Does Bisphenol A (BPA) Exposure Cause Human Diseases?
Abstract
1. Introduction
2. Disease Prevalence
3. Association of Diseases with BPA
BPA as a Toxic Xenobiotic
4. BPA Metabolism
4.1. Step 1: Uptake of BPA into Cells
4.2. Step 2. Glucuronidation by UGTs
4.3. Step 3: Efflux Transporters
5. BPA Glucuronidation and Human Diseases
5.1. Study #1: Polycystic Ovary Disease (PCOS)
5.2. Study #2: Parkinson’s Disease (PD)
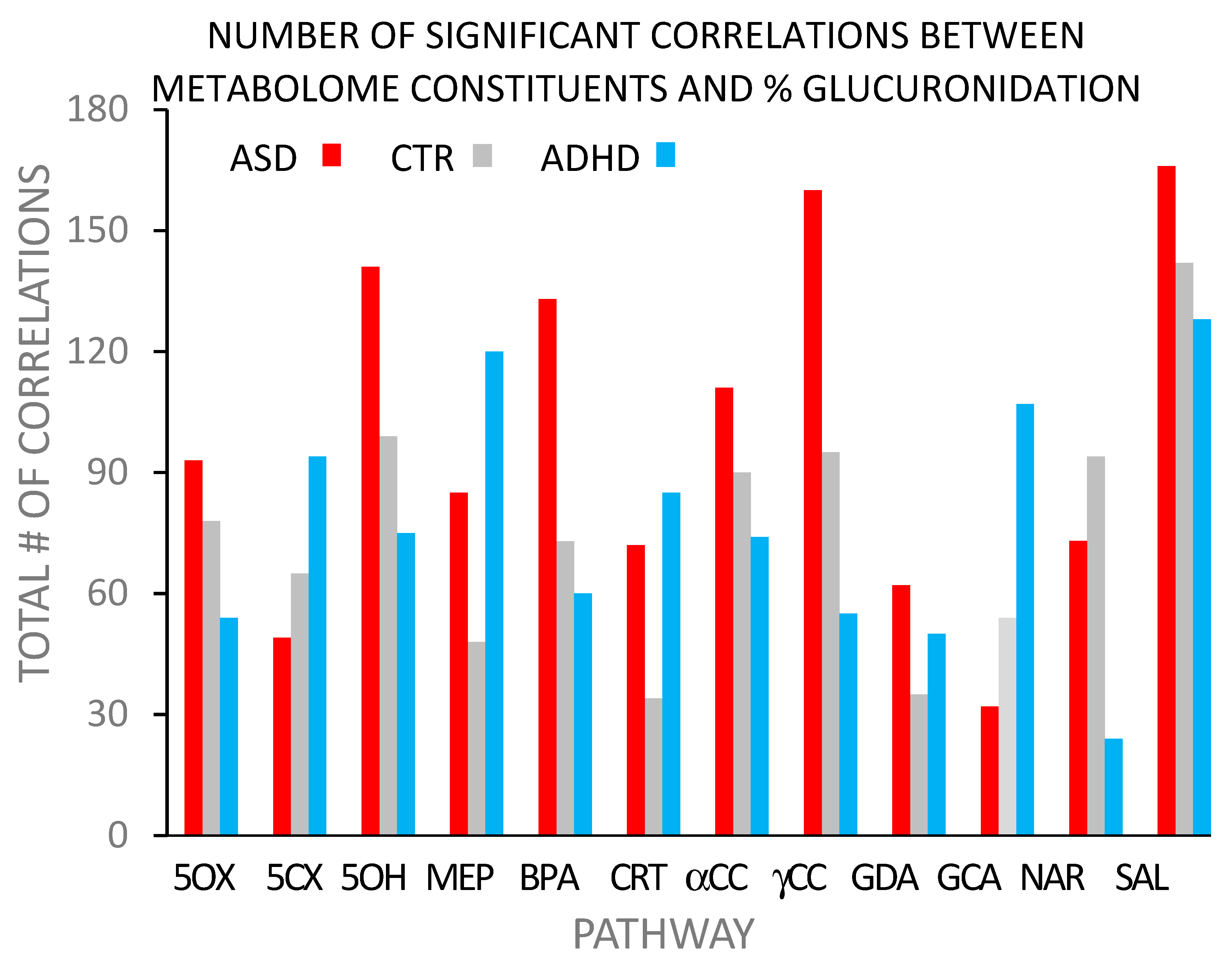
5.3. Studies #3 and #4: ASD and ADHD
6. Discussion
6.1. The Direct Pathway
6.2. The ‘Indirect’ Pathway
6.3. Evidence Favoring the ‘Indirect’ Pathway
- (1)
- BPA is a relatively inert chemical. Environmental concentrations and exposure are very low, and hence, tissue concentrations are also low. It takes high doses of BPA to produce ‘effects’ in rodents, and no rodent studies have been able to faithfully replicate any of the human diseases listed in Table 1 [43,44,45].
- (2)
- The association of BPA with disease is not unique to BPA. Some of the newer polyphenol analogs of BPA, introduced to replace BPA, are also associated with ADHD [130]. Zebrafish are one of the animal models used to study BPA and phthalates, and parallels in their neurologic effects have been noted. All Zebrafish phthalates and several BPA variants interfere with dopaminergic pathways [131,132]. How many other compounds with similar effects are there? The more compounds there are, the less support there is for the simple direct pathway of elevated levels of BPA somehow interfering with a specific metabolic step to yield a disease.The disease differentiating step would probably be either BPA (and MEHP) acting as an endocrine disruptor at different sites (the direct pathway) or differences in the glucuronidation isoenzyme distribution and, hence, a slightly different effect on the metabolism of whatever endogenous compound BPA and MEHP glucuronidation are markers for (the indirect pathway).
- (3)
- The metabolome reflects metabolism within the body. The findings from the previously mentioned studies of ASD (study #3) and ADHD (study #4) on the relationships between toxinogen glucuronidation and the urinary metabolome and ASD and ADHD support the ‘indirect’ pathway. The expected findings from metabolomic studies for the ‘direct’ pathway are either evidence of a simple lesion or an uninterpretable mess. Uninterpretable data is a possibility because of the wealth of data provided by the metabolome, the relatively small number of subjects, and disease heterogeneity. Disease heterogeneity is particularly true for ASD, which is an umbrella term for a variety of closely related diseases. The dataset is complex but not an ‘uninterpretable mess’.For the ‘indirect’ pathway, the expected findings show evidence of relationships with BPA at multiple metabolic sites or an uninterpretable mess. The metabolomic results presented above, although complex, are not random, nor are they unique. A metabolomic study on the effects of low levels of BPA on the metabolome of disease-free African-American women also found low-dose BPA exposure to affect multiple metabolic pathways [133]. Findings of widespread effects on the metabolome from low-dose BPA exposure have also been found in rodent studies [134,135,136]. These observations are consistent with BPA having a well-defined, systemic rather than a specific effect on intermediary metabolism.
- (4)
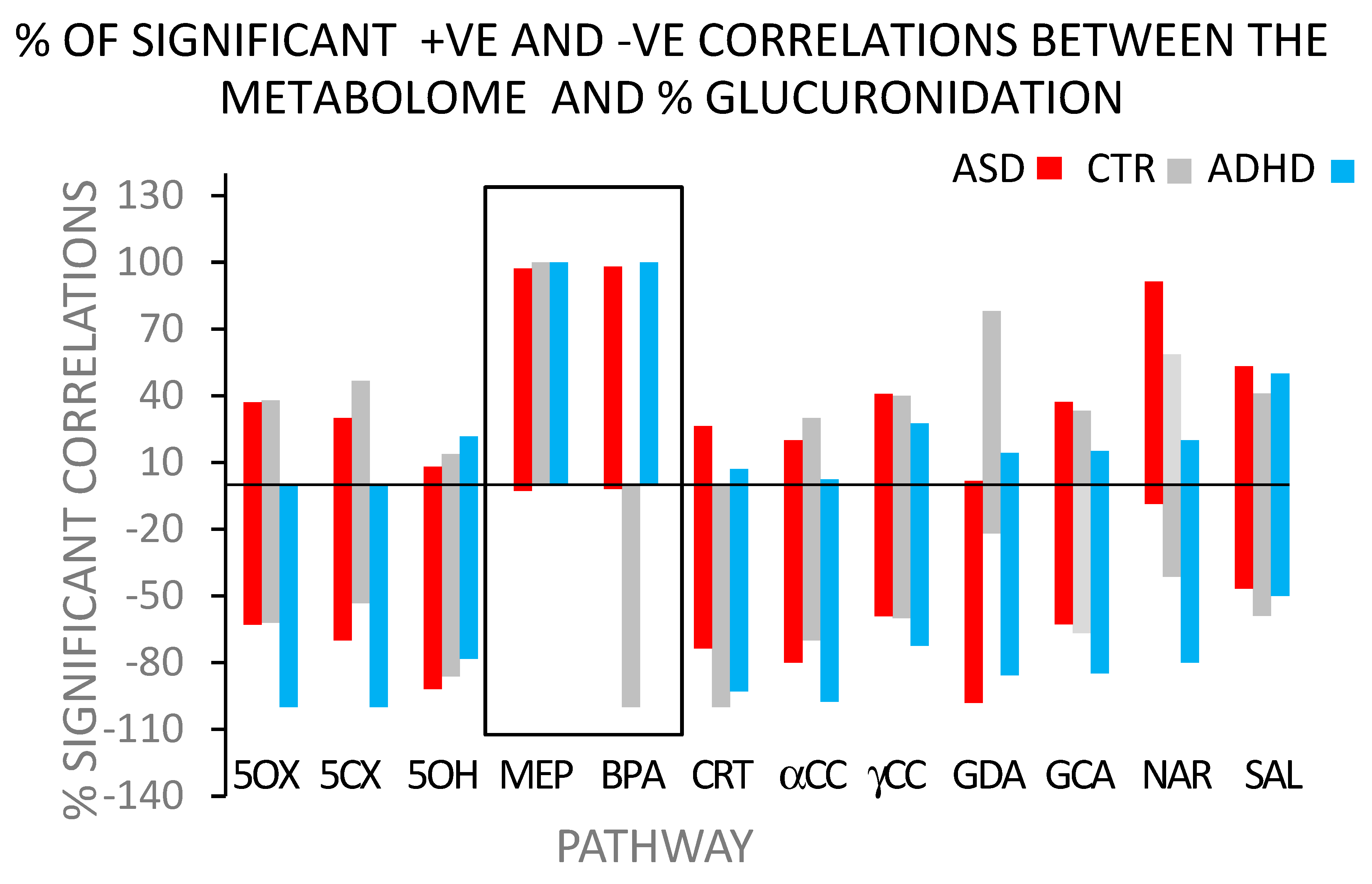
- Support for the concept that BPA glucuronidation detects systemic metabolic disease-related differences comes from a comparison of the correlation distribution patterns found with ASD and ADHD (Figure 2). Finding so many correlations paralleling each other (i.e., >95% correlations in the same direction) is indicative of a systemic effect of BPA on intermediary metabolism.Further support for a clear distinction between how BPA and MEHP affect glucuronidation efficiency is that there was no correlation between the glucuronidation efficiencies of BPA and MEHP (r2 < 0.02), indicating different systemic effects on the metabolome. There is a clear difference between the pathways of MEHP and BPA glucuronidation. The metabolome has a complex but clearly defined structure.The ‘indirect’ pathway explains these differences by attributing them to differences in UGT isoenzyme distribution patterns. More than one pattern can metabolize BPA, but the configurations may differ in their specificity for other substrates. The direct pathway cannot easily explain the systemic effect of low-dose BPA on the metabolome.
- (5)
- This variability in the components of the glucuronidation pathway can account for the observations of multiple diseases correlating with BPA exposure. Just as UGT and transporter distributions can determine the therapeutic effectiveness of drugs metabolized by the glucuronidation pathway [66,67,87], the same process can also lead to endocrine environments favoring disease development (ASD, ADHD, PCOS, PD, and AD), with the actual disease being a function of a particular endocrine environment.
- (6)
- The indirect pathway provides a simple explanation of how a single, relatively inert compound can be associated with several major diseases. Multiple combinations of isoenzymes can be expected to show BPA glucuronidation capability. This variability may not affect overall BPA glucuronidation, but different variants could affect the degradative glucuronidation of various products of intermediary metabolism. The disease potential depends on the product. Plasticity could account for BPA being associated with multiple disparate diseases. The direct pathway does not provide a simple explanation for how BPA could be associated with multiple diseases.
6.4. The Role of Steroids
6.5. Limitations
- (1).
- Firstly, and most importantly, the above analysis only applies to situations where there is evidence of compromised BPA (and/or MEHP) glucuronidation. The proportion of the total number of patients with each disease fitting into this category is not known, but, as previously pointed out, it must be a significant proportion because evidence for compromised glucuronidation is easily detected in moderate-sized studies [105,107,122,147].
- (2).
- The currently available data are fragmented. Proof of the hypothesis requires the measurement of glucuronidation enzymes’ distribution patterns (UGTs and efflux transporters) plus the glucuronidation efficiency in the same subject. This has not yet been carried out. However, what is available is enough fragmentary information to support a distinctive pattern of events that could lead to disease.
- (3).
- For the two neurodevelopmental and two neurodegenerative diseases, the site of injury is the brain. The fifth disease, PCOS, is not a neurological disease. If the disease-causing lesion is hepatic glucuronidation, either free BPA (the direct hypothesis) or an unknown endogenous compound (the indirect hypothesis) will be in the circulation and will attack vulnerable tissues (e.g., the brain or ovaries). There are many steps between a problematic isoenzyme configuration in the liver and disease in a tissue. The analysis presented here provides no information on those details.
- (4).
- The analysis treated the five diseases as single entities. This is certainly not true for ASD, which is a family of closely related diseases, but it is probably true for AD and PD, where there is a single biochemical product (AD; amyloid) or impacted pathway (PD; dopaminergic).
- (5).
- The evidence supporting an association of AD with BPA is not as strong as with the other four diseases.
- (6).
- The potential role of host disease defense mechanisms in the etiology of the disease has not been considered.
7. Conclusions
Funding
Conflicts of Interest
References
- Mir, R.H.; Sawhney, G.; Pottoo, F.H.; Mohi-Ud-Din, R.; Madishetti, S.; Jachak, S.M.; Ahmed, Z.; Masoodi, M.H. Role of environmental pollutants in Alzheimer’s disease: A review. Environ. Sci. Pollut. Res. 2021, 27, 44724–44742. [Google Scholar] [CrossRef] [PubMed]
- Lahiri, D.K.; Maloney, B. The “LEARn” [latent early-life associated regulation] model: An epigenetic pathway linking metabolic and cognitive disorders. J. Alzheimer’s Dis. 2012, 30, S15–S30. [Google Scholar] [CrossRef] [PubMed]
- McCaffery, P.; Deutsch, C.K. Macrocephaly and the control of brain growth in autistic disorders. Prog. Neurobiol. 2005, 77, 38–56. [Google Scholar] [CrossRef] [PubMed]
- Ishihara, K.; Takata, K.; Mizutani, K.I. Involvement of an Aberrant Vascular System in Neurodevelopmental, Neuropsychiatric, and Neuro-Degenerative Diseases. Life 2023, 13, 221. [Google Scholar] [CrossRef] [PubMed]
- Bleuze, L.; Triaca, V.; Borreca, A. FMRP-Driven Neuropathology in Autistic Spectrum Disorder and Alzheimer’s disease: A Losing Game. Front. Mol. Biosci. 2021, 8, 699613. [Google Scholar] [CrossRef]
- Triaca, V.; Calissano, P. Impairment of the nerve growth factor pathway driving amyloid accumulation in cholinergic neurons: The incipit of the Alzheimer’s disease story? Neural Regen. Res. 2016, 11, 1553–1556. [Google Scholar] [CrossRef]
- Masuo, Y.; Ishido, M. Neurotoxicity of endocrine disruptors: Possible involvement in brain development and neurodegeneration. J. Toxicol. Environ. Health Part B Crit. Rev. 2011, 14, 346–369. [Google Scholar] [CrossRef]
- Zeliger, H.I.; Lipinski, B. Physiochemical basis of human degenerative disease. Interdiscip. Toxicol. 2015, 8, 15–21. [Google Scholar] [CrossRef][Green Version]
- Sokol, D.K.; Maloney, B.; Long, J.M.; Ray, B.; Lahiri, D.K. Autism, Alzheimer disease, and fragile X: APP, FMRP, and mGluR5 are molecular links. Neurology 2011, 76, 1344–1352. [Google Scholar] [CrossRef]
- Weiss, B. Endocrine disruptors as a threat to neurological function. J. Neurol. Sci. 2011, 305, 11–21. [Google Scholar] [CrossRef]
- NIA National Institute on Aging-Division of Neuroscience. Can Autism Spectrum Disorders Tell Us Anything About Alzheimer’s Disease? National Institute on Aging-Division of Neuroscience: Washinton, DC, USA, 2022.
- Merchant Research & Consulting, Ltd. A Closer Look at the Global Bisphenol A Market; Merchant Research & Consulting, Ltd. 2021. Available online: https://mcgroup.co.uk/news/20230711/a-closer-look-at-the-global-bisphenol-a-market-demand-regulations-sustainability.html (accessed on 19 October 2024).
- Wikipedia. Bisphenol A. 2024. Available online: https://en.wikipedia.org/wiki/Bisphenol_A (accessed on 19 October 2024).
- Mordor Intelligence. Bisphenol-A Market Size & Share Analysis-Growth Trends & Forecasts 2024–2029. 2024. Available online: https://www.mordorintelligence.com/industry-reports/bisphenol-a-bpa-market (accessed on 19 October 2024).
- Maenner, M.J.; Warren, Z.; Williams, A.R.; Amoakohene, E.; Bakian, A.V.; Bilder, D.A.; Durkin, M.S.; Fitzgerald, R.T.; Furnier, S.M.; Hughes, M.M.; et al. Prevalence and Characteristics of Autism Spectrum Disorder Among Children Aged 8 Years-Autism and Developmental Disabilities Monitoring Network, 11 Sites, United States, 2020. Morb. Mortal. Wkly. Report. Surveill. Summ. 2023, 72, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Xu, G.; Strathearn, L.; Liu, B.; Yang, B.; Bao, W. Twenty-Year Trends in Diagnosed Attention-Deficit/Hyperactivity Disorder Among US Children and Adolescents, 1997–2016. JAMA Netw. Open 2018, 1, e181471. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Yan, X.; Li, Q.; Li, Q.; Xu, G.; Lu, J.; Yang, W. Prevalence and Trends in Diagnosed ADHD Among US Children and Adolescents, 2017–2022. JAMA Netw. Open 2023, 6, e2336872. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Cui, Y.; Zhang, J.; Yan, R.; Su, D.; Zhao, D.; Wang, A.; Feng, T. Temporal trends in the prevalence of Parkinson’s disease from 1980 to 2023: A systematic review and meta-analysis. Lancet Healthy Longev. 2024, 5, e464–e479. [Google Scholar] [CrossRef] [PubMed]
- Alzheimer, A.; Stelzmann, R.A.; Schnitzlein, H.N.; Murtagh, F.R. An English translation of Alzheimer’s 1907 paper, “Uber eine eigenartige Erkankung der Hirnrinde”. Clin. Anat. 1995, 8, 429–431. [Google Scholar]
- National Center for Health Statistics. Age-adjusted death rates for selected causes of death, by sex, race, and Hispanic origin: United States, selected years 1950–2018. In Health, United States-Data Finder; National Center for Health Statistics: Hyattsville, MD, USA, 2024. [Google Scholar]
- Gao, Y.; Liu, H.; Qiao, L.; Liang, J.; Yao, H.; Lin, X.; Gao, Y. Study of Burden in Polycystic Ovary Syndrome at Global, Regional, and National Levels from 1990 to 2019. Healthcare 2023, 11, 562. [Google Scholar] [CrossRef]
- Berni, T.R.; Morgan, C.L.; Rees, D.A. Rising incidence, health resource utilization and costs of Polycystic Ovary Syndrome in the United Kingdom. J. Clin. Endocrinol. Metab. 2024, 25, dgae518. [Google Scholar] [CrossRef]
- Herbert, M.R. Contributions of the environment and environmentally vulnerable physiology to autism spectrum disorders. Curr. Opin. Neurol. 2010, 23, 103–110. [Google Scholar] [CrossRef]
- Pessah, I.N.; Lein, P.J. Evidence for environmental susceptbility in autism: What we need to know about gene x environment interations. In Autism: Current Theories and Evidence; Zimmerman, A., Ed.; Humana Press: New York, NY, USA, 2008; pp. 406–464. [Google Scholar]
- NTP-CERHR. Expert Panel Report on the Reproductive and Developmental Toxicity of BPA; CDC, DHSS: Washington, DC, USA, 2007.
- Ahmed, A.E.; Jacob, S.; Campbell, G.A.; Harirah, H.M.; Perez-Polo, J.R.; Johnson, K.M. Fetal origin of adverse pregnancy outcome: The water disinfectant by-product chloroacetonitrile induces oxidative stress and apoptosis in mouse fetal brain. Brain Res. Dev. Brain Res. 2005, 159, 1–11. [Google Scholar] [CrossRef]
- Mesnil, M.; Defamie, N.; Naus, C.; Sarrouilhe, D. Brain Disorders and Chemical Pollutants: A Gap Junction Link? Biomolecules 2020, 11, 51. [Google Scholar] [CrossRef]
- National Toxicology Program. NTP Monograph on Health Effects of Low-Level Lead; National Toxicology Program: Research Triangle Park, NC, USA, 2012; pp. iii, xv–xvii.
- Farmani, R.; Mehrpour, O.; Kooshki, A.; Nakhaee, S. Exploring the link between toxic metal exposure and ADHD: A systematic review of pb and hg. J. Neurodev. Disord. 2024, 16, 44. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.H.; Kabir, E.; Jahan, S.A. Exposure to pesticides and the associated human health effects. Sci. Total Environ. 2017, 575, 525–535. [Google Scholar] [CrossRef] [PubMed]
- Di Cicco, M.E.; Ragazzo, V.; Jacinto, T. Mortality in relation to smoking: The British Doctors Study. Breathe 2016, 12, 275–276. [Google Scholar] [CrossRef] [PubMed]
- Braun, J.M.; Hauser, R. Bisphenol A and Children’s Health. Curr. Opin. Pediatr. 2011, 23, 233–239. [Google Scholar] [CrossRef] [PubMed]
- Braun, J.M.; Kalkbrenner, A.E.; Calafat, A.M.; Bernert, J.T.; Ye, X.; Silva, M.J.; Barr, D.B.; Sathyanarayana, S.; Lanphear, B.P. Variability and predictors of urinary bisphenol A concentrations during pregnancy. Environ. Health Perspect. 2011, 119, 131–137. [Google Scholar] [CrossRef]
- Makris, K.C.; Andra, S.S.; Jia, A.; Herrick, L.; Christophi, C.A.; Snyder, S.A.; Hauser, R. Association between water consumption from polycarbonate containers and bisphenol A intake during harsh environmental conditions in summer. Environ. Sci. Technol. 2013, 47, 3333–3343. [Google Scholar] [CrossRef]
- Hauser, R.; Calafat, A.M. Phthalates and human health. Occup. Environ. Med. 2005, 62, 806–818. [Google Scholar] [CrossRef]
- Hahladakis, J.N.; Velis, C.A.; Weber, R.; Iacovidou, E.; Purnell, P. An overview of chemical additives present in plastics: Migration, release, fate and environmental impact during their use, disposal and recycling. J. Hazard. Mater. 2018, 344, 179–199. [Google Scholar] [CrossRef]
- Hermabessiere, L.; Dehaut, A.; Paul-Pont, I.; Lacroix, C.; Jezequel, R.; Soudant, P.; Duflos, G. Occurrence and effects of plastic additives on marine environments and organisms: A review. Chemosphere 2017, 182, 781–793. [Google Scholar] [CrossRef]
- Tastet, V.; Le Vee, M.; Bruyere, A.; Fardel, O. Interactions of human drug transporters with chemical additives present in plastics: Potential consequences for toxicokinetics and health. Environ. Pollut. 2023, 331, 121882. [Google Scholar] [CrossRef]
- Sangkham, S.; Faikhaw, O.; Munkong, N.; Sakunkoo, P.; Arunlertaree, C.; Chavali, M.; Mousazadeh, M.; Tiwari, A. A review on microplastics and nanoplastics in the environment: Their occurrence, exposure routes, toxic studies, and potential effects on human health. Mar. Pollut. Bull. 2022, 181, 113832. [Google Scholar] [CrossRef] [PubMed]
- Vandenberg, L.N.; Maffini, M.V.; Sonnenschein, C.; Rubin, B.S.; Soto, A.M. Bisphenol-A and the great divide: A review of controversies in the field of endocrine disruption. Endocr. Rev. 2009, 30, 75–95. [Google Scholar] [CrossRef] [PubMed]
- Saal, F.S.V.; Vandenberg, L.N. Update on the Health Effects of Bisphenol A: Overwhelming Evidence of Harm. Endocrinology 2021, 162, bqaa171. [Google Scholar] [CrossRef] [PubMed]
- Camacho, L.; Lewis, S.; Vanlandingham, M.; Olson, G.R.; Davis, K.J.; Patton, R.E.; Twaddle, N.C.; Doerge, D.R.; Churchwell, M.I.; Bryant, M.S.; et al. A two-year toxicology study of bisphenol A [BPA] in Sprague-Dawley rats: CLARITY-BPA core study results. Food Chem. Toxicol. 2019, 132, 110728. [Google Scholar] [CrossRef] [PubMed]
- Vogel, S.A. The politics of plastics: The making and unmaking of bisphenol a “safety”. Am. J. Public Health 2009, 99, S559–S566. [Google Scholar] [CrossRef]
- Myers, J.P.; vom Saal, F.S.; Akingbemi, B.T.; Arizono, K.; Belcher, S.; Colborn, T.; Chahoud, I.; Crain, D.A.; Farabollini, F.; Guillette, L.; et al. Why Public Health Agencies Cannot Depend on Good Laboratory Practices as a Criterion for Selecting Data: The Case of Bisphenol A. Environ. Health Perspect. 2009, 117, 309–315. [Google Scholar] [CrossRef]
- vom Saal, F.S.; Akingbemi, B.T.; Belcher, S.M.; Birnbaum, L.S.; Crain, D.A.; Eriksen, M.; Farabollini, F.; Guillette, L.J., Jr.; Hauser, R.; Heindel, J.J. Chapel Hill bisphenol A expert panel consensus statement: Integration of mechanisms, effects in animals and potential to impact human health at current levels of exposure. Reprod. Toxicol. 2007, 24, 131–138. [Google Scholar] [CrossRef]
- Cimmino, I.; Fiory, F.; Perruolo, G.; Miele, C.; Beguinot, F.; Formisano, P.; Oriente, F. Potential Mechanisms of Bisphenol A [BPA] Contributing to Human Disease. Int. J. Mol. Sci. 2020, 21, 5761. [Google Scholar] [CrossRef]
- Costa, H.E.; Cairrao, E. Effect of bisphenol A on the neurological system: A review update. Arch. Toxicol. 2024, 98, 1–73. [Google Scholar] [CrossRef]
- Salehabadi, A.; Farkhondeh, T.; Harifi-Mood, M.S.; Aschner, M.; Samarghandian, S. Role of Nrf2 in bisphenol effects: A review study. Environ. Sci. Pollut. Res. 2022, 29, 55457–55472. [Google Scholar] [CrossRef]
- Rebolledo-Solleiro, D.; Castillo Flores, L.Y.; Solleiro-Villavicencio, H. Impact of BPA on behavior, neurodevelopment and neurodegeneration. Front. Biosci. 2021, 26, 363–400. [Google Scholar] [CrossRef] [PubMed]
- vom Saal, F.S.; Myers, J.P. Bisphenol A and risk of metabolic disorders. JAMA 2008, 300, 1353–1355. [Google Scholar] [CrossRef] [PubMed]
- Kim, I.W.; Peng, X.H.; Sauna, Z.E.; FitzGerald, P.C.; Xia, D.; Muller, M.; Nandigama, K.; Ambudkar, S.V. The conserved tyrosine residues 401 and 1044 in ATP sites of human P-glycoprotein are critical for ATP binding and hydrolysis: Evidence for a conserved subdomain, the A-loop in the ATP-binding cassette. Biochemistry 2006, 45, 7605–7616. [Google Scholar] [CrossRef] [PubMed]
- Giacomini, K.M.; Huang, S.M.; Tweedie, D.J.; Benet, L.Z.; Brouwer, K.L.; Chu, X.; Dahlin, A.; Evers, R.; Fischer, V.; Hillgren, K.M. Membrane transporters in drug development. Nature Reviews. Drug Discov. 2010, 9, 215–236. [Google Scholar]
- Hillgren, K.M.; Keppler, D.; Zur, A.A.; Giacomini, K.M.; Stieger, B.; Cass, C.E.; Zhang, L. Emerging transporters of clinical importance: An update from the International Transporter Consortium. Clin. Pharmacol. Ther. 2013, 94, 52–63. [Google Scholar] [CrossRef]
- Jarvinen, E.; Deng, F.; Kiander, W.; Sinokki, A.; Kidron, H.; Sjostedt, N. The Role of Uptake and Efflux Transporters in the Disposition of Glucuronide and Sulfate Conjugates. Front. Pharmacol. 2022, 12, 802539. [Google Scholar] [CrossRef]
- Liao, C.; Kannan, K. Determination of free and conjugated forms of bisphenol A in human urine and serum by liquid chromatography-tandem mass spectrometry. Environ. Sci. Technol. 2012, 46, 5003–5009. [Google Scholar] [CrossRef]
- Volkel, W.; Bittner, N.; Dekant, W. Quantitation of bisphenol A and bisphenol A glucuronide in biological samples by high performance liquid chromatography-tandem mass spectrometry. Drug Metab. Dispos. 2005, 33, 1748–1757. [Google Scholar] [CrossRef]
- Jeong, E.J.; Liu, X.; Jia, X.; Chen, J.; Hu, M. Coupling of conjugating enzymes and efflux transporters: Impact on bioavailability and drug interactions. Curr. Drug Metab. 2005, 6, 455–468. [Google Scholar] [CrossRef]
- Carmeci, C.; Thompson, D.A.; Ring, H.Z.; Francke, U.; Weigel, R.J. Identification of a gene [GPR30] with homology to the G-protein-coupled receptor superfamily associated with estrogen receptor expression in breast cancer. Genomics 1997, 45, 607–617. [Google Scholar] [CrossRef]
- Nohynek, G.J.; Borgert, C.J.; Dietrich, D.; Rozman, K.K. Endocrine disruption: Fact or urban legend? Toxicol. Lett. 2013, 223, 295–305. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Ding, Z.; Shi, Q.M.; Ge, X.; Wang, H.X.; Li, M.X.; Chen, G.; Wang, Q.; Ju, Q.; Zhang, J.P. Anti-androgenic mechanisms of Bisphenol A involve androgen receptor signaling pathway. Toxicology 2017, 387, 10–16. [Google Scholar] [CrossRef] [PubMed]
- Kuiper, G.G.; Carlsson, B.; Grandien, K.; Enmark, E.; Haggblad, J.; Nilsson, S.; Gustafsson, J.A. Comparison of the ligand binding specificity and transcript tissue distribution of estrogen receptors alpha and beta. Endocrinology 1997, 138, 863–870. [Google Scholar] [CrossRef] [PubMed]
- Mathieu-Denoncourt, J.; Wallace, S.J.; de Solla, S.R.; Langlois, V.S. Influence of Lipophilicity on the Toxicity of Bisphenol A and Phthalates to Aquatic Organisms. Bull. Environ. Contam. Toxicol. 2016, 97, 4–10. [Google Scholar] [CrossRef] [PubMed]
- Molina-Molina, J.M.; Amaya, E.; Grimaldi, M.; Saenz, J.M.; Real, M.; Fernandez, M.F.; Balaguer, P.; Olea, N. In vitro study on the agonistic and antagonistic activities of bisphenol-S and other bisphenol-A congeners and derivatives via nuclear receptors. Toxicol. Appl. Pharmacol. 2013, 272, 127–136. [Google Scholar] [CrossRef]
- Sohoni, P.; Sumpter, J.P. Several environmental oestrogens are also anti-androgens. J. Endocrinol. 1998, 158, 327–339. [Google Scholar] [CrossRef]
- Wells, P.G.; Mackenzie, P.I.; Chowdhury, J.R.; Guillemette, C.; Gregory, P.A.; Ishii, Y.; Hansen, A.J.; Kessler, F.K.; Kim, P.M.; Chowdhury, N.R.; et al. Glucuronidation and the UDP-glucuronosyltransferases in health and disease. Drug Metab. Dispos. 2004, 32, 281–290. [Google Scholar] [CrossRef]
- Meech, R.; Hu, D.G.; McKinnon, R.A.; Mubarokah, S.N.; Haines, A.Z.; Nair, P.C.; Rowland, A.; Mackenzie, P.I. The UDP-Glycosyltransferase [UGT] Superfamily: New Members, New Functions, and Novel Paradigms. Physiol. Rev. 2019, 99, 1153–1222. [Google Scholar] [CrossRef]
- Stingl, J.C.; Bartels, H.; Viviani, R.; Lehmann, M.L.; Brockmoller, J. Relevance of UDP-glucuronosyltransferase polymorphisms for drug dosing: A quantitative systematic review. Pharmacol. Ther. 2013, 141, 92–116. [Google Scholar] [CrossRef]
- Kroemer, H.K.; Klotz, U. Glucuronidation of drugs. A re-evaluation of the pharmacological significance of the conjugates and modulating factors. Clin. Pharmacokinet. 1992, 23, 292–310. [Google Scholar] [CrossRef]
- Yang, G.; Ge, S.; Singh, R.; Basu, S.; Shatzer, K.; Zen, M.; Liu, J.; Tu, Y.; Zhang, C.; Wei, J.; et al. Glucuronidation: Driving factors and their impact on glucuronide disposition. Drug Metab. Rev. 2017, 49, 105–138. [Google Scholar] [CrossRef] [PubMed]
- Volkel, W.; Colnot, T.; Csanady, G.A.; Filser, J.G.; Dekant, W. Metabolism and kinetics of bisphenol a in humans at low doses following oral administration. Chem. Res. Toxicol. 2002, 15, 1281–1287. [Google Scholar] [CrossRef] [PubMed]
- Thayer, K.A.; Doerge, D.R.; Hunt, D.; Schurman, S.H.; Twaddle, N.C.; Churchwell, M.I.; Garantziotis, S.; Kissling, G.E.; Easterling, M.R. Pharmacokinetics of bisphenol A in humans following a single oral administration. Environ. Int. 2015, 83, 107–115. [Google Scholar] [CrossRef] [PubMed]
- Ginsberg, G.; Rice, D.C. Does rapid metabolism ensure negligible risk from bisphenol A? Environ. Health Perspect. 2009, 117, 1639–1643. [Google Scholar] [CrossRef]
- Evers, R.; Piquette-Miller, M.; Polli, J.W.; Russel, F.G.M.; Sprowl, J.A.; Tohyama, K.; Ware, J.A.; de Wildt, S.N.; Xie, W.; Brouwer, K.L.R. Disease-Associated Changes in Drug Transporters May Impact the Pharmacokinetics and/or Toxicity of Drugs: A White Paper From the International Transporter Consortium. Clin. Pharmacol. Ther. 2018, 104, 900–915. [Google Scholar] [CrossRef]
- National Technology Program. Center for the Evaluation of Risks to Human Reproduction. Expert Panel Evalution of Bisphenol A; NIH Publication No. 08–5994; National Technology Program: Durhan, NC, USA, 2008.
- Mazur, C.S.; Marchitti, S.A.; Dimova, M.; Kenneke, J.F.; Lumen, A.; Fisher, J. Human and rat ABC transporter efflux of bisphenol a and bisphenol a glucuronide: Interspecies comparison and implications for pharmacokinetic assessment. Toxicol. Sci. 2012, 128, 317–325. [Google Scholar] [CrossRef][Green Version]
- Bock, K.W. Human UDP-glucuronosyltransferases: Feedback loops between substrates and ligands of their transcription factors. Biochem. Pharmacol. 2012, 84, 1000–1006. [Google Scholar] [CrossRef]
- Steventon, G.B.; Heafield, M.T.; Sturman, S.; Waring, R.H.; Williams, A.C. Xenobiotic metabolism in Alzheimer’s disease. Neurology 1990, 40, 1095–1098. [Google Scholar] [CrossRef]
- Oda, S.; Fukami, T.; Yokoi, T.; Nakajima, M. A comprehensive review of UDP-glucuronosyltransferase and esterases for drug development. Drug Metab. Pharmacokinet. 2015, 30, 30–51. [Google Scholar] [CrossRef]
- Mackenzie, P.I.; Bock, K.W.; Burchell, B.; Guillemette, C.; Ikushiro, S.; Iyanagi, T.; Miners, J.O.; Owens, I.S.; Nebert, D.W. Nomenclature update for the mammalian UDP glycosyltransferase [UGT] gene superfamily. Pharmacogenet. Genom. 2005, 15, 677–685. [Google Scholar] [CrossRef]
- Allain, E.P.; Rouleau, M.; Levesque, E.; Guillemette, C. Emerging roles for UDP-glucuronosyltransferases in drug resistance and cancer progression. Br. J. Cancer 2020, 122, 1277–1287. [Google Scholar] [CrossRef] [PubMed]
- Bandyopadhyay, D.; Biswas, K.; Bhattacharyya, M.; Reiter, R.J.; Banerjee, R.K.; Bandyopadhyay, D.; Biswas, K.; Bhattacharyya, M.; Reiter, R.J.; Banerjee, R.K. Involvement of reactive oxygen species in gastric ulceration: Protection by melatonin. Indian J. Exp. Biol. 2002, 40, 693–705. [Google Scholar] [PubMed]
- Kasteel, E.E.J.; Darney, K.; Kramer, N.I. Human variability in isoform-specific UDP-glucuronosyltransferases: Markers of acute and chronic exposure, polymorphisms and uncertainty factors. Arch. Toxicol. 2020, 94, 2637–2661. [Google Scholar] [CrossRef] [PubMed]
- Hanioka, N.; Isobe, T.; Tanaka-Kagawa, T.; Dorne, J.; Lautz, L.S. In vitro glucuronidation of bisphenol A in liver and intestinal microsomes: Interspecies differences in humans and laboratory animals. Drug Chem. Toxicol. 2022, 45, 1565–1569. [Google Scholar] [CrossRef] [PubMed]
- Hanioka, N.; Naito, T.; Narimatsu, S. Human UDP-glucuronosyltransferase isoforms involved in bisphenol A glucuronidation. Chemosphere 2008, 74, 33–36. [Google Scholar] [CrossRef] [PubMed]
- Swanson, C.; Lorentzon, M.; Vandenput, L.; Labrie, F.; Rane, A.; Jakobsson, J.; Chouinard, S.; Belanger, A.; Ohlsson, C. Sex steroid levels and cortical bone size in young men are associated with a uridine diphosphate glucuronosyltransferase 2B7 polymorphism [H268Y]. J. Clin. Endocrinol. Metab. 2007, 92, 3697–3704. [Google Scholar] [CrossRef]
- Wang, H.; Yuan, L.; Zeng, S. Characterizing the effect of UDP-glucuronosyltransferase [UGT] 2B7 and UGT1A9 genetic polymorphisms on enantioselective glucuronidation of flurbiprofen. Biochem. Pharmacol. 2011, 82, 1757–1763. [Google Scholar] [CrossRef]
- Argikar, U.A.; Iwuchukwu, O.F.; Nagar, S. Update on tools for evaluation of uridine diphosphoglucuronosyltransferase polymorphisms. Expert Opin. Drug Metab. Toxicol. 2008, 4, 879–894. [Google Scholar] [CrossRef]
- Nie, Y.L.; He, H.; Li, J.F.; Meng, X.G.; Yan, L.; Wang, P.; Wang, S.J.; Bi, H.Z.; Zhang, L.R.; Kan, Q.C. Hepatic expression of transcription factors affecting developmental regulation of UGT1A1 in the Han Chinese population. Eur. J. Clin. Pharmacol. 2017, 73, 29–37. [Google Scholar] [CrossRef]
- Oswald, S.; Grube, M.; Siegmund, W.; Kroemer, H.K. Transporter-mediated uptake into cellular compartments. Xenobiotica 2007, 37, 1171–1195. [Google Scholar] [CrossRef]
- Schinkel, A.H.; Jonker, J.W. Mammalian drug efflux transporters of the ATP binding cassette [ABC] family: An overview. Adv. Drug Deliv. Rev. 2003, 55, 3–29. [Google Scholar] [CrossRef] [PubMed]
- Glavinas, H.; Krajcsi, P.; Cserepes, J.; Sarkadi, B. The role of ABC transporters in drug resistance, metabolism and toxicity. Curr. Drug Deliv. 2004, 1, 27–42. [Google Scholar] [CrossRef] [PubMed]
- Amawi, H.; Sim, H.M.; Tiwari, A.K.; Ambudkar, S.V.; Shukla, S. ABC Transporter-Mediated Multidrug-Resistant Cancer. Adv. Exp. Med. Biol. 2019, 1141, 549–580. [Google Scholar] [PubMed]
- Schwabedissen, H.E.M.Z.; Kroemer, H.K. In vitro and in vivo evidence for the importance of breast cancer resistance protein transporters [BCRP/MXR/ABCP/ABCG2]. In Handbook of Experimental Pharmacology; Springer-Nature: New York, NY, USA, 2011; pp. 325–371. [Google Scholar]
- Bera, T.K.; Iavarone, C.; Kumar, V.; Lee, S.; Lee, B.; Pastan, I. MRP9, an unusual truncated member of the ABC transporter superfamily, is highly expressed in breast cancer. Proc. Natl. Acad. Sci. USA 2002, 99, 6997–7002. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Ma, L.; Lin, Y.; Liu, X.; Xiao, L.; Zhang, Y.; Xu, Y.; Zhou, H.; Pan, G. Leflunomide Increases Hepatic Exposure to Methotrexate and Its Metabolite by Differentially Regulating Multidrug Resistace-Associated Protein Mrp2/3/4 Transporters via Peroxisome Proliferator-Activated Receptor alpha Activation. Mol. Pharmacol. 2018, 93, 563–574. [Google Scholar] [CrossRef]
- Wang, J.Q.; Yang, Y.; Cai, C.Y.; Teng, Q.X.; Cui, Q.; Lin, J.; Assaraf, Y.G.; Chen, Z.S. Multidrug resistance proteins [MRPs]: Structure, function and the overcoming of cancer multidrug resistance. Drug Resist. Updates 2021, 54, 100743. [Google Scholar] [CrossRef]
- McLean, C.; Wilson, A.; Kim, R.B. Impact of Transporter Polymorphisms on Drug Development: Is It Clinically Significant? J. Clin. Pharmacol. 2016, 56, 40–58. [Google Scholar] [CrossRef]
- Droge, C.; Bonus, M.; Baumann, U.; Klindt, C.; Lainka, E.; Kathemann, S.; Brinkert, F.; Grabhorn, E.; Pfister, E.D.; Wenning, D.; et al. Sequencing of FIC1, BSEP and MDR3 in a large cohort of patients with cholestasis revealed a high number of different genetic variants. J. Hepatol. 2017, 67, 1253–1264. [Google Scholar] [CrossRef]
- Kock, K.; Brouwer, K.L. A perspective on efflux transport proteins in the liver. Clin. Pharmacol. Ther. 2012, 92, 599–612. [Google Scholar] [CrossRef]
- Sissung, T.M.; Troutman, S.M.; Campbell, T.J.; Pressler, H.M.; Sung, H.; Bates, S.E.; Figg, W. Transporter pharmacogenetics: Transporter polymorphisms affect normal physiology, diseases, and pharmacotherapy. Discov. Med. 2012, 13, 19–34. [Google Scholar]
- Patel, S. Polycystic ovary syndrome [PCOS], an inflammatory, systemic, lifestyle endocrinopathy. J. Steroid Biochem. Mol. Biol. 2018, 182, 27–36. [Google Scholar] [CrossRef] [PubMed]
- Vahedi, M.; Saeedi, A.; Poorbaghi, S.L.; Sepehrimanesh, M.; Fattahi, M. Metabolic and endocrine effects of bisphenol A exposure in market seller women with polycystic ovary syndrome. Environ. Sci. Pollut. Res. 2016, 23, 23546–23550. [Google Scholar] [CrossRef] [PubMed]
- Prabhu, N.B.; Vasishta, S.; Bhat, S.K.; Joshi, M.B.; Kabekkodu, S.P.; Satyamoorthy, K.; Rai, P.S. Distinct metabolic signatures in blood plasma of bisphenol A-exposed women with polycystic ovarian syndrome. Environ. Sci. Pollut. Res. 2023, 30, 64025–64035. [Google Scholar] [CrossRef] [PubMed]
- Zhan, W.; Tang, W.; Shen, X.; Xu, H.; Zhang, J. Exposure to bisphenol A and its analogs and polycystic ovarian syndrome in women of childbearing age: A multicenter case-control study. Chemosphere 2023, 313, 137463. [Google Scholar] [CrossRef]
- Luo, Y.; Nie, Y.; Tang, L.; Xu, C.C.; Xu, L. The correlation between UDP-glucuronosyltransferase polymorphisms and environmental endocrine disruptors levels in polycystic ovary syndrome patients. Medicine 2020, 99, e19444. [Google Scholar] [CrossRef]
- Yoest, K.E.; Cummings, J.A.; Becker, J.B. Estradiol, dopamine and motivation. Cent. Nerv. Syst. Agents Med. Chem. 2023, 14, 83–89. [Google Scholar] [CrossRef]
- Landolfi, A.; Troisi, J.; Savanelli, M.C.; Vitale, C.; Barone, P.; Amboni, M. Bisphenol A glucuronidation in patients with Parkinson’s disease. Neurotoxicology 2017, 63, 90–96. [Google Scholar] [CrossRef]
- Landrigan, P.J. What causes autism? Exploring the environmental contribution. Curr. Opin. Pediatr. 2010, 22, 219–225. [Google Scholar] [CrossRef]
- Muhle, R.; Trentacoste, S.V.; Rapin, I. The genetics of autism. Pediatrics 2004, 113, e472–e486. [Google Scholar] [CrossRef]
- Zigman, T.; Petkovic Ramadza, D.; Baric, I. Inborn Errors of Metabolism Associated with Autism Spectrum Disorders: Approaches to Intervention. Front. Neurosci. 2021, 15, 673600. [Google Scholar] [CrossRef]
- Minatoya, M.; Kishi, R. A Review of Recent Studies on Bisphenol A and Phthalate Exposures and Child Neurodevelopment. Int. J. Environ. Res. Public Health 2021, 18, 30. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.X.; Liu, B.; Zhou, L.; Xiong, X.; Fu, J.; Huang, Z.F.; Tan, T.; Tang, M.; Wang, J.; Tang, Y.P. De novo mutations within metabolism networks of amino acid/protein/energy in Chinese autistic children with intellectual disability. Hum. Genom. 2022, 16, 52. [Google Scholar] [CrossRef] [PubMed]
- Santos, J.X.; Rasga, C.; Marques, A.R.; Martiniano, H.; Asif, M.; Vilela, J.; Oliveira, G.; Sousa, L.; Nunes, A.; Vicente, A.M. A Role for Gene-Environment Interactions in Autism Spectrum Disorder Is Supported by Variants in Genes Regulating the Effects of Exposure to Xenobiotics. Front. Neurosci. 2022, 16, 862315. [Google Scholar] [CrossRef] [PubMed]
- Moore, S.; Paalanen, L.; Melymuk, L.; Katsonouri, A.; Kolossa-Gehring, M.; Tolonen, H. The Association between ADHD and Environmental Chemicals-A Scoping Review. Int. J. Environ. Res. Public Health 2022, 19, 2849. [Google Scholar] [CrossRef] [PubMed]
- Praveena, S.M.; Munisvaradass, R.; Masiran, R.; Rajendran, R.K.; Lin, C.C.; Kumar, S. Phthalates exposure and attention-deficit/hyperactivity disorder in children: A systematic review of epidemiological literature. Environ. Sci. Pollut. Res. 2020, 27, 44757–44770. [Google Scholar] [CrossRef]
- Flaws, J.D.P.; Patisaul, H.B.; Gore, A.; Raetzman, L.; Vandenberg, L.N. Plastics, EDCs and Health; A guide for public interest and policy-makers on endocrine disrupting chemicals and plastics; The Endocrine Society: Washington, DC, USA, 2020. [Google Scholar]
- Tewar, S.; Auinger, P.; Braun, J.M.; Lanphear, B.; Yolton, K.; Epstein, J.N.; Ehrlich, S.; Froehlich, T.E. Association of Bisphenol A exposure and Attention-Deficit/Hyperactivity Disorder in a national sample of U.S. children. Environ. Res. 2016, 150, 112–118. [Google Scholar] [CrossRef]
- Shoaff, J.R.; Coull, B.; Weuve, J.; Bellinger, D.C.; Calafat, A.M.; Schantz, S.L.; Korrick, S.A. Association of Exposure to Endocrine-Disrupting Chemicals During Adolescence with Attention-Deficit/Hyperactivity Disorder-Related Behaviors. JAMA Netw. Open 2020, 3, e2015041. [Google Scholar] [CrossRef]
- MacKay, H.; Abizaid, A. A plurality of molecular targets: The receptor ecosystem for bisphenol-A [BPA]. Horm. Behav. 2018, 101, 59–67. [Google Scholar] [CrossRef]
- Rubin, A.M.; Seebacher, F. Bisphenols impact hormone levels in animals: A meta-analysis. Sci. Total Environ. 2022, 828, 154533. [Google Scholar] [CrossRef]
- Welch, C.; Mulligan, K. Does Bisphenol A Confer Risk of Neurodevelopmental Disorders? What We Have Learned from Developmental Neurotoxicity Studies in Animal Models. Int. J. Mol. Sci. 2022, 23, 2894. [Google Scholar] [CrossRef]
- Stein, T.P.; Schluter, M.D.; Steer, R.A.; Ming, X. Bisphenol-A and phthalate metabolism in children with neurodevelopmental disorders. PLoS ONE 2023, 18, e0289841. [Google Scholar] [CrossRef] [PubMed]
- Bustamante-Barrientos, F.A.; Mendez-Ruette, M.; Ortloff, A.; Luz-Crawford, P.; Rivera, F.J.; Figueroa, C.D.; Molina; Batiz, L.F. The Impact of Estrogen and Estrogen-Like Molecules in Neurogenesis and Neurodegeneration: Beneficial or Harmful? Front. Cell. Neurosci. 2013, 15, 636176. [Google Scholar] [CrossRef] [PubMed]
- Zeliger, H.I. Exposure to lipophilic chemicals as a cause of neurological impairments, neurodevelopmental disorders and neurodegenerative diseases. Interdiscip. Toxicol. 2013, 6, 103–110. [Google Scholar] [CrossRef] [PubMed]
- Hong, D.; Li, X.W.; Lian, Q.Q.; Lamba, P.; Bernard, D.J.; Hardy, D.O.; Chen, H.X.; Ge, R.S. Mono-(2-ethylhexyl) phthalate (MEHP) regulates glucocorticoid metabolism through 11beta-hydroxysteroid dehydrogenase 2 in murine gonadotrope cells. Biochem. Biophys. Res. Commun. 2009, 389, 305–309. [Google Scholar] [CrossRef] [PubMed]
- Huber, W.W.; Grasl-Kraupp, B.; Schulte-Hermann, R. Hepatocarcinogenic potential of di [2-ethylhexyl]phthalate in rodents and its implications on human risk. Crit. Rev. Toxicol. 1996, 26, 365–481. [Google Scholar] [CrossRef]
- Hanet, N.; Lancon, A.; Delmas, D.; Jannin, B.; Chagnon, M.C.; Cherkaoui-Malki, M.; Latruffe, N.; Artur, Y.; Heydel, J.M. Effects of endocrine disruptors on genes associated with 17beta-estradiol metabolism and excretion. Steroids 2008, 73, 1242–1251. [Google Scholar] [CrossRef]
- Nuttall, J.R. The plausibility of maternal toxicant exposure and nutritional status as contributing factors to the risk of autism spectrum disorders. Nutr. Neurosci. 2017, 20, 209–218. [Google Scholar] [CrossRef]
- Lind, P.M.; Lind, L. Circulating levels of bisphenol A and phthalates are related to carotid atherosclerosis in the elderly. Atherosclerosis 2011, 218, 207–213. [Google Scholar] [CrossRef]
- Kim, J.I.; Lee, Y.A.; Shin, C.H.; Hong, Y.C.; Kim, B.N.; Lim, Y.H. Association of bisphenol A, bisphenol F, and bisphenol S with ADHD symptoms in children. Environ. Int. 2022, 161, 107093. [Google Scholar] [CrossRef]
- Vutukuru, S.S.; Ganugapati, J.; Ganesh, V.; Atheeksha, P.; Potti, R.B. Endocrine disruption by Bisphenol A, polychlorinated biphenyls and polybrominated diphenyl ether, in zebra fish [Danio rerio] model: An in silico approach. Fish Physiol. Biochem. 2016, 42, 1541–1555. [Google Scholar] [CrossRef]
- Scaramella, C.; Alzagatiti, J.B.; Creighton, C.; Mankatala, S.; Licea, F.; Winter, G.M.; Emtage, J.; Wisnieski, J.R.; Salazar, L.; Hussain, A.; et al. Bisphenol A Exposure Induces Sensory Processing Deficits in Larval Zebrafish during Neurodevelopment. eNeuro 2022, 9, ENEURO.0020-22.2022. [Google Scholar] [CrossRef] [PubMed]
- Tchen, R.; Tan, Y.; Boyd Barr, D.; Tran, V.; Li, Z.; Hu, Y.J.; Smith, A.K.; Jones, D.P.; Dunlop, A.L.; Liang, D. Use of high-resolution metabolomics to assess the biological perturbations associated with maternal exposure to Bisphenol A and Bisphenol F among pregnant African American women. Environ. Int. 2022, 169, 107530. [Google Scholar] [CrossRef] [PubMed]
- Cabaton, N.J.; Canlet, C.; Wadia, P.R.; Tremblay-Franco, M.; Gautier, R.; Molina, J.; Sonnenschein, C.; Cravedi, J.P.; Rubin, B.S. Effects of low doses of bisphenol A on the metabolome of perinatally exposed CD-1 mice. Env. Health Perspect 2013, 121, 586–593. [Google Scholar] [CrossRef] [PubMed]
- Mao, L.; Fang, S.; Zhao, M.; Liu, W.; Jin, H. Effects of Bisphenol A and Bisphenol S Exposure at Low Doses on the Metabolome of Adolescent Male Sprague-Dawley Rats. Chem. Res. Toxicol. 2021, 34, 1578–1587. [Google Scholar] [CrossRef] [PubMed]
- Zeng, J.; Kuang, H.; Hu, C.; Shi, X.; Yan, M.; Xu, L.; Wang, L.; Xu, C.; Xu, G. Effect of bisphenol A on rat metabolic profiling studied by using capillary electrophoresis time-of-flight mass spectrometry. Environ. Sci. Technol. 2013, 47, 7457–7465. [Google Scholar] [CrossRef]
- Ihde, E.S.; Loh, J.M.; Rosen, L. Association of environmental chemicals & estrogen metabolites in children. BMC Endocr. Disord. 2015, 15, 83. [Google Scholar]
- Yong, M.; Schwartz, S.M.; Atkinson, C.C.; Makar, K.W.; Thomas, S.S.; Stanczyk, F.Z.; Westerlind, K.C.; Newton, K.M.; Holt, V.L.; Leisenring, W.M. Associations between polymorphisms in glucuronidation and sulfation enzymes and sex steroid concentrations in premenopausal women in the United States. J. Steroid Biochem. Mol. Biol. 2011, 124, 10–18. [Google Scholar] [CrossRef]
- Jansakova, K.; Hill, M.; Celarova, D.; Celusakova, H.; Repiska, G.; Bicikova, M.; Macova, L.; Ostatnikova, D. Alteration of the steroidogenesis in boys with autism spectrum disorders. Transl. Psychiatry 2020, 10, 340. [Google Scholar] [CrossRef]
- Gasser, B.A.; Kurz, J.; Dick, B.; Mohaupt, M.G. A reply to ‘Alteration of steroidogenesis in boys with autism spectrum disorders’. Transl. Psychiatry 2021, 11, 278. [Google Scholar] [CrossRef]
- Dumesic, D.A.; Oberfield, E.; Stener-Victorin, E.; Marshall, J.C.; Laven, J.S.; Legro, R.S. Scientific Statement on the Diagnostic Criteria, Epidemiology, Pathophysiology, and Molecular Genetics of Polycystic Ovary Syndrome. Endocr. Rev. 2015, 36, 487–525. [Google Scholar] [CrossRef]
- Bourque, M.; Di Paolo, T.; Morissette, M. Neuroactive steroids and Parkinson’s disease: Review of human and animal studies. Neurosci. Biobehav. Rev. 2024, 156, 105479. [Google Scholar] [CrossRef] [PubMed]
- Shao, Y.; Li, T.; Liu, Z.; Wang, X.; Xu, X.; Li, S.; Xu, G. Comprehensive metabolic profiling of Parkinson’s disease by liquid chromatography-mass spectrometry. Mol. Neurodegener. 2016, 16, 4. [Google Scholar] [CrossRef] [PubMed]
- Barth, C.; Crestol, A.; de Lange, A.G.; Galea, L.A.M. Sex steroids and the female brain across the lifespan: Insights into risk of depression and Alzheimer’s disease. Lancet Diabetes Endocrinol. 2023, 11, 926–942. [Google Scholar] [CrossRef] [PubMed]
- Akwa, Y. Steroids and Alzheimer’s Disease: Changes Associated with Pathology and Therapeutic Potential. Int. J. Mol. Sci. 2020, 21, 4812. [Google Scholar] [CrossRef]
- Stein, T.P.; Schluter, M.D.; Steer, R.A.; Ming, X. Autism and Phthalate Metabolite Glucuronidation. J. Autism Dev. Disord. 2013, 43, 2677–2685. [Google Scholar] [CrossRef]
- Stein, T.P.; Schluter, M.D.; Steer, R.A.; Ming, X. Bisphenol A Exposure in Children with Autism Spectrum Disorders. Autism Res. 2015, 8, 272–283. [Google Scholar] [CrossRef]
| Disease | Period | % Increase | Ref. |
|---|---|---|---|
| ASD | 2000–2020 | 15.7 | [15] |
| ADHD | 1997–2022 | 3.4 | [16,17] |
| PCOS | 1990–2019 | 1.8 | [21] |
| PD | 2000–2020 | 7.7 | [18] |
| AD | 2000–2018 | 4.0 | [20] |
| DISEASES |
| Behavioral effects caused by BPA Memory and learning disorders Anxiety and depressive-like behaviors Socio-sexual behavior |
| BPA and neurodegenerative disorders Parkinson’s disease (PD) Amyotrophic lateral sclerosis (ALS) Multiple sclerosis (MS) Alzheimer’s disease (AD) |
| BPA and neurodevelopmental disorders Autism spectrum disorders (ASDs) Attention-deficit/hyperactivity disorder (ADHD) |
| Diseases of the reproductive system Polycystic ovary syndrome (PCOS) |
| POTENTIAL MECHANISMS IDENTIFIED FROM ANIMAL STUDIES |
| Endocrine-related mechanisms Epigenetic mechanisms Mitochondrial pathways Calcium and oxidative stress pathways Inflammatory response pathways |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stein, T.P. Does Bisphenol A (BPA) Exposure Cause Human Diseases? Biomedicines 2024, 12, 2678. https://doi.org/10.3390/biomedicines12122678
Stein TP. Does Bisphenol A (BPA) Exposure Cause Human Diseases? Biomedicines. 2024; 12(12):2678. https://doi.org/10.3390/biomedicines12122678
Chicago/Turabian StyleStein, T. Peter. 2024. "Does Bisphenol A (BPA) Exposure Cause Human Diseases?" Biomedicines 12, no. 12: 2678. https://doi.org/10.3390/biomedicines12122678
APA StyleStein, T. P. (2024). Does Bisphenol A (BPA) Exposure Cause Human Diseases? Biomedicines, 12(12), 2678. https://doi.org/10.3390/biomedicines12122678






