Pan-Cancer Analysis Reveals the Potential of PLOD1 as a Prognostic and Immune Biomarker for Human Cancer
Abstract
1. Introduction
2. Materials and Methods
2.1. Gene Expression Analysis of PLOD1 at the Pan-Cancer Level
2.2. Prognostic Analysis
2.3. Analysis of the Immune Microenvironment and Immune Cell Infiltration
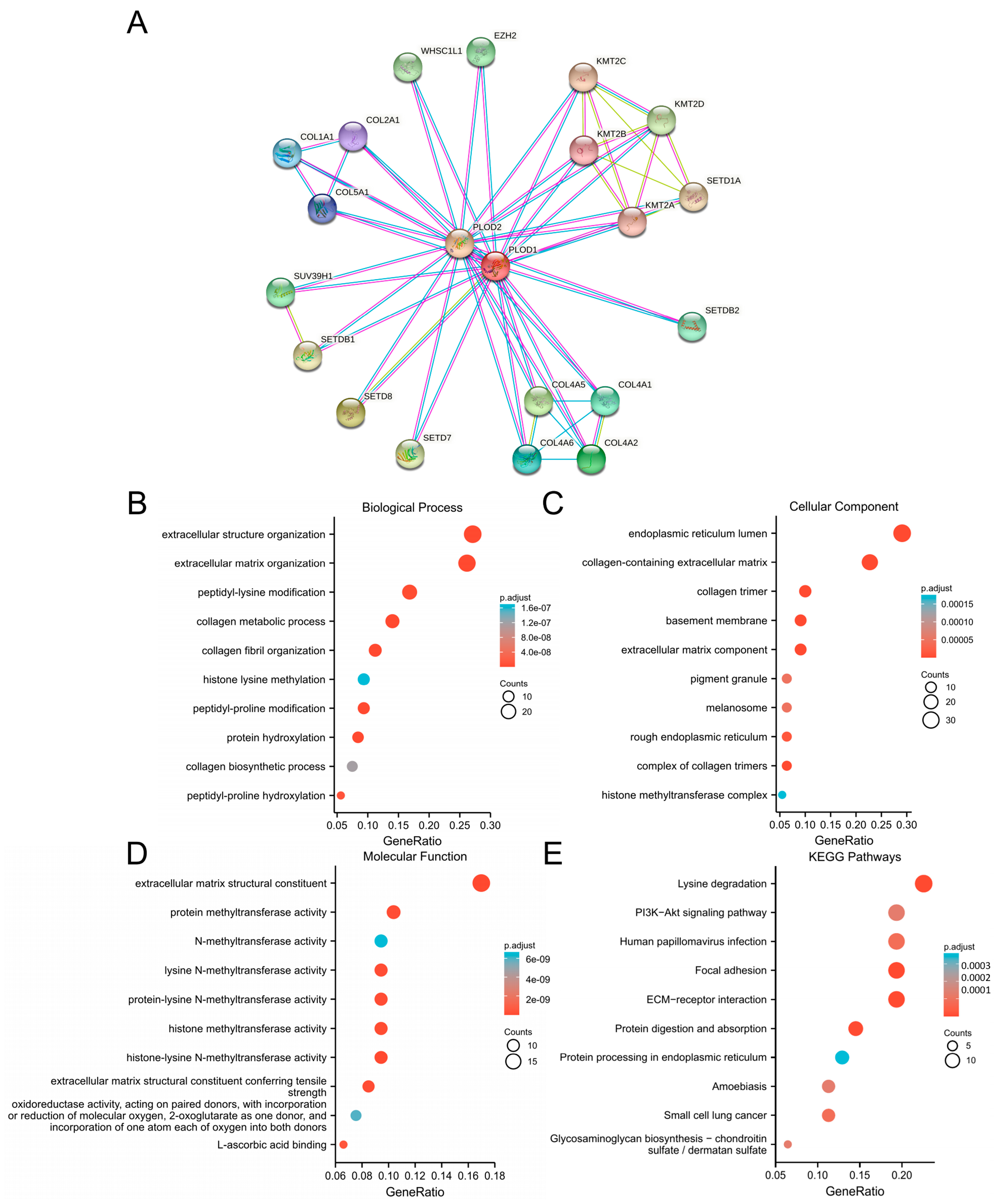
2.4. Enrichment Analysis
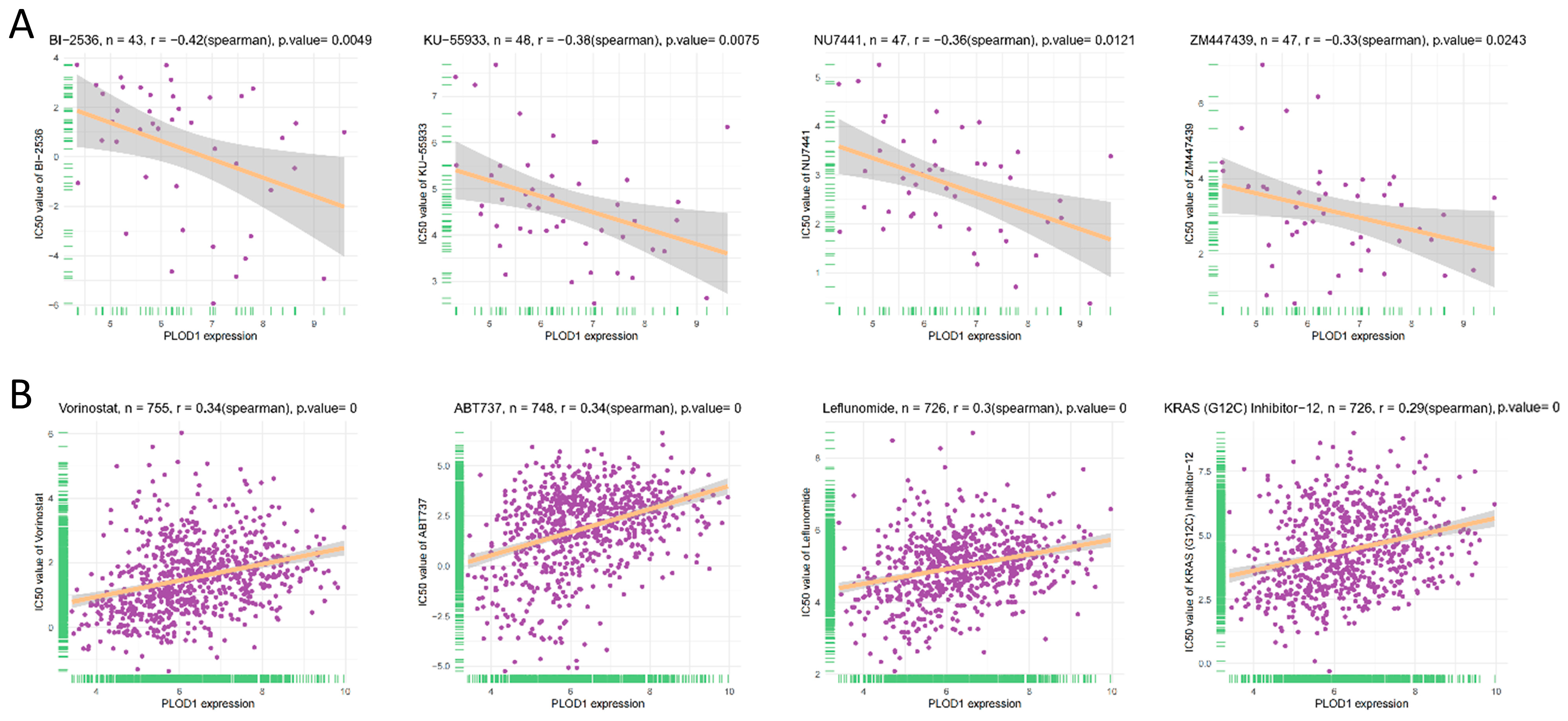
2.5. Correlation Analysis Between PLOD1 Expression and the Half Inhibitory Concentration (IC50) of Anticancer Drugs
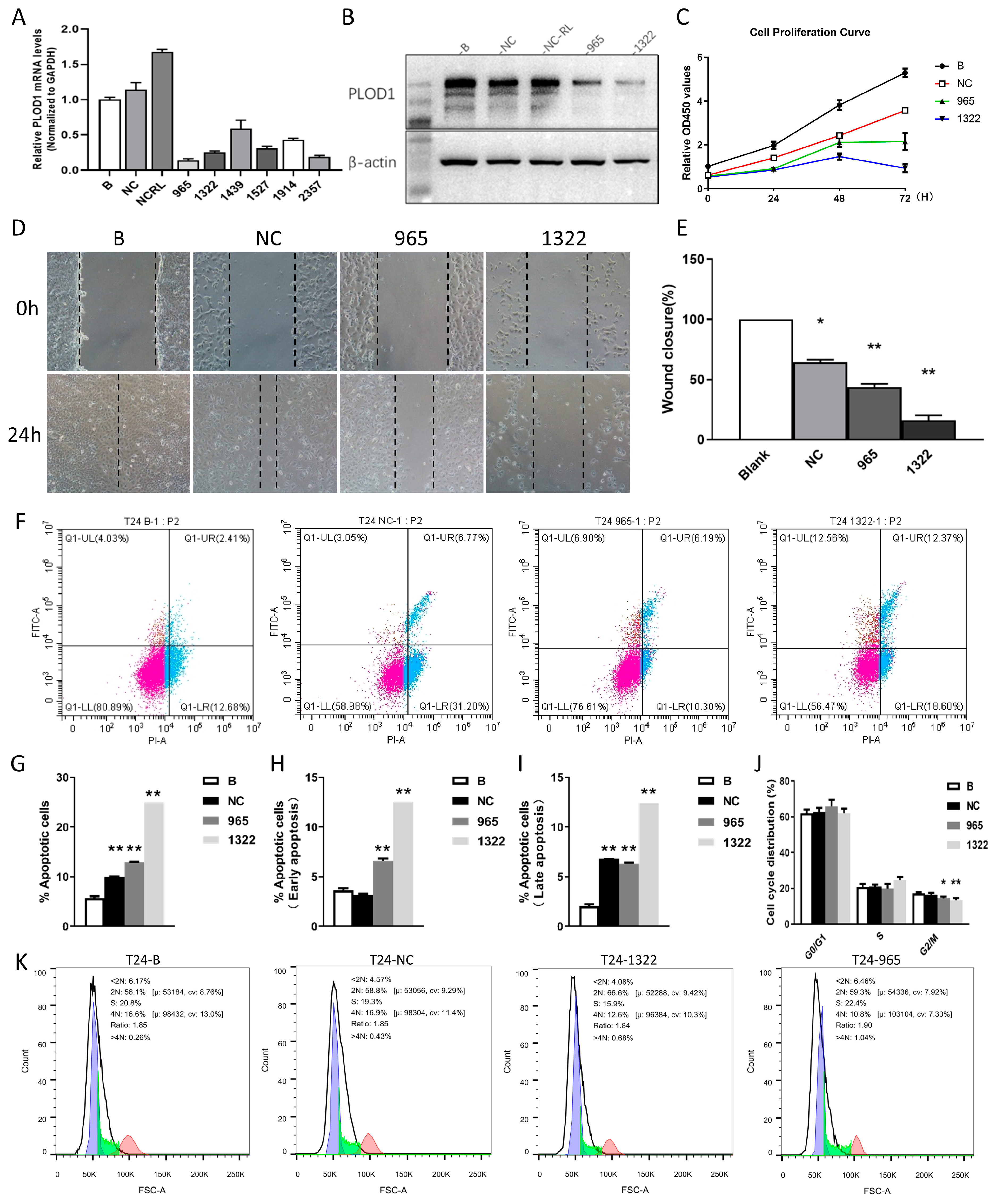
2.6. Cell Culture and siRNA Transfection
2.7. RNA Extraction and Real-Time Quantitative PCR (RT-qPCR)
2.8. Western Blotting
2.9. The CCK-8 Experiment
2.10. The Wound-Healing Experiment
2.11. The Apoptosis Assay and the Cell Cycle Assay
2.12. Statistical Analyses
3. Results
3.1. Aberrant Expression of PLOD1 in Pan-Cancer
3.2. Mutations in PLOD1
3.3. Prognostic Analysis
3.4. Analysis of the TME and Immune Cell Infiltration
3.5. Enrichment Analysis
3.6. Drug Resistance Analysis
3.7. Functional Analysis of PLOD1 in T24 Cells
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA A Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Wagle, N.S.; Jemal, A. Cancer statistics, 2023. CA A Cancer J. Clin. 2023, 73, 17–48. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Severson, E.; Pignon, J.C.; Zhao, H.; Li, T.; Novak, J.; Jiang, P.; Shen, H.; Aster, J.C.; Rodig, S.; et al. Comprehensive analyses of tumor immunity: Implications for cancer immunotherapy. Genome Biol. 2016, 17, 174. [Google Scholar] [CrossRef] [PubMed]
- Binnewies, M.; Roberts, E.W.; Kersten, K.; Chan, V.; Fearon, D.F.; Merad, M.; Coussens, L.M.; Gabrilovich, D.I.; Ostrand-Rosenberg, S.; Hedrick, C.C.; et al. Understanding the tumor immune microenvironment (TIME) for effective therapy. Nat. Med. 2018, 24, 541–550. [Google Scholar] [CrossRef]
- Topalian, S.L.; Forde, P.M.; Emens, L.A.; Yarchoan, M.; Smith, K.N.; Pardoll, D.M. Neoadjuvant immune checkpoint blockade: A window of opportunity to advance cancer immunotherapy. Cancer Cell 2023, 41, 1551–1566. [Google Scholar] [CrossRef]
- Forde, P.M.; Spicer, J.; Lu, S.; Provencio, M.; Mitsudomi, T.; Awad, M.M.; Felip, E.; Broderick, S.R.; Brahmer, J.R.; Swanson, S.J.; et al. Neoadjuvant Nivolumab plus Chemotherapy in Resectable Lung Cancer. N. Engl. J. Med. 2022, 386, 1973–1985. [Google Scholar] [CrossRef]
- Schmid, P.; Cortes, J.; Dent, R.; Pusztai, L.; McArthur, H.; Kümmel, S.; Bergh, J.; Denkert, C.; Park, Y.H.; Hui, R.; et al. Event-free Survival with Pembrolizumab in Early Triple-Negative Breast Cancer. N. Engl. J. Med. 2022, 386, 556–567. [Google Scholar] [CrossRef]
- Lee, J.K.; Sivakumar, S.; Schrock, A.B.; Madison, R.; Fabrizio, D.; Gjoerup, O.; Ross, J.S.; Frampton, G.M.; Napalkov, P.; Montesion, M.; et al. Comprehensive pan-cancer genomic landscape of KRAS altered cancers and real-world outcomes in solid tumors. NPJ Precis. Oncol. 2022, 6, 91. [Google Scholar] [CrossRef]
- Tian, L.; Zhou, H.; Wang, G.; Wang, W.Y.; Li, Y.; Xue, X. The relationship between PLOD1 expression level and glioma prognosis investigated using public databases. PeerJ 2021, 9, e11422. [Google Scholar] [CrossRef]
- Petitprez, F.; Meylan, M.; de Reyniès, A.; Sautès-Fridman, C.; Fridman, W.H. The Tumor Microenvironment in the Response to Immune Checkpoint Blockade Therapies. Front. Immunol. 2020, 11, 784. [Google Scholar] [CrossRef]
- Du, H.; Pang, M.; Hou, X.; Yuan, S.; Sun, L. PLOD2 in cancer research. Biomed. Pharmacother. 2017, 90, 670–676. [Google Scholar] [CrossRef] [PubMed]
- Jover, E.; Silvente, A.; Marín, F.; Martínez-González, J.; Orriols, M.; Martinez, C.M.; Puche, C.M.; Valdés, M.; Rodriguez, C.; Hernández-Romero, D. Inhibition of enzymes involved in collagen cross-linking reduces vascular smooth muscle cell calcification. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2018, 32, 4459–4469. [Google Scholar] [CrossRef] [PubMed]
- Qi, Y.; Xu, R. Roles of PLODs in Collagen Synthesis and Cancer Progression. Front. Cell Dev. Biol. 2018, 6, 66. [Google Scholar] [CrossRef] [PubMed]
- Kurozumi, A.; Kato, M.; Goto, Y.; Matsushita, R.; Nishikawa, R.; Okato, A.; Fukumoto, I.; Ichikawa, T.; Seki, N. Regulation of the collagen cross-linking enzymes LOXL2 and PLOD2 by tumor-suppressive microRNA-26a/b in renal cell carcinoma. Int. J. Oncol. 2016, 48, 1837–1846. [Google Scholar] [CrossRef] [PubMed]
- Giunta, C.; Randolph, A.; Steinmann, B. Mutation analysis of the PLOD1 gene: An efficient multistep approach to the molecular diagnosis of the kyphoscoliotic type of Ehlers-Danlos syndrome (EDS VIA). Mol. Genet. Metab. 2005, 86, 269–276. [Google Scholar] [CrossRef]
- Ganga, M.; Espinoza, H.M.; Cox, C.J.; Morton, L.; Hjalt, T.A.; Lee, Y.; Amendt, B.A. PITX2 isoform-specific regulation of atrial natriuretic factor expression: Synergism and repression with Nkx2.5. J. Biol. Chem. 2003, 278, 22437–22445. [Google Scholar] [CrossRef]
- Rohrbach, M.; Vandersteen, A.; Yiş, U.; Serdaroglu, G.; Ataman, E.; Chopra, M.; Garcia, S.; Jones, K.; Kariminejad, A.; Kraenzlin, M.; et al. Phenotypic variability of the kyphoscoliotic type of Ehlers-Danlos syndrome (EDS VIA): Clinical, molecular and biochemical delineation. Orphanet J. Rare Dis. 2011, 6, 46. [Google Scholar] [CrossRef]
- Li, B.; Yang, H.; Shen, B.; Huang, J.; Qin, Z. Procollagen-lysine, 2-oxoglutarate 5-dioxygenase 1 increases cellular proliferation and colony formation capacity in lung cancer via activation of E2F transcription factor 1. Oncol. Lett. 2021, 22, 851. [Google Scholar] [CrossRef]
- Li, L.; Wang, W.; Li, X.; Gao, T. Association of ECRG4 with PLK1, CDK4, PLOD1 and PLOD2 in esophageal squamous cell carcinoma. Am. J. Transl. Res. 2017, 9, 3741–3748. [Google Scholar]
- Wang, D.; Zhang, S.; Chen, F. High Expression of PLOD1 Drives Tumorigenesis and Affects Clinical Outcome in Gastrointestinal Carcinoma. Genet. Test. Mol. Biomark. 2018, 22, 366–373. [Google Scholar] [CrossRef]
- Geng, F.; Wenzel, S.; Tansey, W.P. Ubiquitin and proteasomes in transcription. Annu. Rev. Biochem. 2012, 81, 177–201. [Google Scholar] [CrossRef] [PubMed]
- Bellí, G.; Colomina, N.; Castells-Roca, L.; Lorite, N.P. Post-Translational Modifications of PCNA: Guiding for the Best DNA Damage Tolerance Choice. J. Fungi 2022, 8, 621. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Jiang, M.; Hu, B.; Fu, B.; Sun, T. Comprehensive Analysis of the Expression, Prognosis, and Biological Significance of PLOD Family in Bladder Cancer. Int. J. Gen. Med. 2023, 16, 707–722. [Google Scholar] [CrossRef]
- Oudin, M.J.; Jonas, O.; Kosciuk, T.; Broye, L.C.; Guido, B.C.; Wyckoff, J.; Riquelme, D.; Lamar, J.M.; Asokan, S.B.; Whittaker, C.; et al. Tumor Cell-Driven Extracellular Matrix Remodeling Drives Haptotaxis during Metastatic Progression. Cancer Discov. 2016, 6, 516–531. [Google Scholar] [CrossRef]
- Sun, L.; Wang, D.; Li, X.; Zhang, L.; Zhang, H.; Zhang, Y. Extracellular matrix protein ITGBL1 promotes ovarian cancer cell migration and adhesion through Wnt/PCP signaling and FAK/SRC pathway. Biomed. Pharmacother. 2016, 81, 145–151. [Google Scholar] [CrossRef]
- Jiang, H.; Guo, W.; Yuan, S.; Song, L. PLOD1 Is a Prognostic Biomarker and Mediator of Proliferation and Invasion in Osteosarcoma. Biomed. Res. Int. 2020, 2020, 3418398. [Google Scholar] [CrossRef]
- Mosele, F.; Remon, J.; Mateo, J.; Westphalen, C.B.; Barlesi, F.; Lolkema, M.P.; Normanno, N.; Scarpa, A.; Robson, M.; Meric-Bernstam, F.; et al. Recommendations for the use of next-generation sequencing (NGS) for patients with metastatic cancers: A report from the ESMO Precision Medicine Working Group. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2020, 31, 1491–1505. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.H.; Xu, Y.; Wang, J.; Tian, X.; Wu, J.; Wan, F.N.; Wang, H.K.; Qu, Y.Y.; Zhang, H.L.; Ye, D.W. Procollagen-lysine, 2-oxoglutarate 5-dioxygenases 1, 2, and 3 are potential prognostic indicators in patients with clear cell renal cell carcinoma. Aging 2019, 11, 6503–6521. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Xiang, H.; Cong, W.; Yang, H.; Zhang, G.; Wang, Y.; Guo, Z.; Shen, Y.; Chen, B. PLOD1, a target of miR-34c, contributes to cell growth and metastasis via repressing LATS1 phosphorylation and inactivating Hippo pathway in osteosarcoma. Biochem. Biophys. Res. Commun. 2020, 527, 29–36. [Google Scholar] [CrossRef]
- Gawel, D.R.; Lee, E.J.; Li, X.; Lilja, S.; Matussek, A.; Schäfer, S.; Olsen, R.S.; Stenmarker, M.; Zhang, H.; Benson, M. An algorithm-based meta-analysis of genome- and proteome-wide data identifies a combination of potential plasma biomarkers for colorectal cancer. Sci. Rep. 2019, 9, 15575. [Google Scholar]
- Zeng, H.; Li, H.; Zhao, Y.; Chen, L.; Ma, X. Transcripto-based network analysis reveals a model of gene activation in tongue squamous cell carcinomas. Head Neck 2019, 41, 4098–4110. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Lu, Q.; Chang, C. Epigenetics in Health and Disease. Adv. Exp. Med. Biol. 2020, 1253, 3–55. [Google Scholar] [PubMed]
- Mehdi, A.; Rabbani, S.A. Role of Methylation in Pro- and Anti-Cancer Immunity. Cancers 2021, 13, 545. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Ngo, V.; Wang, W. Deciphering the genetic code of DNA methylation. Brief. Bioinform. 2021, 22, bbaa424. [Google Scholar] [CrossRef]
- Li, C.; Tang, Y.; Li, Q.; Liu, H.; Ma, X.; He, L.; Shi, H. The prognostic and immune significance of C15orf48 in pan-cancer and its relationship with proliferation and apoptosis of thyroid carcinoma. Front. Immunol. 2023, 14, 1131870. [Google Scholar] [CrossRef]
- Feng, D.; Zhang, F.; Li, D.; Shi, X.; Xiong, Q.; Wei, Q.; Yang, L. Developing an immune-related gene prognostic index associated with progression and providing new insights into the tumor immune microenvironment of prostate cancer. Immunology 2022, 166, 197–209. [Google Scholar] [CrossRef]
- Guo, S.; Deng, C.X. Effect of Stromal Cells in Tumor Microenvironment on Metastasis Initiation. Int. J. Biol. Sci. 2018, 14, 2083–2093. [Google Scholar] [CrossRef] [PubMed]
- Schwab, C.L.; English, D.P.; Roque, D.M.; Pasternak, M.; Santin, A.D. Past, present and future targets for immunotherapy in ovarian cancer. Immunotherapy 2014, 6, 1279–1293. [Google Scholar] [CrossRef]
- Cui, X.; Zhang, X.; Liu, M.; Zhao, C.; Zhang, N.; Ren, Y.; Su, C.; Zhang, W.; Sun, X.; He, J.; et al. A pan-cancer analysis of the oncogenic role of staphylococcal nuclease domain-containing protein 1 (SND1) in human tumors. Genomics 2020, 112, 3958–3967. [Google Scholar] [CrossRef]
- Curtis, M.; Kenny, H.A.; Ashcroft, B.; Mukherjee, A.; Johnson, A.; Zhang, Y.; Helou, Y.; Batlle, R.; Liu, X.; Gutierrez, N.; et al. Fibroblasts Mobilize Tumor Cell Glycogen to Promote Proliferation and Metastasis. Cell Metab. 2019, 29, 141–155.e149. [Google Scholar] [CrossRef]
- Galkina, S.I.; Fedorova, N.V.; Ksenofontov, A.L.; Stadnichuk, V.I.; Baratova, L.A.; Sud’Ina, G.F. Neutrophils as a source of branched-chain, aromatic and positively charged free amino acids. Cell Adhes. Migr. 2019, 13, 98–105. [Google Scholar] [CrossRef] [PubMed]
- Fan, T.; Kuang, G.; Long, R.; Han, Y.; Wang, J. The overall process of metastasis: From initiation to a new tumor. Biochim. Biophys. Acta Rev. Cancer 2022, 1877, 188750. [Google Scholar] [CrossRef] [PubMed]
- Farhood, B.; Najafi, M.; Mortezaee, K. CD8(+) cytotoxic T lymphocytes in cancer immunotherapy: A review. J. Cell. Physiol. 2019, 234, 8509–8521. [Google Scholar] [CrossRef] [PubMed]
- Topalian, S.L.; Drake, C.G.; Pardoll, D.M. Immune checkpoint blockade: A common denominator approach to cancer therapy. Cancer Cell 2015, 27, 450–461. [Google Scholar] [CrossRef]
- Uzawa, K.; Grzesik, W.J.; Nishiura, T.; Kuznetsov, S.A.; Robey, P.G.; Brenner, D.A.; Yamauchi, M. Differential expression of human lysyl hydroxylase genes, lysine hydroxylation, and cross-linking of type I collagen during osteoblastic differentiation in vitro. J. Bone Miner. Res. Off. J. Am. Soc. Bone Miner. Res. 1999, 14, 1272–1280. [Google Scholar] [CrossRef]
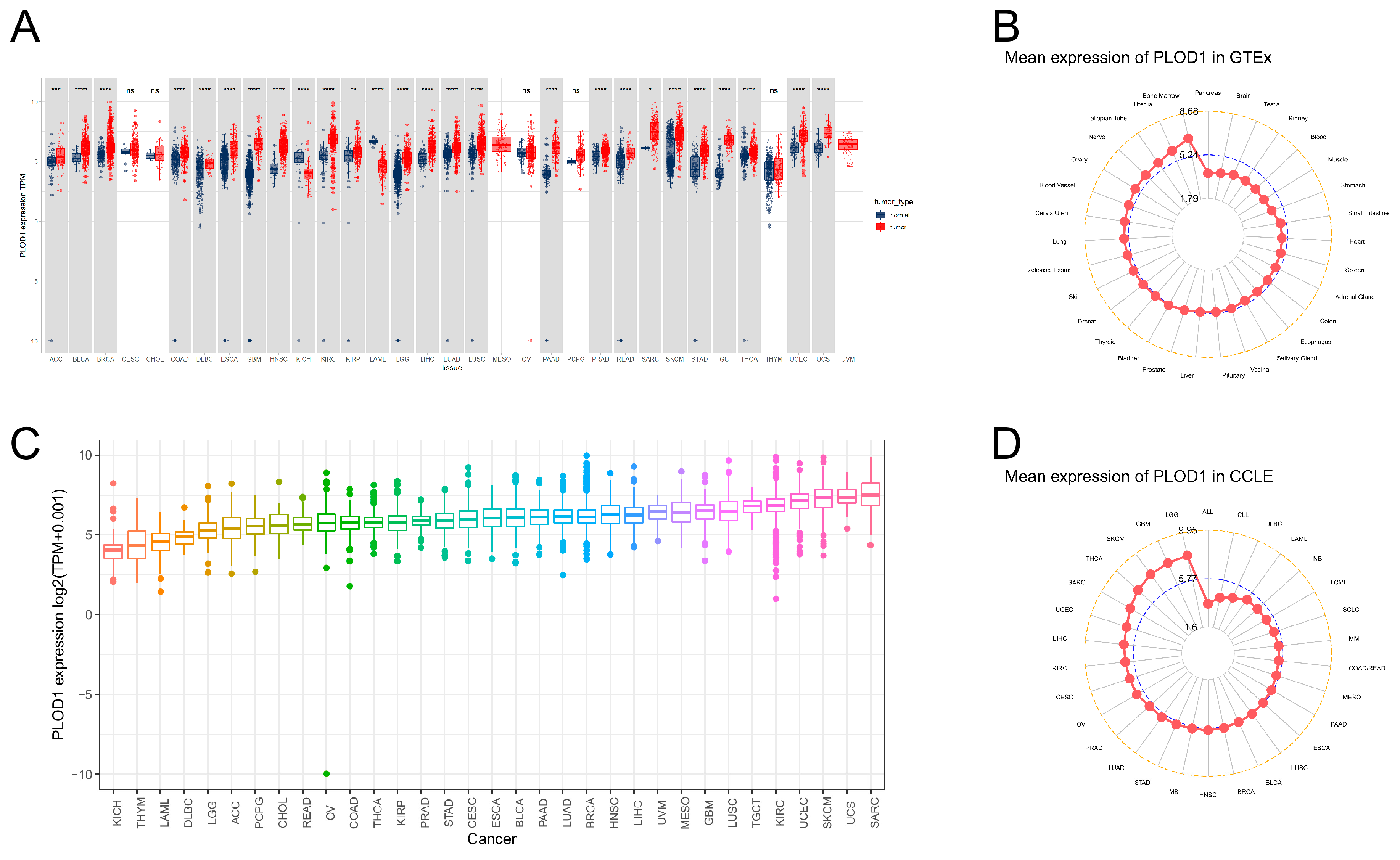
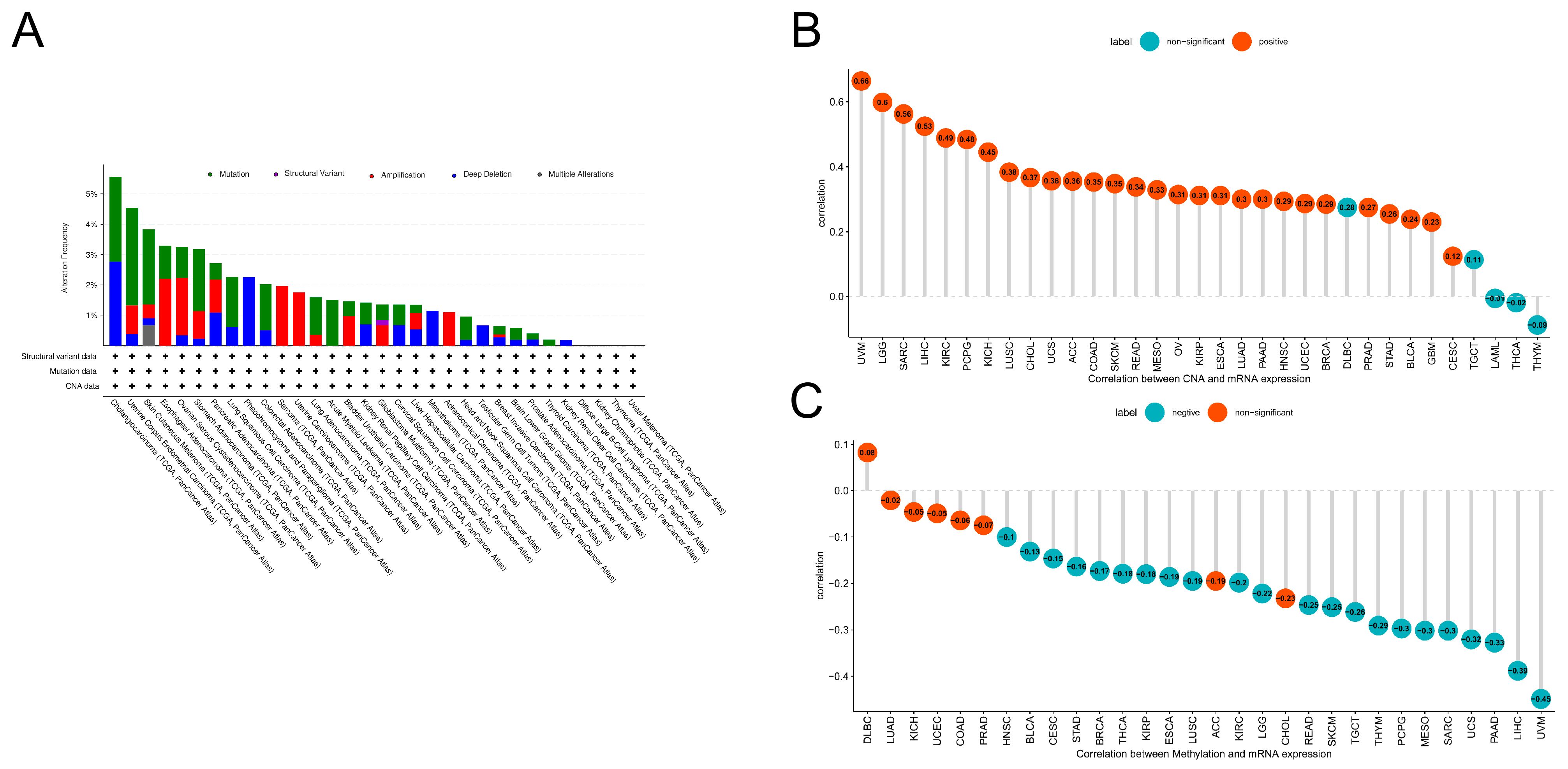

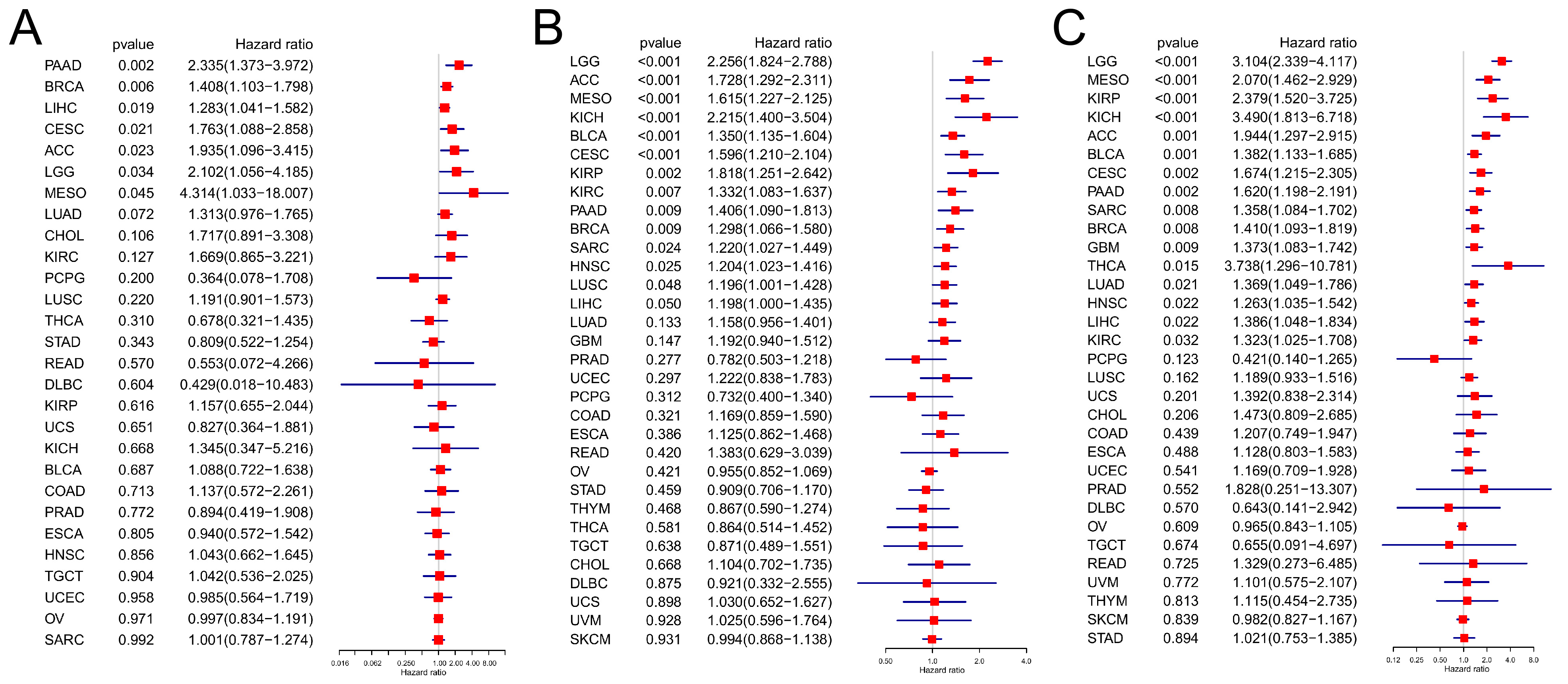
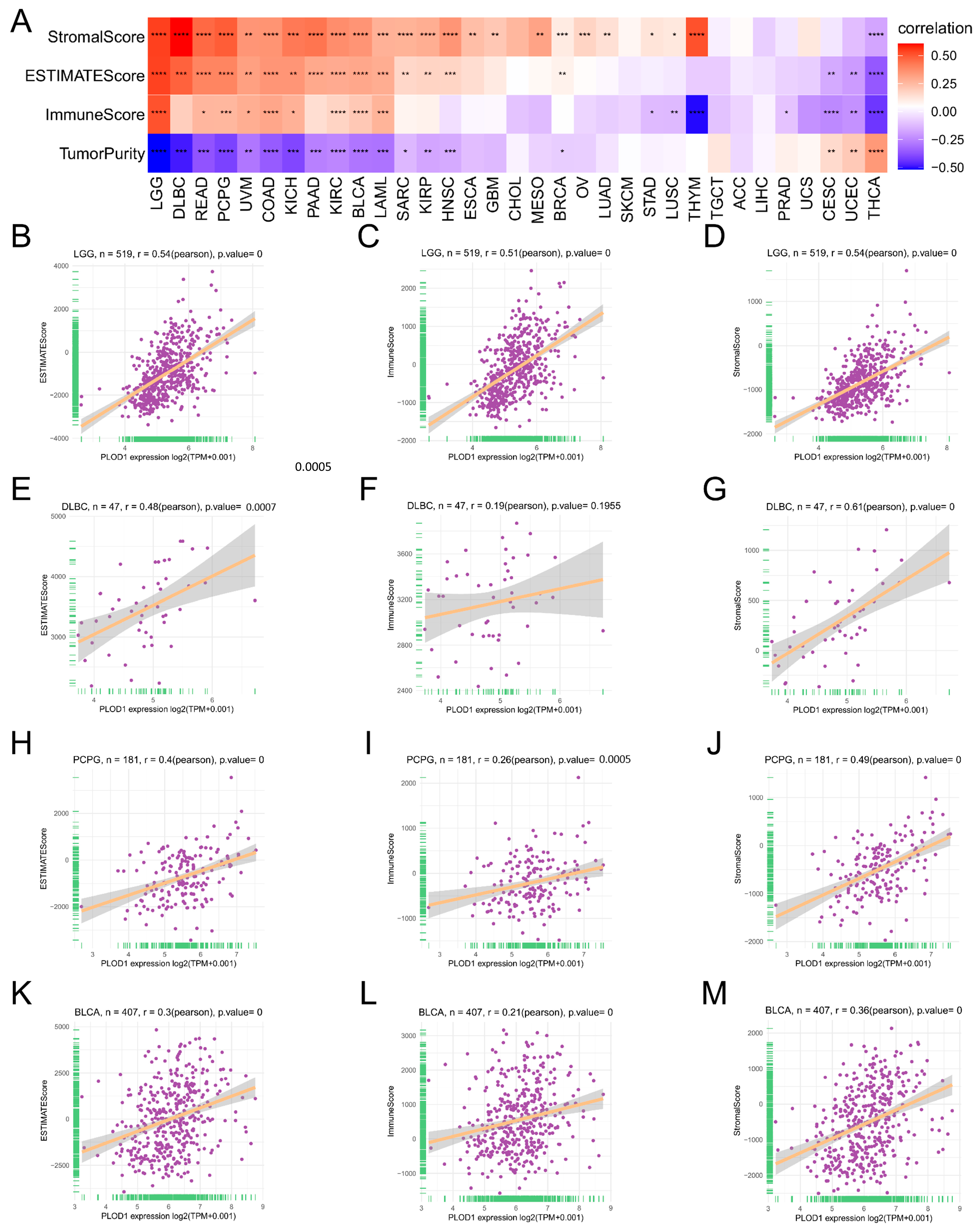


| Characteristics | Low Expression of PLOD1 | High Expression of PLOD1 | p Value |
|---|---|---|---|
| n | 206 | 206 | |
| Pathologic T stage, n (%) | 0.023 | ||
| T1 | 4 (1.1%) | 1 (0.3%) | |
| T2 | 68 (18%) | 50 (13.2%) | |
| T3 | 83 (22%) | 113 (29.9%) | |
| T4 | 32 (8.5%) | 27 (7.1%) | |
| Pathologic stage, n (%) | 0.006 | ||
| Stage I | 4 (1%) | 0 (0%) | |
| Stage II | 77 (18.8%) | 52 (12.7%) | |
| Stage III | 64 (15.6%) | 78 (19%) | |
| Stage IV | 59 (14.4%) | 76 (18.5%) | |
| Gender, n (%) | 0.502 | ||
| Female | 51 (12.4%) | 57 (13.8%) | |
| Male | 155 (37.6%) | 149 (36.2%) | |
| Primary therapy outcome, n (%) | 0.024 | ||
| PD | 25 (7%) | 45 (12.7%) | |
| SD | 14 (3.9%) | 16 (4.5%) | |
| CR | 131 (36.9%) | 102 (28.7%) | |
| PR | 12 (3.4%) | 10 (2.8%) | |
| Age, n (%) | 0.551 | ||
| ≤70 | 119 (28.9%) | 113 (27.4%) | |
| >70 | 87 (21.1%) | 93 (22.6%) | |
| Histologic grade, n (%) | <0.001 | ||
| High-grade | 183 (44.7%) | 205 (50.1%) | |
| Low-grade | 20 (4.9%) | 1 (0.2%) | |
| Smoker, n (%) | 0.279 | ||
| No | 60 (15%) | 49 (12.3%) | |
| Yes | 142 (35.6%) | 148 (37.1%) | |
| OS event, n (%) | <0.001 | ||
| Alive | 132 (32%) | 98 (23.8%) | |
| Dead | 74 (18%) | 108 (26.2%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhai, Z.; Wang, S.; Cao, Y.; Liu, J.; Zhao, Q.; Ji, Y.; Yang, X.; Tang, X.; Ma, J.; Du, P. Pan-Cancer Analysis Reveals the Potential of PLOD1 as a Prognostic and Immune Biomarker for Human Cancer. Biomedicines 2024, 12, 2653. https://doi.org/10.3390/biomedicines12122653
Zhai Z, Wang S, Cao Y, Liu J, Zhao Q, Ji Y, Yang X, Tang X, Ma J, Du P. Pan-Cancer Analysis Reveals the Potential of PLOD1 as a Prognostic and Immune Biomarker for Human Cancer. Biomedicines. 2024; 12(12):2653. https://doi.org/10.3390/biomedicines12122653
Chicago/Turabian StyleZhai, Zhao, Shuo Wang, Yudong Cao, Jia Liu, Qiang Zhao, Yongpeng Ji, Xiao Yang, Xingxing Tang, Jinchao Ma, and Peng Du. 2024. "Pan-Cancer Analysis Reveals the Potential of PLOD1 as a Prognostic and Immune Biomarker for Human Cancer" Biomedicines 12, no. 12: 2653. https://doi.org/10.3390/biomedicines12122653
APA StyleZhai, Z., Wang, S., Cao, Y., Liu, J., Zhao, Q., Ji, Y., Yang, X., Tang, X., Ma, J., & Du, P. (2024). Pan-Cancer Analysis Reveals the Potential of PLOD1 as a Prognostic and Immune Biomarker for Human Cancer. Biomedicines, 12(12), 2653. https://doi.org/10.3390/biomedicines12122653







