Radixin: Roles in the Nervous System and Beyond
Abstract
Simple Summary
Abstract
1. Introduction
2. Activation of Radixin
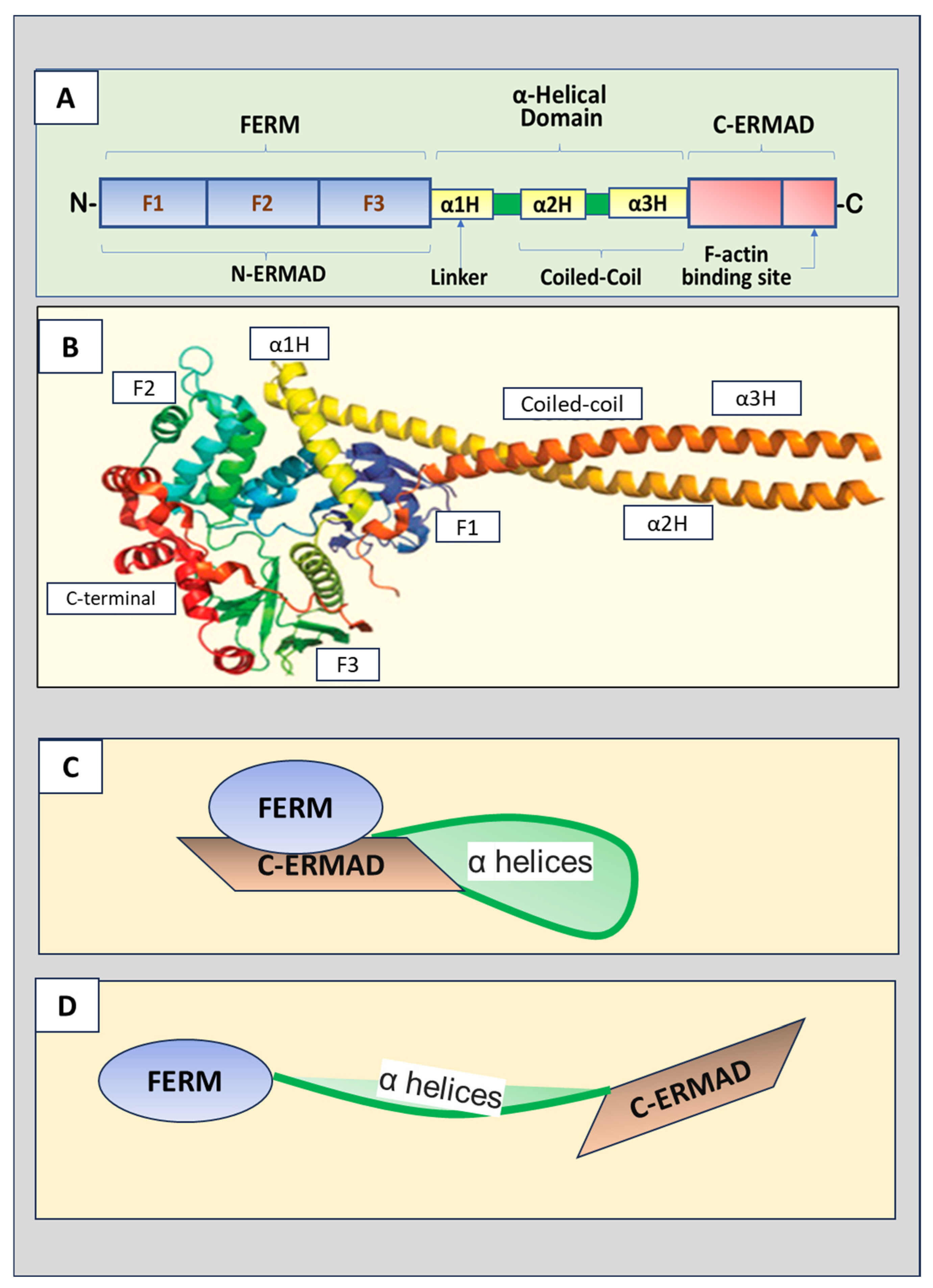
2.1. Radixin Structure
2.2. Radixin Activation
2.3. Kinases and Radixin Phosphorylation
2.4. Radixin Binding to the Cell Membrane
3. Expression of Radixin
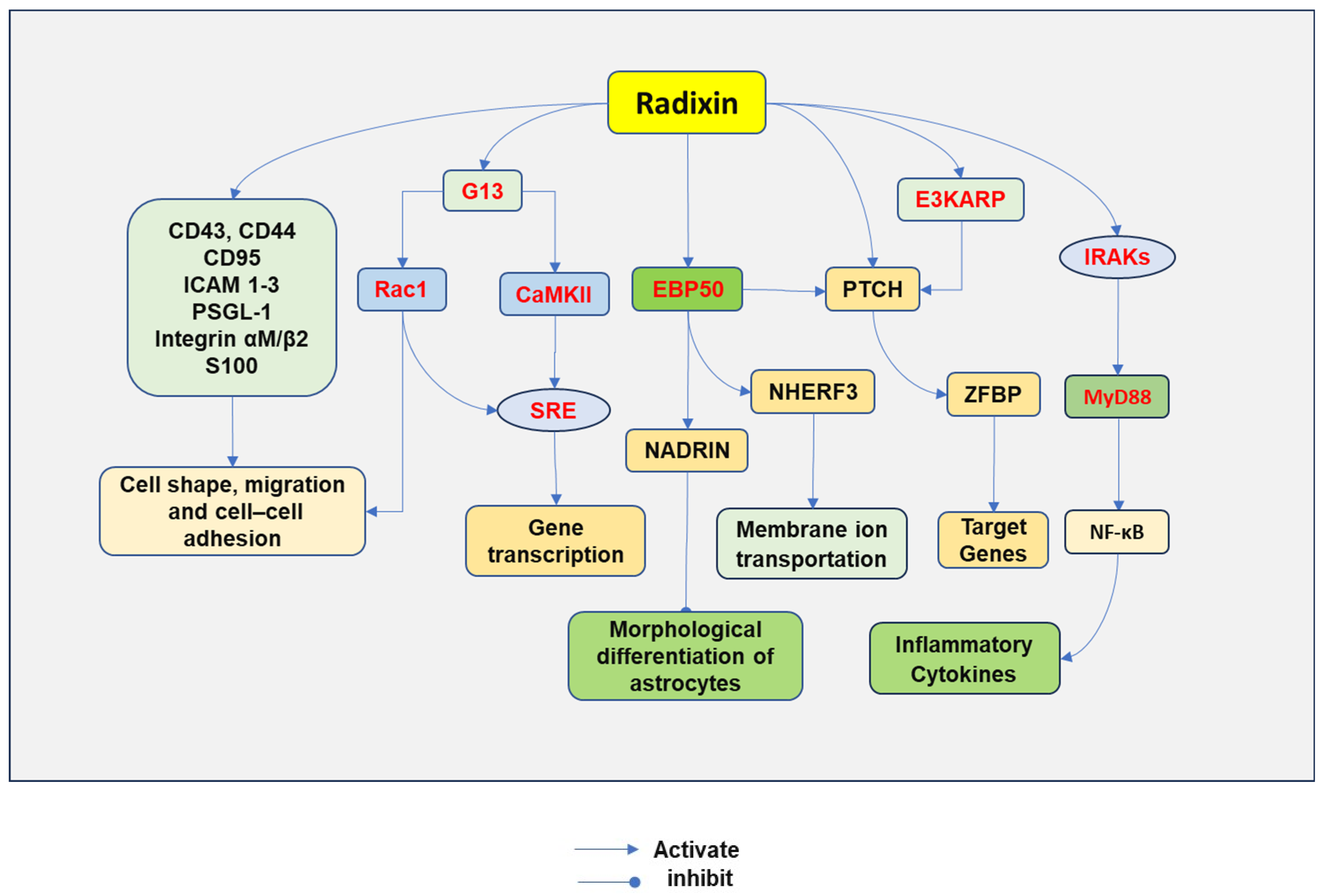
4. The Role of Radixin and Cell Signaling Pathways
4.1. Relaying the Cell Signaling
4.2. Binging through Adaptor Proteins
4.3. Regulating PTCH Cell Signaling
4.4. Mediating the Differentiation of Astrocytes
4.5. Regulating Inflammatory Cascade
4.6. Regulating G13-Induced Cell Signaling
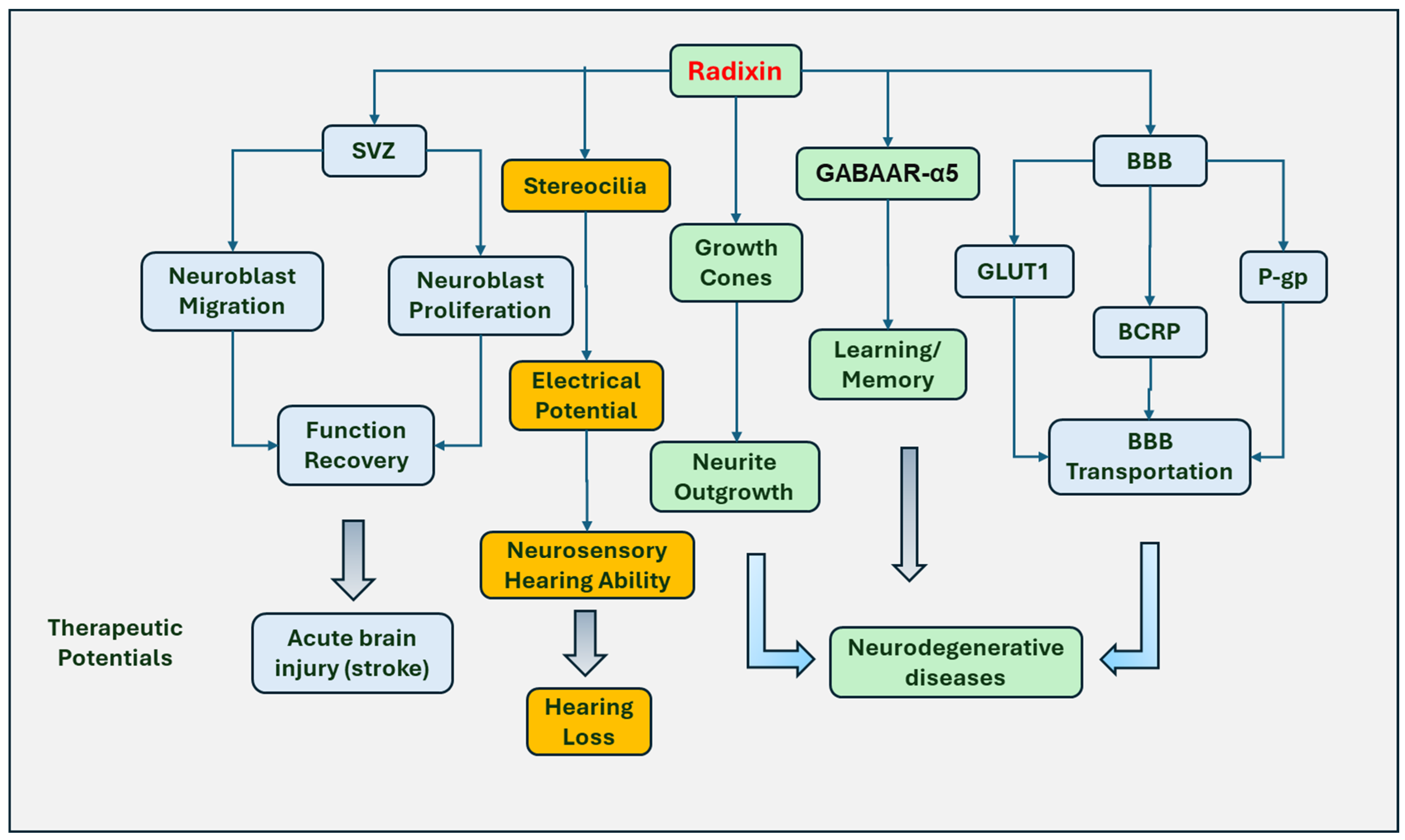
5. The Roles of Radixin in the Nervous System
5.1. Promoting the Growth of Neurons
5.2. Regulating Hearing Function
5.3. Involved in Learning and Memory Processing
5.4. Regulating Transport through the BBB
5.5. Involved in Peripheral Nerve Injury
6. The Roles of Radixin in Cancer
6.1. Cancer Growth
6.2. Drug Resistance
7. The Role of Radixin in Diabetes Mellitus
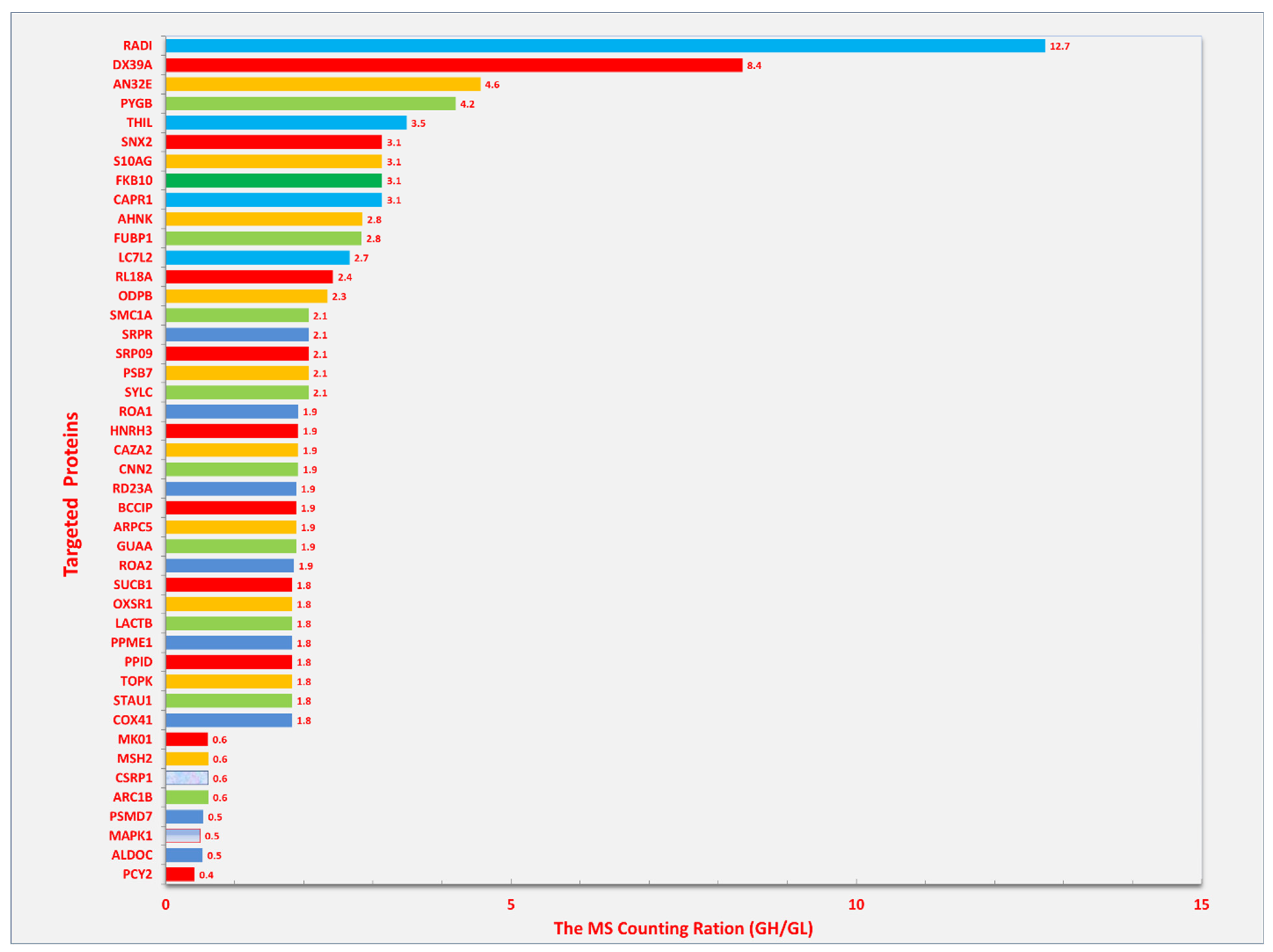
8. Other Possible Roles of Radixin
9. Targeting Radixin for Therapeutic Applications
9.1. Neurodegenerative Diseases
9.2. Cancers
10. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bretscher, A.; Edwards, K.; Fehon, R.G. ERM proteins and merlin: Integrators at the cell cortex. Nat. Rev. Mol. Cell Biol. 2002, 3, 586–599. [Google Scholar] [CrossRef] [PubMed]
- Ponuwei, G.A. Aglimpse of the ERM proteins. J. Biomed. Sci. 2016, 23, 35. [Google Scholar] [CrossRef] [PubMed]
- Michie, K.A.; Bermeister, A.; Robertson, N.O.; Goodchild, S.C.; Curmi, P.M.G. Two Sides of the Coin: Ezrin/Radixin/Moesin and Merlin Control Membrane Structure and Contact Inhibition. Int. J. Mol. Sci. 2019, 20, 1996. [Google Scholar] [CrossRef]
- Neisch, A.L.; Fehon, R.G. Ezrin, Radixin and Moesin: Key regulators of membrane-cortex interactions and signaling. Curr. Opin. Cell Biol. 2011, 23, 377–382. [Google Scholar] [CrossRef] [PubMed]
- Pearson, M.A.; Reczek, D.; Bretscher, A.; Karplus, P.A. Structure of the ERM protein moesin reveals the FERM domain fold masked by an extended actin binding tail domain. Cell 2000, 101, 259–270. [Google Scholar] [CrossRef]
- Li, Q.; Nance, M.R.; Kulikauskas, R.; Nyberg, K.; Fehon, R.; Karplus, P.A.; Bretscher, A.; Tesmer, J.J. Self-masking in an intact ERM-merlin protein: An active role for the central alpha-helical domain. J. Mol. Biol. 2007, 365, 1446–1459. [Google Scholar] [CrossRef]
- Pelaseyed, T.; Viswanatha, R.; Sauvanet, C.; Filter, J.J.; Goldberg, M.L.; Bretscher, A. Ezrin activation by LOK phosphorylation involves a PIP(2)-dependent wedge mechanism. eLife 2017, 6, e22759. [Google Scholar] [CrossRef]
- Fehon, R.G.; McClatchey, A.I.; Bretscher, A. Organizing the cell cortex: The role of ERM proteins. Nat. Rev. Mol. Cell Biol. 2010, 11, 276–287. [Google Scholar] [CrossRef]
- Jin, E.J.; Ko, H.R.; Hwang, I.; Kim, B.S.; Choi, J.Y.; Park, K.W.; Cho, S.W.; Ahn, J.Y. Akt regulates neurite growth by phosphorylation-dependent inhibition of radixin proteasomal degradation. Sci. Rep. 2018, 8, 2557. [Google Scholar] [CrossRef]
- Kahsai, A.W.; Wisler, J.W.; Lee, J.; Ahn, S.; Cahill Iii, T.J.; Dennison, S.M.; Staus, D.P.; Thomsen, A.R.; Anasti, K.M.; Pani, B.; et al. Conformationally selective RNA aptamers allosterically modulate the beta2-adrenoceptor. Nat. Chem. Biol. 2016, 12, 709–716. [Google Scholar] [CrossRef]
- Cant, S.H.; Pitcher, J.A. G protein-coupled receptor kinase 2-mediated phosphorylation of ezrin is required for G protein-coupled receptor-dependent reorganization of the actin cytoskeleton. Mol. Biol. Cell 2005, 16, 3088–3099. [Google Scholar] [CrossRef] [PubMed]
- Kahsai, A.W.; Zhu, S.; Fenteany, G. G protein-coupled receptor kinase 2 activates radixin, regulating membrane protrusion and motility in epithelial cells. Biochim. Biophys. Acta 2010, 1803, 300–310. [Google Scholar] [CrossRef] [PubMed]
- Baumgartner, M.; Sillman, A.L.; Blackwood, E.M.; Srivastava, J.; Madson, N.; Schilling, J.W.; Wright, J.H.; Barber, D.L. The Nck-interacting kinase phosphorylates ERM proteins for formation of lamellipodium by growth factors. Proc. Natl. Acad. Sci. USA 2006, 103, 13391–13396. [Google Scholar] [CrossRef] [PubMed]
- Belkina, N.V.; Liu, Y.; Hao, J.J.; Karasuyama, H.; Shaw, S. LOK is a major ERM kinase in resting lymphocytes and regulates cytoskeletal rearrangement through ERM phosphorylation. Proc. Natl. Acad. Sci. USA 2009, 106, 4707–4712. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.H.; Osborne, T.; Ren, L.; Briggs, J.; Mazcko, C.; Burkett, S.S.; Khanna, C. Protein kinase C regulates ezrin-radixin-moesin phosphorylation in canine osteosarcoma cells. Vet. Comp. Oncol. 2011, 9, 207–218. [Google Scholar] [CrossRef]
- Ng, T.; Parsons, M.; Hughes, W.E.; Monypenny, J.; Zicha, D.; Gautreau, A.; Arpin, M.; Gschmeissner, S.; Verveer, P.J.; Bastiaens, P.I.; et al. Ezrin is a downstream effector of trafficking PKC-integrin complexes involved in the control of cell motility. EMBO J. 2001, 20, 2723–2741. [Google Scholar] [CrossRef]
- Pietromonaco, S.F.; Simons, P.C.; Altman, A.; Elias, L. Protein kinase C-theta phosphorylation of moesin in the actin-binding sequence. J. Biol. Chem. 1998, 273, 7594–7603. [Google Scholar] [CrossRef]
- Wald, F.A.; Oriolo, A.S.; Mashukova, A.; Fregien, N.L.; Langshaw, A.H.; Salas, P.J. Atypical protein kinase C (iota) activates ezrin in the apical domain of intestinal epithelial cells. J. Cell Sci. 2008, 121, 644–654. [Google Scholar] [CrossRef]
- Hoshi, Y.; Uchida, Y.; Kuroda, T.; Tachikawa, M.; Couraud, P.O.; Suzuki, T.; Terasaki, T. Distinct roles of ezrin, radixin and moesin in maintaining the plasma membrane localizations and functions of human blood-brain barrier transporters. J. Cereb. Blood Flow. Metab. 2020, 40, 1533–1545. [Google Scholar] [CrossRef]
- Adyshev, D.M.; Dudek, S.M.; Moldobaeva, N.; Kim, K.M.; Ma, S.F.; Kasa, A.; Garcia, J.G.; Verin, A.D. Ezrin/radixin/moesin proteins differentially regulate endothelial hyperpermeability after thrombin. Am. J. Physiol. Lung Cell. Mol. Physiol. 2013, 305, L240–L255. [Google Scholar] [CrossRef]
- Yang, H.S.; Hinds, P.W. Phosphorylation of ezrin by cyclin-dependent kinase 5 induces the release of Rho GDP dissociation inhibitor to inhibit Rac1 activity in senescent cells. Cancer Res. 2006, 66, 2708–2715. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Yang, H.S.; Alexander, K.; Santiago, P.; Hinds, P.W. ERM proteins and Cdk5 in cellular senescence. Cell Cycle 2003, 2, 517–520. [Google Scholar] [CrossRef] [PubMed]
- Oshiro, N.; Fukata, Y.; Kaibuchi, K. Phosphorylation of moesin by rho-associated kinase (Rho-kinase) plays a crucial role in the formation of microvilli-like structures. J. Biol. Chem. 1998, 273, 34663–34666. [Google Scholar] [CrossRef] [PubMed]
- Jeon, S.; Kim, S.; Park, J.B.; Suh, P.G.; Kim, Y.S.; Bae, C.D.; Park, J. RhoA and Rho kinase-dependent phosphorylation of moesin at Thr-558 in hippocampal neuronal cells by glutamate. J. Biol. Chem. 2002, 277, 16576–16584. [Google Scholar] [CrossRef]
- Haas, M.A.; Vickers, J.C.; Dickson, T.C. Rho kinase activates ezrin-radixin-moesin (ERM) proteins and mediates their function in cortical neuron growth, morphology and motility in vitro. J. Neurosci. Res. 2007, 85, 34–46. [Google Scholar] [CrossRef]
- Jeon, S.; Park, J.K.; Bae, C.D.; Park, J. NGF-induced moesin phosphorylation is mediated by the PI3K, Rac1 and Akt and required for neurite formation in PC12 cells. Neurochem. Int. 2010, 56, 810–818. [Google Scholar] [CrossRef]
- Hebert, M.; Potin, S.; Sebbagh, M.; Bertoglio, J.; Breard, J.; Hamelin, J. Rho-ROCK-dependent ezrin-radixin-moesin phosphorylation regulates Fas-mediated apoptosis in Jurkat cells. J. Immunol. 2008, 181, 5963–5973. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, N.; Oshiro, N.; Fukata, Y.; Amano, M.; Fukata, M.; Kuroda, S.; Matsuura, Y.; Leung, T.; Lim, L.; Kaibuchi, K. Phosphorylation of ERM proteins at filopodia induced by Cdc42. Genes. Cells 2000, 5, 571–581. [Google Scholar] [CrossRef]
- Jaleel, M.; Nichols, R.J.; Deak, M.; Campbell, D.G.; Gillardon, F.; Knebel, A.; Alessi, D.R. LRRK2 phosphorylates moesin at threonine-558: Characterization of how Parkinson’s disease mutants affect kinase activity. Biochem. J. 2007, 405, 307–317. [Google Scholar] [CrossRef]
- Parisiadou, L.; Xie, C.; Cho, H.J.; Lin, X.; Gu, X.L.; Long, C.X.; Lobbestael, E.; Baekelandt, V.; Taymans, J.M.; Sun, L.; et al. Phosphorylation of ezrin/radixin/moesin proteins by LRRK2 promotes the rearrangement of actin cytoskeleton in neuronal morphogenesis. J. Neurosci. 2009, 29, 13971–13980. [Google Scholar] [CrossRef]
- Parameswaran, N.; Enyindah-Asonye, G.; Bagheri, N.; Shah, N.B.; Gupta, N. Spatial coupling of JNK activation to the B cell antigen receptor by tyrosine-phosphorylated ezrin. J. Immunol. 2013, 190, 2017–2026. [Google Scholar] [CrossRef] [PubMed]
- Coffey, G.P.; Rajapaksa, R.; Liu, R.; Sharpe, O.; Kuo, C.C.; Krauss, S.W.; Sagi, Y.; Davis, R.E.; Staudt, L.M.; Sharman, J.P.; et al. Engagement of CD81 induces ezrin tyrosine phosphorylation and its cellular redistribution with filamentous actin. J. Cell Sci. 2009, 122, 3137–3144. [Google Scholar] [CrossRef] [PubMed]
- Elliott, B.E.; Meens, J.A.; SenGupta, S.K.; Louvard, D.; Arpin, M. The membrane cytoskeletal crosslinker ezrin is required for metastasis of breast carcinoma cells. Breast Cancer Res. 2005, 7, R365–R373. [Google Scholar] [CrossRef]
- Vaiskunaite, R.; Adarichev, V.; Furthmayr, H.; Kozasa, T.; Gudkov, A.; Voyno-Yasenetskaya, T.A. Conformational activation of radixin by G13 protein alpha subunit. J. Biol. Chem. 2000, 275, 26206–26212. [Google Scholar] [CrossRef]
- Ben-Aissa, K.; Patino-Lopez, G.; Belkina, N.V.; Maniti, O.; Rosales, T.; Hao, J.J.; Kruhlak, M.J.; Knutson, J.R.; Picart, C.; Shaw, S. Activation of moesin, a protein that links actin cytoskeleton to the plasma membrane, occurs by phosphatidylinositol 4,5-bisphosphate (PIP2) binding sequentially to two sites and releasing an autoinhibitory linker. J. Biol. Chem. 2012, 287, 16311–16323. [Google Scholar] [CrossRef]
- Chen, X.; Khajeh, J.A.; Ju, J.H.; Gupta, Y.K.; Stanley, C.B.; Do, C.; Heller, W.T.; Aggarwal, A.K.; Callaway, D.J.; Bu, Z. Phosphatidylinositol 4,5-bisphosphate clusters the cell adhesion molecule CD44 and assembles a specific CD44-Ezrin heterocomplex, as revealed by small angle neutron scattering. J. Biol. Chem. 2015, 290, 6639–6652. [Google Scholar] [CrossRef]
- Ehret, T.; Heissenberg, T.; de Buhr, S.; Aponte-Santamaria, C.; Steinem, C.; Grater, F. FERM domains recruit ample PI(4,5)P(2)s to form extensive protein-membrane attachments. Biophys. J. 2023, 122, 1325–1333. [Google Scholar] [CrossRef] [PubMed]
- Hoeflich, K.P.; Ikura, M. Radixin: Cytoskeletal adopter and signaling protein. Int. J. Biochem. Cell Biol. 2004, 36, 2131–2136. [Google Scholar] [CrossRef]
- Wakayama, T.; Nakata, H.; Kurobo, M.; Sai, Y.; Iseki, S. Expression, localization, and binding activity of the ezrin/radixin/moesin proteins in the mouse testis. J. Histochem. Cytochem. 2009, 57, 351–362. [Google Scholar] [CrossRef]
- Ramoni, C.; Luciani, F.; Spadaro, F.; Lugini, L.; Lozupone, F.; Fais, S. Differential expression and distribution of ezrin, radixin and moesin in human natural killer cells. Eur. J. Immunol. 2002, 32, 3059–3065. [Google Scholar] [CrossRef]
- Scherer, S.S.; Xu, T.; Crino, P.; Arroyo, E.J.; Gutmann, D.H. Ezrin, radixin, and moesin are components of Schwann cell microvilli. J. Neurosci. Res. 2001, 65, 150–164. [Google Scholar] [CrossRef] [PubMed]
- Persson, A.; Lindwall, C.; Curtis, M.A.; Kuhn, H.G. Expression of ezrin radixin moesin proteins in the adult subventricular zone and the rostral migratory stream. Neuroscience 2010, 167, 312–322. [Google Scholar] [CrossRef] [PubMed]
- Persson, A.; Osman, A.; Bolouri, H.; Mallard, C.; Kuhn, H.G. Radixin expression in microglia after cortical stroke lesion. Glia 2013, 61, 790–799. [Google Scholar] [CrossRef] [PubMed]
- Hausrat, T.J.; Muhia, M.; Gerrow, K.; Thomas, P.; Hirdes, W.; Tsukita, S.; Heisler, F.F.; Herich, L.; Dubroqua, S.; Breiden, P.; et al. Radixin regulates synaptic GABAA receptor density and is essential for reversal learning and short-term memory. Nat. Commun. 2015, 6, 6872. [Google Scholar] [CrossRef]
- He, X.J.; Wang, W.R.; Zhang, Y.; Yang, Q. The effect of radixin knockdown on the expression and efflux function of MRP2 in SGC-7901 cells. Eur. J. Pharm. Sci. 2012, 46, 426–434. [Google Scholar] [CrossRef]
- Takai, Y.; Kitano, K.; Terawaki, S.; Maesaki, R.; Hakoshima, T. Structural basis of PSGL-1 binding to ERM proteins. Genes. Cells 2007, 12, 1329–1338. [Google Scholar] [CrossRef]
- Tang, P.; Cao, C.; Xu, M.; Zhang, L. Cytoskeletal protein radixin activates integrin alpha(M)beta(2) by binding to its cytoplasmic tail. FEBS Lett. 2007, 581, 1103–1108. [Google Scholar] [CrossRef]
- Austermann, J.; Nazmi, A.R.; Muller-Tidow, C.; Gerke, V. Characterization of the Ca2+ -regulated ezrin-S100P interaction and its role in tumor cell migration. J. Biol. Chem. 2008, 283, 29331–29340. [Google Scholar] [CrossRef]
- Dransfield, D.T.; Bradford, A.J.; Goldenring, J.R. Distribution of A-kinase anchoring proteins in parietal cells. Biochim. Biophys. Acta 1995, 1269, 215–220. [Google Scholar] [CrossRef][Green Version]
- Terawaki, S.; Kitano, K.; Aoyama, M.; Hakoshima, T. Crystallographic characterization of the radixin FERM domain bound to the cytoplasmic tail of membrane-type 1 matrix metalloproteinase (MT1-MMP). Acta Crystallogr. Sect. F Struct. Biol. Cryst. Commun. 2008, 64, 911–913. [Google Scholar] [CrossRef]
- Bretscher, A.; Chambers, D.; Nguyen, R.; Reczek, D. ERM-Merlin and EBP50 protein families in plasma membrane organization and function. Annu. Rev. Cell Dev. Biol. 2000, 16, 113–143. [Google Scholar] [CrossRef] [PubMed]
- Weinman, E.J.; Hall, R.A.; Friedman, P.A.; Liu-Chen, L.Y.; Shenolikar, S. The association of NHERF adaptor proteins with g protein-coupled receptors and receptor tyrosine kinases. Annu. Rev. Physiol. 2006, 68, 491–505. [Google Scholar] [CrossRef] [PubMed]
- LaLonde, D.P.; Bretscher, A. The scaffold protein PDZK1 undergoes a head-to-tail intramolecular association that negatively regulates its interaction with EBP50. Biochemistry 2009, 48, 2261–2271. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zachos, N.C.; Li, X.; Kovbasnjuk, O.; Hogema, B.; Sarker, R.; Lee, L.J.; Li, M.; de Jonge, H.; Donowitz, M. NHERF3 (PDZK1) contributes to basal and calcium inhibition of NHE3 activity in Caco-2BBe cells. J. Biol. Chem. 2009, 284, 23708–23718. [Google Scholar] [CrossRef]
- Ingham, P.W.; McMahon, A.P. Hedgehog signaling in animal development: Paradigms and principles. Genes. Dev. 2001, 15, 3059–3087. [Google Scholar] [CrossRef] [PubMed]
- McClatchey, A.I.; Fehon, R.G. Merlin and the ERM proteins--regulators of receptor distribution and signaling at the cell cortex. Trends Cell Biol. 2009, 19, 198–206. [Google Scholar] [CrossRef]
- Kobayashi, Y.; Harada, A.; Furuta, B.; Asou, H.; Kato, U.; Umeda, M. The role of NADRIN, a Rho GTPase-activating protein, in the morphological differentiation of astrocytes. J. Biochem. 2013, 153, 389–398. [Google Scholar] [CrossRef]
- Ding, N.; Li, P.; Li, H.; Lei, Y.; Zhang, Z. The ROCK-ezrin signaling pathway mediates LPS-induced cytokine production in pulmonary alveolar epithelial cells. Cell Commun. Signal 2022, 20, 65. [Google Scholar] [CrossRef]
- Zawawi, K.H.; Kantarci, A.; Schulze-Spate, U.; Fujita, T.; Batista, E.L., Jr.; Amar, S.; Van Dyke, T.E. Moesin-induced signaling in response to lipopolysaccharide in macrophages. J. Periodontal Res. 2010, 45, 589–601. [Google Scholar] [CrossRef]
- Liu, G.; Voyno-Yasenetskaya, T.A. Radixin stimulates Rac1 and Ca2+/calmodulin-dependent kinase, CaMKII: Cross-talk with Galpha13 signaling. J. Biol. Chem. 2005, 280, 39042–39049. [Google Scholar] [CrossRef]
- Valderrama, F.; Thevapala, S.; Ridley, A.J. Radixin regulates cell migration and cell-cell adhesion through Rac1. J. Cell Sci. 2012, 125, 3310–3319. [Google Scholar] [CrossRef] [PubMed]
- Arvidsson, A.; Collin, T.; Kirik, D.; Kokaia, Z.; Lindvall, O. Neuronal replacement from endogenous precursors in the adult brain after stroke. Nat. Med. 2002, 8, 963–970. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Yu, S.P.; Mohamad, O.; Genetta, T.; Wei, L. Sublethal transient global ischemia stimulates migration of neuroblasts and neurogenesis in mice. Transl. Stroke Res. 2010, 1, 184–196. [Google Scholar] [CrossRef] [PubMed]
- Osman, A.M.; Neumann, S.; Kuhn, H.G.; Blomgren, K. Caspase inhibition impaired the neural stem/progenitor cell response after cortical ischemia in mice. Oncotarget 2016, 7, 2239–2248. [Google Scholar] [CrossRef]
- Paglini, G.; Kunda, P.; Quiroga, S.; Kosik, K.; Caceres, A. Suppression of radixin and moesin alters growth cone morphology, motility, and process formation in primary cultured neurons. J. Cell Biol. 1998, 143, 443–455. [Google Scholar] [CrossRef]
- Castelo, L.; Jay, D.G. Radixin is involved in lamellipodial stability during nerve growth cone motility. Mol. Biol. Cell 1999, 10, 1511–1520. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Schafer, M.K.; Frotscher, M. Role of L1CAM for axon sprouting and branching. Cell Tissue Res. 2012, 349, 39–48. [Google Scholar] [CrossRef]
- Haas, M.A.; Vickers, J.C.; Dickson, T.C. Binding partners L1 cell adhesion molecule and the ezrin-radixin-moesin (ERM) proteins are involved in development and the regenerative response to injury of hippocampal and cortical neurons. Eur. J. Neurosci. 2004, 20, 1436–1444. [Google Scholar] [CrossRef]
- Prasad, S.; Vona, B.; Dineiro, M.; Costales, M.; Gonzalez-Aguado, R.; Fontalba, A.; Diego-Perez, C.; Subasioglu, A.; Bademci, G.; Tekin, M.; et al. Radixin modulates the function of outer hair cell stereocilia. Commun. Biol. 2020, 3, 792. [Google Scholar] [CrossRef]
- Kitajiri, S.; Fukumoto, K.; Hata, M.; Sasaki, H.; Katsuno, T.; Nakagawa, T.; Ito, J.; Tsukita, S.; Tsukita, S. Radixin deficiency causes deafness associated with progressive degeneration of cochlear stereocilia. J. Cell Biol. 2004, 166, 559–570. [Google Scholar] [CrossRef]
- Khan, S.Y.; Ahmed, Z.M.; Shabbir, M.I.; Kitajiri, S.; Kalsoom, S.; Tasneem, S.; Shayiq, S.; Ramesh, A.; Srisailpathy, S.; Khan, S.N.; et al. Mutations of the RDX gene cause nonsyndromic hearing loss at the DFNB24 locus. Hum. Mutat. 2007, 28, 417–423. [Google Scholar] [CrossRef] [PubMed]
- Hausrat, T.J.; Vogl, C.; Neef, J.; Schweizer, M.; Yee, B.K.; Strenzke, N.; Kneussel, M. Monoallelic loss of the F-actin-binding protein radixin facilitates startle reactivity and pre-pulse inhibition in mice. Front. Cell Dev. Biol. 2022, 10, 987691. [Google Scholar] [CrossRef] [PubMed]
- Loebrich, S.; Bahring, R.; Katsuno, T.; Tsukita, S.; Kneussel, M. Activated radixin is essential for GABAA receptor alpha5 subunit anchoring at the actin cytoskeleton. EMBO J. 2006, 25, 987–999. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Wang, S.; Wang, Z.; Dong, J.; Zhang, M.; Wang, Y.; Wang, J.; Jia, B.; Luo, Y.; Yin, Y. The changing of alpha5-GABAA receptors expression and distribution participate in sevoflurane-induced learning and memory impairment in young mice. CNS Neurosci. Ther. 2024, 30, e14716. [Google Scholar] [CrossRef]
- Kashimoto, R.; Yamanaka, H.; Kobayashi, K.; Okubo, M.; Yagi, H.; Mimura, O.; Noguchi, K. Phosphorylation of ezrin/radixin/moesin (ERM) protein in spinal microglia following peripheral nerve injury and lysophosphatidic acid administration. Glia 2013, 61, 338–348. [Google Scholar] [CrossRef]
- Qin, J.J.; Wang, J.M.; Du, J.; Zeng, C.; Han, W.; Li, Z.D.; Xie, J.; Li, G.L. Radixin knockdown by RNA interference suppresses human glioblastoma cell growth in vitro and in vivo. Asian Pac. J. Cancer Prev. 2014, 15, 9805–9812. [Google Scholar] [CrossRef][Green Version]
- Bartholow, T.L.; Becich, M.J.; Chandran, U.R.; Parwani, A.V. Immunohistochemical analysis of ezrin-radixin-moesin-binding phosphoprotein 50 in prostatic adenocarcinoma. BMC Urol. 2011, 11, 12. [Google Scholar] [CrossRef]
- Zhu, Y.W.; Yan, J.K.; Li, J.J.; Ou, Y.M.; Yang, Q. Knockdown of Radixin Suppresses Gastric Cancer Metastasis In Vitro by Up-Regulation of E-Cadherin via NF-kappaB/Snail Pathway. Cell Physiol. Biochem. 2016, 39, 2509–2521. [Google Scholar] [CrossRef]
- Jiang, Q.H.; Wang, A.X.; Chen, Y. Radixin enhances colon cancer cell invasion by increasing MMP-7 production via Rac1-ERK pathway. Sci. World J. 2014, 2014, 340271. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.D.; Song, M.M.; Zhong, Z.Q.; Li, N.; Wang, P.L.; Cheng, S.; Bai, R.X.; Yuan, H.S. Knockdown of radixin by RNA interference suppresses the growth of human pancreatic cancer cells in vitro and in vivo. Asian Pac. J. Cancer Prev. 2012, 13, 753–759. [Google Scholar] [CrossRef][Green Version]
- Kobori, T.; Ito, Y.; Doukuni, R.; Urashima, Y.; Ito, T.; Obata, T. Radixin modulates the plasma membrane localization of CD47 in human uterine cervical adenocarcinoma cells. J. Reprod. Immunol. 2023, 158, 103982. [Google Scholar] [CrossRef] [PubMed]
- Kobori, T.; Ito, Y.; Sawada, Y.; Urashima, Y.; Ito, T.; Obata, T. Cellular Membrane Localization of Innate Immune Checkpoint Molecule CD47 Is Regulated by Radixin in Human Pancreatic Ductal Adenocarcinoma Cells. Biomedicines 2023, 11, 1117. [Google Scholar] [CrossRef] [PubMed]
- Tokunou, M.; Niki, T.; Saitoh, Y.; Imamura, H.; Sakamoto, M.; Hirohashi, S. Altered expression of the ERM proteins in lung adenocarcinoma. Lab. Investig. 2000, 80, 1643–1650. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kobori, T.; Tanaka, C.; Tameishi, M.; Urashima, Y.; Ito, T.; Obata, T. Role of Ezrin/Radixin/Moesin in the Surface Localization of Programmed Cell Death Ligand-1 in Human Colon Adenocarcinoma LS180 Cells. Pharmaceuticals 2021, 14, 864. [Google Scholar] [CrossRef]
- Kawase, A.; Inoue, Y.; Nakazaki, S.; Koizumi, E.; Iwaki, M. Radixin knockdown improves the accumulation and efficiency of methotrexate in tumor cells. Oncol. Rep. 2019, 42, 283–290. [Google Scholar] [CrossRef]
- Yang, Q.; Onuki, R.; Nakai, C.; Sugiyama, Y. Ezrin and radixin both regulate the apical membrane localization of ABCC2 (MRP2) in human intestinal epithelial Caco-2 cells. Exp. Cell Res. 2007, 313, 3517–3525. [Google Scholar] [CrossRef]
- Kojima, H.; Nies, A.T.; Konig, J.; Hagmann, W.; Spring, H.; Uemura, M.; Fukui, H.; Keppler, D. Changes in the expression and localization of hepatocellular transporters and radixin in primary biliary cirrhosis. J. Hepatol. 2003, 39, 693–702. [Google Scholar] [CrossRef] [PubMed]
- Kamioka, H.; Edaki, K.; Kasahara, H.; Tomono, T.; Yano, K.; Ogihara, T. Drug resistance via radixin-mediated increase of P-glycoprotein membrane expression during SNAI1-induced epithelial-mesenchymal transition in HepG2 cells. J. Pharm. Pharmacol. 2021, 73, 1609–1616. [Google Scholar] [CrossRef]
- McRobert, E.A.; Gallicchio, M.; Jerums, G.; Cooper, M.E.; Bach, L.A. The amino-terminal domains of the ezrin, radixin, and moesin (ERM) proteins bind advanced glycation end products, an interaction that may play a role in the development of diabetic complications. J. Biol. Chem. 2003, 278, 25783–25789. [Google Scholar] [CrossRef]
- Bach, L.A.; Gallicchio, M.A.; McRobert, E.A.; Tikoo, A.; Cooper, M.E. Effects of advanced glycation end products on ezrin-dependent functions in LLC-PK1 proximal tubule cells. Ann. N. Y. Acad. Sci. 2005, 1043, 609–616. [Google Scholar] [CrossRef]
- McRobert, E.A.; Tikoo, A.; Cooper, M.E.; Bach, L.A. Localization of the ezrin binding epitope for advanced glycation endproducts. Int. J. Biochem. Cell Biol. 2008, 40, 1570–1580. [Google Scholar] [CrossRef] [PubMed]
- McRobert, E.A.; Young, A.N.; Bach, L.A. Advanced glycation end-products induce calpain-mediated degradation of ezrin. FEBS J. 2012, 279, 3240–3250. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Fan, A.; Yuan, Y.; Chen, L.; Guo, X.; Huang, X.; Huang, Q. Role of Moesin in Advanced Glycation End Products-Induced Angiogenesis of Human Umbilical Vein Endothelial Cells. Sci. Rep. 2016, 6, 22749. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.S.; Hu, J.Q.; Liu, X.H.; Chen, L.X.; Chen, H.; Guo, X.H.; Huang, Q.B. Role of Moesin Phosphorylation in Retinal Pericyte Migration and Detachment Induced by Advanced Glycation Endproducts. Front. Endocrinol. 2020, 11, 603450. [Google Scholar] [CrossRef]
- Kawase, A.; Sakata, M.; Yada, N.; Nakasaka, M.; Shimizu, T.; Kato, Y.; Iwaki, M. Decreased radixin function for ATP-binding cassette transporters in liver in adjuvant-induced arthritis rats. J. Pharm. Sci. 2014, 103, 4058–4065. [Google Scholar] [CrossRef]
- Kawase, A.; Nakasaka, M.; Bando, H.; Yasuda, S.; Shimada, H.; Iwaki, M. Changes in Radixin Expression and Interaction with Efflux Transporters in the Liver of Adjuvant-Induced Arthritic Rats. Inflammation 2020, 43, 85–94. [Google Scholar] [CrossRef] [PubMed]
- Dellbrugge, F.; Jesse, L.D.; Medyukhina, A.; Liu, N.; Neugebauer, S.; Freissmuth, M.; Hoppener, S.; Figge, M.T.; Morrison, H.; Riecken, L.B.; et al. Contribution of radixin and ezrin to the maintenance of hepatocytes’ excretory function in health and disease. Heliyon 2023, 9, e21009. [Google Scholar] [CrossRef]
- Suda, J.; Zhu, L.; Karvar, S. Phosphorylation of radixin regulates cell polarity and Mrp-2 distribution in hepatocytes. Am. J. Physiol. Cell Physiol. 2011, 300, C416–C424. [Google Scholar] [CrossRef]
- Wang, W.; Soroka, C.J.; Mennone, A.; Rahner, C.; Harry, K.; Pypaert, M.; Boyer, J.L. Radixin is required to maintain apical canalicular membrane structure and function in rat hepatocytes. Gastroenterology 2006, 131, 878–884. [Google Scholar] [CrossRef]
- Bukong, T.N.; Kodys, K.; Szabo, G. Human ezrin-moesin-radixin proteins modulate hepatitis C virus infection. Hepatology 2013, 58, 1569–1579. [Google Scholar] [CrossRef]
- Bukong, T.N.; Kodys, K.; Szabo, G. A Novel Human Radixin Peptide Inhibits Hepatitis C Virus Infection at the Level of Cell Entry. Int. J. Pept. Res. Ther. 2014, 20, 269–276. [Google Scholar] [CrossRef] [PubMed]
- Rayaprolu, S.; Gao, T.; Xiao, H.; Ramesha, S.; Weinstock, L.D.; Shah, J.; Duong, D.M.; Dammer, E.B.; Webster, J.A., Jr.; Lah, J.J.; et al. Flow-cytometric microglial sorting coupled with quantitative proteomics identifies moesin as a highly-abundant microglial protein with relevance to Alzheimer’s disease. Mol. Neurodegener. 2020, 15, 28. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; Bradshaw, W.J.; Leisner, T.M.; Annor-Gyamfi, J.K.; Qian, K.; Bashore, F.M.; Sikdar, A.; Nwogbo, F.O.; Ivanov, A.A.; Frye, S.V.; et al. Discovery of FERM domain protein-protein interaction inhibitors for MSN and CD44 as a potential therapeutic approach for Alzheimer’s disease. J. Biol. Chem. 2023, 299, 105382. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.; Huang, J.; Jiang, Y.; Qiu, J.; Li, T.; Li, W.; Chen, Z.; Huang, Z.; Yu, X.; Yang, T.; et al. Intercellular adhesion molecule 2 as a novel prospective tumor suppressor induced by ERG promotes ubiquitination-mediated radixin degradation to inhibit gastric cancer tumorigenicity and metastasis. J. Transl. Med. 2023, 21, 670. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.Y.; Wei, P.L.; Batzorig, U.; Makondi, P.T.; Lee, C.C.; Chang, Y.J. Identification of Moesin (MSN) as a Potential Therapeutic Target for Colorectal Cancer via the beta-Catenin-RUNX2 Axis. Int. J. Mol. Sci. 2023, 24, 10951. [Google Scholar] [CrossRef]
- Song, J.Y.; Stastny, J.; Fosslien, E.; Robertson, A.L., Jr. Aging of human aortic intima proteins. Zhonghua Xin Xue Guan Bing Za Zhi 1987, 15, 356–360, 70, 16. [Google Scholar]
| Phosphorylated Residues | Kinases | Function | References |
|---|---|---|---|
| Thr567 of Ezrin | GRK2 | G protein-coupled receptor-dependent reorganization of the actin cytoskeleton. Regulates membrane protrusion and motility in epithelial cells. | [11,12] |
| NIK | Lamellipodium extension induced by growth factors. | [13] | |
| LOK | Regulates cytoskeletal rearrangement of lymphocytes. | [14] | |
| PKC | Regulates osteosarcoma cell migration. Regulates endothelial permeability. | [19,20] | |
| Thr235 of Ezrin | CDK5 | Mediates pRb-induced cell shape changes in senescent cells. | [21,22] |
| Thr558 of Moesin | RhoK | Mediates the formation of microvilli-like structures. Mediates glutamate-induced phosphorylation in neurons and post-injury regeneration of neurons. | [23,24,25] |
| Akt | Mediates neurite formation in vitro. | [26] | |
| Thr567 of Ezrin Thr558 of Moesin | ROCK | Involved in the early steps of apoptotic signaling following Fas triggering and regulates apoptosis induction. | [27] |
| Cdc42 | Involved in the formation of filopodia. | [28] | |
| PKC | Regulates endothelial permeability. | [20] | |
| Thr564 of Radixin | Akt | Involves neurite outgrowth and growth cone formation. | [9] |
| GRK2 | Regulates membrane protrusion and motility in epithelial cells. | [12] | |
| PKC | Regulates endothelial permeability. | [20] | |
| Thr573 of Radixin | Akt | Stabilizes radixin interactions with F-actin to regulate neurite outgrowth. Inhibits ubiquitin-dependent proteasomal degradation of radixin. | [9] |
| Thr567 of Ezrin Thr558 of Moesin Thr564 of Radixin | LRRK2 | Involved in neuronal growth cone development. | [29,30] |
| Tyr145, 353, and 477 of Ezrin | SK ITK | Phosphorylation of Tyr145 and 477 involves cell adhesion and migration, whereas Tyr353 phosphorylation regulates the reorganization of the actin cytoskeleton and activation of B cells. | [31,32,33] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chong, Z.Z.; Souayah, N. Radixin: Roles in the Nervous System and Beyond. Biomedicines 2024, 12, 2341. https://doi.org/10.3390/biomedicines12102341
Chong ZZ, Souayah N. Radixin: Roles in the Nervous System and Beyond. Biomedicines. 2024; 12(10):2341. https://doi.org/10.3390/biomedicines12102341
Chicago/Turabian StyleChong, Zhao Zhong, and Nizar Souayah. 2024. "Radixin: Roles in the Nervous System and Beyond" Biomedicines 12, no. 10: 2341. https://doi.org/10.3390/biomedicines12102341
APA StyleChong, Z. Z., & Souayah, N. (2024). Radixin: Roles in the Nervous System and Beyond. Biomedicines, 12(10), 2341. https://doi.org/10.3390/biomedicines12102341





