Tumor Suppressor miR-27a-5p and Its Significance for Breast Cancer
Abstract
1. Introduction
2. Material and Methods
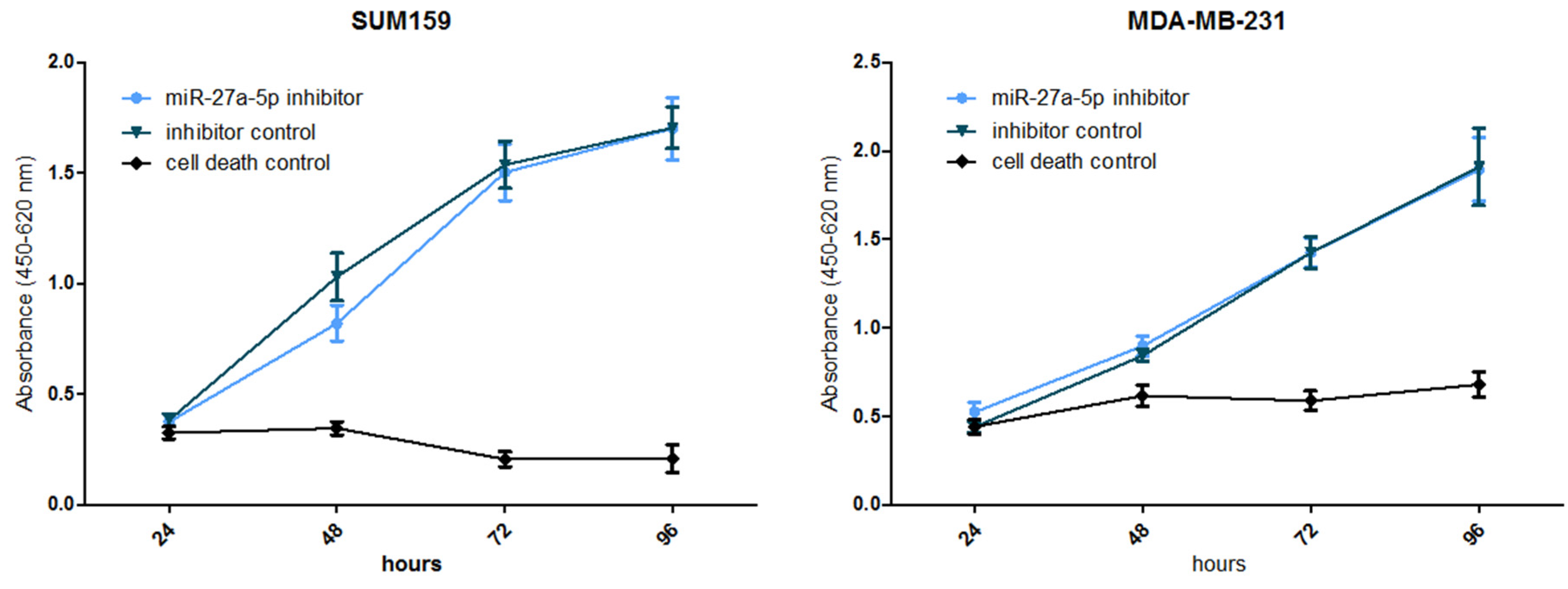
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Benitez Fuentes, J.D.; Morgan, E.; de Luna Aguilar, A.; Mafra, A.; Shah, R.; Giusti, F.; Vignat, J.; Znaor, A.; Musetti, C.; Yip, C.-H.; et al. Global Stage Distribution of Breast Cancer at Diagnosis: A Systematic Review and Meta-Analysis. JAMA Oncol. 2024, 10, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Gomis, R.R.; Gawrzak, S. Tumor cell dormancy. Mol. Oncol. 2017, 11, 62–78. [Google Scholar] [CrossRef] [PubMed]
- Voets, M.M.; Hassink, N.S.; Veltman, J.; Slump, C.H.; Koffijberg, H.; Siesling, S. Opportunities for personalised follow-up in breast cancer: The gap between daily practice and recurrence risk. Breast Cancer Res. Treat. 2024, 205, 313–322. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, R.N.; Esen, B.Ö.; Mellemkjær, L.; Christiansen, P.; Ejlertsen, B.; Lash, T.L.; Nørgaard, M.; Cronin-Fenton, D. The Incidence of Breast Cancer Recurrence 10–32 Years After Primary Diagnosis. J. Natl. Cancer Inst. 2022, 114, 391–399. [Google Scholar] [CrossRef]
- Riggio, A.I.; Varley, K.E.; Welm, A.L. The lingering mysteries of metastatic recurrence in breast cancer. Br. J. Cancer 2021, 124, 13–26. [Google Scholar] [CrossRef]
- Duff, M.J.; Harbeck, N.; Nap, M.; Molina, R.; Nicolini, A.; Senkus, E.; Cardoso, F. Clinical use of biomarkers in breast cancer: Updated guidelines from the European Group on Tumor Markers (EGTM). Eur. J. Cancer 2017, 75, 284–298. [Google Scholar] [CrossRef]
- Perou, C.M.; Sørlie, T.; Eisen, M.B.; van de Rijn, M.; Jeffrey, S.S.; Rees, C.A.; Pollack, J.R.; Ross, D.T.; Johnsen, H.; Akslen, L.A.; et al. Molecular portraits of human breast tumors. Nature 2000, 406, 747–752. [Google Scholar] [CrossRef]
- Courtney, D.; Davey, M.G.; Moloney, B.M.; Barry, M.K.; Sweeney, K.; McLaughlin, R.P.; Malone, C.M.; Lowery, A.J.; Kerin, M.J. Breast cancer recurrence: Factors impacting occurrence and survival. Ir. J. Med. Sci. 2022, 191, 2501–2510. [Google Scholar] [CrossRef]
- Grinda, T.; Antoine, A.; Jacot, W.; Cottu, P.-H.; Rouge, T.d.l.M.; Frenel, J.-S.; Mailliez, A.; Dalenc, F.; Goncalves, A.; Clatot, F.; et al. Real-world clinical and survival outcomes of patients with early relapsed triple-negative breast cancer from the ESME national cohort. Eur. J. Cancer 2023, 189, 112935. [Google Scholar] [CrossRef]
- Leone, J.P.; Vallejo, C.T.; Hassett, M.J.; Leone, J.; Graham, N.; Tayob, N.; Freedman, R.A.; Tolaney, S.M.; Leone, B.A.; Winer, E.P.; et al. Factors associated with late risks of breast cancer-specific mortality in the SEER registry. Breast Cancer Res. Treat. 2021, 189, 203–212. [Google Scholar] [CrossRef] [PubMed]
- Van Mechelen, M.; Van Herck, A.; Punie, K.; Nevelsteen, I.; Smeets, A.; Neven, P.; Weltens, C.; Han, S.; Vanderstichele, A.; Floris, G.; et al. Behavior of metastatic breast cancer according to subtype. Breast Cancer Res. Treat. 2020, 181, 115–125. [Google Scholar] [CrossRef] [PubMed]
- Dexheimer, P.J.; Cochella, L. MicroRNAs: From Mechanism to Organism. Front. Cell Dev. Biol. 2020, 8, 409. [Google Scholar] [CrossRef] [PubMed]
- Berindan-Neagoe, I.; Monroig Pdel, C.; Pasculli, B.; Calin, G.A. MicroRNAome genome: A treasure for cancer diagnosis and therapy. CA Cancer J. Clin. 2014, 64, 311–336. [Google Scholar] [CrossRef]
- Pasculli, B.; Barbano, R.; Parrella, P. Epigenetics of breast cancer: Biology and clinical implication in the era of precision medicine. Semin. Cancer Biol. 2018, 51, 22–35. [Google Scholar] [CrossRef]
- Abdul Manap, A.S.; Wisham, A.A.; Wong, F.W.; Ahmad Najmi, H.R.; Ng, Z.F.; Diba, R.S. Mapping the function of MicroRNAs as a critical regulator of tumor-immune cell communication in breast cancer and potential treatment strategies. Front. Cell Dev. Biol. 2024, 12, 1390704. [Google Scholar] [CrossRef]
- Schwarzenbacher, D.; Klec, C.; Pasculli, B.; Cerk, S.; Rinner, B.; Karbiener, M.; Ivan, C.; Barbano, R.; Ling, H.; Wulf-Goldenberg, A.; et al. MiR-1287-5p inhibits triple negative breast cancer growth by interaction with phosphoinositide 3-kinase CB, thereby sensitizing cells for PI3Kinase inhibitors. Breast Cancer Res. 2019, 21, 20. [Google Scholar] [CrossRef]
- Cancer Genome Atlas Network. Comprehensive molecular portraits of human breast tumors. Nature 2012, 490, 61–70. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Pasculli, B.; Barbano, R.; Fontana, A.; Biagini, T.; Di Viesti, M.P.; Rendina, M.; Valori, V.M.; Morritti, M.; Bravaccini, S.; Ravaioli, S.; et al. Hsa-miR-155-5p Up-Regulation in Breast Cancer and Its Relevance for Treatment with Poly[ADP-Ribose] Polymerase 1 (PARP-1) Inhibitors. Front. Oncol. 2020, 10, 1415. [Google Scholar] [CrossRef]
- Lampugnani, M.G. Cell migration into a wounded area in vitro. Methods Mol. Biol. 1999, 96, 177–182. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, L.G.; Wu, X.; Guan, J.L. Wound-healing assay. Methods Mol. Biol. 2005, 294, 23–29. [Google Scholar] [CrossRef] [PubMed]
- Liang, C.C.; Park, A.; Guan, J.L. In vitro scratch assay: A convenient and inexpensive method for analysis of cell migration in vitro. Nat. Protoc. 2007, 2, 329–333. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Decker, C.C.; Zechner, L.; Krstin, S.; Wink, M. In vitro wound healing of tumor cells: Inhibition of cell migration by selected cytotoxic alkaloids. BMC Pharmacol. Toxicol. 2019, 20, 4. [Google Scholar] [CrossRef] [PubMed]
- McShane, L.M.; Altman, D.G.; Sauerbrei, W.; Taube, S.E.; Gion, M.; Clark, G.M. Statistics Subcommittee of the NCI-EORTC Working Group on Cancer Diagnostics. Reporting recommendations for tumor marker prognostic studies (REMARK). J. Natl. Cancer Inst. 2005, 97, 1180–1184. [Google Scholar] [CrossRef]
- Allison, K.H.; Hammond, M.E.H.; Dowsett, M.; McKernin, S.E.; Carey, L.A.; Fitzgibbons, P.L.; Hayes, D.F.; Lakhani, S.R.; Chavez-MacGregor, M.; Perlmutter, J.; et al. Estrogen and Progesterone Receptor Testing in Breast Cancer: American Society of Clinical Oncology/College of American Pathologists Guideline Update. Arch. Pathol. Lab. Med. 2020, 144, 545–563. [Google Scholar] [CrossRef]
- Wolff, A.C.; Hammond, M.E.H.; Allison, K.H.; Harvey, B.E.; Mangu, P.B.; Bartlett, J.M.S.; Bilous, M.; Ellis, I.O.; Fitzgibbons, P.; Hanna, W.; et al. Human Epidermal Growth Factor Receptor 2 Testing in Breast Cancer: American Society of Clinical Oncology/College of American Pathologists Clinical Practice Guideline Focused Update. J. Clin. Oncol. 2018, 36, 2105–2122. [Google Scholar] [CrossRef]
- Edge, S.B.; Byrd, D.R.; Compton, C.C.; Fritz, A.G.; Greene, F.L.; Trotti, A. (Eds.) AJCC Cancer Staging Manual, 7th ed.; Springer: New York, NY, USA, 2010. [Google Scholar]
- Barbano, R.; Pasculli, B.; Rendina, M.; Fontana, A.; Fusilli, C.; Copetti, M.; Castellana, S.; Valori, V.M.; Morritti, M.; Graziano, P.; et al. Stepwise analysis of MIR9 loci identifies miR-9-5p to be involved in Oestrogen regulated pathways in breast cancer patients. Sci. Rep. 2017, 7, 45283. [Google Scholar] [CrossRef]
- He, H.; Song, F.; Gao, Q.; Lu, Z.; Yuan, Y.; Li, X.; Chen, L.; Jia, C.; Yang, R.; Yang, J.; et al. The APEX1/miRNA-27a-5p axis plays key roles in progression, metastasis and targeted chemotherapy of gastric cancer. Int. J. Pharm. 2021, 599, 120446. [Google Scholar] [CrossRef]
- Barros-Silva, D.; Costa-Pinheiro, P.; Duarte, H.; Sousa, E.J.; Evangelista, A.F.; Graça, I.; Carneiro, I.; Martins, A.T.; Oliveira, J.; Carvalho, A.L.; et al. MicroRNA-27a-5p regulation by promoter methylation and MYC signaling in prostate carcinogenesis. Cell Death Dis. 2018, 9, 167. [Google Scholar] [CrossRef]
- Mizuno, K.; Mataki, H.; Arai, T.; Okato, A.; Kamikawaji, K.; Kumamoto, T.; Hiraki, T.; Hatanaka, K.; Inoue, H.; Seki, N. The microRNA expression signature of small cell lung cancer: Tumor suppressors of miR-27a-5p and miR-34b-3p and their targeted oncogenes. J. Hum. Genet. 2017, 62, 671–678. [Google Scholar] [CrossRef] [PubMed]
- Chari, N.S.; Ivan, C.; Le, X.; Li, J.; Mijiti, A.; Patel, A.A.; Osman, A.A.; Peterson, C.B.; Williams, M.D.; Pickering, C.R.; et al. Disruption of TP63-miR-27a* Feedback Loop by Mutant TP53 in Head and Neck Cancer. J. Natl. Cancer Inst. 2020, 112, 266–277. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Che, W.; Liang, N.; Deng, S.; Song, Z.; Yang, L. Silent FOSL1 Enhances the Radiosensitivity of Glioma Stem Cells by Down-Regulating miR-27a-5p. Neurochem. Res. 2021, 46, 3222–3246. [Google Scholar] [CrossRef] [PubMed]
- Ueda, S.; Takanashi, M.; Sudo, K.; Kanekura, K.; Kuroda, M. miR-27a ameliorates chemoresistance of breast cancer cells by disruption of reactive oxygen species homeostasis and impairment of autophagy. Lab. Investig. 2020, 100, 863–873. [Google Scholar] [CrossRef] [PubMed]
- de Resende, M.F.; Vieira, S.; Chinen, L.T.; Chiappelli, F.; da Fonseca, F.P.; Guimarães, G.C.; Soares, F.A.; Neves, I.; Pagotty, S.; Pellionisz, P.A.; et al. Prognostication of prostate cancer based on TOP2A protein and gene assessment: TOP2A in prostate cancer. J. Transl. Med. 2013, 11, 36. [Google Scholar] [CrossRef]
- Kuner, R.; Fälth, M.; Pressinotti, N.C.; Brase, J.C.; Puig, S.B.; Metzger, J.; Gade, S.; Schäfer, G.; Bartsch, G.; Steiner, E.; et al. The maternal embryonic leucine zipper kinase (MELK) is upregulated in high-grade prostate cancer. J. Mol. Med. 2013, 91, 237–248. [Google Scholar] [CrossRef]
- Aytes, A.; Mitrofanova, A.; Lefebvre, C.; Alvarez, M.J.; Castillo-Martin, M.; Zheng, T.; Eastham, J.A.; Gopalan, A.; Pienta, K.J.; Shen, M.M.; et al. Cross-species regulatory network analysis identifies a synergistic interaction between FOXM1 and CENPF that drives prostate cancer malignancy. Cancer Cell 2014, 25, 638–651. [Google Scholar] [CrossRef]


| Sample | Group | miR-27a-5p Median [IQR] | Test for Linear Trend (on Log Values) | |
|---|---|---|---|---|
| R | p-Value | |||
| Overall (N = 256) | 0- NBT (N = 13) | 2.28 [1.50–5.40] | −0.13 | 0.038 |
| 1- Non-invasive tumors (N = 11) | 3.32 [1.68–4.32] | |||
| 2- M0 (N = 178) | 2.17 [1.19–3.64] | |||
| 3- M0->M1 (N = 45) | 1.83 [1.29–3.17] | |||
| 4- M1: Synchronous metastasis (N = 9) | 1.03 [0.83–1.58] | |||
| Clinical Variable | Level | Number of Patients # | Median [IQR] of miR-27a-5p or Correlation Coefficient (R) | p-Value * |
|---|---|---|---|---|
| Age (years) | R correlation coefficient | 242 | R = −0.02 | 0.719 |
| Menopause—N (%) | No | 72 | 2.24 (1.26–3.33) | 0.594 |
| Yes | 164 | 1.94 (1.10–3.76) | ||
| TH—N (%) | IDC | 212 | 2.28 (1.20–3.78) | 0.101 |
| ILC | 14 | 1.26 (0.70–1.95) | ||
| IDC + ILC | 5 | 1.33 (1.23–1.58) | ||
| Others | 11 | 3.32 (1.68–4.32) | ||
| Site of onset—N (%) | Right | 113 | 1.76 (1.18–3.63) | 0.402 |
| Left | 117 | 2.34 (1.14–3.58) | ||
| Bilateral | 1 | 2.78 (2.78–2.78) | ||
| Type of Surgery—N (%) | MAST + LND | 125 | 2.65 (1.30–4.31) | 0.003 |
| MAST + SN | 28 | 2.26 (1.63–3.79) | ||
| QUADR | 4 | 2.18 (1.38–3.30) | ||
| QUADR + LND | 54 | 1.49 (0.78–2.10) | ||
| QUADR + SN | 20 | 1.88 (1.16–2.70) | ||
| Tumor size (cm) | R correlation coefficient | 231 | R = 0.01 | 0.923 |
| T Classification—N (%) | T1c | 61 | 1.93 (1.14–3.33) | 0.995 |
| T2 | 124 | 1.96 (1.16–3.39) | ||
| T3 | 13 | 1.82 (1.33–3.68) | ||
| T4 | 33 | 2.32 (1.30–4.31) | ||
| N classification—N (%) | N0 | 94 | 1.93 (1.19–3.33) | 0.003 |
| N1 | 77 | 2.68 (1.48–4.31) | ||
| N2 | 26 | 1.40 (1.02–2.37) | ||
| N3 | 35 | 1.56 (0.83–3.17) | ||
| Lymph node status—N (%) | Negative | 94 | 1.93 (1.19–3.33) | 0.944 |
| Positive | 148 | 2.14 (1.11–3.78) | ||
| M classification—N (%) | M0 | 222 | 2.11 (1.21–3.61) | 0.240 |
| M1 | 9 | 1.03 (0.83–1.58) | ||
| Tumor stage—N (%) | 1 | 35 | 1.77 (0.85–3.14) | 0.028 |
| 2A | 82 | 2.28 (1.23–3.25) | ||
| 2B | 37 | 2.61 (1.55–3.95) | ||
| 3A | 15 | 1.64 (1.08–2.37) | ||
| 3B | 23 | 3.58 (1.49–5.19) | ||
| 3C | 32 | 1.65 (0.83–3.17) | ||
| 4 | 7 | 1.03 (0.83–1.58) | ||
| Tumor grading—N (%) | 1 | 23 | 2.41 (1.16–3.81) | 0.618 |
| 2 | 119 | 1.96 (1.22–3.64) | ||
| 3 | 89 | 1.83 (1.14–3.60) | ||
| ERc—N (%) | Negative | 53 | 1.91 (1.14–3.95) | 0.637 |
| Positive | 178 | 1.96 (1.19–3.58) | ||
| PgR—N (%) | Negative | 71 | 1.83 (1.18–3.43) | 0.896 |
| Positive | 160 | 2.15 (1.12–3.64) | ||
| HER2—N (%) | AMP | 54 | 2.02 (1.16–3.51) | 0.990 |
| NEG | 165 | 1.94 (1.17–3.60) | ||
| Ki67 | R correlation coefficient | 216 | R = 0.166 | 0.015 |
| OT—N (%) | No | 54 | 1.86 (1.14–3.07) | 0.988 |
| Yes | 159 | 2.12 (1.21–3.61) | ||
| CT-ADJ—N (%) | No | 40 | 2.36 (1.73–3.40) | 0.421 |
| Yes | 171 | 1.82 (1.11–3.60) | ||
| RT—N (%) | No | 55 | 2.44 (1.19–3.43) | 0.660 |
| Yes | 152 | 1.80 (1.16–3.59) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Parrella, P.; Barbano, R.; Jonas, K.; Fontana, A.; Barile, S.; Rendina, M.; lo Mele, A.; Prencipe, G.; Ciuffreda, L.; Morritti, M.G.; et al. Tumor Suppressor miR-27a-5p and Its Significance for Breast Cancer. Biomedicines 2024, 12, 2625. https://doi.org/10.3390/biomedicines12112625
Parrella P, Barbano R, Jonas K, Fontana A, Barile S, Rendina M, lo Mele A, Prencipe G, Ciuffreda L, Morritti MG, et al. Tumor Suppressor miR-27a-5p and Its Significance for Breast Cancer. Biomedicines. 2024; 12(11):2625. https://doi.org/10.3390/biomedicines12112625
Chicago/Turabian StyleParrella, Paola, Raffaela Barbano, Katharina Jonas, Andrea Fontana, Serena Barile, Michelina Rendina, Antonio lo Mele, Giuseppina Prencipe, Luigi Ciuffreda, Maria Grazia Morritti, and et al. 2024. "Tumor Suppressor miR-27a-5p and Its Significance for Breast Cancer" Biomedicines 12, no. 11: 2625. https://doi.org/10.3390/biomedicines12112625
APA StyleParrella, P., Barbano, R., Jonas, K., Fontana, A., Barile, S., Rendina, M., lo Mele, A., Prencipe, G., Ciuffreda, L., Morritti, M. G., Valori, V. M., Graziano, P., Maiello, E., Copetti, M., Pichler, M., & Pasculli, B. (2024). Tumor Suppressor miR-27a-5p and Its Significance for Breast Cancer. Biomedicines, 12(11), 2625. https://doi.org/10.3390/biomedicines12112625







