Age-Related Choroidal Involution Is Associated with the Senescence of Endothelial Progenitor Cells in the Choroid
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal Care
2.2. Rat Model of Age-Related Choroidal Involution and Isolation of EPCs
2.3. Immunohistochemistry of Choroidal Vessels
2.4. Senescence Analysis Using β-Gal Assay
2.5. EPC Capillary-Like Tubulogenesis and Choroidal Angiogenic Sprouting Assay on Matrigel
2.6. Reverse Transcription–Quantitative Polymerase Chain Reaction (RT-qPCR) Analyses
2.7. Next-Generation Sequencing (NGS) and Predictive Pathway Analysis
2.8. Statistical Analysis
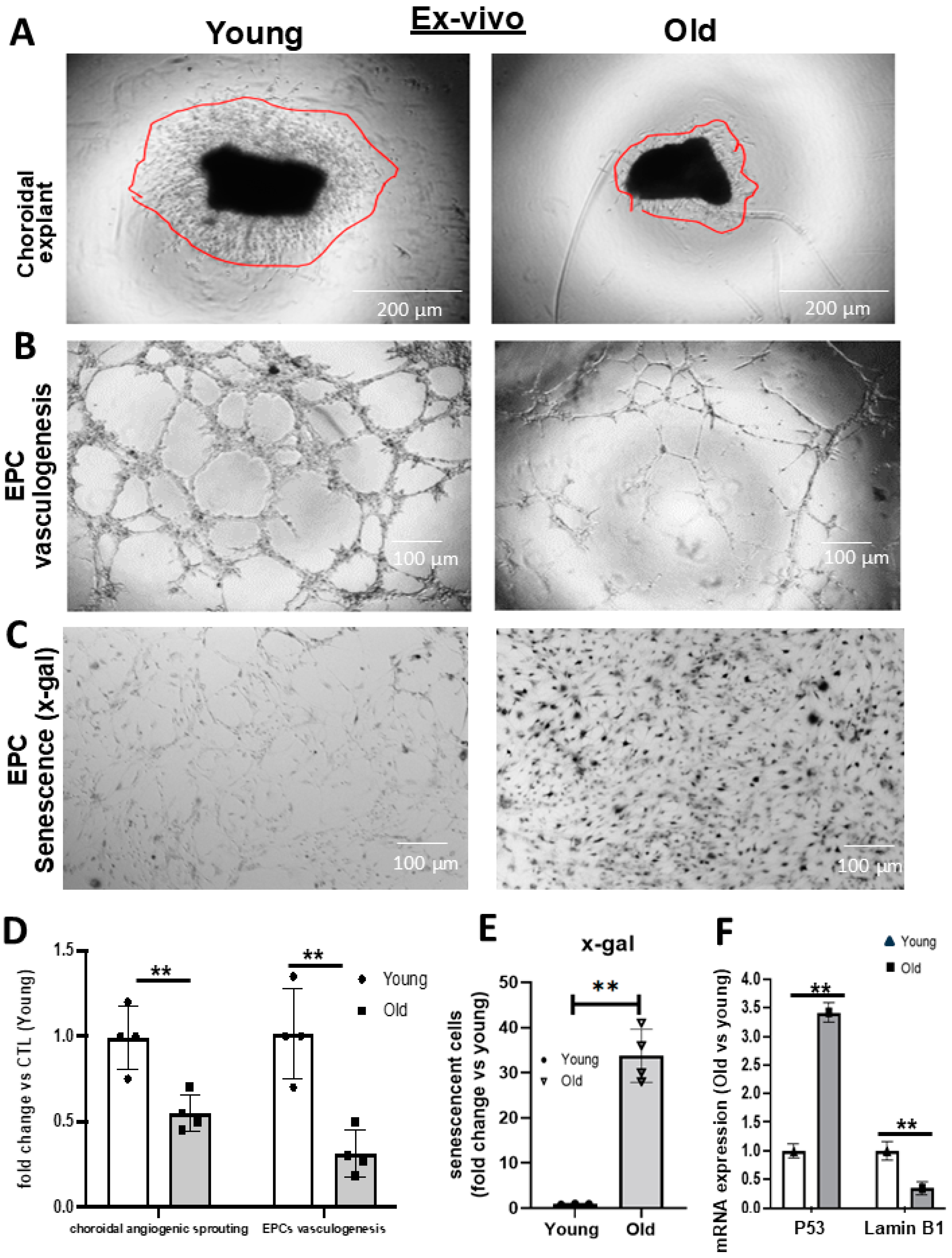
3. Results
Age-Related Alterations in EPC Cellular Pathways
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tisi, A.; Feligioni, M.; Passacantando, M.; Ciancaglini, M.; Maccarone, R. The Impact of Oxidative Stress on Blood-Retinal Barrier Physiology in Age-Related Macular Degeneration. Cells 2021, 10, 64. [Google Scholar] [CrossRef] [PubMed]
- Harris, A.; Bingaman, D.; Ciulla, T.A.; Martin, B. Chapter 5—Retinal and Choroidal Blood Flow in Health and Disease. In Retina, 4th ed.; Ryan, S.J., Hinton, D.R., Schachat, A.P., Wilkinson, C.P., Eds.; Mosby: Edinburgh, UK, 2006; pp. 83–102. [Google Scholar] [CrossRef]
- Desjarlais, M.; Rivera, J.C.; Lahaie, I.; Cagnone, G.; Wirt, M.; Omri, S.; Chemtob, S. MicroRNA expression profile in retina and choroid in oxygen-induced retinopathy model. PLoS ONE 2019, 14, e0218282. [Google Scholar] [CrossRef] [PubMed]
- Fragiotta, S.; Scuderi, L.; Iodice, C.M.; Rullo, D.; Di Pippo, M.; Maugliani, E.; Abdolrahimzadeh, S. Choroidal Vasculature Changes in Age-Related Macular Degeneration: From a Molecular to a Clinical Perspective. Int. J. Mol. Sci. 2022, 23, 12010. [Google Scholar] [CrossRef] [PubMed]
- Zhou, T.E.; Rivera, J.C.; Bhosle, V.K.; Lahaie, I.; Shao, Z.; Tahiri, H.; Zhu, T.; Polosa, A.; Dorfman, A.; Beaudry-Richard, A.; et al. Choroidal Involution Is Associated with a Progressive Degeneration of the Outer Retinal Function in a Model of Retinopathy of Prematurity: Early Role for IL-1β. Am. J. Pathol. 2016, 186, 3100–3116. [Google Scholar] [CrossRef]
- Hassanpour, M.; Salybekov, A.A.; Kobayashi, S.; Asahara, T. CD34 positive cells as endothelial progenitor cells in biology and medicine. Front. Cell Dev. Biol. 2023, 11, 1128134. [Google Scholar] [CrossRef]
- Ma, F.; Morancho, A.; Montaner, J.; Rosell, A. Endothelial progenitor cells and revascularization following stroke. Brain Res. 2015, 1623, 150–159. [Google Scholar] [CrossRef]
- Ruknudin, P.; Nazari, A.R.; Wirth, M.; Lahaie, I.; Bajon, E.; Rivard, A.; Chemtob, S.; Desjarlais, M. Novel Function of Nogo-A as Negative Regulator of Endothelial Progenitor Cell Angiogenic Activity: Impact in Oxygen-Induced Retinopathy. Int. J. Mol. Sci. 2023, 24, 13185. [Google Scholar] [CrossRef]
- Peters, E.B. Endothelial Progenitor Cells for the Vascularization of Engineered Tissues. Tissue Eng. Part B Rev. 2018, 24, 1–24. [Google Scholar] [CrossRef]
- Desjarlais, M.; Dussault, S.; Dhahri, W.; Mathieu, R.; Rivard, A. MicroRNA-150 Modulates Ischemia-Induced Neovascularization in Atherosclerotic Conditions. Arterioscler. Thromb. Vasc. Biol. 2017, 37, 900–908. [Google Scholar] [CrossRef]
- Desjarlais, M.; Ruknudin, P.; Wirth, M.; Lahaie, I.; Dabouz, R.; Rivera, J.C.; Habelrih, T.; Omri, S.; Hardy, P.; Rivard, A.; et al. Tyrosine-Protein Phosphatase Non-receptor Type 9 (PTPN9) Negatively Regulates the Paracrine Vasoprotective Activity of Bone-Marrow Derived Pro-angiogenic Cells: Impact on Vascular Degeneration in Oxygen-Induced Retinopathy. Front. Cell Dev. 2021, 9, 679906. [Google Scholar] [CrossRef]
- Liguori, I.; Russo, G.; Curcio, F.; Bulli, G.; Aran, L.; Della-Morte, D.; Gargiulo, G.; Testa, G.; Cacciatore, F.; Bonaduce, D.; et al. Oxidative stress, aging, and diseases. Clin. Interv. Aging 2018, 13, 757–772. [Google Scholar] [CrossRef] [PubMed]
- Kokame, G.T.; Yannuzzi, N.A.; Shantha, J.G.; Yamane, M.; Relhan, N.; Gross, J.; Ryan, E.H.; Flynn, H.W., Jr. Involution of neovascular age-related macular degeneration after endophthalmitis. Retin. Cases Brief Rep. 2021, 15, 495–499. [Google Scholar] [CrossRef] [PubMed]
- Desjarlais, M.; Dussault, S.; Rivera, J.C.; Chemtob, S.; Rivard, A. MicroRNA Expression Profiling of Bone Marrow-Derived Proangiogenic Cells (PACs) in a Mouse Model of Hindlimb Ischemia: Modulation by Classical Cardiovascular Risk Factors. Front. Genet. 2020, 11, 947. [Google Scholar] [CrossRef] [PubMed]
- Di Micco, R.; Krizhanovsky, V.; Baker, D.; d’Adda di Fagagna, F. Cellular senescence in ageing: From mechanisms to therapeutic opportunities. Nat. Rev. Mol. Cell. Biol. 2021, 22, 75–95. [Google Scholar] [CrossRef]
- Coppé, J.P.; Desprez, P.Y.; Krtolica, A.; Campisi, J. The senescence-associated secretory phenotype: The dark side of tumor suppression. Annu. Rev. Pathol. 2010, 5, 99–118. [Google Scholar] [CrossRef]
- Li, X.; Li, C.; Zhang, W.; Wang, Y.; Qian, P.; Huang, H. Inflammation and aging: Signaling pathways and intervention therapies. Signal Transduct. Target. Ther. 2023, 8, 239. [Google Scholar] [CrossRef]
- Zhou, T.E.; Zhu, T.; Rivera, J.C.; Omri, S.; Tahiri, H.; Lahaie, I.; Rouget, R.; Wirth, M.; Nattel, S.; Lodygensky, G.; et al. The Inability of the Choroid to Revascularize in Oxygen-Induced Retinopathy Results from Increased p53/miR-Let-7b Activity. Am. J. Pathol. 2019, 189, 2340–2356. [Google Scholar] [CrossRef]
- Desjarlais, M.; Wirth, M.; Rivera, J.C.; Lahaie, I.; Dabouz, R.; Omri, S.; Ruknudin, P.; Borras, C.; Chemtob, S. MicroRNA-96 Promotes Vascular Repair in Oxygen-Induced Retinopathy-A Novel Uncovered Vasoprotective Function. Front. Pharmacol. 2020, 11, 13. [Google Scholar] [CrossRef]
- Kuleshov, M.V.; Jones, M.R.; Rouillard, A.D.; Fernandez, N.F.; Duan, Q.; Wang, Z.; Koplev, S.; Jenkins, S.L.; Jagodnik, K.M.; Lachmann, A.; et al. Enrichr: A comprehensive gene set enrichment analysis web server 2016 update. Nucleic Acids Res. 2016, 44, W90–W97. [Google Scholar] [CrossRef]
- Vlachos, I.S.; Zagganas, K.; Paraskevopoulou, M.D.; Georgakilas, G.; Karagkouni, D.; Vergoulis, T.; Dalamagas, T.; Hatzigeorgiou, A.G. DIANA-miRPath v3.0: Deciphering microRNA function with experimental support. Nucleic Acids Res. 2015, 43, W460–W466. [Google Scholar] [CrossRef]
- Yang, D.; Zhou, Q.; Labroska, V.; Qin, S.; Darbalaei, S.; Wu, Y.; Yuliantie, E.; Xie, L.; Tao, H.; Cheng, J.; et al. G protein-coupled receptors: Structure- and function-based drug discovery. Signal Transduct. Target Ther. 2021, 6, 7. [Google Scholar] [CrossRef] [PubMed]
- Pluquet, O.; Pourtier, A.; Abbadie, C. The unfolded protein response and cellular senescence. A review in the theme: Cellular mechanisms of endoplasmic reticulum stress signaling in health and disease. Am. J. Physiol. Cell Physiol. 2015, 308, C415–C425. [Google Scholar] [CrossRef] [PubMed]
- Ren, J.L.; Pan, J.S.; Lu, Y.P.; Sun, P.; Han, J. Inflammatory signaling and cellular senescence. Cell Signal 2009, 21, 378–383. [Google Scholar] [CrossRef] [PubMed]
- Takagi, H. Aging and retinal vascular diseases. Nippon Ganka Gakkai Zasshi 2007, 111, 207–230; discussion 231. [Google Scholar]
- Li, Z.; Zhang, Z.; Ren, Y.; Wang, Y.; Fang, J.; Yue, H.; Ma, S.; Guan, F. Aging and age-related diseases: From mechanisms to therapeutic strategies. Biogerontology 2021, 22, 165–187. [Google Scholar] [CrossRef]
- Werner, N.; Nickenig, G. Influence of cardiovascular risk factors on endothelial progenitor cells: Limitations for therapy? Arterioscler. Thromb. Vasc. Biol. 2006, 26, 257–266. [Google Scholar] [CrossRef]
- Xiao, Q.; Kiechl, S.; Patel, S.; Oberhollenzer, F.; Weger, S.; Mayr, A.; Metzler, B.; Reindl, M.; Hu, Y.; Willeit, J.; et al. Endothelial progenitor cells, cardiovascular risk factors, cytokine levels and atherosclerosis—Results from a large population-based study. PLoS ONE 2007, 2, e975. [Google Scholar] [CrossRef]
- Tousoulis, D.; Andreou, I.; Antoniades, C.; Tentolouris, C.; Stefanadis, C. Role of inflammation and oxidative stress in endothelial progenitor cell function and mobilization: Therapeutic implications for cardiovascular diseases. Atherosclerosis 2008, 201, 236–247. [Google Scholar] [CrossRef]
- Dhahri, W.; Dussault, S.; Légaré, É.; Rivard, F.; Desjarlais, M.; Mathieu, R.; Rivard, A. Reduced expression of microRNA-130a promotes endothelial cell senescence and age-dependent impairment of neovascularization. Aging 2020, 12, 10180–10193. [Google Scholar] [CrossRef]
- Saito, Y.; Yamamoto, S.; Chikenji, T.S. Role of cellular senescence in inflammation and regeneration. Inflamm. Regen. 2024, 44, 28. [Google Scholar] [CrossRef]
- Kundu, N.; Domingues, C.C.; Chou, C.; Ahmadi, N.; Houston, S.; Jerry, D.J.; Sen, S. Use of p53-Silenced Endothelial Progenitor Cells to Treat Ischemia in Diabetic Peripheral Vascular Disease. J. Am. Heart Assoc. 2017, 6, e005146. [Google Scholar] [CrossRef] [PubMed]
- bin Imtiaz, M.K.; Jaeger, B.N.; Bottes, S.; Machado, R.A.C.; Vidmar, M.; Moore, D.L.; Jessberger, S. Declining lamin B1 expression mediates age-dependent decreases of hippocampal stem cell activity. Cell Stem Cell 2021, 28, 967–977.e968. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Lan, W.; Miller, D. Next-Generation Sequencing for MicroRNA Expression Profile. Methods Mol. Biol. 2017, 1617, 169–177. [Google Scholar] [CrossRef]
- Kanehisa, M.; Furumichi, M.; Tanabe, M.; Sato, Y.; Morishima, K. KEGG: New perspectives on genomes, pathways, diseases and drugs. Nucleic Acids Res. 2017, 45, D353–D361. [Google Scholar] [CrossRef]
- De Francesco, E.M.; Sotgia, F.; Clarke, R.B.; Lisanti, M.P.; Maggiolini, M. G Protein-Coupled Receptors at the Crossroad between Physiologic and Pathologic Angiogenesis: Old Paradigms and Emerging Concepts. Int. J. Mol. Sci. 2017, 18, 2713. [Google Scholar] [CrossRef]
- Hu, J.; Li, T.; Du, X.; Wu, Q.; Le, Y.Z. G protein-coupled receptor 91 signaling in diabetic retinopathy and hypoxic retinal diseases. Vis. Res. 2017, 139, 59–64. [Google Scholar] [CrossRef]
- Li, T.; Hu, J.; Gao, F.; Du, X.; Chen, Y.; Wu, Q. Transcription factors regulate GPR91-mediated expression of VEGF in hypoxia-induced retinopathy. Sci. Rep. 2017, 7, 45807. [Google Scholar] [CrossRef]
- Hakim, M.A.; Chum, P.P.; Buchholz, J.N.; Behringer, E.J. Aging Alters Cerebrovascular Endothelial GPCR and K+ Channel Function: Divergent Role of Biological Sex. J. Gerontol. A Biol. Sci. Med. Sci. 2020, 75, 2064–2073. [Google Scholar] [CrossRef]
- Layden, B.T.; Newman, M.; Chen, F.; Fisher, A.; Lowe, W.L., Jr. G protein coupled receptors in embryonic stem cells: A role for Gs-alpha signaling. PLoS ONE 2010, 5, e9105. [Google Scholar] [CrossRef]
- New, D.C.; Wu, K.; Kwok, A.W.; Wong, Y.H. G protein-coupled receptor-induced Akt activity in cellular proliferation and apoptosis. FEBS J. 2007, 274, 6025–6036. [Google Scholar] [CrossRef]
- Kunwar, P.S.; Sano, H.; Renault, A.D.; Barbosa, V.; Fuse, N.; Lehmann, R. Tre1 GPCR initiates germ cell transepithelial migration by regulating Drosophila melanogaster E-cadherin. J. Cell Biol. 2008, 183, 157–168. [Google Scholar] [CrossRef] [PubMed]
- Kremer, K.N.; Clift, I.C.; Miamen, A.G.; Bamidele, A.O.; Qian, N.X.; Humphreys, T.D.; Hedin, K.E. Stromal cell-derived factor-1 signaling via the CXCR4-TCR heterodimer requires phospholipase C-β3 and phospholipase C-γ1 for distinct cellular responses. J. Immunol. 2011, 187, 1440–1447. [Google Scholar] [CrossRef] [PubMed]
- Tilling, L.; Chowienczyk, P.; Clapp, B. Progenitors in motion: Mechanisms of mobilization of endothelial progenitor cells. Br. J. Clin. Pharmacol. 2009, 68, 484–492. [Google Scholar] [CrossRef]
- Rivera, J.C.; Sitaras, N.; Noueihed, B.; Hamel, D.; Madaan, A.; Zhou, T.; Honoré, J.C.; Quiniou, C.; Joyal, J.S.; Hardy, P.; et al. Microglia and interleukin-1β in ischemic retinopathy elicit microvascular degeneration through neuronal semaphorin-3A. Arterioscler. Thromb. Vasc. Biol. 2013, 33, 1881–1891. [Google Scholar] [CrossRef]
- Dabouz, R.; Cheng, C.W.H.; Abram, P.; Omri, S.; Cagnone, G.; Sawmy, K.V.; Joyal, J.S.; Desjarlais, M.; Olson, D.; Weil, A.G.; et al. An allosteric interleukin-1 receptor modulator mitigates inflammation and photoreceptor toxicity in a model of retinal degeneration. J. Neuroinflam. 2020, 17, 359. [Google Scholar] [CrossRef]
- Romaniello, D.; Gelfo, V.; Pagano, F.; Sgarzi, M.; Morselli, A.; Girone, C.; Filippini, D.M.; D’Uva, G.; Lauriola, M. IL-1 and senescence: Friends and foe of EGFR neutralization and immunotherapy. Front. Cell Dev. Biol. 2022, 10, 1083743. [Google Scholar] [CrossRef]
- Frisch, S.M. Interleukin-1α: Novel functions in cell senescence and antiviral response. Cytokine 2022, 154, 155875. [Google Scholar] [CrossRef]
- Lau, L.; Porciuncula, A.; Yu, A.; Iwakura, Y.; David, G. Uncoupling the Senescence-Associated Secretory Phenotype from Cell Cycle Exit via Interleukin-1 Inactivation Unveils Its Protumorigenic Role. Mol. Cell Biol. 2019, 39, e00586-18. [Google Scholar] [CrossRef]
- Robson, R.L.; Westwick, J.; Brown, Z. Interleukin-1-induced IL-8 and IL-6 gene expression and production in human mesangial cells is differentially regulated by cAMP. Kidney Int. 1995, 48, 1767–1777. [Google Scholar] [CrossRef]
- Flak, M.B.; Koenis, D.S.; Gonzalez-Nunez, M.; Chopo-Pizarro, A.; Dalli, J. Deletion of macrophage Gpr101 disrupts their phenotype and function dysregulating host immune responses in sterile and infectious inflammation. Biochem. Pharmacol. 2023, 207, 115348. [Google Scholar] [CrossRef]
- Iwasa, K.; Yamagishi, A.; Yamamoto, S.; Haruta, C.; Maruyama, K.; Yoshikawa, K. GPR137 Inhibits Cell Proliferation and Promotes Neuronal Differentiation in the Neuro2a Cells. Neurochem. Res. 2023, 48, 996–1008. [Google Scholar] [CrossRef] [PubMed]
- Saliminejad, K.; Khorram Khorshid, H.R.; Soleymani Fard, S.; Ghaffari, S.H. An overview of microRNAs: Biology, functions, therapeutics, and analysis methods. J. Cell. Physiol. 2019, 234, 5451–5465. [Google Scholar] [CrossRef] [PubMed]
- Celic, T.; Metzinger-Le Meuth, V.; Six, I.; Massy, Z.A.; Metzinger, L. The mir-221/222 Cluster is a Key Player in Vascular Biology via the Fine-Tuning of Endothelial Cell Physiology. Curr. Vasc. Pharmacol. 2017, 15, 40–46. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Yan, C.; Fan, J.; Hou, Z.; Han, Y. MiR-221-3p targets Hif-1α to inhibit angiogenesis in heart failure. Lab. Investig. 2021, 101, 104–115. [Google Scholar] [CrossRef]
- Zhou, E.; Zou, Y.; Mao, C.; Li, D.; Wang, C.; Zhang, Z. MicroRNA-221 inhibits the transition of endothelial progenitor cells to mesenchymal cells via the PTEN/FoxO3a signaling pathway. Adv. Clin. Exp. Med. 2021, 30, 1263–1270. [Google Scholar] [CrossRef]
- Ren, H.; Guo, Z.; Liu, Y.; Song, C. Stem Cell-derived Exosomal MicroRNA as Therapy for Vascular Age-related Diseases. Aging Dis. 2022, 13, 852–867. [Google Scholar] [CrossRef]
- Liu, Y.; Hu, X.; Hu, L.; Xu, C.; Liang, X. Let-7i-5p enhances cell proliferation, migration and invasion of ccRCC by targeting HABP4. BMC Urol. 2021, 21, 49. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nazari, A.R.; Gresseau, L.; Habelrih, T.; Zia, A.; Lahaie, I.; Er-Reguyeg, Y.; Coté, F.; Annabi, B.; Rivard, A.; Chemtob, S.; et al. Age-Related Choroidal Involution Is Associated with the Senescence of Endothelial Progenitor Cells in the Choroid. Biomedicines 2024, 12, 2669. https://doi.org/10.3390/biomedicines12122669
Nazari AR, Gresseau L, Habelrih T, Zia A, Lahaie I, Er-Reguyeg Y, Coté F, Annabi B, Rivard A, Chemtob S, et al. Age-Related Choroidal Involution Is Associated with the Senescence of Endothelial Progenitor Cells in the Choroid. Biomedicines. 2024; 12(12):2669. https://doi.org/10.3390/biomedicines12122669
Chicago/Turabian StyleNazari, Ali Riza, Loraine Gresseau, Tiffany Habelrih, Aliabbas Zia, Isabelle Lahaie, Yosra Er-Reguyeg, France Coté, Borhane Annabi, Alain Rivard, Sylvain Chemtob, and et al. 2024. "Age-Related Choroidal Involution Is Associated with the Senescence of Endothelial Progenitor Cells in the Choroid" Biomedicines 12, no. 12: 2669. https://doi.org/10.3390/biomedicines12122669
APA StyleNazari, A. R., Gresseau, L., Habelrih, T., Zia, A., Lahaie, I., Er-Reguyeg, Y., Coté, F., Annabi, B., Rivard, A., Chemtob, S., & Desjarlais, M. (2024). Age-Related Choroidal Involution Is Associated with the Senescence of Endothelial Progenitor Cells in the Choroid. Biomedicines, 12(12), 2669. https://doi.org/10.3390/biomedicines12122669






