Altered PLCβ/IP3/Ca2+ Signaling Pathway Activated by GPRCs in Olfactory Neuronal Precursor Cells Derived from Patients Diagnosed with Schizophrenia
Abstract
1. Introduction
2. Materials and Methods
2.1. Human Olfactory Neural Precursor Cells
2.2. Protein Detection by Immunofluorescence
2.3. Quantification of Cytosolic Calcium Concentration by Microfluorometry
2.4. Calcium Imaging by Fluorescence Microscopy
2.5. Measurement of IP3 Concentration by ELISA
2.6. Statistical Analysis
3. Results
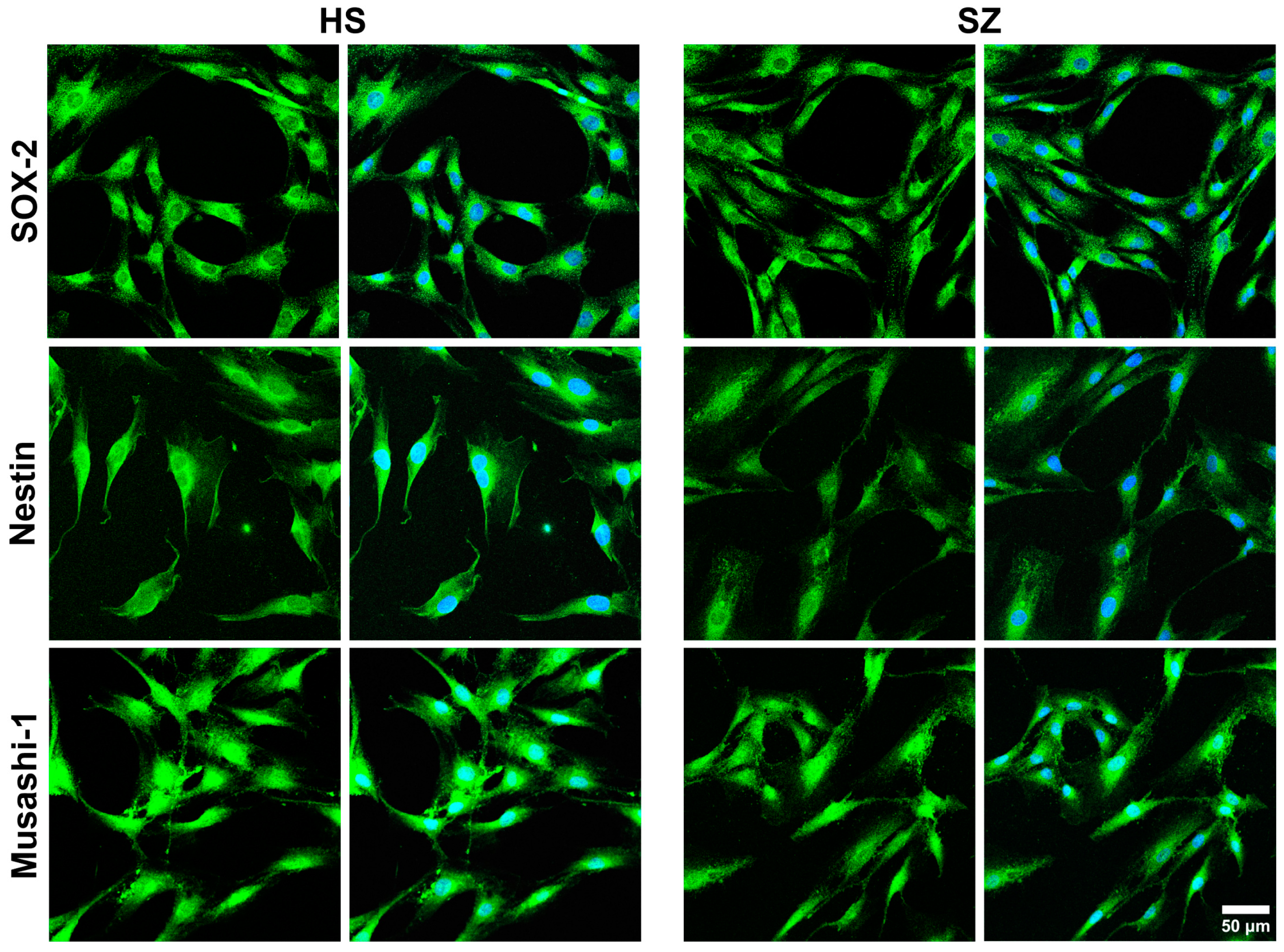
3.1. Expression of Multipotency Markers in Cells from HS and SZ Patients
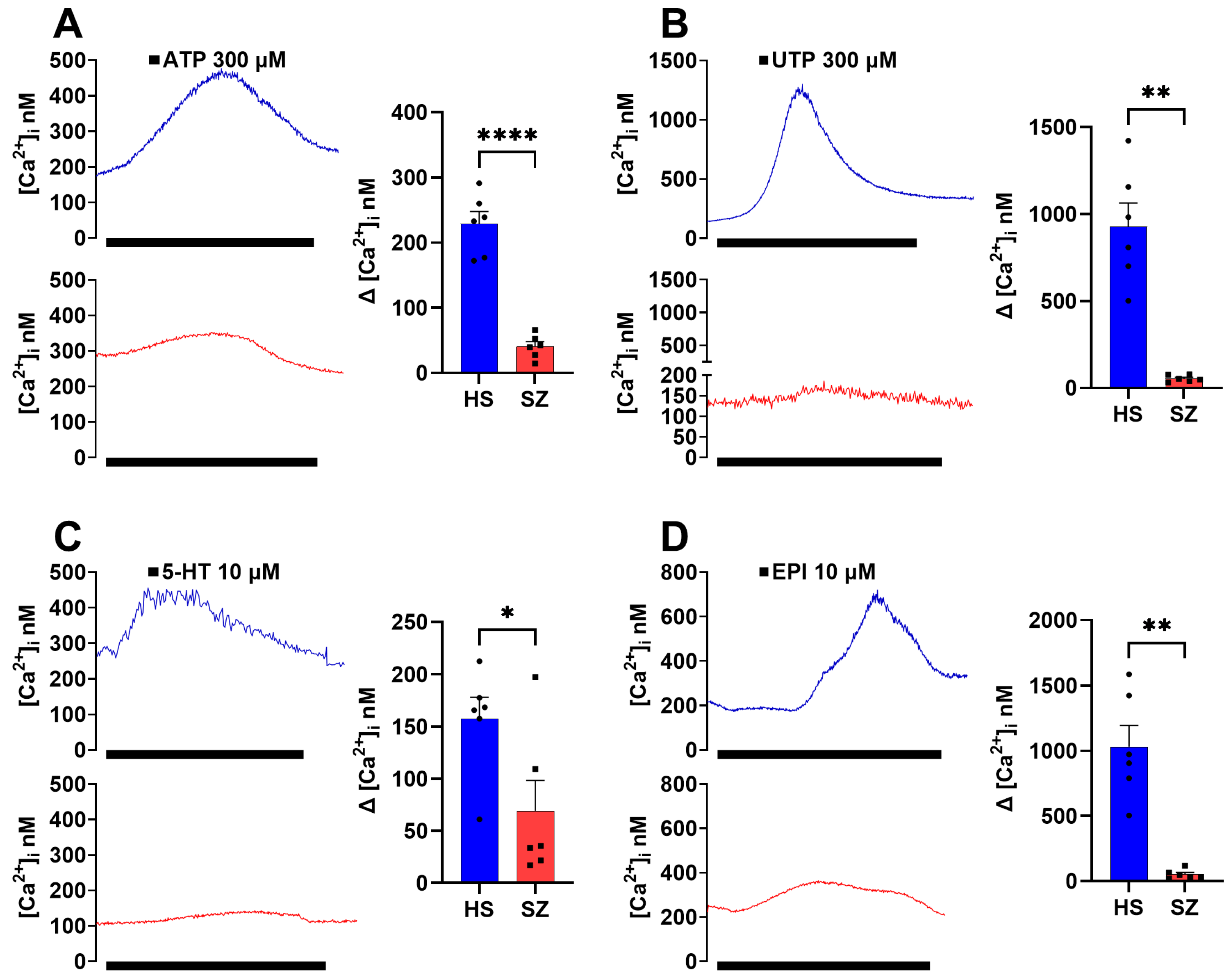
3.2. Olfactory Epithelium Single-Cell Ca2+ Response Induced by Gq-Coupled Agonists in HS and SZ Patients
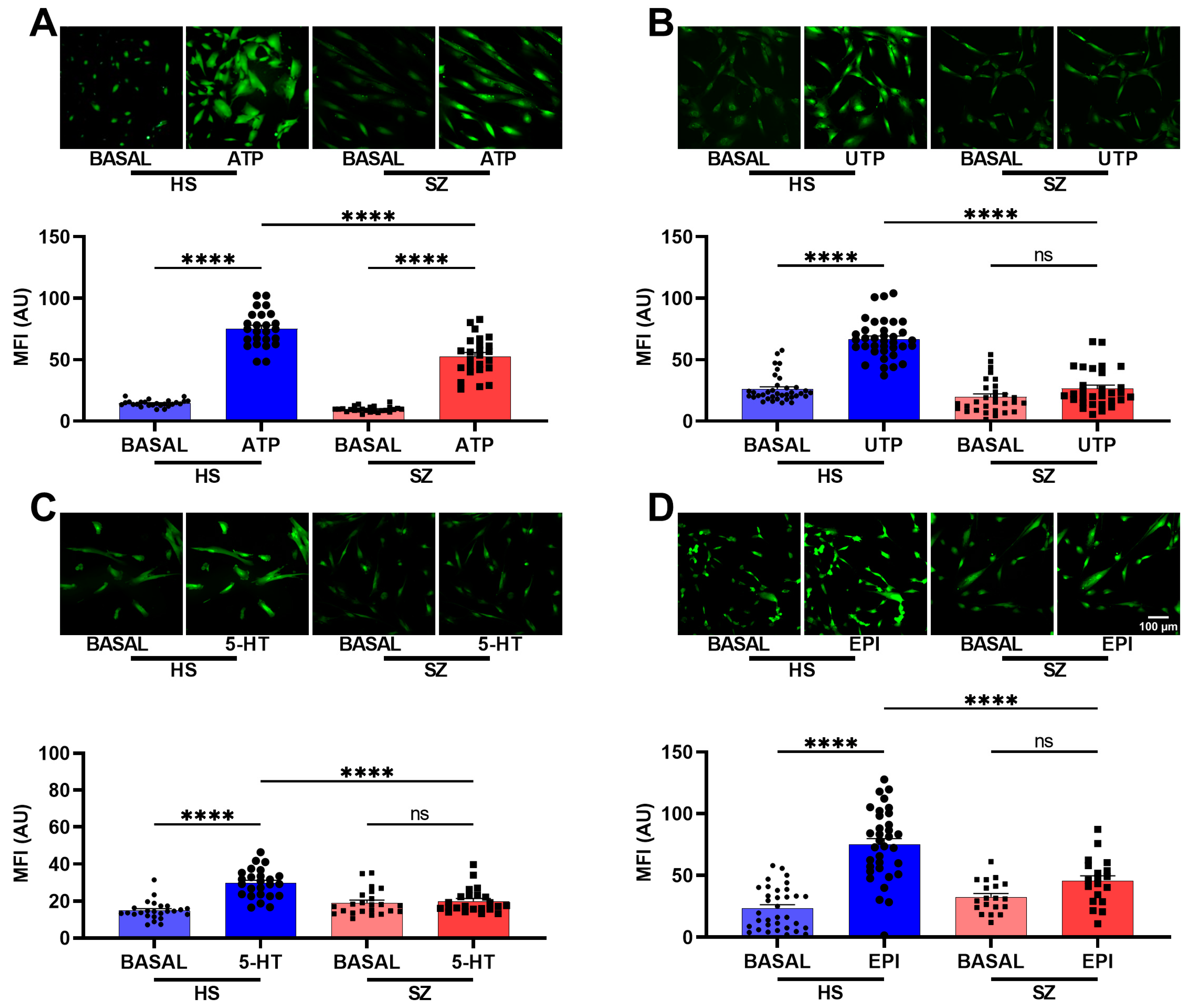
3.3. Cellular Population Ca2+ Imaging after GPCR Agonist Stimulation in hONPCs from HS and SZ Patients
3.4. Function and Expression of the PLCβ Protein in hONPCs
3.5. Production of IP3 and IP3 Receptor Function in hONPCs
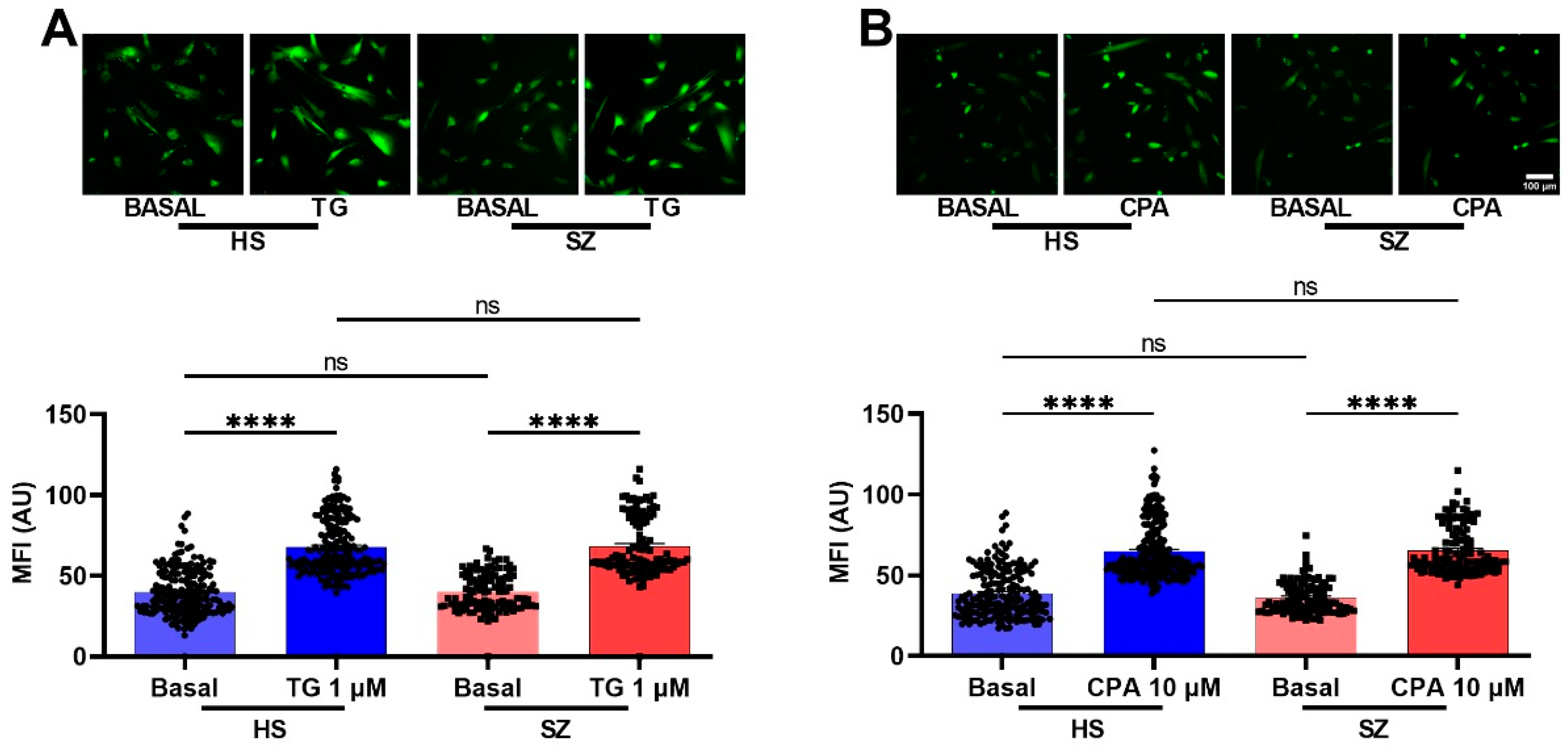
3.6. Olfactory Epithelium Single Cell Ca2+ Response Independent of the IP3/IP3R/Ca2+ Pathway in HS and SZ Patients
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jauhar, S.; Johnstone, M.; McKenna, P.J. Schizophrenia. Lancet 2022, 399, 473–486. [Google Scholar] [CrossRef] [PubMed]
- Stilo, S.A.; Murray, R.M. Non-Genetic Factors in Schizophrenia. Curr. Psychiatry Rep. 2019, 21, 100. [Google Scholar] [CrossRef] [PubMed]
- Howes, O.D.; Mccutcheon, R.; Owen, M.J.; Murray, R.M. The Role of Genes, Stress, and Dopamine in the Development of Schizophrenia. Biol. Psychiatry 2017, 81, 9–20. [Google Scholar] [CrossRef] [PubMed]
- Lewis, D.A.; Moghaddam, B. Cognitive dysfunction in schizophrenia: Convergence of gamma-aminobutyric acid and glutamate alterations. Arch. Neurol. 2006, 63, 1372–1376. [Google Scholar] [CrossRef]
- Stahl, S.M. Beyond the dopamine hypothesis of schizophrenia to three neural networks of psychosis: Dopamine, serotonin, and glutamate. CNS Spectr. 2018, 23, 187–191. [Google Scholar] [CrossRef]
- Berridge, M.J. Calcium signalling and psychiatric disease: Bipolar disorder and schizophrenia. Cell Tissue Res. 2014, 357, 477–492. [Google Scholar] [CrossRef]
- Yang, A.; Tsai, S.-J. New Targets for Schizophrenia Treatment beyond the Dopamine Hypothesis. Int. J. Mol. Sci. 2017, 18, 1689. [Google Scholar] [CrossRef]
- Lo Vasco, V.R.; Longo, L.; Polonia, P. Phosphoinositide-specific Phospholipase C β1 gene deletion in bipolar disorder affected patient. J. Cell Commun. Signal 2013, 7, 25–29. [Google Scholar] [CrossRef] [PubMed]
- Udawela, M.; Scarr, E.; Hannan, A.J.; Thomas, E.A.; Dean, B. Phospholipase C beta 1 expression in the dorsolateral prefrontal cortex from patients with schizophrenia at different stages of illness. Aust. N. Z. J. Psychiatry 2011, 45, 140–147. [Google Scholar] [CrossRef]
- Udawela, M.; Scarr, E.; Boer, S.; Um, J.Y.; Hannan, A.J.; Mcomish, C.; Felder, C.C.; Thomas, E.A.; Dean, B. Isoform specific differences in phospholipase C beta 1 expression in the prefrontal cortex in schizophrenia and suicide. NPJ Schizophr. 2017, 3, 19. [Google Scholar] [CrossRef]
- Féron, F.; Perry, C.; Mcgrath, J.J.; Mackay-Sim, A. New Techniques for Biopsy and Culture of Human Olfactory Epithelial Neurons. Arch. Otolaryngol.–Head Neck Surg. 1998, 124, 861. [Google Scholar] [CrossRef] [PubMed]
- Benitez-King, G.; Riquelme, A.; Ortiz-Lopez, L.; Berlanga, C.; Rodriguez-Verdugo, M.S.; Romo, F.; Calixto, E.; Solis-Chagoyan, H.; Jimenez, M.; Montano, L.M.; et al. A non-invasive method to isolate the neuronal linage from the nasal epithelium from schizophrenic and bipolar diseases. J. Neurosci. Methods 2011, 201, 35–45. [Google Scholar] [CrossRef] [PubMed]
- Estrada-Reyes, R.; Quero-Chávez, D.B.; Alarcón-Elizalde, S.; Cercós, M.G.; Trueta, C.; Constantino-Jonapa, L.A.; Oikawa-Sala, J.; Argueta, J.; Cruz-Garduño, R.; Dubocovich, M.L.; et al. Antidepressant Low Doses of Ketamine and Melatonin in Combination Produce Additive Neurogenesis in Human Olfactory Neuronal Precursors. Molecules 2022, 27, 5650. [Google Scholar] [CrossRef]
- Riquelme, A.; Valdés-Tovar, M.; Ugalde, O.; Maya-Ampudia, V.; Fernández, M.; Mendoza-Durán, L.; Rodríguez-Cárdenas, L.; Benítez-King, G. Potential Use of Exfoliated and Cultured Olfactory Neuronal Precursors for In Vivo Alzheimer’s Disease Diagnosis: A Pilot Study. Cell Mol. Neurobiol. 2020, 40, 87–98. [Google Scholar] [CrossRef]
- Rantanen, L.M.; Bitar, M.; Lampinen, R.; Stewart, R.; Quek, H.; Oikari, L.E.; Cunί-Lόpez, C.; Sutharsan, R.; Thillaiyampalam, G.; Iqbal, J.; et al. An Alzheimer’s Disease Patient-Derived Olfactory Stem Cell Model Identifies Gene Expression Changes Associated with Cognition. Cells 2022, 11, 3258. [Google Scholar] [CrossRef]
- Santillán-Morales, V.; Rodriguez-Espinosa, N.; Muñoz-Estrada, J.; Alarcón-Elizalde, S.; Acebes, Á.; Benítez-King, G. Biomarkers in Alzheimer’s Disease: Are Olfactory Neuronal Precursors Useful for Antemortem Biomarker Research? Brain Sci. 2024, 14, 46. [Google Scholar] [CrossRef] [PubMed]
- Solís-Chagoyán, H.; Calixto, E.; Figueroa, A.; Montaño, L.M.; Berlanga, C.; Rodríguez-Verdugo, M.S.; Romo, F.; Jiménez, M.; Gurrola, C.Z.; Riquelme, A.; et al. Microtubule organization and L-type voltage-activated calcium current in olfactory neuronal cells obtained from patients with schizophrenia and bipolar disorder. Schizophr. Res. 2013, 143, 384–389. [Google Scholar] [CrossRef]
- Matigian, N.; Abrahamsen, G.; Sutharsan, R.; Cook, A.L.; Vitale, A.M.; Nouwens, A.; Bellette, B.; An, J.; Anderson, M.; Beckhouse, A.G.; et al. Disease-specific, neurosphere-derived cells as models for brain disorders. Dis. Models Mech. 2010, 3, 785–798. [Google Scholar] [CrossRef] [PubMed]
- Galván-Arrieta, T.; Trueta, C.; Cercós, M.G.; Valdés-Tovar, M.; Alarcón, S.; Oikawa, J.; Zamudio-Meza, H.; Benítez-King, G. The role of melatonin in the neurodevelopmental etiology of schizophrenia: A study in human olfactory neuronal precursors. J. Pineal Res. 2017, 63, e12421. [Google Scholar] [CrossRef]
- Evgrafov, O.V.; Armoskus, C.; Wrobel, B.B.; Spitsyna, V.N.; Souaiaia, T.; Herstein, J.S.; Walker, C.P.; Nguyen, J.D.; Camarena, A.; Weitz, J.R.; et al. Gene Expression in Patient-Derived Neural Progenitors Implicates WNT5A Signaling in the Etiology of Schizophrenia. Biol. Psychiatry 2020, 88, 236–247. [Google Scholar] [CrossRef]
- Mihaljevic, M.; Lam, M.; Ayala-Grosso, C.; Davis-Batt, F.; Schretlen, D.J.; Ishizuka, K.; Yang, K.; Sawa, A. Olfactory neuronal cells as a promising tool to realize the "druggable genome" approach for drug discovery in neuropsychiatric disorders. Front. Neurosci. 2022, 16, 1081124. [Google Scholar] [CrossRef] [PubMed]
- Abrams, M.T.; Kaufmann, W.E.; Rousseau, F.; Oostra, B.A.; Wolozin, B.; Taylor, C.V.; Lishaa, N.; Morel, M.L.; Hoogeveen, A.; Reiss, A.L. FMR1 gene expression in olfactory neuroblasts from two males with fragile X syndrome. Am. J. Med. Genet. 1999, 82, 25–30. [Google Scholar] [CrossRef]
- Galindo, L.; Moreno, E.; López-Armenta, F.; Guinart, D.; Cuenca-Royo, A.; Izquierdo-Serra, M.; Xicota, L.; Fernandez, C.; Menoyo, E.; Fernández-Fernández, J.M.; et al. Cannabis Users Show Enhanced Expression of CB(1)-5HT(2A) Receptor Heteromers in Olfactory Neuroepithelium Cells. Mol. Neurobiol. 2018, 55, 6347–6361. [Google Scholar] [CrossRef]
- Gunder, N.; Dörig, P.; Witt, M.; Welge-Lüssen, A.; Menzel, S.; Hummel, T. Future therapeutic strategies for olfactory disorders: Electrical stimulation, stem cell therapy, and transplantation of olfactory epithelium—An overview. Hno 2023, 71, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.; Evgrafov, O.V. Editorial: Olfactory neuroepithelium-derived cellular models to study neurological and psychiatric disorders. Front. Neurosci. 2023, 17, 1203466. [Google Scholar] [CrossRef]
- Borgmann-Winter, K.; Willard, S.L.; Sinclair, D.; Mirza, N.; Turetsky, B.; Berretta, S.; Hahn, C.-G. Translational potential of olfactory mucosa for the study of neuropsychiatric illness. Transl. Psychiatry 2015, 5, e527. [Google Scholar] [CrossRef]
- Gao, Y.; Winstead, W.; Lei, Z.; Lu, C.; Roisen, F.J.; El-Mallakh, R.S. Olfactory Neuroepithelial Neural Progenitor Cells from Subjects with Bipolar I Disorder. J. Cent. Nerv. Syst. Dis. 2017, 9, 117957351769452. [Google Scholar] [CrossRef]
- Solis-Chagoyan, H.; Flores-Soto, E.; Valdes-Tovar, M.; Cercos, M.G.; Calixto, E.; Montano, L.M.; Barajas-Lopez, C.; Sommer, B.; Aquino-Galvez, A.; Trueta, C.; et al. Purinergic Signaling Pathway in Human Olfactory Neuronal Precursor Cells. Stem Cells Int. 2019, 2019, 2728786. [Google Scholar] [CrossRef]
- Harding, S.D.; Armstrong, J.F.; Faccenda, E.; Southan, C.; Alexander, S.P.; Davenport, A.P.; Spedding, M.; Davies, J.A. The IUPHAR/BPS Guide to PHARMACOLOGY in 2024. Nucleic Acids Res. 2024, 52, D1438–D1449. [Google Scholar] [CrossRef]
- Borgmann-Winter, K.E.; Rawson, N.E.; Wang, H.Y.; Wang, H.; Macdonald, M.L.; Ozdener, M.H.; Yee, K.K.; Gomez, G.; Xu, J.; Bryant, B.; et al. Human olfactory epithelial cells generated in vitro express diverse neuronal characteristics. Neuroscience 2009, 158, 642–653. [Google Scholar] [CrossRef]
- Grynkiewicz, G.; Poenie, M.; Tsien, R.Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J. Biol. Chem. 1985, 260, 3440–3450. [Google Scholar] [CrossRef] [PubMed]
- Bae, Y.S.; Lee, T.G.; Park, J.C.; Hur, J.H.; Kim, Y.; Heo, K.; Kwak, J.Y.; Suh, P.G.; Ryu, S.H. Identification of a compound that directly stimulates phospholipase C activity. Mol. Pharmacol. 2003, 63, 1043–1050. [Google Scholar] [CrossRef] [PubMed]
- Hirota, J.; Michikawa, T.; Miyawaki, A.; Takahashi, M.; Tanzawa, K.; Okura, I.; Furuichi, T.; Mikoshiba, K. Adenophostin-medicated quantal Ca2+ release in the purified and reconstituted inositol 1,4,5-trisphosphate receptor type 1. FEBS Lett. 1995, 368, 248–252. [Google Scholar] [CrossRef] [PubMed]
- Kang, H. Sample size determination and power analysis using the G*Power software. J. Educ. Eval. Health Prof. 2021, 18, 17. [Google Scholar] [CrossRef]
- Fiume, R.; Keune, W.J.; Faenza, I.; Bultsma, Y.; Ramazzotti, G.; Jones, D.R.; Martelli, A.M.; Somner, L.; Follo, M.Y.; Divecha, N.; et al. Nuclear Phosphoinositides: Location, Regulation and Function. In Phosphoinositides II: The Diverse Biological Functions; Balla, T., Wymann, M., York, J.D., Eds.; Springer: Dordrecht, The Netherlands, 2012; pp. 335–361. [Google Scholar]
- Jang, H.-J.; Yang, Y.R.; Cocco, L.; Ryu, S.H.; Suh, P.-G. Phosphoinositide-Specific Phospholipase C (PI-PLC). In Encyclopedia of Signaling Molecules; Choi, S., Ed.; Springer International Publishing: Cham, Switzerland, 2018; pp. 3973–3988. [Google Scholar]
- Cocco, L.; Rubbini, S.; Manzoli, L.; Billi, A.M.; Faenza, I.; Peruzzi, D.; Matteucci, A.; Artico, M.; Gilmour, R.S.; Rhee, S.G. Inositides in the nucleus: Presence and characterisation of the isozymes of phospholipase β family in NIH 3T3 cells. Biochim. Et Biophys. Acta (BBA)—Mol. Cell Biol. Lipids 1999, 1438, 295–299. [Google Scholar] [CrossRef]
- Durante, M.A.; Kurtenbach, S.; Sargi, Z.B.; Harbour, J.W.; Choi, R.; Goss, G.M.; Matsunami, H.; Goldstein, B.J. Single-cell analysis of olfactory neurogenesis and differentiation in adult humans. Nat. Neurosci. 2020, 23, 323–326. [Google Scholar] [CrossRef]
- Ellis, P.; Fagan, B.M.; Magness, S.T.; Hutton, S.; Taranova, O.; Hayashi, S.; McMahon, A.; Rao, M.; Pevny, L. SOX2, a persistent marker for multipotential neural stem cells derived from embryonic stem cells, the embryo or the adult. Dev. Neurosci. 2004, 26, 148–165. [Google Scholar] [CrossRef]
- Berridge, M.J.; Bootman, M.D.; Roderick, H.L. Calcium signalling: Dynamics, homeostasis and remodelling. Nat. Rev. Mol. Cell Biol. 2003, 4, 517–529. [Google Scholar] [CrossRef]
- Berridge, M.J. The Inositol Trisphosphate/Calcium Signaling Pathway in Health and Disease. Physiol. Rev. 2016, 96, 1261–1296. [Google Scholar] [CrossRef]
- Matsumoto, I.; Inoue, Y.; Iwazaki, T.; Pavey, G.; Dean, B. 5-HT2A and muscarinic receptors in schizophrenia: A postmortem study. Neurosci. Lett. 2005, 379, 164–168. [Google Scholar] [CrossRef]
- Kang, K.; Huang, X.F.; Wang, Q.; Deng, C. Decreased density of serotonin 2A receptors in the superior temporal gyrus in schizophrenia--a postmortem study. Prog. Neuropsychopharmacol. Biol. Psychiatry 2009, 33, 867–871. [Google Scholar] [CrossRef] [PubMed]
- Matosin, N.; Newell, K.A. Metabotropic glutamate receptor 5 in the pathology and treatment of schizophrenia. Neurosci. Biobehav. Rev. 2013, 37, 256–268. [Google Scholar] [CrossRef] [PubMed]
- Scarr, E.; Dean, B. Muscarinic receptors: Do they have a role in the pathology and treatment of schizophrenia? J. Neurochem. 2008, 107, 1188–1195. [Google Scholar] [CrossRef] [PubMed]
- Vasco, V.R.L.; Cardinale, G.; Polonia, P. Deletion of PLCB1 gene in schizophrenia-affected patients. J. Cell. Mol. Med. 2012, 16, 844–851. [Google Scholar] [CrossRef]
- García del Caño, G.; Montaña, M.; Aretxabala, X.; González-Burguera, I.; López de Jesús, M.; Barrondo, S.; Sallés, J. Nuclear phospholipase C-β1 and diacylglycerol LIPASE-α in brain cortical neurons. Adv. Biol. Regul. 2014, 54, 12–23. [Google Scholar] [CrossRef]
- Kurian, M.A.; Meyer, E.; Vassallo, G.; Morgan, N.V.; Prakash, N.; Pasha, S.; Hai, N.A.; Shuib, S.; Rahman, F.; Wassmer, E.; et al. Phospholipase C beta 1 deficiency is associated with early-onset epileptic encephalopathy. Brain 2010, 133, 2964–2970. [Google Scholar] [CrossRef] [PubMed]
- Vakalopoulos, C. The effect of deficient muscarinic signaling on commonly reported biochemical effects in schizophrenia and convergence with genetic susceptibility loci in explaining symptom dimensions of psychosis. Front. Pharmacol. 2014, 5, 277. [Google Scholar] [CrossRef]
- McOmish, C.E.; Burrows, E.; Howard, M.; Scarr, E.; Kim, D.; Shin, H.S.; Dean, B.; van den Buuse, M.; Hannan, A.J. Phospholipase C-beta1 knockout mice exhibit endophenotypes modeling schizophrenia which are rescued by environmental enrichment and clozapine administration. Mol. Psychiatry 2008, 13, 661–672. [Google Scholar] [CrossRef]
- Koh, H.Y. Phospholipase C-β1 and schizophrenia-related behaviors. Adv. Biol. Regul. 2013, 53, 242–248. [Google Scholar] [CrossRef]
- Wagner, L.E., 2nd; Yule, D.I. Differential regulation of the InsP₃ receptor type-1 and -2 single channel properties by InsP₃, Ca²⁺ and ATP. J. Physiol. 2012, 590, 3245–3259. [Google Scholar] [CrossRef]
- Mikoshiba, K. Role of IP3 receptor signaling in cell functions and diseases. Adv. Biol. Regul. 2015, 57, 217–227. [Google Scholar] [CrossRef] [PubMed]
- Lago, S.G.; Tomasik, J.; Van Rees, G.F.; Steeb, H.; Cox, D.A.; Rustogi, N.; Ramsey, J.M.; Bishop, J.A.; Petryshen, T.; Haggarty, S.J.; et al. Drug discovery for psychiatric disorders using high-content single-cell screening of signaling network responses ex vivo. Sci. Adv. 2019, 5, eaau9093. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.R.; Jung, J.H.; Kim, S.-J.; Hamada, K.; Suzuki, A.; Kim, H.J.; Lee, J.H.; Kwon, O.-B.; Lee, Y.K.; Kim, J.; et al. Forebrain-specific ablation of phospholipase Cγ1 causes manic-like behavior. Mol. Psychiatry 2017, 22, 1473–1482. [Google Scholar] [CrossRef] [PubMed]
- Gordon-Smith, K.; Green, E.; Grozeva, D.; Tavadia, S.; Craddock, N.; Jones, L. Genotype–phenotype correlations in Darier disease: A focus on the neuropsychiatric phenotype. Am. J. Med. Genet. Part. B Neuropsychiatr. Genet. 2018, 177, 717–726. [Google Scholar] [CrossRef]
- Hayashi, A.; Kasahara, T.; Kametani, M.; Toyota, T.; Yoshikawa, T.; Kato, T. Aberrant endoplasmic reticulum stress response in lymphoblastoid cells from patients with bipolar disorder. Int. J. Neuropsychopharmacol. 2009, 12, 33. [Google Scholar] [CrossRef]
- Arranz, B.; Rosel, P.; Sarró, S.; Ramirez, N.; Dueñas, R.; Cano, R.; María Sanchez, J.; San, L. Altered platelet serotonin 5-HT2A receptor density but not second messenger inositol trisphosphate levels in drug-free schizophrenic patients. Psychiatry Res. 2003, 118, 165–174. [Google Scholar] [CrossRef]
- Rípová, D.; Strunecká, A.; Platilová, V.; Höschl, C. Phosphoinositide signalling system in platelets of schizophrenic patients and the effect of neuroleptic therapy. Prostaglandins Leukot. Essent. Fat. Acids 1999, 61, 125–129. [Google Scholar] [CrossRef]
- Rípová, D.; Strunecká, A.; Nemcová, V.; Farská, I. Phospholipids and calcium alterations in platelets of schizophrenic patients. Physiol. Res. 1997, 46, 59–68. [Google Scholar]
- Bondy, B.; Ackenheil, M.; Birzle, W.; Elbers, R.; Fröhler, M. Catecholamines and their receptors in blood: Evidence for alterations in schizophrenia. Biol. Psychiatry 1984, 19, 1377–1393. [Google Scholar]
- Dreux, C.; Launay, J.M. Blood platelets: Neuronal model in psychiatric disorders. Encephale 1985, 11, 57–64. [Google Scholar]
- Rosati, B.; Mckinnon, D. Regulation of Ion Channel Expression. Circ. Res. 2004, 94, 874–883. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Florentino, Z.A.; Romero-Martínez, B.S.; Flores-Soto, E.; Serrano, H.; Montaño, L.M.; Valdés-Tovar, M.; Calixto, E.; Aquino-Gálvez, A.; López-Riquelme, G.O.; Alvarado, R.; et al. Potential of olfactory neuroepithelial cells as a model to study schizophrenia: A focus on GPCRs (Review). Int. J. Mol. Med. 2024, 53, 7. [Google Scholar] [CrossRef] [PubMed]
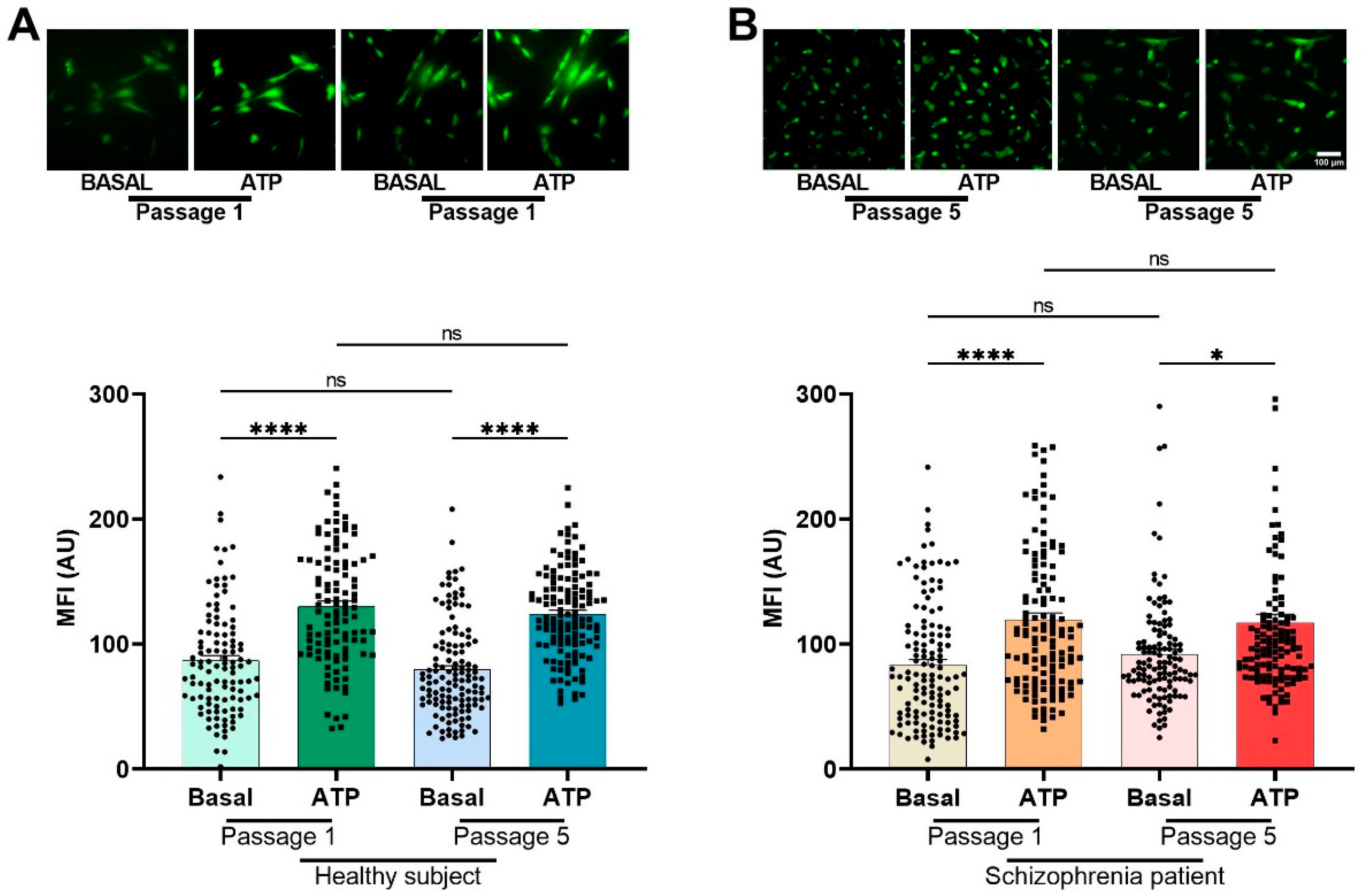
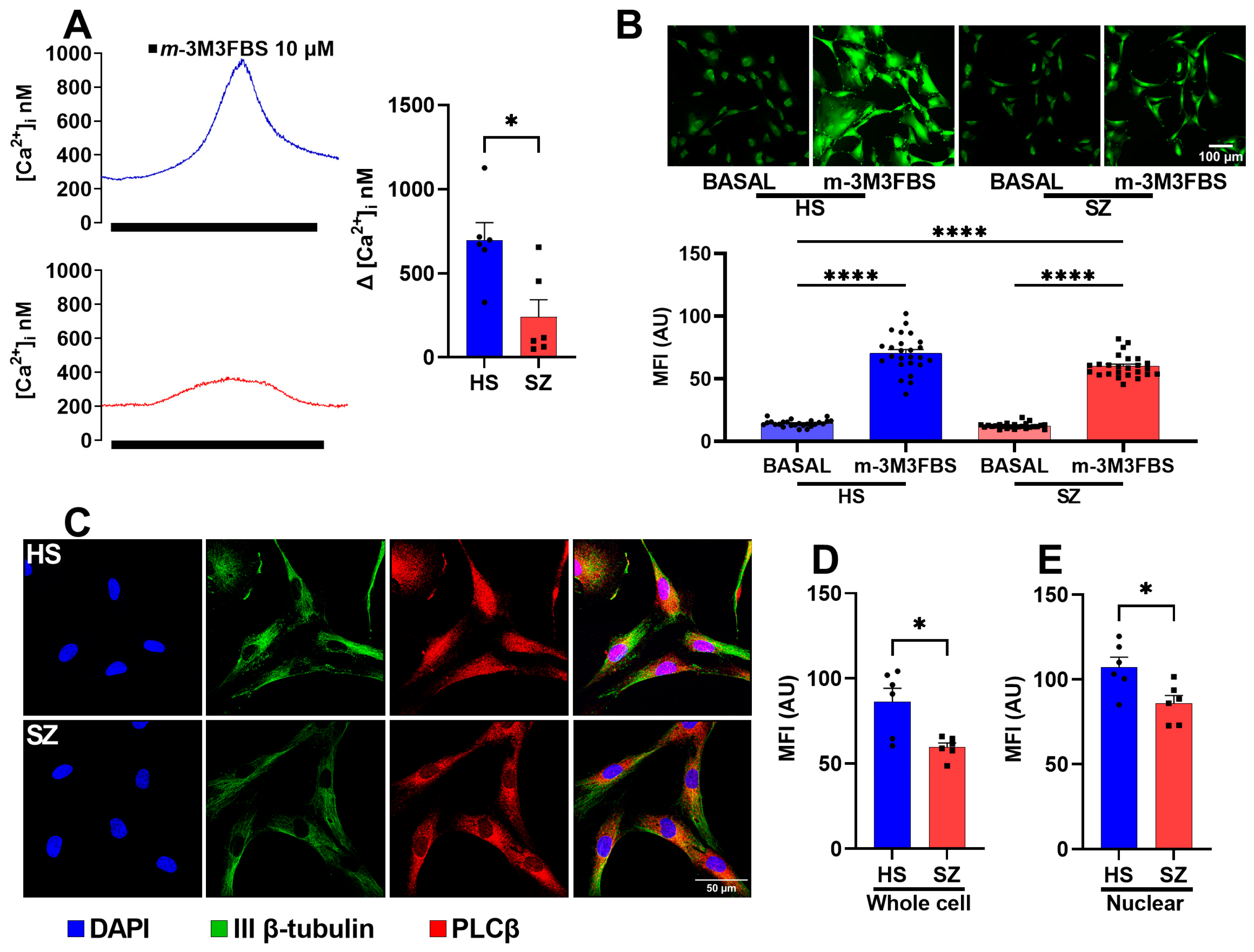
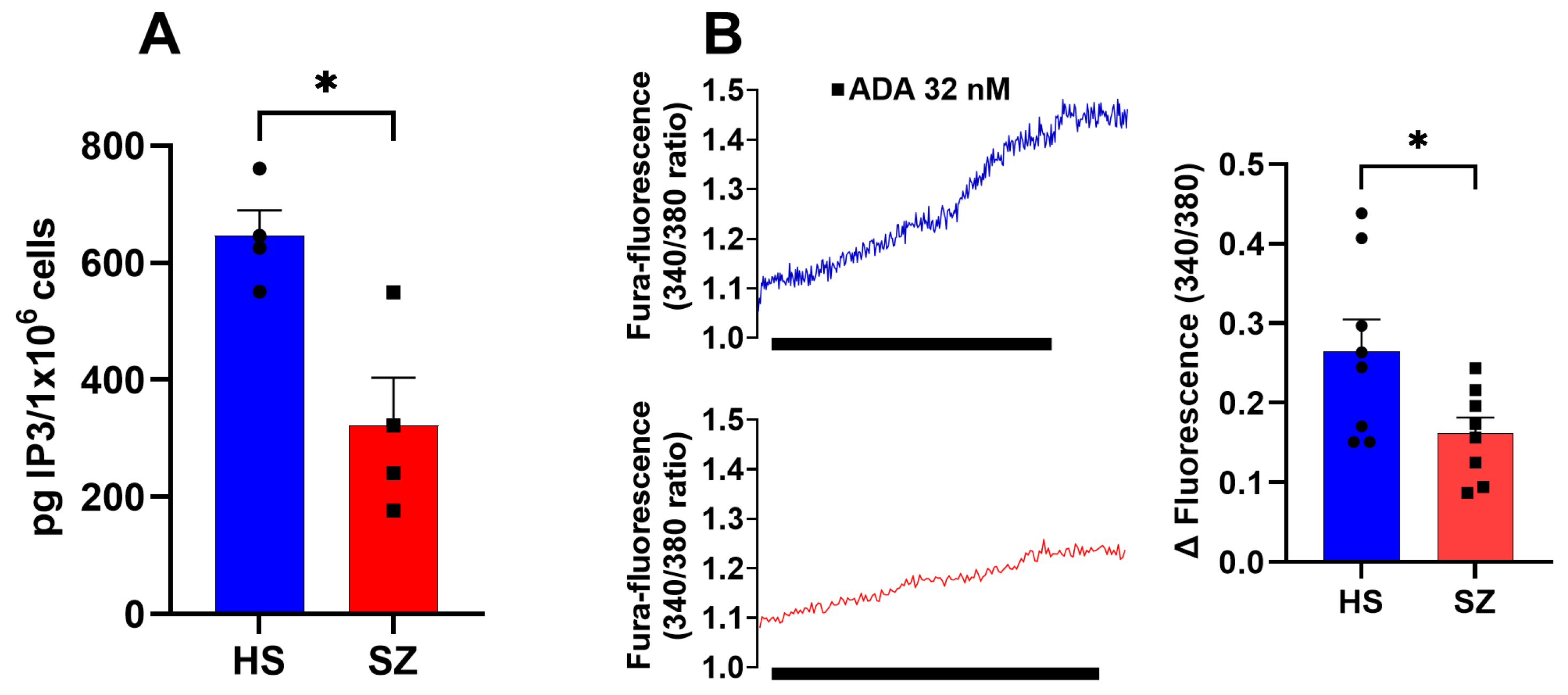
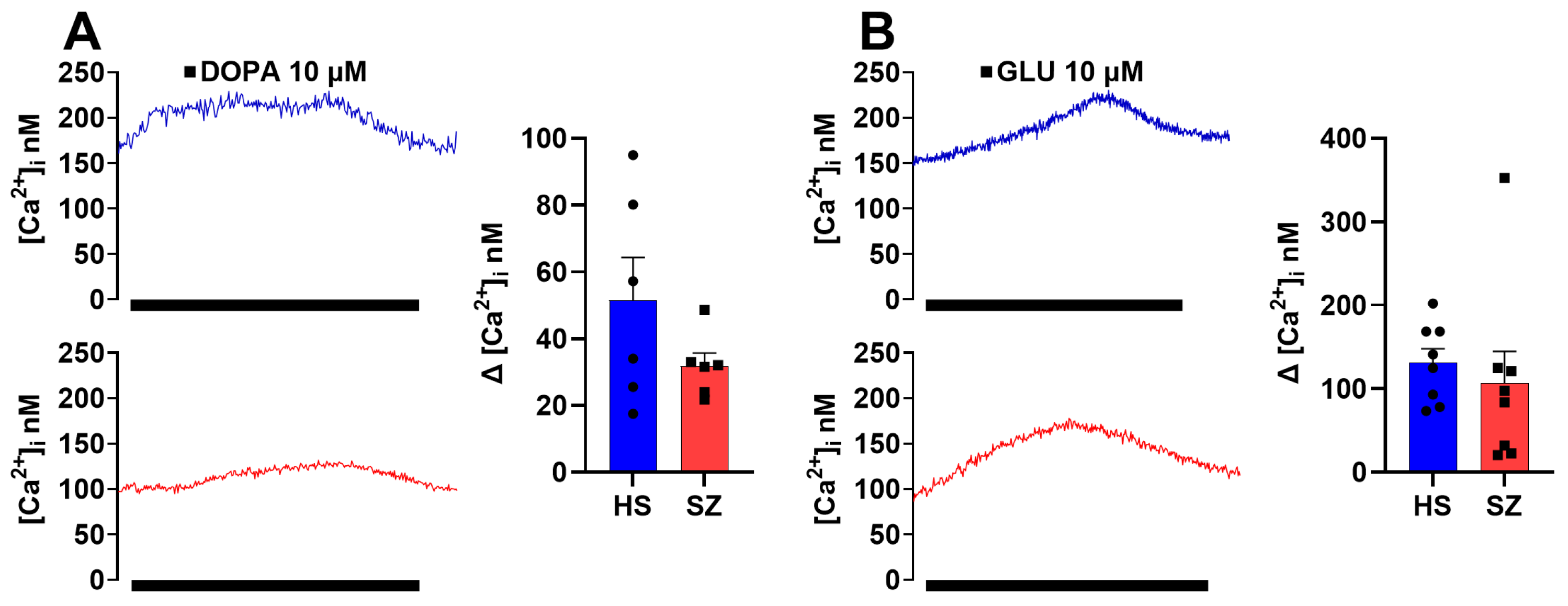
| Diagnosis | Sex | Age | Age at Diagnosis | Pre-Existing Conditions | Family Psychiatric History | Treatment | Time of Evolution | Alcoholism | Smoking |
|---|---|---|---|---|---|---|---|---|---|
| HS | F | 28 | |||||||
| HS | F | 23 | Yes | ||||||
| HS | F | 27 | Ibuprofen Vitamin C | Yes | |||||
| HS | M | 23 | |||||||
| HS | M | 27 | Hypoglycemia | Yes | |||||
| HS | M | 28 | Hypothyroidism | Levothyroxine Coenzyme Q Carnitine | |||||
| SZ | F | 32 | 25 | Haloperidol 50 mg, biperiden 4 mg, fluoxetine 20 mg | 7 | ||||
| SZ | F | 27 | 26 | Risperidone 2 mg, fluoxetine 20 mg, biperiden 2 mg | 1 | ||||
| SZ | M | 20 | 20 | ADHD care at age 8 | Naïve | 0 | |||
| SZ | M | 28 | 27 | Naïve | 1 | Yes | Yes | ||
| SZ | M | 31 | 28 | Risperidone 2 mg | 3 | ||||
| SZ | F | 33 | 15 | Obesity and hypothyroidism | Yes | Haloperidol 10 mg, fluoxetine 40 mg, akineton 2 mg, eutirox | 18 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sánchez-Florentino, Z.A.; Romero-Martínez, B.S.; Flores-Soto, E.; Montaño, L.M.; Sommer, B.; Valdés-Tovar, M.; Argueta, J.; Calixto, E.; Aquino-Gálvez, A.; Castillejos-López, M.; et al. Altered PLCβ/IP3/Ca2+ Signaling Pathway Activated by GPRCs in Olfactory Neuronal Precursor Cells Derived from Patients Diagnosed with Schizophrenia. Biomedicines 2024, 12, 2343. https://doi.org/10.3390/biomedicines12102343
Sánchez-Florentino ZA, Romero-Martínez BS, Flores-Soto E, Montaño LM, Sommer B, Valdés-Tovar M, Argueta J, Calixto E, Aquino-Gálvez A, Castillejos-López M, et al. Altered PLCβ/IP3/Ca2+ Signaling Pathway Activated by GPRCs in Olfactory Neuronal Precursor Cells Derived from Patients Diagnosed with Schizophrenia. Biomedicines. 2024; 12(10):2343. https://doi.org/10.3390/biomedicines12102343
Chicago/Turabian StyleSánchez-Florentino, Zuly A., Bianca S. Romero-Martínez, Edgar Flores-Soto, Luis M. Montaño, Bettina Sommer, Marcela Valdés-Tovar, Jesús Argueta, Eduardo Calixto, Arnoldo Aquino-Gálvez, Manuel Castillejos-López, and et al. 2024. "Altered PLCβ/IP3/Ca2+ Signaling Pathway Activated by GPRCs in Olfactory Neuronal Precursor Cells Derived from Patients Diagnosed with Schizophrenia" Biomedicines 12, no. 10: 2343. https://doi.org/10.3390/biomedicines12102343
APA StyleSánchez-Florentino, Z. A., Romero-Martínez, B. S., Flores-Soto, E., Montaño, L. M., Sommer, B., Valdés-Tovar, M., Argueta, J., Calixto, E., Aquino-Gálvez, A., Castillejos-López, M., Serrano, H., Gomez-Verjan, J. C., López-Riquelme, G. O., Benítez-King, G. A., Jaimez, R., & Solís-Chagoyán, H. (2024). Altered PLCβ/IP3/Ca2+ Signaling Pathway Activated by GPRCs in Olfactory Neuronal Precursor Cells Derived from Patients Diagnosed with Schizophrenia. Biomedicines, 12(10), 2343. https://doi.org/10.3390/biomedicines12102343







