Metabolic Crossroad Between Macrophages and Cancer Cells: Overview of Hepatocellular Carcinoma
Abstract
1. Introduction
2. Macrophages—Origin
3. Macrophage Classification and Function
4. Link Between the Metabolism of Macrophages and HCC Cells
4.1. Glucose Metabolism
4.2. Lipid Metabolism
4.3. Amino Acid Metabolism
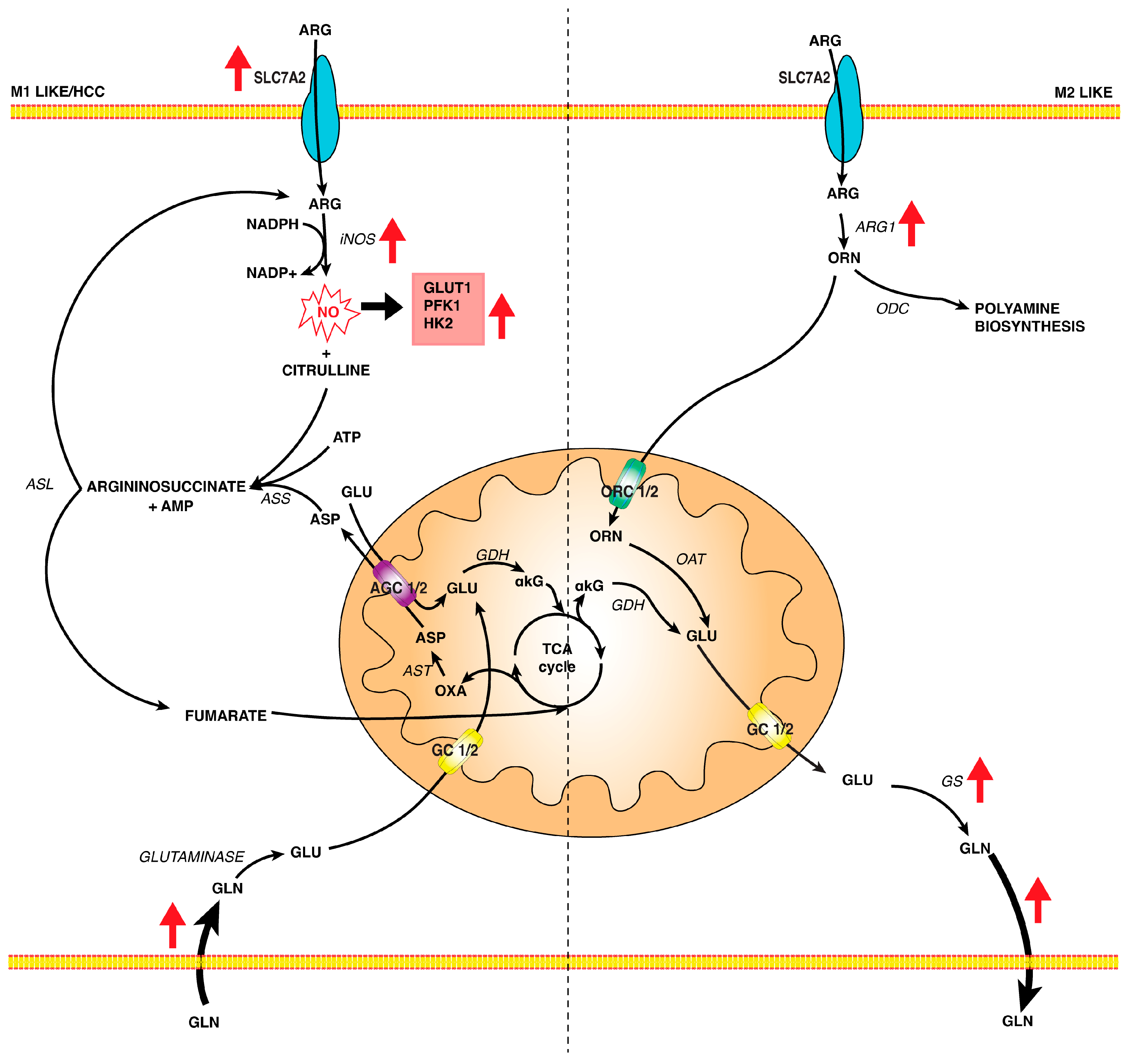
4.3.1. L-Arginine Metabolism
4.3.2. L-Glutamine Metabolism
5. Metabolites of TCA Cycle and Macrophage Reprogramming
5.1. Citrate
5.2. Alpha-Ketoglutarate, Succinate, and Fumarate
6. Metabolic Crosstalk Between HCC Cells and TAMs
7. A Clinical Viewpoint on Therapeutic Interventions
8. Future Perspectives and Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Rumgay, H.; Ferlay, J.; de Martel, C.; Georges, D.; Ibrahim, A.S.; Zheng, R.; Wei, W.; Lemmens, V.E.P.P.; Soerjomataram, I. Global, Regional and National Burden of Primary Liver Cancer by Subtype. Eur. J. Cancer 2022, 161, 108–118. [Google Scholar] [CrossRef] [PubMed]
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global Cancer Statistics 2022: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef] [PubMed]
- Ioannou, G.N.; Splan, M.F.; Weiss, N.S.; McDonald, G.B.; Beretta, L.; Lee, S.P. Incidence and Predictors of Hepatocellular Carcinoma in Patients with Cirrhosis. Clin. Gastroenterol. Hepatol. Off. Clin. Pract. J. Am. Gastroenterol. Assoc. 2007, 5, 938–945.e4. [Google Scholar] [CrossRef] [PubMed]
- Todisco, S.; Convertini, P.; Iacobazzi, V.; Infantino, V. TCA Cycle Rewiring as Emerging Metabolic Signature of Hepatocellular Carcinoma. Cancers 2019, 12, 68. [Google Scholar] [CrossRef] [PubMed]
- Agosti, P.; Sabbà, C.; Mazzocca, A. Emerging Metabolic Risk Factors in Hepatocellular Carcinoma and Their Influence on the Liver Microenvironment. Biochim. Biophys. Acta Mol. Basis Dis. 2018, 1864, 607–617. [Google Scholar] [CrossRef]
- Rumgay, H.; Arnold, M.; Ferlay, J.; Lesi, O.; Cabasag, C.J.; Vignat, J.; Laversanne, M.; McGlynn, K.A.; Soerjomataram, I. Global Burden of Primary Liver Cancer in 2020 and Predictions to 2040. J. Hepatol. 2022, 77, 1598–1606. [Google Scholar] [CrossRef]
- Liberti, M.V.; Locasale, J.W. The Warburg Effect: How Does It Benefit Cancer Cells? Trends Biochem. Sci. 2016, 41, 211–218. [Google Scholar] [CrossRef]
- Pascale, R.M.; Calvisi, D.F.; Simile, M.M.; Feo, C.F.; Feo, F. The Warburg Effect 97 Years after Its Discovery. Cancers 2020, 12, 2819. [Google Scholar] [CrossRef]
- Cassim, S.; Raymond, V.-A.; Lacoste, B.; Lapierre, P.; Bilodeau, M. Metabolite Profiling Identifies a Signature of Tumorigenicity in Hepatocellular Carcinoma. Oncotarget 2018, 9, 26868–26883. [Google Scholar] [CrossRef]
- Gnocchi, D.; Sabbà, C.; Massimi, M.; Mazzocca, A. Metabolism as a New Avenue for Hepatocellular Carcinoma Therapy. Int. J. Mol. Sci. 2023, 24, 3710. [Google Scholar] [CrossRef]
- de Visser, K.E.; Joyce, J.A. The Evolving Tumor Microenvironment: From Cancer Initiation to Metastatic Outgrowth. Cancer Cell 2023, 41, 374–403. [Google Scholar] [CrossRef] [PubMed]
- Shi, R.; Tang, Y.-Q.; Miao, H. Metabolism in Tumor Microenvironment: Implications for Cancer Immunotherapy. MedComm 2020, 1, 47–68. [Google Scholar] [CrossRef] [PubMed]
- Antonio, M.J.; Zhang, C.; Le, A. Different Tumor Microenvironments Lead to Different Metabolic Phenotypes. Adv. Exp. Med. Biol. 2021, 1311, 137–147. [Google Scholar] [CrossRef] [PubMed]
- Biswas, S.K.; Sica, A.; Lewis, C.E. Plasticity of Macrophage Function during Tumor Progression: Regulation by Distinct Molecular Mechanisms. J. Immunol. 2008, 180, 2011–2017. [Google Scholar] [CrossRef] [PubMed]
- Wan, S.; Zhao, E.; Kryczek, I.; Vatan, L.; Sadovskaya, A.; Ludema, G.; Simeone, D.M.; Zou, W.; Welling, T.H. Tumor-Associated Macrophages Produce Interleukin 6 and Signal via STAT3 to Promote Expansion of Human Hepatocellular Carcinoma Stem Cells. Gastroenterology 2014, 147, 1393–1404. [Google Scholar] [CrossRef]
- Colegio, O.R.; Chu, N.-Q.; Szabo, A.L.; Chu, T.; Rhebergen, A.M.; Jairam, V.; Cyrus, N.; Brokowski, C.E.; Eisenbarth, S.C.; Phillips, G.M.; et al. Functional Polarization of Tumour-Associated Macrophages by Tumour-Derived Lactic Acid. Nature 2014, 513, 559–563. [Google Scholar] [CrossRef]
- Merien, F. A Journey with Elie Metchnikoff: From Innate Cell Mechanisms in Infectious Diseases to Quantum Biology. Front. Public Health 2016, 4, 125. [Google Scholar] [CrossRef]
- Teti, G.; Biondo, C.; Beninati, C. The Phagocyte, Metchnikoff, and the Foundation of Immunology. Microbiol. Spectr. 2016, 4, 17–29. [Google Scholar] [CrossRef]
- Gasteiger, G.; D’Osualdo, A.; Schubert, D.A.; Weber, A.; Bruscia, E.M.; Hartl, D. Cellular Innate Immunity: An Old Game with New Players. J. Innate Immun. 2017, 9, 111–125. [Google Scholar] [CrossRef]
- van Furth, R.; Cohn, Z.A.; Hirsch, J.G.; Humphrey, J.H.; Spector, W.G.; Langevoort, H.L. The Mononuclear Phagocyte System: A New Classification of Macrophages, Monocytes, and Their Precursor Cells. Bull. World Health Organ. 1972, 46, 845–852. [Google Scholar]
- Ginhoux, F.; Guilliams, M. Tissue-Resident Macrophage Ontogeny and Homeostasis. Immunity 2016, 44, 439–449. [Google Scholar] [CrossRef] [PubMed]
- van Furth, R.; Cohn, Z.A. The Origin and Kinetics of Mononuclear Phagocytes. J. Exp. Med. 1968, 128, 415–435. [Google Scholar] [CrossRef] [PubMed]
- Epelman, S.; Lavine, K.J.; Randolph, G.J. Origin and Functions of Tissue Macrophages. Immunity 2014, 41, 21–35. [Google Scholar] [CrossRef] [PubMed]
- Guilliams, M.; Thierry, G.R.; Bonnardel, J.; Bajenoff, M. Establishment and Maintenance of the Macrophage Niche. Immunity 2020, 52, 434–451. [Google Scholar] [CrossRef]
- Blériot, C.; Chakarov, S.; Ginhoux, F. Determinants of Resident Tissue Macrophage Identity and Function. Immunity 2020, 52, 957–970. [Google Scholar] [CrossRef]
- Pollard, J.W. Trophic Macrophages in Development and Disease. Nat. Rev. Immunol. 2009, 9, 259–270. [Google Scholar] [CrossRef]
- Mass, E.; Nimmerjahn, F.; Kierdorf, K.; Schlitzer, A. Tissue-Specific Macrophages: How They Develop and Choreograph Tissue Biology. Nat. Rev. Immunol. 2023, 23, 563–579. [Google Scholar] [CrossRef]
- Colonna, M.; Butovsky, O. Microglia Function in the Central Nervous System During Health and Neurodegeneration. Annu. Rev. Immunol. 2017, 35, 441–468. [Google Scholar] [CrossRef]
- Peiseler, M.; Araujo David, B.; Zindel, J.; Surewaard, B.G.J.; Lee, W.-Y.; Heymann, F.; Nusse, Y.; Castanheira, F.V.S.; Shim, R.; Guillot, A.; et al. Kupffer Cell-like Syncytia Replenish Resident Macrophage Function in the Fibrotic Liver. Science 2023, 381, eabq5202. [Google Scholar] [CrossRef]
- Mantovani, A.; Sica, A.; Sozzani, S.; Allavena, P.; Vecchi, A.; Locati, M. The Chemokine System in Diverse Forms of Macrophage Activation and Polarization. Trends Immunol. 2004, 25, 677–686. [Google Scholar] [CrossRef]
- Martinez, F.O.; Gordon, S. The M1 and M2 Paradigm of Macrophage Activation: Time for Reassessment. F1000Prime Rep. 2014, 6, 13. [Google Scholar] [CrossRef] [PubMed]
- Wilson, H.M. SOCS Proteins in Macrophage Polarization and Function. Front. Immunol. 2014, 5, 357. [Google Scholar] [CrossRef] [PubMed]
- Günthner, R.; Anders, H.-J. Interferon-Regulatory Factors Determine Macrophage Phenotype Polarization. Mediat. Inflamm. 2013, 2013, 731023. [Google Scholar] [CrossRef] [PubMed]
- Arnold, C.E.; Whyte, C.S.; Gordon, P.; Barker, R.N.; Rees, A.J.; Wilson, H.M. A Critical Role for Suppressor of Cytokine Signalling 3 in Promoting M1 Macrophage Activation and Function in Vitro and in Vivo. Immunology 2014, 141, 96–110. [Google Scholar] [CrossRef] [PubMed]
- Chhabra, J.K.; Chattopadhyay, B.; Paul, B.N. SOCS3 Dictates the Transition of Divergent Time-Phased Events in Granulocyte TNF-α Signaling. Cell. Mol. Immunol. 2014, 11, 105–106. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Xu, X.-H.; Jin, L. Macrophage Polarization in Physiological and Pathological Pregnancy. Front. Immunol. 2019, 10, 792. [Google Scholar] [CrossRef] [PubMed]
- Serbulea, V.; Upchurch, C.M.; Ahern, K.W.; Bories, G.; Voigt, P.; DeWeese, D.E.; Meher, A.K.; Harris, T.E.; Leitinger, N. Macrophages Sensing Oxidized DAMPs Reprogram Their Metabolism to Support Redox Homeostasis and Inflammation through a TLR2-Syk-Ceramide Dependent Mechanism. Mol. Metab. 2018, 7, 23–34. [Google Scholar] [CrossRef]
- Gleissner, C.A. Macrophage Phenotype Modulation by CXCL4 in Atherosclerosis. Front. Physiol. 2012, 3, 1. [Google Scholar] [CrossRef]
- Finn, A.V.; Nakano, M.; Polavarapu, R.; Karmali, V.; Saeed, O.; Zhao, X.; Yazdani, S.; Otsuka, F.; Davis, T.; Habib, A.; et al. Hemoglobin Directs Macrophage Differentiation and Prevents Foam Cell Formation in Human Atherosclerotic Plaques. J. Am. Coll. Cardiol. 2012, 59, 166–177. [Google Scholar] [CrossRef]
- Viola, A.; Munari, F.; Sánchez-Rodríguez, R.; Scolaro, T.; Castegna, A. The Metabolic Signature of Macrophage Responses. Front. Immunol. 2019, 10, 1462. [Google Scholar] [CrossRef]
- Infantino, V.; Santarsiero, A.; Convertini, P.; Todisco, S.; Iacobazzi, V. Cancer Cell Metabolism in Hypoxia: Role of HIF-1 as Key Regulator and Therapeutic Target. Int. J. Mol. Sci. 2021, 22, 5703. [Google Scholar] [CrossRef] [PubMed]
- Qiu, B.; Yuan, P.; Du, X.; Jin, H.; Du, J.; Huang, Y. Hypoxia Inducible Factor-1α Is an Important Regulator of Macrophage Biology. Heliyon 2023, 9, e17167. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.; Li, J.; Wu, L.; Yu, Q.; Ji, J.; Wu, J.; Dai, W.; Guo, C. Emerging Roles and the Regulation of Aerobic Glycolysis in Hepatocellular Carcinoma. J. Exp. Clin. Cancer Res. CR 2020, 39, 126. [Google Scholar] [CrossRef] [PubMed]
- Perrin-Cocon, L.; Vidalain, P.-O.; Jacquemin, C.; Aublin-Gex, A.; Olmstead, K.; Panthu, B.; Rautureau, G.J.P.; André, P.; Nyczka, P.; Hütt, M.-T.; et al. A Hexokinase Isoenzyme Switch in Human Liver Cancer Cells Promotes Lipogenesis and Enhances Innate Immunity. Commun. Biol. 2021, 4, 217. [Google Scholar] [CrossRef] [PubMed]
- Ros, S.; Schulze, A. Balancing Glycolytic Flux: The Role of 6-Phosphofructo-2-Kinase/Fructose 2,6-Bisphosphatases in Cancer Metabolism. Cancer Metab. 2013, 1, 8. [Google Scholar] [CrossRef]
- Alves-Filho, J.C.; Pålsson-McDermott, E.M. Pyruvate Kinase M2: A Potential Target for Regulating Inflammation. Front. Immunol. 2016, 7, 145. [Google Scholar] [CrossRef]
- Shang, R.-Z.; Qu, S.-B.; Wang, D.-S. Reprogramming of Glucose Metabolism in Hepatocellular Carcinoma: Progress and Prospects. World J. Gastroenterol. 2016, 22, 9933–9943. [Google Scholar] [CrossRef]
- Wu, J.; Hu, L.; Chen, M.; Cao, W.; Chen, H.; He, T. Pyruvate Kinase M2 Overexpression and Poor Prognosis in Solid Tumors of Digestive System: Evidence from 16 Cohort Studies. OncoTargets Ther. 2016, 9, 4277–4288. [Google Scholar] [CrossRef]
- Chen, Z.; Lu, X.; Wang, Z.; Jin, G.; Wang, Q.; Chen, D.; Chen, T.; Li, J.; Fan, J.; Cong, W.; et al. Co-Expression of PKM2 and TRIM35 Predicts Survival and Recurrence in Hepatocellular Carcinoma. Oncotarget 2015, 6, 2538–2548. [Google Scholar] [CrossRef]
- Yang, L.; Xie, M.; Yang, M.; Yu, Y.; Zhu, S.; Hou, W.; Kang, R.; Lotze, M.T.; Billiar, T.R.; Wang, H.; et al. PKM2 Regulates the Warburg Effect and Promotes HMGB1 Release in Sepsis. Nat. Commun. 2014, 5, 4436. [Google Scholar] [CrossRef]
- Li, S.; Li, J.; Dai, W.; Zhang, Q.; Feng, J.; Wu, L.; Liu, T.; Yu, Q.; Xu, S.; Wang, W.; et al. Genistein Suppresses Aerobic Glycolysis and Induces Hepatocellular Carcinoma Cell Death. Br. J. Cancer 2017, 117, 1518–1528. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Zhou, J.; Li, J.; Feng, J.; Chen, Z.; Wang, X. Plasma Metabolomic Analysis of Human Hepatocellular Carcinoma: Diagnostic and Therapeutic Study. Oncotarget 2016, 7, 47332–47342. [Google Scholar] [CrossRef] [PubMed]
- Peng, X.; He, Z.; Yuan, D.; Liu, Z.; Rong, P. Lactic Acid: The Culprit behind the Immunosuppressive Microenvironment in Hepatocellular Carcinoma. Biochim. Biophys. Acta Rev. Cancer 2024, 1879, 189164. [Google Scholar] [CrossRef] [PubMed]
- Payen, V.L.; Mina, E.; Van Hée, V.F.; Porporato, P.E.; Sonveaux, P. Monocarboxylate Transporters in Cancer. Mol. Metab. 2020, 33, 48–66. [Google Scholar] [CrossRef]
- Gao, H.-J.; Zhao, M.-C.; Zhang, Y.-J.; Zhou, D.-S.; Xu, L.; Li, G.-B.; Chen, M.-S.; Liu, J. Monocarboxylate Transporter 4 Predicts Poor Prognosis in Hepatocellular Carcinoma and Is Associated with Cell Proliferation and Migration. J. Cancer Res. Clin. Oncol. 2015, 141, 1151–1162. [Google Scholar] [CrossRef]
- Wang, F.; Zhang, S.; Vuckovic, I.; Jeon, R.; Lerman, A.; Folmes, C.D.; Dzeja, P.P.; Herrmann, J. Glycolytic Stimulation Is Not a Requirement for M2 Macrophage Differentiation. Cell Metab. 2018, 28, 463–475.e4. [Google Scholar] [CrossRef]
- Geeraerts, X.; Bolli, E.; Fendt, S.-M.; Van Ginderachter, J.A. Macrophage Metabolism As Therapeutic Target for Cancer, Atherosclerosis, and Obesity. Front. Immunol. 2017, 8, 289. [Google Scholar] [CrossRef]
- Yu, Q.; Wang, Y.; Dong, L.; He, Y.; Liu, R.; Yang, Q.; Cao, Y.; Wang, Y.; Jia, A.; Bi, Y.; et al. Regulations of Glycolytic Activities on Macrophages Functions in Tumor and Infectious Inflammation. Front. Cell. Infect. Microbiol. 2020, 10, 287. [Google Scholar] [CrossRef]
- Nagy, C.; Haschemi, A. Time and Demand Are Two Critical Dimensions of Immunometabolism: The Process of Macrophage Activation and the Pentose Phosphate Pathway. Front. Immunol. 2015, 6, 164. [Google Scholar] [CrossRef]
- Kowalik, M.A.; Columbano, A.; Perra, A. Emerging Role of the Pentose Phosphate Pathway in Hepatocellular Carcinoma. Front. Oncol. 2017, 7, 87. [Google Scholar] [CrossRef]
- Haschemi, A.; Kosma, P.; Gille, L.; Evans, C.R.; Burant, C.F.; Starkl, P.; Knapp, B.; Haas, R.; Schmid, J.A.; Jandl, C.; et al. The Sedoheptulose Kinase CARKL Directs Macrophage Polarization through Control of Glucose Metabolism. Cell Metab. 2012, 15, 813–826. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.; Choksi, S.; Qu, J.; Jang, J.; Choe, M.; Banfi, B.; Engelhardt, J.F.; Liu, Z.-G. NADPH Oxidases Are Essential for Macrophage Differentiation. J. Biol. Chem. 2016, 291, 20030–20041. [Google Scholar] [CrossRef] [PubMed]
- Yeudall, S.; Upchurch, C.M.; Seegren, P.V.; Pavelec, C.M.; Greulich, J.; Lemke, M.C.; Harris, T.E.; Desai, B.N.; Hoehn, K.L.; Leitinger, N. Macrophage Acetyl-CoA Carboxylase Regulates Acute Inflammation through Control of Glucose and Lipid Metabolism. Sci. Adv. 2022, 8, eabq1984. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.; Song, H.; Yin, L.; Rizzo, M.G.; Sidhu, R.; Covey, D.F.; Ory, D.S.; Semenkovich, C.F. Fatty Acid Synthesis Configures the Plasma Membrane for Inflammation in Diabetes. Nature 2016, 539, 294–298. [Google Scholar] [CrossRef] [PubMed]
- Bort, A.; Sánchez, B.G.; de Miguel, I.; Mateos-Gómez, P.A.; Diaz-Laviada, I. Dysregulated Lipid Metabolism in Hepatocellular Carcinoma Cancer Stem Cells. Mol. Biol. Rep. 2020, 47, 2635–2647. [Google Scholar] [CrossRef]
- Wang, M.-D.; Wu, H.; Fu, G.-B.; Zhang, H.-L.; Zhou, X.; Tang, L.; Dong, L.-W.; Qin, C.-J.; Huang, S.; Zhao, L.-H.; et al. Acetyl-Coenzyme A Carboxylase Alpha Promotion of Glucose-Mediated Fatty Acid Synthesis Enhances Survival of Hepatocellular Carcinoma in Mice and Patients. Hepatology 2016, 63, 1272–1286. [Google Scholar] [CrossRef]
- Calle, P.; Muñoz, A.; Sola, A.; Hotter, G. CPT1a Gene Expression Reverses the Inflammatory and Anti-Phagocytic Effect of 7-Ketocholesterol in RAW264.7 Macrophages. Lipids Health Dis. 2019, 18, 215. [Google Scholar] [CrossRef]
- Feingold, K.R.; Shigenaga, J.K.; Kazemi, M.R.; McDonald, C.M.; Patzek, S.M.; Cross, A.S.; Moser, A.; Grunfeld, C. Mechanisms of Triglyceride Accumulation in Activated Macrophages. J. Leukoc. Biol. 2012, 92, 829–839. [Google Scholar] [CrossRef]
- Zang, S.; Ma, X.; Wu, Y.; Liu, W.; Cheng, H.; Li, J.; Liu, J.; Huang, A. PGE2 Synthesis and Signaling in Malignant Transformation and Progression of Human Hepatocellular Carcinoma. Hum. Pathol. 2017, 63, 120–127. [Google Scholar] [CrossRef]
- Mazzone, M.; Menga, A.; Castegna, A. Metabolism and TAM Functions-It Takes Two to Tango. FEBS J. 2018, 285, 700–716. [Google Scholar] [CrossRef]
- Im, S.-S.; Yousef, L.; Blaschitz, C.; Liu, J.Z.; Edwards, R.A.; Young, S.G.; Raffatellu, M.; Osborne, T.F. Linking Lipid Metabolism to the Innate Immune Response in Macrophages through Sterol Regulatory Element Binding Protein-1a. Cell Metab. 2011, 13, 540–549. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Yang, W.; Zhang, J.; Zheng, X.; Yao, Y.; Tu, K.; Liu, Q. SREBP-1 Has a Prognostic Role and Contributes to Invasion and Metastasis in Human Hepatocellular Carcinoma. Int. J. Mol. Sci. 2014, 15, 7124–7138. [Google Scholar] [CrossRef] [PubMed]
- Santarsiero, A.; Convertini, P.; Todisco, S.; Pierri, C.L.; De Grassi, A.; Williams, N.C.; Iacobazzi, D.; De Stefano, G.; O’Neill, L.A.J.; Infantino, V. ACLY Nuclear Translocation in Human Macrophages Drives Proinflammatory Gene Expression by NF-κB Acetylation. Cells 2021, 10, 2962. [Google Scholar] [CrossRef] [PubMed]
- Shimano, H.; Sato, R. SREBP-Regulated Lipid Metabolism: Convergent Physiology—Divergent Pathophysiology. Nat. Rev. Endocrinol. 2017, 13, 710–730. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Yang, S.; Zhang, M.; Gao, D.; He, T.; Guo, M. ZNF545 Suppresses Human Hepatocellular Carcinoma Growth by Inhibiting NF-kB Signaling. Genes Cancer 2017, 8, 528–535. [Google Scholar] [CrossRef]
- Batista-Gonzalez, A.; Vidal, R.; Criollo, A.; Carreño, L.J. New Insights on the Role of Lipid Metabolism in the Metabolic Reprogramming of Macrophages. Front. Immunol. 2019, 10, 2993. [Google Scholar] [CrossRef]
- Remmerie, A.; Scott, C.L. Macrophages and Lipid Metabolism. Cell. Immunol. 2018, 330, 27–42. [Google Scholar] [CrossRef]
- Mossmann, D.; Park, S.; Ryback, B.; Weißenberger, D.; Colombi, M.; Hindupur, S.K.; Dazert, E.; Coto-Llerena, M.; Caner, E.; Cenzano, V.J.; et al. Elevated Arginine Levels in Liver Tumors Promote Metabolic Reprogramming and Tumor Growth 2022. bioRxiv 2022, bioRxiv:2022.04.26.489545. [Google Scholar]
- Momma, T.Y.; Ottaviani, J.I. There Is No Direct Competition between Arginase and Nitric Oxide Synthase for the Common Substrate L-Arginine. Nitric Oxide Biol. Chem. 2022, 129, 16–24. [Google Scholar] [CrossRef]
- Rath, M.; Müller, I.; Kropf, P.; Closs, E.I.; Munder, M. Metabolism via Arginase or Nitric Oxide Synthase: Two Competing Arginine Pathways in Macrophages. Front. Immunol. 2014, 5, 532. [Google Scholar] [CrossRef]
- Nicholson, B.; Manner, C.K.; Kleeman, J.; MacLeod, C.L. Sustained Nitric Oxide Production in Macrophages Requires the Arginine Transporter CAT2. J. Biol. Chem. 2001, 276, 15881–15885. [Google Scholar] [CrossRef] [PubMed]
- Hammami, I.; Bertrand, M.; Chen, J.; Bronte, V.; De Crescenzo, G.; Jolicoeur, M. Nitric Oxide Affects Immune Cells Bioenergetics: Long-Term Effects of Nitric-Oxide Derivatives on Leukaemic Jurkat Cell Metabolism. Immunobiology 2012, 217, 808–815. [Google Scholar] [CrossRef] [PubMed]
- Caneba, C.A.; Yang, L.; Baddour, J.; Curtis, R.; Win, J.; Hartig, S.; Marini, J.; Nagrath, D. Nitric Oxide Is a Positive Regulator of the Warburg Effect in Ovarian Cancer Cells. Cell Death Dis. 2014, 5, e1302. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Geller, D.A.; Wink, D.A.; Cheng, B.; Billiar, T.R. NO and Hepatocellular Cancer. Br. J. Pharmacol. 2020, 177, 5459–5466. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Li, Y.; Tsung, A.; Huang, H.; Du, Q.; Yang, M.; Deng, M.; Xiong, S.; Wang, X.; Zhang, L.; et al. iNOS Promotes CD24+CD133+ Liver Cancer Stem Cell Phenotype through a TACE/ADAM17-Dependent Notch Signaling Pathway. Proc. Natl. Acad. Sci. USA 2018, 115, E10127–E10136. [Google Scholar] [CrossRef]
- Ding, Z.; Ogata, D.; Roszik, J.; Qin, Y.; Kim, S.-H.; Tetzlaff, M.T.; Lazar, A.J.; Davies, M.A.; Ekmekcioglu, S.; Grimm, E.A. iNOS Associates With Poor Survival in Melanoma: A Role for Nitric Oxide in the PI3K-AKT Pathway Stimulation and PTEN S-Nitrosylation. Front. Oncol. 2021, 11, 631766. [Google Scholar] [CrossRef]
- Zhan, R.; He, W.; Wang, F.; Yao, Z.; Tan, J.; Xu, R.; Zhou, J.; Wang, Y.; Li, H.; Wu, J.; et al. Nitric Oxide Promotes Epidermal Stem Cell Migration via cGMP-Rho GTPase Signalling. Sci. Rep. 2016, 6, 30687. [Google Scholar] [CrossRef]
- Vergadi, E.; Ieronymaki, E.; Lyroni, K.; Vaporidi, K.; Tsatsanis, C. Akt Signaling Pathway in Macrophage Activation and M1/M2 Polarization. J. Immunol. 2017, 198, 1006–1014. [Google Scholar] [CrossRef]
- Tian, L.-Y.; Smit, D.J.; Jücker, M. The Role of PI3K/AKT/mTOR Signaling in Hepatocellular Carcinoma Metabolism. Int. J. Mol. Sci. 2023, 24, 2652. [Google Scholar] [CrossRef]
- Mitsuishi, Y.; Taguchi, K.; Kawatani, Y.; Shibata, T.; Nukiwa, T.; Aburatani, H.; Yamamoto, M.; Motohashi, H. Nrf2 Redirects Glucose and Glutamine into Anabolic Pathways in Metabolic Reprogramming. Cancer Cell 2012, 22, 66–79. [Google Scholar] [CrossRef]
- He, F.; Antonucci, L.; Karin, M. NRF2 as a Regulator of Cell Metabolism and Inflammation in Cancer. Carcinogenesis 2020, 41, 405–416. [Google Scholar] [CrossRef] [PubMed]
- Patra, K.C.; Hay, N. The Pentose Phosphate Pathway and Cancer. Trends Biochem. Sci. 2014, 39, 347–354. [Google Scholar] [CrossRef] [PubMed]
- Hoxhaj, G.; Manning, B.D. The PI3K-AKT Network at the Interface of Oncogenic Signalling and Cancer Metabolism. Nat. Rev. Cancer 2020, 20, 74–88. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Wu, Q.; Geller, D.A.; Yan, Y. Macrophage Metabolism, Phenotype, Function, and Therapy in Hepatocellular Carcinoma (HCC). J. Transl. Med. 2023, 21, 815. [Google Scholar] [CrossRef]
- Mao, Y.; Shi, D.; Li, G.; Jiang, P. Citrulline Depletion by ASS1 Is Required for Proinflammatory Macrophage Activation and Immune Responses. Mol. Cell 2022, 82, 527–541.e7. [Google Scholar] [CrossRef]
- Monné, M.; Vozza, A.; Lasorsa, F.M.; Porcelli, V.; Palmieri, F. Mitochondrial Carriers for Aspartate, Glutamate and Other Amino Acids: A Review. Int. J. Mol. Sci. 2019, 20, 4456. [Google Scholar] [CrossRef]
- Arlauckas, S.P.; Garren, S.B.; Garris, C.S.; Kohler, R.H.; Oh, J.; Pittet, M.J.; Weissleder, R. Arg1 Expression Defines Immunosuppressive Subsets of Tumor-Associated Macrophages. Theranostics 2018, 8, 5842–5854. [Google Scholar] [CrossRef]
- Van den Bossche, J.; O’Neill, L.A.; Menon, D. Macrophage Immunometabolism: Where Are We (Going)? Trends Immunol. 2017, 38, 395–406. [Google Scholar] [CrossRef]
- Infantino, V.; Dituri, F.; Convertini, P.; Santarsiero, A.; Palmieri, F.; Todisco, S.; Mancarella, S.; Giannelli, G.; Iacobazzi, V. Epigenetic Upregulation and Functional Role of the Mitochondrial Aspartate/Glutamate Carrier Isoform 1 in Hepatocellular Carcinoma. Biochim. Biophys. Acta Mol. Basis Dis. 2019, 1865, 38–47. [Google Scholar] [CrossRef]
- Convertini, P.; Todisco, S.; De Santis, F.; Pappalardo, I.; Iacobazzi, D.; Castiglione Morelli, M.A.; Fondufe-Mittendorf, Y.N.; Martelli, G.; Palmieri, F.; Infantino, V. Transcriptional Regulation Factors of the Human Mitochondrial Aspartate/Glutamate Carrier Gene, Isoform 2 (SLC25A13): USF1 as Basal Factor and FOXA2 as Activator in Liver Cells. Int. J. Mol. Sci. 2019, 20, 1888. [Google Scholar] [CrossRef]
- Iacobazzi, V.; Infantino, V.; Castegna, A.; Menga, A.; Palmieri, E.M.; Convertini, P.; Palmieri, F. Mitochondrial Carriers in Inflammation Induced by Bacterial Endotoxin and Cytokines. Biol. Chem. 2017, 398, 303–317. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Q.; Qiu, Y.; Kurland, I.J.; Drlica, K.; Subbian, S.; Tyagi, S.; Shi, L. Glutamine Is Required for M1-like Polarization of Macrophages in Response to Mycobacterium Tuberculosis Infection. mBio 2022, 13, e0127422. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.-S.; Wang, H.; Li, X.; Chao, T.; Teav, T.; Christen, S.; Di Conza, G.; Cheng, W.-C.; Chou, C.-H.; Vavakova, M.; et al. α-Ketoglutarate Orchestrates Macrophage Activation through Metabolic and Epigenetic Reprogramming. Nat. Immunol. 2017, 18, 985–994. [Google Scholar] [CrossRef] [PubMed]
- Marsico, M.; Santarsiero, A.; Pappalardo, I.; Convertini, P.; Chiummiento, L.; Sardone, A.; Di Noia, M.A.; Infantino, V.; Todisco, S. Mitochondria-Mediated Apoptosis of HCC Cells Triggered by Knockdown of Glutamate Dehydrogenase 1: Perspective for Its Inhibition through Quercetin and Permethylated Anigopreissin A. Biomedicines 2021, 9, 1664. [Google Scholar] [CrossRef] [PubMed]
- Fiermonte, G.; Palmieri, L.; Todisco, S.; Agrimi, G.; Palmieri, F.; Walker, J.E. Identification of the Mitochondrial Glutamate Transporter. Bacterial Expression, Reconstitution, Functional Characterization, and Tissue Distribution of Two Human Isoforms. J. Biol. Chem. 2002, 277, 19289–19294. [Google Scholar] [CrossRef]
- Koo, M.-S.; Subbian, S.; Kaplan, G. Strain Specific Transcriptional Response in Mycobacterium Tuberculosis Infected Macrophages. Cell Commun. Signal. CCS 2012, 10, 2. [Google Scholar] [CrossRef]
- Menga, A.; Serra, M.; Todisco, S.; Riera-Domingo, C.; Ammarah, U.; Ehling, M.; Palmieri, E.M.; Di Noia, M.A.; Gissi, R.; Favia, M.; et al. Glufosinate Constrains Synchronous and Metachronous Metastasis by Promoting Anti-Tumor Macrophages. EMBO Mol. Med. 2020, 12, e11210. [Google Scholar] [CrossRef]
- Iacobazzi, V.; Infantino, V. Citrate—New Functions for an Old Metabolite. Biol. Chem. 2014, 395, 387–399. [Google Scholar] [CrossRef]
- Mosaoa, R.; Kasprzyk-Pawelec, A.; Fernandez, H.R.; Avantaggiati, M.L. The Mitochondrial Citrate Carrier SLC25A1/CIC and the Fundamental Role of Citrate in Cancer, Inflammation and Beyond. Biomolecules 2021, 11, 141. [Google Scholar] [CrossRef]
- Schlichtholz, B.; Turyn, J.; Goyke, E.; Biernacki, M.; Jaskiewicz, K.; Sledzinski, Z.; Swierczynski, J. Enhanced Citrate Synthase Activity in Human Pancreatic Cancer. Pancreas 2005, 30, 99–104. [Google Scholar] [CrossRef]
- Wellen, K.E.; Hatzivassiliou, G.; Sachdeva, U.M.; Bui, T.V.; Cross, J.R.; Thompson, C.B. ATP-Citrate Lyase Links Cellular Metabolism to Histone Acetylation. Science 2009, 324, 1076–1080. [Google Scholar] [CrossRef]
- Migita, T.; Narita, T.; Nomura, K.; Miyagi, E.; Inazuka, F.; Matsuura, M.; Ushijima, M.; Mashima, T.; Seimiya, H.; Satoh, Y.; et al. ATP Citrate Lyase: Activation and Therapeutic Implications in Non-Small Cell Lung Cancer. Cancer Res. 2008, 68, 8547–8554. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, Y.; Shen, L.; Pang, Y.; Qiao, Z.; Liu, P. Prognostic and Therapeutic Implications of Increased ATP Citrate Lyase Expression in Human Epithelial Ovarian Cancer. Oncol. Rep. 2012, 27, 1156–1162. [Google Scholar] [CrossRef] [PubMed]
- Han, Q.; Chen, C.-A.; Yang, W.; Liang, D.; Lv, H.-W.; Lv, G.-S.; Zong, Q.-N.; Wang, H.-Y. ATP-Citrate Lyase Regulates Stemness and Metastasis in Hepatocellular Carcinoma via the Wnt/β-Catenin Signaling Pathway. Hepatobiliary Pancreat. Dis. Int. HBPD INT 2021, 20, 251–261. [Google Scholar] [CrossRef] [PubMed]
- Pirozzi, C.J.; Yan, H. The Implications of IDH Mutations for Cancer Development and Therapy. Nat. Rev. Clin. Oncol. 2021, 18, 645–661. [Google Scholar] [CrossRef] [PubMed]
- Heinz, A.; Nonnenmacher, Y.; Henne, A.; Khalil, M.-A.; Bejkollari, K.; Dostert, C.; Hosseini, S.; Goldmann, O.; He, W.; Palorini, R.; et al. Itaconate Controls Its Own Synthesis via Feedback-Inhibition of Reverse TCA Cycle Activity at IDH2. Biochim. Biophys. Acta Mol. Basis Dis. 2022, 1868, 166530. [Google Scholar] [CrossRef] [PubMed]
- Peace, C.G.; O’Neill, L.A. The Role of Itaconate in Host Defense and Inflammation. J. Clin. Investig. 2022, 132, e148548. [Google Scholar] [CrossRef]
- Chen, J.-Y.; Lai, Y.-S.; Tsai, H.-J.; Kuo, C.-C.; Yen, B.L.; Yeh, S.-P.; Sun, H.S.; Hung, W.-C. The Oncometabolite R-2-Hydroxyglutarate Activates NF-κB-Dependent Tumor-Promoting Stromal Niche for Acute Myeloid Leukemia Cells. Sci. Rep. 2016, 6, 32428. [Google Scholar] [CrossRef]
- Harber, K.J.; de Goede, K.E.; Verberk, S.G.S.; Meinster, E.; de Vries, H.E.; van Weeghel, M.; de Winther, M.P.J.; Van den Bossche, J. Succinate Is an Inflammation-Induced Immunoregulatory Metabolite in Macrophages. Metabolites 2020, 10, 372. [Google Scholar] [CrossRef]
- Kuo, C.-C.; Wu, J.-Y.; Wu, K.K. Cancer-Derived Extracellular Succinate: A Driver of Cancer Metastasis. J. Biomed. Sci. 2022, 29, 93. [Google Scholar] [CrossRef]
- Trauelsen, M.; Hiron, T.K.; Lin, D.; Petersen, J.E.; Breton, B.; Husted, A.S.; Hjorth, S.A.; Inoue, A.; Frimurer, T.M.; Bouvier, M.; et al. Extracellular Succinate Hyperpolarizes M2 Macrophages through SUCNR1/GPR91-Mediated Gq Signaling. Cell Rep. 2021, 35, 109246. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, C.; Sciacovelli, M.; Frezza, C. Fumarate Hydratase in Cancer: A Multifaceted Tumour Suppressor. Semin. Cell Dev. Biol. 2020, 98, 15–25. [Google Scholar] [CrossRef] [PubMed]
- Hooftman, A.; Peace, C.G.; Ryan, D.G.; Day, E.A.; Yang, M.; McGettrick, A.F.; Yin, M.; Montano, E.N.; Huo, L.; Toller-Kawahisa, J.E.; et al. Macrophage Fumarate Hydratase Restrains mtRNA-Mediated Interferon Production. Nature 2023, 615, 490–498. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Liu, G.; Li, Y.; Pan, Y. Metabolic Reprogramming Induces Macrophage Polarization in the Tumor Microenvironment. Front. Immunol. 2022, 13, 840029. [Google Scholar] [CrossRef] [PubMed]
- Bailey, J.D.; Diotallevi, M.; Nicol, T.; McNeill, E.; Shaw, A.; Chuaiphichai, S.; Hale, A.; Starr, A.; Nandi, M.; Stylianou, E.; et al. Nitric Oxide Modulates Metabolic Remodeling in Inflammatory Macrophages through TCA Cycle Regulation and Itaconate Accumulation. Cell Rep. 2019, 28, 218–230.e7. [Google Scholar] [CrossRef]
- Lee, W.J.; Tateya, S.; Cheng, A.M.; Rizzo-DeLeon, N.; Wang, N.F.; Handa, P.; Wilson, C.L.; Clowes, A.W.; Sweet, I.R.; Bomsztyk, K.; et al. M2 Macrophage Polarization Mediates Anti-Inflammatory Effects of Endothelial Nitric Oxide Signaling. Diabetes 2015, 64, 2836–2846. [Google Scholar] [CrossRef]
- Almatroudi, A.; Alsahli, M.A.; Syed, M.A.; Khan, A.A.; Rahmani, A.H. Regulation of Pro-Inflammatory Macrophage Polarization via Lipid Nanoparticles Mediated Delivery of Anti-Prostaglandin-E2 siRNA. Curr. Issues Mol. Biol. 2022, 45, 1–11. [Google Scholar] [CrossRef]
- Wang, W.; Liang, M.; Wang, L.; Bei, W.; Rong, X.; Xu, J.; Guo, J. Role of Prostaglandin E2 in Macrophage Polarization: Insights into Atherosclerosis. Biochem. Pharmacol. 2023, 207, 115357. [Google Scholar] [CrossRef]
- Wang, D.; Dubois, R.N. Eicosanoids and Cancer. Nat. Rev. Cancer 2010, 10, 181–193. [Google Scholar] [CrossRef]
- Chen, C.; Guan, J.; Gu, X.; Chu, Q.; Zhu, H. Prostaglandin E2 and Receptors: Insight Into Tumorigenesis, Tumor Progression, and Treatment of Hepatocellular Carcinoma. Front. Cell Dev. Biol. 2022, 10, 834859. [Google Scholar] [CrossRef]
- Forner, A.; Reig, M.; Bruix, J. Hepatocellular Carcinoma. Lancet 2018, 391, 1301–1314. [Google Scholar] [CrossRef] [PubMed]
- Vogel, A.; Cervantes, A.; Chau, I.; Daniele, B.; Llovet, J.M.; Meyer, T.; Nault, J.-C.; Neumann, U.; Ricke, J.; Sangro, B.; et al. Hepatocellular Carcinoma: ESMO Clinical Practice Guidelines for Diagnosis, Treatment and Follow-Up. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2018, 29, iv238–iv255. [Google Scholar] [CrossRef] [PubMed]
- Finn, R.S.; Qin, S.; Ikeda, M.; Galle, P.R.; Ducreux, M.; Kim, T.-Y.; Kudo, M.; Breder, V.; Merle, P.; Kaseb, A.O.; et al. Atezolizumab plus Bevacizumab in Unresectable Hepatocellular Carcinoma. N. Engl. J. Med. 2020, 382, 1894–1905. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Singh, P.P.; Singh, A.G.; Murad, M.H.; Sanchez, W. Statins Are Associated with a Reduced Risk of Hepatocellular Cancer: A Systematic Review and Meta-Analysis. Gastroenterology 2013, 144, 323–332. [Google Scholar] [CrossRef] [PubMed]
- Souk, K.; Al-Badri, M.; Azar, S.T. The Safety and Benefit of Statins in Liver Cirrhosis: A Review. Exp. Clin. Endocrinol. Diabetes Off. J. Ger. Soc. Endocrinol. Ger. Diabetes Assoc. 2015, 123, 577–580. [Google Scholar] [CrossRef]
- Li, S.; Saviano, A.; Erstad, D.J.; Hoshida, Y.; Fuchs, B.C.; Baumert, T.; Tanabe, K.K. Risk Factors, Pathogenesis, and Strategies for Hepatocellular Carcinoma Prevention: Emphasis on Secondary Prevention and Its Translational Challenges. J. Clin. Med. 2020, 9, 3817. [Google Scholar] [CrossRef]
- Goldberg, F.W.; Kettle, J.G.; Lamont, G.M.; Buttar, D.; Ting, A.K.T.; McGuire, T.M.; Cook, C.R.; Beattie, D.; Morentin Gutierrez, P.; Kavanagh, S.L.; et al. Discovery of Clinical Candidate AZD0095, a Selective Inhibitor of Monocarboxylate Transporter 4 (MCT4) for Oncology. J. Med. Chem. 2023, 66, 384–397. [Google Scholar] [CrossRef]
- Cervello, M.; Montalto, G. Cyclooxygenases in Hepatocellular Carcinoma. World J. Gastroenterol. 2006, 12, 5113–5121. [Google Scholar] [CrossRef]
- Finetti, F.; Travelli, C.; Ercoli, J.; Colombo, G.; Buoso, E.; Trabalzini, L. Prostaglandin E2 and Cancer: Insight into Tumor Progression and Immunity. Biology 2020, 9, 434. [Google Scholar] [CrossRef]
- Elwood, P.; Morgan, G.; Watkins, J.; Protty, M.; Mason, M.; Adams, R.; Dolwani, S.; Pickering, J.; Delon, C.; Longley, M. Aspirin and Cancer Treatment: Systematic Reviews and Meta-Analyses of Evidence: For and Against. Br. J. Cancer 2024, 130, 3–8. [Google Scholar] [CrossRef]
- Wang, P.; Chen, B.; Huang, Y.; Li, J.; Cao, D.; Chen, Z.; Li, J.; Ran, B.; Yang, J.; Wang, R.; et al. The Relationship between Nonsteroidal Anti-Inflammatory Drugs and Cancer Incidence: An Umbrella Review. Heliyon 2024, 10, e23203. [Google Scholar] [CrossRef] [PubMed]
- Tai, Y.; Zhang, L.-H.; Gao, J.-H.; Zhao, C.; Tong, H.; Ye, C.; Huang, Z.-Y.; Liu, R.; Tang, C.-W. Suppressing Growth and Invasion of Human Hepatocellular Carcinoma Cells by Celecoxib through Inhibition of Cyclooxygenase-2. Cancer Manag. Res. 2019, 11, 2831–2848. [Google Scholar] [CrossRef] [PubMed]
- Morisaki, T.; Umebayashi, M.; Kiyota, A.; Koya, N.; Tanaka, H.; Onishi, H.; Katano, M. Combining Celecoxib with Sorafenib Synergistically Inhibits Hepatocellular Carcinoma Cells in Vitro. Anticancer Res. 2013, 33, 1387–1395. [Google Scholar] [PubMed]
- Abdallah, F.M.; Helmy, M.W.; Katary, M.A.; Ghoneim, A.I. Synergistic Antiproliferative Effects of Curcumin and Celecoxib in Hepatocellular Carcinoma HepG2 Cells. Naunyn. Schmiedebergs Arch. Pharmacol. 2018, 391, 1399–1410. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.-W.; Chen, B.; Zhang, J.; Qi, Y.-P.; Liang, J.-H.; Zhong, J.-H.; Xiang, B.-D. Novel Combination of Celecoxib and Metformin Improves the Antitumor Effect by Inhibiting the Growth of Hepatocellular Carcinoma. J. Cancer 2020, 11, 6437–6444. [Google Scholar] [CrossRef]
- Xun, X.; Zhang, C.; Wang, S.; Hu, S.; Xiang, X.; Cheng, Q.; Li, Z.; Wang, Y.; Zhu, J. Cyclooxygenase-2 Expressed Hepatocellular Carcinoma Induces Cytotoxic T Lymphocytes Exhaustion through M2 Macrophage Polarization. Am. J. Transl. Res. 2021, 13, 4360–4375. [Google Scholar]
- Ge, Z.; Ding, S. The Crosstalk Between Tumor-Associated Macrophages (TAMs) and Tumor Cells and the Corresponding Targeted Therapy. Front. Oncol. 2020, 10, 590941. [Google Scholar] [CrossRef]
- Zheng, H.; Peng, X.; Yang, S.; Li, X.; Huang, M.; Wei, S.; Zhang, S.; He, G.; Liu, J.; Fan, Q.; et al. Targeting Tumor-Associated Macrophages in Hepatocellular Carcinoma: Biology, Strategy, and Immunotherapy. Cell Death Discov. 2023, 9, 65. [Google Scholar] [CrossRef]
- Ho, D.W.-H.; Lo, R.C.-L.; Chan, L.-K.; Ng, I.O.-L. Molecular Pathogenesis of Hepatocellular Carcinoma. Liver Cancer 2016, 5, 290–302. [Google Scholar] [CrossRef]
- Llovet, J.M.; Fuster, J.; Bruix, J. Barcelona-Clínic Liver Cancer Group The Barcelona Approach: Diagnosis, Staging, and Treatment of Hepatocellular Carcinoma. Liver Transplant. Off. Publ. Am. Assoc. Study Liver Dis. Int. Liver Transplant. Soc. 2004, 10, S115–S120. [Google Scholar] [CrossRef]
- Torbenson, M.S. Morphologic Subtypes of Hepatocellular Carcinoma. Gastroenterol. Clin. N. Am. 2017, 46, 365–391. [Google Scholar] [CrossRef] [PubMed]

| M1 | M2 | Mox | M4 | Mhem | ||||
|---|---|---|---|---|---|---|---|---|
| M2a | M2b | M2c | M2d | |||||
| Stimuli | IFN γ LPS IRF1 IRF5 IRF8 | IL-4 IL-13 | IL-1β IL-1R IC + TLR agonists | IL-10 TGF-β Glucocorticoids | IL-6, LPS+ A2R agonists | OxPL | CLCX4 | Hb |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santarsiero, A.; Convertini, P.; Iacobazzi, D.; Infantino, V.; Todisco, S. Metabolic Crossroad Between Macrophages and Cancer Cells: Overview of Hepatocellular Carcinoma. Biomedicines 2024, 12, 2684. https://doi.org/10.3390/biomedicines12122684
Santarsiero A, Convertini P, Iacobazzi D, Infantino V, Todisco S. Metabolic Crossroad Between Macrophages and Cancer Cells: Overview of Hepatocellular Carcinoma. Biomedicines. 2024; 12(12):2684. https://doi.org/10.3390/biomedicines12122684
Chicago/Turabian StyleSantarsiero, Anna, Paolo Convertini, Dominga Iacobazzi, Vittoria Infantino, and Simona Todisco. 2024. "Metabolic Crossroad Between Macrophages and Cancer Cells: Overview of Hepatocellular Carcinoma" Biomedicines 12, no. 12: 2684. https://doi.org/10.3390/biomedicines12122684
APA StyleSantarsiero, A., Convertini, P., Iacobazzi, D., Infantino, V., & Todisco, S. (2024). Metabolic Crossroad Between Macrophages and Cancer Cells: Overview of Hepatocellular Carcinoma. Biomedicines, 12(12), 2684. https://doi.org/10.3390/biomedicines12122684





