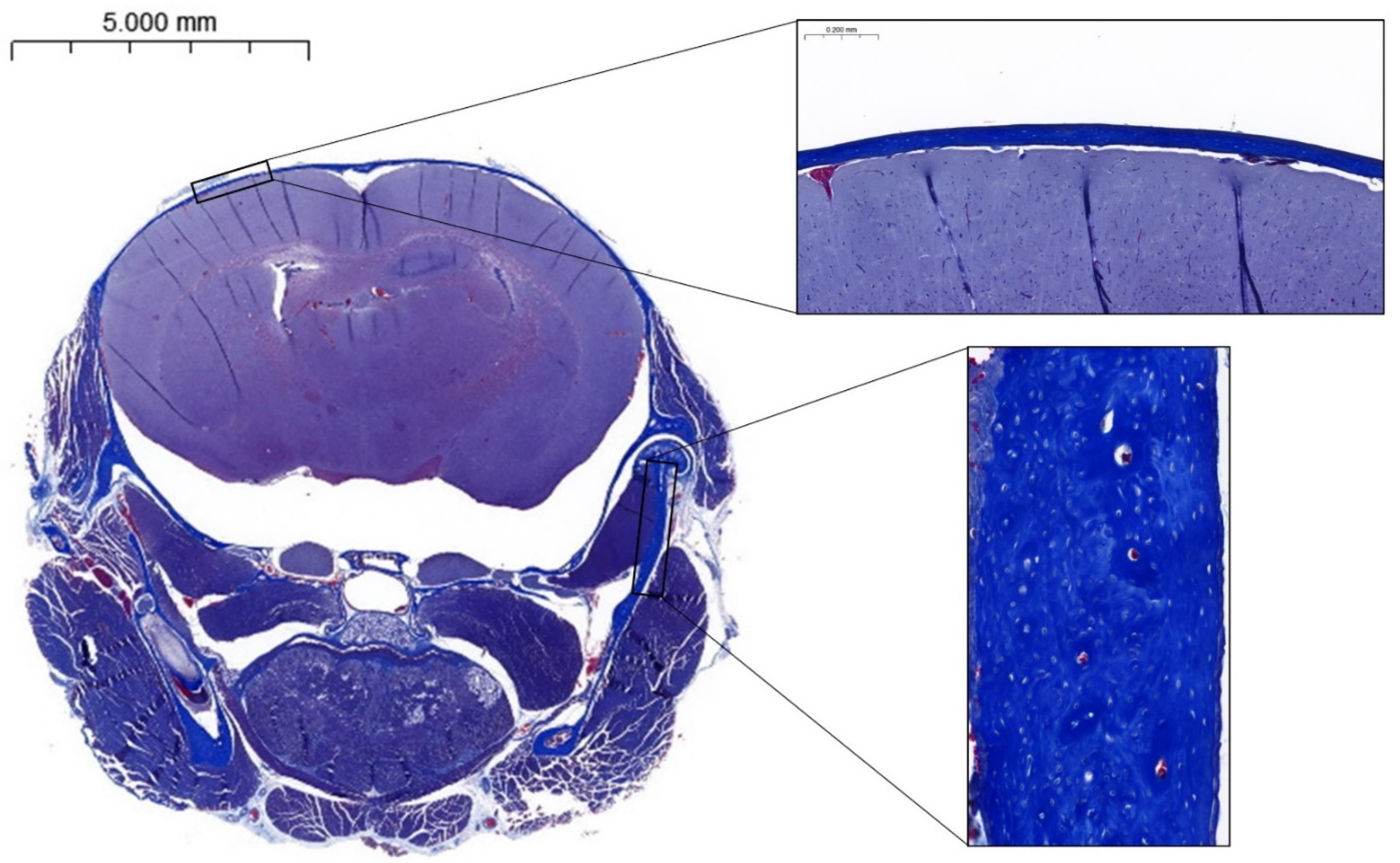
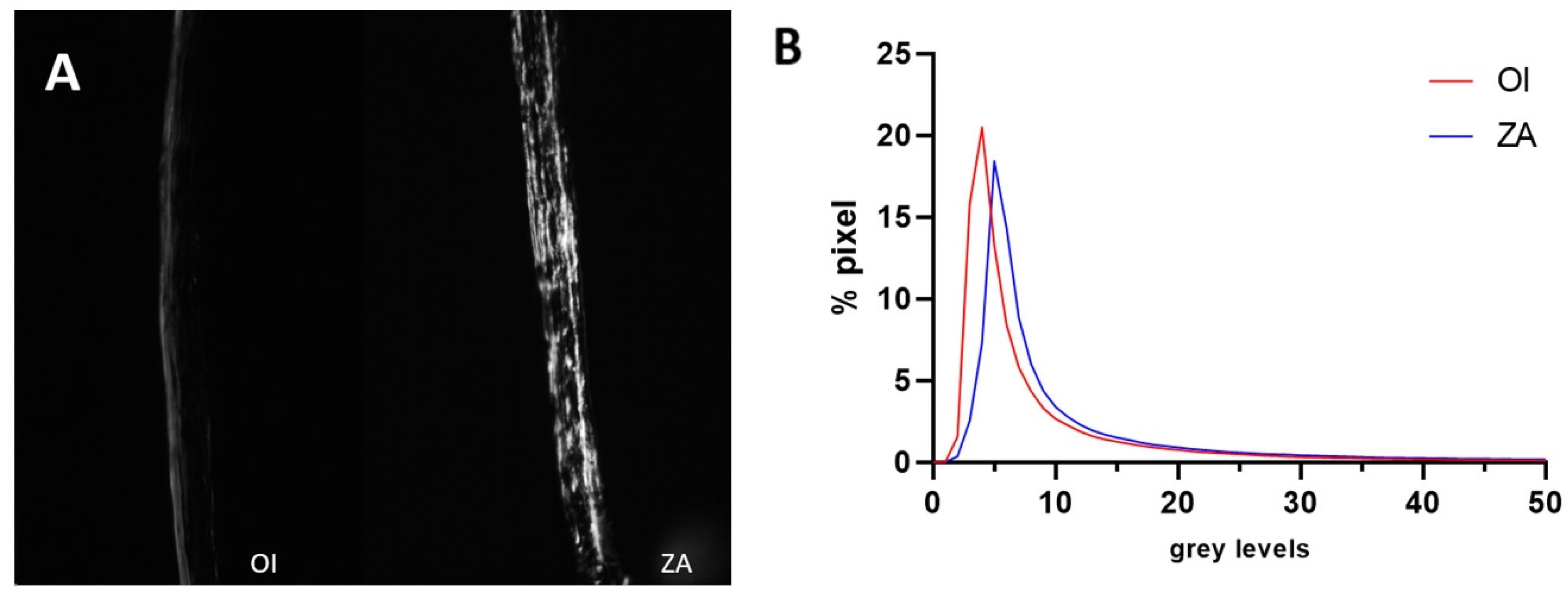
3.4. Histomorphometry and Birefringence
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chretien, A.; Couchot, M.; Mabilleau, G.; Behets, C. Biomechanical, Microstructural and Material Properties of Tendon and Bone in the Young Oim Mice Model of Osteogenesis Imperfecta. Int. J. Mol. Sci. 2022, 23, 9928. [Google Scholar] [CrossRef] [PubMed]
- Van Dijk, F.S.; Sillence, D.O. Osteogenesis imperfecta: Clinical diagnosis, nomenclature and severity assessment. Am. J. Med. Genet. A 2014, 164A, 1470–1481. [Google Scholar] [CrossRef] [PubMed]
- Jovanovic, M.; Guterman-Ram, G.; Marini, J.C. Osteogenesis Imperfecta: Mechanisms and Signaling Pathways Connecting Classical and Rare OI Types. Endocr. Rev. 2022, 43, 61–90. [Google Scholar] [CrossRef] [PubMed]
- Khandanpour, N.; Connolly, D.J.; Raghavan, A.; Griffiths, P.D.; Hoggard, N. Craniospinal abnormalities and neurologic complications of osteogenesis imperfecta: Imaging overview. Radiographics 2012, 32, 2101–2112. [Google Scholar] [CrossRef] [PubMed]
- Arponen, H.; Mäkitie, O.; Haukka, J.; Ranta, H.; Ekholm, M.; Mäyränpää, M.K.; Kaitila, I.; Waltimo-Sirén, J. Prevalence and natural course of craniocervical junction anomalies during growth in patients with osteogenesis imperfecta. J. Bone Miner. Res. 2012, 27, 1142–1149. [Google Scholar] [CrossRef] [PubMed]
- Dwan, K.; Phillipi, C.A.; Steiner, R.D.; Basel, D. Bisphosphonate therapy for osteogenesis imperfecta. Cochrane Database Syst. Rev. 2014, CD005088. [Google Scholar] [CrossRef]
- Drake, M.; Clarke, B.; Khosla, S. Bisphosphonates: Mechanism of action and role in clinical practice. Mayo Clin. Proc. 2008, 83, 1032–1045. [Google Scholar] [CrossRef]
- Wei, Z.; Hong, C.; Tu, C.; Ge, W.; Hu, Y.; Lin, S. Development and validation of a clinical prediction model for osteonecrosis of the jaw in patients receiving zoledronic acid using FAERS and canadian databases. Front. Pharmacol. 2024, 15, 1456900. [Google Scholar] [CrossRef]
- Patel, V.; McLeod, N.M.; Rogers, S.N.; Brennan, P.A. Bisphosphonate osteonecrosis of the jaw—A literature review of UK policies versus international policies on bisphosphonates, risk factors and prevention. Br. J. Oral. Maxillofac. Surg. 2011, 49, 251–257. [Google Scholar] [CrossRef]
- Saito, M.; Marumo, K. Effects of Collagen Crosslinking on Bone Material Properties in Health and Disease. Calcif. Tissue Int. 2015, 97, 242–261. [Google Scholar] [CrossRef]
- Egawa, S.; Miura, S.; Yokoyama, H.; Endo, T.; Tamura, K. Growth and differentiation of a long bone in limb development, repair and regeneration. Dev. Growth Differ. 2014, 56, 410–424. [Google Scholar] [CrossRef] [PubMed]
- Waltimo-Siren, J.; Kolkka, M.; Pynnonen, S.; Kuurila, K.; Kaitila, I.; Kovero, O. Craniofacial features in osteogenesis imperfecta: A cephalometric study. Am. J. Med. Genet. A 2005, 133A, 142–150. [Google Scholar] [CrossRef] [PubMed]
- Eimar, H.; Tamimi, F.; Retrouvey, J.M.; Rauch, F.; Aubin, J.E.; McKee, M.D. Craniofacial and Dental Defects in the Col1a1Jrt/+ Mouse Model of Osteogenesis Imperfecta. J. Dent. Res. 2016, 95, 761–768. [Google Scholar] [CrossRef] [PubMed]
- Fosséprez, J.; Roels, T.; Manicourt, D.; Behets, C. Craniofacial dysmorphism of osteogenesis imperfecta mouse and effect of cathepsin K knockout: Preliminary craniometry observations. Morphologie 2024, 108, 100785. [Google Scholar] [CrossRef] [PubMed]
- Fosséprez, J. Ostéogenèse imparfaite: Anomalies craniofaciales chez la souris oim. Session commune Association des Morphologites—Collège des Histologistes, Embryologistes et Cytogénéticiens. Morphologie 2021, 350, S1. [Google Scholar] [CrossRef]
- Cardinal, M.; Chretien, A.; Roels, T.; Lafont, S.; Ominsky, M.S.; Devogelaer, J.P.; Manicourt, D.H.; Behets, C. Gender-Related Impact of Sclerostin Antibody on Bone in the Osteogenesis Imperfecta Mouse. Front. Genet. 2021, 12, 705505. [Google Scholar] [CrossRef]
- Chang, P.C.; Lin, S.Y.; Hsu, K.H. The craniofacial characteristics of osteogenesis imperfecta patients. Eur. J. Orthod. 2007, 29, 232–237. [Google Scholar] [CrossRef]
- Menegaz, R.A.; Ladd, S.H.; Organ, J.M. Craniofacial allometry in the OIM−/− mouse model of osteogenesis imperfecta. FASEB J. 2020, 34, 10850–10859. [Google Scholar] [CrossRef]
- Irving, M.; AlSayed, M.; Arundel, P.; Baujat, G.; Ben-Omran, T.; Boero, S.; Cormier-Daire, V.; Fredwall, S.; Guillen-Navarro, E.; Hoyer-Kuhn, H.; et al. European Achondroplasia Forum guiding principles for the detection and management of foramen magnum stenosis. Orphanet J. Rare Dis. 2023, 18, 219. [Google Scholar] [CrossRef]
- Kovero, O. Skull base abnormalities in osteogenesis imperfecta: A cephalometric evaluation of 54 patients and 108 control volunteers. J. Neurosurg. 2006, 105, 361–370. [Google Scholar] [CrossRef]
- Pantoja, L.L.Q.; Lustosa, M.; Yamaguti, P.M.; Rosa, L.S.; Leite, A.F.; Figueiredo, P.T.; Castro, L.C.; Acevedo, A.C. Pamidronate Therapy Increases Trabecular Bone Complexity of Mandibular Condyles in Individuals with Osteogenesis Imperfecta. Calcif. Tissue Int. 2022, 110, 303–312. [Google Scholar] [CrossRef] [PubMed]
- Moore, J.C.; Husain, T.S.; Huston, L.A.; Steele, A.T.; Organ, J.M.; Gonzales, L.A.; Menegaz, R.A.; Handler, E.K. Dental tissue changes in juvenile and adult mice with osteogenesis imperfecta. Anat. Rec. 2023, 307, 600–610. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, C.S.; Santos Felippe, M.C.; Tadeu Felippe, W.; Silva-Sousa, Y.T.; Sousa-Neto, M.D. The role of dentists in diagnosing osteogenesis imperfecta in patients with dentinogenesis imperfecta. J. Am. Dent. Assoc. 2008, 139, 906–914; quiz 94. [Google Scholar] [CrossRef] [PubMed]
- Vatsa, A.; Breuls, R.G.; Semeins, C.M.; Salmon, P.L.; Smit, T.H.; Klein-Nulend, J. Osteocyte morphology in fibula and calvaria—Is there a role for mechanosensing? Bone 2008, 43, 452–458. [Google Scholar] [CrossRef] [PubMed]
- Himeno-Ando, A.; Izumi, Y.; Yamaguchi, A.; Iimura, T. Structural differences in the osteocyte network between the calvaria and long bone revealed by three-dimensional fluorescence morphometry, possibly reflecting distinct mechano-adaptations and sensitivities. Biochem. Biophys. Res. Commun. 2012, 417, 765–770. [Google Scholar] [CrossRef]
- Gao, M.; Zhu, B.; Fan, J.; Gao, Y.; Xue, F.; Li, G.; Hubbard, A.; Gao, X.; Sun, J.; Ling, J.; et al. Distinct differences between calvarial and long bone osteocytes in cell morphologies, gene expression and aging responses. FEBS J. 2023, 290, 4074–4091. [Google Scholar] [CrossRef]
- Lee, S.K.; Kim, Y.S.; Oh, H.S.; Yang, K.H.; Kim, E.C.; Chi, J.G. Prenatal development of the human mandible. Anat. Rec. 2001, 263, 314–325. [Google Scholar] [CrossRef]
- Parada, C.; Chai, Y. Mandible and Tongue Development. Curr. Top. Dev. Biol. 2015, 115, 31–58. [Google Scholar]
- Rengasamy Venugopalan, S.; Allareddy, V. Craniofacial Growth and Development. In Peterson’s Principles of Oral and Maxillofacial Surgery; Miloro, M., Ghali, G.E., Larsen, P.E., Waite, P., Eds.; Springer International Publishing: Cham, Switzerland, 2022; pp. 1729–1765. [Google Scholar]
- Liang, C.; Profico, A.; Buzi, C.; Khonsari, R.H.; Johnson, D.; O’Higgins, P.; Moazen, M. Normal human craniofacial growth and development from 0 to 4 years. Sci. Rep. 2023, 13, 9641. [Google Scholar] [CrossRef]

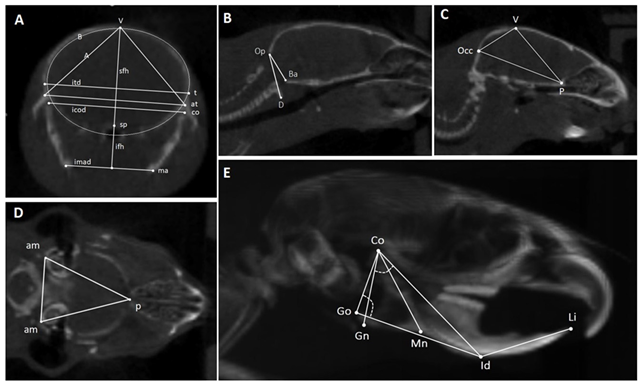 | ||||
| Landmarks | Linear Measurements | Angle | Area | |
| A: Coronal slice through mandibular ramus, temporomandibular joints, and calvaria | t = temporo-parietal suture; sp = sphenoid bone; co = condylar process; ma = mandibular angle; at = articular tubercle. | sfh: superior frontal height; ifh: inferior frontal height; itd: intertemporal distance; icod: intercondylar distance; imad: intermandibular angles distance. | None | A = area of the triangle between V and both At; B is the frontal skull area. |
| B, C: Midsagittal slices | V = vertex; Occ = posterior point of the occipital bone; P = posterior point of the palatal bone; Op = occipital segment of the foramen magnum; Ba = basilar segment of the foramen magnum; D = anterior arch of atlas. | Occ-p = distance between the posterior point of the occipital bone and the posterior point of the palatal bone; Op-Ba = foramen magnum diameter. | D-Op-Ba angle = basilar angulation. | Sagittal skull area (Ssa) = area of the triangle between Occ, V, and P. |
| D: Transversal slice through both auditory meatuses | am = auditory meatus; p = posterior point of the palatal bone. | None | None | Transversal skull area (Tsa) = area of the triangle between Pp and both Am |
| E: 3D lateral view of the head | Co = condylar process; Go = the most posterior point of the gonial angle or of the angular process; Gn = the lowest point of the mandibular corpus; Mn = point in the deepest part of the antegonial notch curvature; Id = the most anterior point of the vestibular mandibular alveolar process; Li = upper incisor edge. | Co-Gn = mandibular ramus length; Co-Id = mandibular length; Go-Id = mandibular corpus length; Id-Li = extra-bone part of the lower incisor; Co-Mn = mandibular height. | Co-Go-Id = gonial angle; Gn-Co-Id = condylar angle. | None |
| (Distance in mm; Area in mm2; Angle in °) | OI | ZA | p |
|---|---|---|---|
| Coronal cut Superior frontal height (sfh) Inferior frontal height (ifh) Intertemporal distance (itd) Intercondylar distance (icod) Intermandibular angles distance (imad) Triangle vertex—both articular tubercules (A) Frontal skull area (B) | 6.06 ± 0.23 3.86 ± 0.18 9.92 ± 0.21 9.60 ± 0.23 6.92 ± 0.36 23.34 ± 1.15 49.09 ± 2.47 | 6.24 ± 0.38 4.02 ± 0.34 9.88 ± 0.20 9.59 ± 0.24 6.94 ± 0.29 24.24 ± 1.50 49.41 ± 3.55 | 0.11 0.11 0.36 0.45 0.45 0.08 0.41 |
| Midsagittal cut Sagittal skull area (Ssa) Sagittal length (Occ-P) Foramen magnum diameter (Op-Ba) Basilar angulation (D-Op-Ba, °) | 26.06 ± 2.97 11.52 ± 0.38 3.70 ± 0.29 163.3 ± 27.7 | 28.37 ± 1.32 11.46 ± 0.41 3.74 ± 0.14 152.1 ± 29.1 | <0.05 0.38 0.35 0.23 |
| Transversal cut Transversal skull area (Tsa) | 36.7 ± 1.8 | 37.6 ± 3.3 | 0.26 |
| Lateral view of the head Extra-bone part of the lower incisor (Id-Li) Mandibular ramus length (Co-Gn) Mandibular length; (Co-Id) Mandibular corpus length (Go-Id) Mandibular height (Co-Mn) Gonial angle (Co-Go-Id, °) Condylar angle (Gn-Co-Id, °) | 5.13 ± 0.47 4.40 ± 0.35 9.23 ± 0.67 8.59 ± 0.68 5.36 ± 0.37 87.7 ± 2.7 58.8 ± 3.9 | 5.97 ± 0.39 4.86 ± 0.31 9.38 ± 0.58 8.80 ± 0.49 5.61 ± 0.24 85.6 ± 3.6 55.6 ± 3.2 | <0.01 <0.01 0.28 0.25 0.07 0.09 <0.05 |
| OI | ZA | p | |
|---|---|---|---|
Cross section area (mm2)
| 3.03 ± 0.20 1.33 ± 0.29 1.54 ± 0.16 | 3.08 ± 0.39 1.37 ± 0.14 1.66 ± 0.23 | 0.71 0.64 0.11 |
Total mineral density (mg/cm³)
| 525.0 ± 24.6 729.0 ± 41.6 692.6 ± 51.3 | 541.8 ± 52.2 785.5 ± 45.6 734.4 ± 55.9 | 0.29 <0.01 0.05 |
Cortical mineral density (mg/cm³)
| 712.7 ± 15.8 931.1 ± 33.8 885.1 ± 51.9 | 730.3 ± 32.1 972.8 ± 39.7 926.5 ± 42.1 | 0.08 <0.01 0.03 |
| OI | ZA | p-Value | |
|---|---|---|---|
| Calvaria thickness (mm) | 0.61 ± 0.23 | 0.77 ± 0.22 | 0.08 |
| Mean grey level (0–255) | 10.69 ± 4.67 | 17.34 ± 12.52 | 0.20 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jeannerod, G.; Chretien, A.; André, G.; Mabilleau, G.; Behets, C. Craniofacial Effects of Zoledronic Acid on the Osteogenesis Imperfecta Mouse (−/−) Model of Severe Osteogenesis Imperfecta. Biomedicines 2024, 12, 2692. https://doi.org/10.3390/biomedicines12122692
Jeannerod G, Chretien A, André G, Mabilleau G, Behets C. Craniofacial Effects of Zoledronic Acid on the Osteogenesis Imperfecta Mouse (−/−) Model of Severe Osteogenesis Imperfecta. Biomedicines. 2024; 12(12):2692. https://doi.org/10.3390/biomedicines12122692
Chicago/Turabian StyleJeannerod, Gaspard, Antoine Chretien, Grégoire André, Guillaume Mabilleau, and Catherine Behets. 2024. "Craniofacial Effects of Zoledronic Acid on the Osteogenesis Imperfecta Mouse (−/−) Model of Severe Osteogenesis Imperfecta" Biomedicines 12, no. 12: 2692. https://doi.org/10.3390/biomedicines12122692
APA StyleJeannerod, G., Chretien, A., André, G., Mabilleau, G., & Behets, C. (2024). Craniofacial Effects of Zoledronic Acid on the Osteogenesis Imperfecta Mouse (−/−) Model of Severe Osteogenesis Imperfecta. Biomedicines, 12(12), 2692. https://doi.org/10.3390/biomedicines12122692






