Three-Dimensional Planimetry Assessment of Dental Plaque-Covered Area Reduction after Rinsing with 0.2% Sodium Hypochlorite Solution as Part of a Guided Biofilm Therapy® Protocol—Pilot Longitudinal Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
- Patients systemically healthy with at least 20 teeth present (natural or artificial crowns), evenly distributed between the two arches;
- Patients willing to participate and who signed the informed consent;
- Patients not having practiced oral hygiene for 24 h before the procedure.
- Patients with known intolerances, allergies, and hypersensitivity to the components of the plaque-disclosing agent (glycine, etil paraben, Cetilpiridiniumclorid, Povidone Iodine, Erytrosine, blue dye) and NaOCl.
2.2. Clinical Procedures
2.3. Data Acquisition
2.4. Data Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gunsolley, J.C. Clinical efficacy of antimicrobial mouthrinses. J. Dent. 2010, 38, S6–S10. [Google Scholar] [CrossRef] [PubMed]
- Hossainian, N.; Slot, D.; Afennich, F.; Van der Weijden, G. The effects of hydrogen peroxide mouthwashes on the prevention of plaque and gingival inflammation: A systematic review. Int. J. Dent. Hyg. 2011, 9, 171–181. [Google Scholar] [CrossRef] [PubMed]
- Chaves, E.S.; Kornman, K.S.; Manwell, M.A.; Jones, A.A.; Newbold, D.A.; Wood, R.C. Mechanism of irrigation effects on gingivitis. J. Periodontol. 1994, 65, 1016–1021. [Google Scholar] [CrossRef] [PubMed]
- Hase, J.; Edwardsson, S.; Rundegren, J.; Attström, R.; Kelty, E. 6-month use of 0.2% delmopinol hydrochloride in comparison with 0.2% chlorhexidine digluconate and placebo (II). Effect on plaque and salivary microflora. J. Clin. Periodontol. 1998, 25, 841–849. [Google Scholar] [CrossRef] [PubMed]
- Van Leeuwen, M.; Rosema, N.; Versteeg, P.; Slot, D.; Van Winkelhoff, A.; Van der Weijden, G. Long-term efficacy of a 0.07% cetylpyridinium chloride mouth rinse in relation to plaque and gingivitis: A 6-month randomized, vehicle-controlled clinical trial. Int. J. Dent. Hyg. 2015, 13, 93–103. [Google Scholar] [CrossRef]
- Farid Ayad, B.; Prado, R.; Dentales, D.E.; Mateo, L.R.; Stewart, B.; BSEng, M.G.S.; Arvanitidou, E.; Panagakos, P.F.S. A comparative investigation to evaluate the clinical efficacy of an alcohol-free CPC-containing mouthwash as compared to a control mouthwash in controlling dental plaque and gingivitis: A six-month clinical study on adults in San Jose, Costa Rica. J. Clin. Dent. 2011, 204. [Google Scholar] [CrossRef]
- Triratana, T.; Kraivaphan, P.; Tandhachoon, K.; Rustogi, K.; Volpe, A.; Petrone, M. Effect of a pre-brush mounthrinse containing triclosan and a copolymer on calculus formation: A three-month clinical study in Thailand. J. Clin. Dent. 1995, 6, 139–141. [Google Scholar]
- Charles, C.H.; Sharma, N.C.; GALUSTIANS, H.J.; Qaqish, J.; MCGUIRE, J.A.; Vincent, J.W. Comparative efficacy of an antiseptic mouthrinse and an antiplaque/antigingivitis dentifrice: A six-month clinical trial. J. Am. Dent. Assoc. 2001, 132, 670–675. [Google Scholar] [CrossRef]
- Stojicic, S.; Zivkovic, S.; Qian, W.; Zhang, H.; Haapasalo, M. Tissue dissolution by sodium hypochlorite: Effect of concentration, temperature, agitation, and surfactant. J. Endod. 2010, 36, 1558–1562. [Google Scholar] [CrossRef]
- Galván, M.; Gonzalez, S.; Cohen, C.L.; Alonaizan, F.A.; Chen, C.T.; Rich, S.K.; Slots, J. Periodontal effects of 0.25% sodium hypochlorite twice-weekly oral rinse. A pilot study. J. Periodontal Res. 2014, 49, 696–702. [Google Scholar] [CrossRef]
- Gonzalez, S.; Cohen, C.L.; Galván, M.; Alonaizan, F.A.; Rich, S.K.; Slots, J. Gingival bleeding on probing: Relationship to change in periodontal pocket depth and effect of sodium hypochlorite oral rinse. J. Periodontal Res. 2015, 50, 397–402. [Google Scholar] [CrossRef] [PubMed]
- Espíndola, L.C.P.; Colombo, A.P.V. Lack of adjunctive effect of 0.1% sodium hypochlorite mouthwash combined to full-mouth ultrasonic debridement on supragingival plaque, gingival inflammation, and subgingival microbiota: A randomized placebo-controlled 6-month trial. Clin. Exp. Dent. Res. 2017, 3, 51–61. [Google Scholar] [CrossRef] [PubMed]
- Kardaras, G.; Marcovici, I.; Rusu, D.; Dehelean, C.; Coricovac, D.; Iorio-Siciliano, V.; Sculean, A.; Stratul, S.I. In-Vitro Safety Evaluation of Sodium Hypochlorite (NaOCl) as Part of Step 2 and Maintenance Therapy Protocols in Patients with Periodontitis Stages III–IV. Oral Health Prev. Dent. 2023, 21, 103–112. [Google Scholar] [PubMed]
- EMS Dental: Guided Biofilm Therapy. Available online: https://www.ems-dental.com/en/guided-biofilm-therapy (accessed on 1 July 2024).
- Sagel, P.A.; Lapujade, P.G.; Miller, J.M.; Sunberg, R.J. Objective quantification of plaque using digital image analysis. Monogr. Oral Sci. 2000, 17, 130–143. [Google Scholar] [PubMed]
- Pretty, I.; Edgar, W.; Smith, P.; Higham, S. Quantification of dental plaque in the research environment. J. Dent. 2005, 33, 193–207. [Google Scholar] [CrossRef]
- Fasoulas, A.; Pavlidou, E.; Petridis, D.; Mantzorou, M.; Seroglou, K.; Giaginis, C. Detection of dental plaque with disclosing agents in the context of preventive oral hygiene training programs. Heliyon 2019, 5, e02064. [Google Scholar] [CrossRef]
- Lang, N.P.; ØStergaard, E.; Löe, H. A fluorescent plaque disclosing agnent. J. Periodontal Res. 1972, 7, 59–67. [Google Scholar] [CrossRef]
- Gonçalves, M.L.L.; Sobral, A.P.T.; Gallo, J.M.A.S.; Gimenez, T.; Ferri, E.P.; Ianello, S.; de Barros Motta, P.; Motta, L.J.; Horliana, A.C.R.T.; Santos, E.M. Antimicrobial photodynamic therapy with erythrosine and blue light on dental biofilm bacteria: Study protocol for randomised clinical trial. BMJ Open 2023, 13, e075084. [Google Scholar] [CrossRef]
- Chowdhary, Z.; Mohan, R.; Sharma, V.; Rai, R.; Das, A. Disclosing agents in periodontics: An update. J. Dent. Coll. Azamgarh 2015, 1, 103–110. [Google Scholar]
- Nayak, D.G.; Upoor, A.; Mahesh, C.P. Textbook of Periodontology and Oral Implantology, 2nd ed.; Reed Elsevier India Pvt. Ltd.: New Delhi, India, 2015. [Google Scholar]
- Chetruş, V.; Ion, I. Dental plaque-classification, formation, and identification. Int. J. Med. Dent. 2013, 3, 139–143. [Google Scholar]
- Silva, D.D.d.; Gonçalo, C.d.S.; Sousa, M.d.L.R.d.; Wada, R.S. Aggregation of plaque disclosing agent in a dentifrice. J. Appl. Oral Sci. 2004, 12, 154–158. [Google Scholar] [CrossRef] [PubMed]
- Yeganeh, S.; Lynch, E.; Jovanovski, V.; Zou, L. Quantification of root surface plaque using a new 3-D laser scanning method. J. Clin. Periodontol. 1999, 26, 692–697. [Google Scholar] [CrossRef] [PubMed]
- Jovanovski, V.; Lynch, E. Analysis of the morphology of oral structures from 3-D co-ordinate data. Monogr. Oral Sci. 2000, 17, 73–129. [Google Scholar] [PubMed]
- Mensi, M.; Scotti, E.; Sordillo, A.; Agosti, R.; Calza, S. Plaque disclosing agent as a guide for professional biofilm removal: A randomized controlled clinical trial. Int. J. Dent. Hyg. 2020, 18, 285–294. [Google Scholar] [CrossRef] [PubMed]
- Giese-Kraft, K.; Jung, K.; Schlueter, N.; Vach, K.; Ganss, C. Detecting and monitoring dental plaque levels with digital 2D and 3D imaging techniques. PLoS ONE 2022, 17, e0263722. [Google Scholar] [CrossRef]
- Jung, K.; Giese-Kraft, K.; Fischer, M.; Schulze, K.; Schlueter, N.; Ganss, C. Visualization of dental plaque with a 3D-intraoral-scanner—A tool for whole mouth planimetry. PLoS ONE 2022, 17, e0276686. [Google Scholar] [CrossRef]
- Guo, S.; Chen, H.; Zhang, Y.; Gao, L.; Wu, F.; Sun, Y. Establishment and evaluation of a 3D quantitative analysis method for dental plaque based on an intraoral scanner technique. Int. J. Comput. Dent. 2024, 27, 141–149. [Google Scholar]
- Doi, K.; Yoshiga, C.; Oue, H.; Kobatake, R.; Kawagoe, M.; Umehara, H.; Wakamatsu, K.; Tsuga, K. Comparison of plaque control record measurements obtained using intraoral scanner and direct visualization. Clin. Exp. Dent. Res. 2024, 10, e852. [Google Scholar] [CrossRef]
- Yoshiga, C.; Doi, K.; Oue, H.; Kobatake, R.; Kawagoe, M.; Umehara, H.; Tsuga, K. Utility of intraoral scanner imaging for dental plaque detection. Imaging Sci. Dent. 2024, 54, 43. [Google Scholar] [CrossRef]
- Jung, K.; Giese-Kraft, K.; Schlenz, M.A.; Wöstmann, B.; Ganss, C. Digital plaque monitoring: An evaluation of different intraoral scanners. J. Dent. 2024, 145, 104978. [Google Scholar] [CrossRef]
- EMS. BIOFILM DISCLOSER. Available online: https://www.ems-dental.com/en/products/biofilm-discloser (accessed on 1 July 2024).
- Furey, E. Mean, Median, Mode Calculator. Available online: https://www.calculatorsoup.com/calculators/statistics/mean-median-mode.php (accessed on 1 July 2024).
- Van Strydonck, D.A.; Slot, D.E.; Van der Velden, U.; Van der Weijden, F. Effect of a chlorhexidine mouthrinse on plaque, gingival inflammation and staining in gingivitis patients: A systematic review. J. Clin. Periodontol. 2012, 39, 1042–1055. [Google Scholar] [CrossRef] [PubMed]
- Stiefel, P.; Rosenberg, U.; Schneider, J.; Mauerhofer, S.; Maniura-Weber, K.; Ren, Q. Is biofilm removal properly assessed? Comparison of different quantification methods in a 96-well plate system. Appl. Microbiol. Biotechnol. 2016, 100, 4135–4145. [Google Scholar] [CrossRef] [PubMed]
- Doll, K.; Jongsthaphongpun, K.L.; Stumpp, N.S.; Winkel, A.; Stiesch, M. Quantifying implant-associated biofilms: Comparison of microscopic, microbiologic and biochemical methods. J. Microbiol. Methods 2016, 130, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Di Domenico, E.G.; Toma, L.; Provot, C.; Ascenzioni, F.; Sperduti, I.; Prignano, G.; Gallo, M.T.; Pimpinelli, F.; Bordignon, V.; Bernardi, T. Development of an in vitro assay, based on the biofilm ring test®, for rapid profiling of biofilm-growing bacteria. Front. Microbiol. 2016, 7, 1429. [Google Scholar] [CrossRef] [PubMed]
- Sanz, M.; Herrera, D.; Kebschull, M.; Chapple, I.; Jepsen, S.; Beglundh, T.; Sculean, A.; Tonetti, M.S.; Participants, E.F.P.W.; Methodological, C. Treatment of stage I-III periodontitis-The EFP S3 level clinical practice guideline. J. Clin. Periodontol. 2020, 47 (Suppl. 22), 4–60. [Google Scholar] [CrossRef] [PubMed]
- Slots, J. Primer on etiology and treatment of progressive/severe periodontitis: A systemic health perspective. Periodontology 2000 2020, 83, 272–276. [Google Scholar] [CrossRef]
- Roseblade, A.; Ung, A.; Bebawy, M. Synthesis and in vitro biological evaluation of thiosulfinate derivatives for the treatment of human multidrug-resistant breast cancer. Acta Pharmacol. Sin. 2017, 38, 1353–1368. [Google Scholar] [CrossRef]
- Slots, J.; Jorgensen, M.G. Effective, safe, practical and affordable periodontal antimicrobial therapy: Where are we going, and are we there yet? Periodontology 2000 2002, 28, 298–312. [Google Scholar] [CrossRef]
- American Dental Association; Council on Dental Therapeutics. Accepted Dental Therapeutics; Council on Dental Therapeutics of the American Dental Association: Chicago, IL, USA, 1984; Volume 40. [Google Scholar]
- Adcock, J.E.; Wc, B., Jr.; Kalkwarf, K.L. Effect of sodium hypochlorite solution on the subgingival microflora of juvenile periodontitis lesions. Pediatr. Dent. 1983, 5, 190–194. [Google Scholar]
- Bizzarro, S.; Van der Velden, U.; Loos, B.G. Local disinfection with sodium hypochlorite as adjunct to basic periodontal therapy: A randomized controlled trial. J. Clin. Periodontol. 2016, 43, 778–788. [Google Scholar] [CrossRef]
- Iorio-Siciliano, V.; Ramaglia, L.; Isola, G.; Blasi, A.; Salvi, G.E.; Sculean, A. Changes in clinical parameters following adjunctive local sodium hypochlorite gel in minimally invasive nonsurgical therapy (MINST) of periodontal pockets: A 6-month randomized controlled clinical trial. Clin. Oral Investig. 2021, 25, 5331–5340. [Google Scholar] [CrossRef] [PubMed]
- Sedghi, L.M.; Bacino, M.; Kapila, Y.L. Periodontal Disease: The Good, The Bad, and The Unknown. Front. Cell. Infect. Microbiol. 2021, 11, 766944. [Google Scholar] [CrossRef] [PubMed]
- Slaughter, R.J.; Watts, M.; Vale, J.A.; Grieve, S., Jr.; Schep, L.J. The clinical toxicology of sodium hypochlorite. Clin. Toxicol. 2019, 57, 303–311. [Google Scholar] [CrossRef] [PubMed]
- Kinoshita, S.; Schait, A.; Brebou, M.; Mühlemann, H. Effects of sucrose on early dental calculus and plaque. Helv. Odontol. Acta 1966, 10, 134–137. [Google Scholar] [PubMed]
- Staudt, C.B.; Kinzel, S.; Haßfeld, S.; Stein, W.; Staehle, H.J.; Dörfer, C.E. Computer-based intraoral image analysis of the clinical plaque removing capacity of 3 manual toothbrushes. J. Clin. Periodontol. 2001, 28, 746–752. [Google Scholar] [CrossRef]
- Pretty, I.; Ingram, G.; Agalamanyi, E.; Edgar, W.; Higham, S. The use of fluorescein-enhanced quantitative light-induced fluorescence to monitor de-and re-mineralization of in vitro root caries. J. Oral Rehabil. 2003, 30, 1151–1156. [Google Scholar] [CrossRef]
- Pretty, I.; Edgar, W.; Higham, S. The validation of quantitative light-induced fluorescence to quantify acid erosion of human enamel. Arch. Oral Biol. 2004, 49, 285–294. [Google Scholar] [CrossRef]
- Kardaras, G.; Christodorescu, R.; Boariu, M.; Rusu, D.; Belova, A.; Chinnici, S.; Vela, O.; Radulescu, V.; Boia, S.; Stratul, S.-I. A Low-Cost Protocol Using the Adjunctive Action of Povidone–Iodine Irrigations and Sodium Hypochlorite Rinsing Solution in Step 2 of Periodontal Therapy for Patients with Stage III–IV Periodontitis: A Single-Blind, Randomized Controlled Trial. Dent. J. 2024, 12, 144. [Google Scholar] [CrossRef]


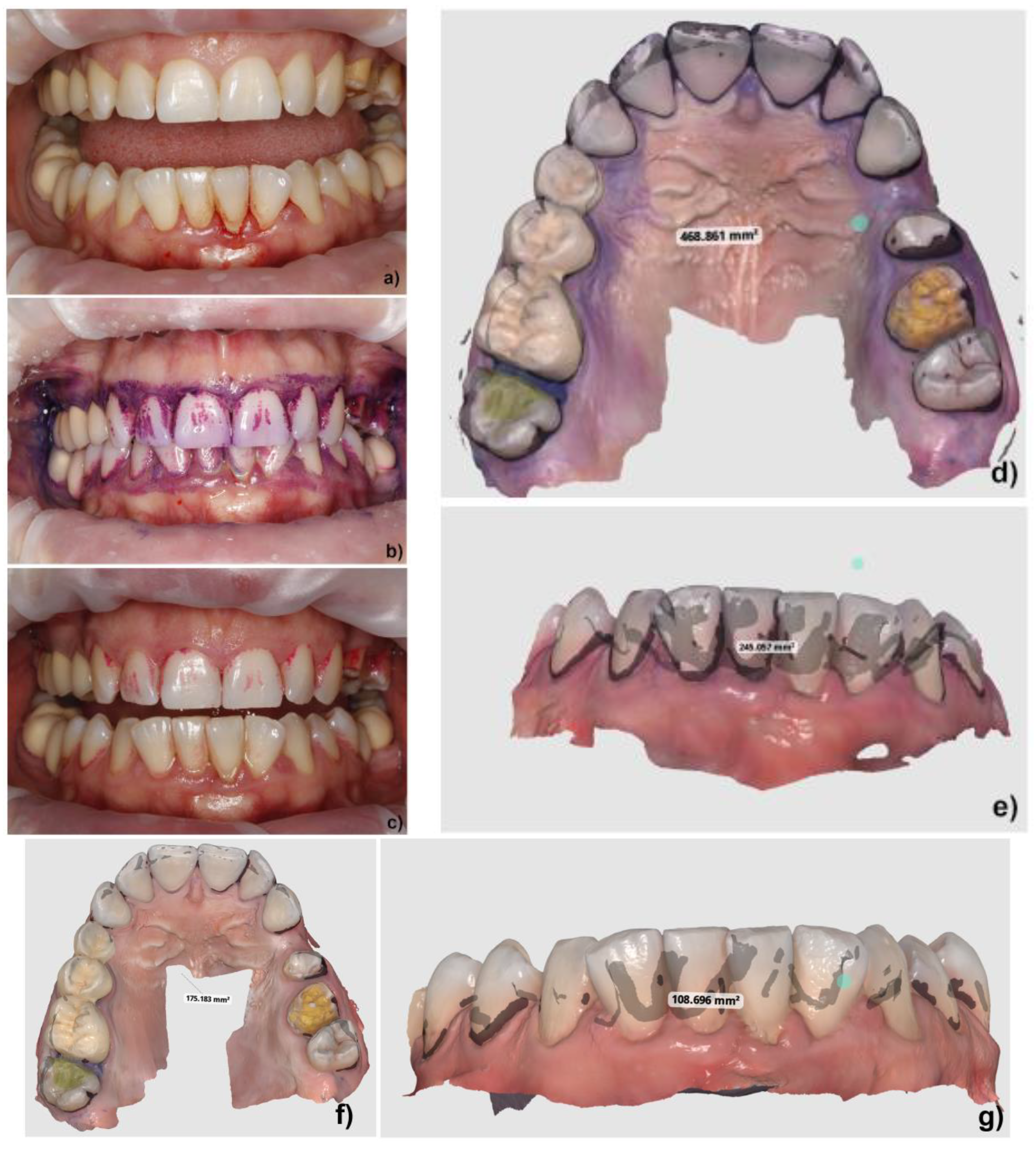
| Upper Arch | Before Rinsing (mm2) | After Rinsing (mm2) | Difference (mm2) | Difference (%) |
|---|---|---|---|---|
| Minimum value | 138.12 mm2 | 69.34 mm2 | 42.98 mm2 | 20.01 mm2 % |
| Maximum value | 875.57 mm2 | 595.11 mm2 | 293.67 mm2 | 62.64 mm2 % |
| Range | 737.45 mm2 | 525.76 mm2 | 250.69 mm2 | 42.63 mm2 % |
| Sum | 7155.55 mm2 | 2356.26 mm2 | 1450.58 mm2 | 317.21 mm2 % |
| Mean | 475.85 mm2 | 294.53 mm2 | 181.32 mm2 | 39.65 mm2 % |
| Median | 512.54 mm2 | 240.19 mm2 | 174.00 mm2 | 40.74 mm2 % |
| Standard deviation | 242.59 mm2 | 187.39 mm2 | 100.87 mm2 | 15.72 mm2 % |
| Variance | 58,852.35 mm2 | 35,118.18 mm2 | 10,175.34 mm2 | 247.22 mm2 % |
| Lower Arch | Before Rinsing (mm2) | After Rinsing (mm2) | Difference (mm2) | Difference (%) |
|---|---|---|---|---|
| Minimum value | 143.36 mm2 | 63.82 mm2 | 12.77 mm2 | 8.91 mm2 % |
| Maximum value | 701.33 mm2 | 595.22 mm2 | 347.93 mm2 | 75.62 mm2 % |
| Range | 557.96 mm2 | 531.39 mm2 | 335.16 mm2 | 66.71 mm2 % |
| Sum | 3348.70 mm2 | 2086.42 mm2 | 1262.28 mm2 | 306.08 mm2 % |
| Mean | 418.58 mm2 | 260.80 mm2 | 157.78 mm2 | 38.26 mm2 % |
| Median | 383.98 mm2 | 270.09 mm2 | 149.81 mm2 | 42.86 mm2 % |
| Standard deviation | 206.42 mm2 | 169.53 mm2 | 107.84 mm2 | 23.64 mm2 % |
| Variance | 42,610.09 mm2 | 28,743.66 mm2 | 11,630.94 mm2 | 559.18 mm2 % |
| Full-Mouth Data | Before Rinsing (mm2) | After Rinsing (mm2) | Difference (mm2) | Difference (%) |
|---|---|---|---|---|
| Minimum value | 138.12 mm2 | 63.82 mm2 | 12.77 mm2 | 8.91 mm2 % |
| Maximum value | 875.57 mm2 | 595.22 mm2 | 347.93 mm2 | 75.62 mm2 % |
| Range | 737.45 mm2 | 531.39 mm2 | 335.16 mm2 | 66.71 mm2 % |
| Sum | 7155.55 mm2 | 4442.68 mm2 | 2712.86 mm2 | 623.29 mm2 % |
| Mean | 447.22 mm2 | 277.66 mm2 | 169.55 mm2 | 38.95 mm2 % |
| Median | 434.34 mm2 | 267.98 mm2 | 156.89 mm2 | 42.86 mm2 % |
| Standard deviation | 219.59 mm2 | 173.50 mm2 | 101.60 mm2 | 19.41 mm2 % |
| Variance | 48,223.69 mm2 | 30,105.57 mm2 | 10,324.01 mm2 | 376.83 mm2 % |
| Basic Statistical Measures | ||||||
|---|---|---|---|---|---|---|
| Location | Variability | |||||
| Mean | 39.65 | Std Deviation | 15.72 | |||
| Median | 40.74 | Variance | 247.23 | |||
| Range | 42.63 | |||||
| Interquartile Range | 25.44 | |||||
| Basic Confidence Limits Assuming Normality | ||||||
| Parameter | Estimate | 95% Confidence Limits | ||||
| Mean | 39.65 | 26.51 | 52.80 | |||
| Tests for Location: Mu0 = 15 | ||||||
| Test | Statistic | p value | ||||
| Student’s t-test | t | 4.43 | Pr > ItI | 0.003 | ||
| Tests for Normality | ||||||
| Test | Statistic | p value | ||||
| Kolmogorov–Smirnov | 0.23 | Pr > D | >0.150 | |||
| Basic Statistical Measures | ||||||
|---|---|---|---|---|---|---|
| Location | Variability | |||||
| Mean | 38.26 | Std Deviation | 23.65 | |||
| Median | 42.86 | Variance | 559.18 | |||
| Range | 66.71 | |||||
| Interquartile Range | 39.02 | |||||
| Basic Confidence Limits Assuming Normality | ||||||
| Parameter | Estimate | 95% Confidence Limits | ||||
| Mean | 38.26 | 18.49 | 58.03 | |||
| Tests for Location: Mu0 = 15 | ||||||
| Test | Statistic | p value | ||||
| Student’s t-test | t | 2.78 | Pr > ItI | 0.027 | ||
| Tests for Normality | ||||||
| Test | Statistic | p value | ||||
| Kolmogorov–Smirnov | D | 0.21 | Pr > D | >0.150 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kardaras, G.; Boariu, M.; Varlamov, V.; Vintila, C.; Boia, S.; Belova, A.; Rusu, D.; Machoy, M.; Solomon, S.M.; Stratul, S.-I. Three-Dimensional Planimetry Assessment of Dental Plaque-Covered Area Reduction after Rinsing with 0.2% Sodium Hypochlorite Solution as Part of a Guided Biofilm Therapy® Protocol—Pilot Longitudinal Study. Biomedicines 2024, 12, 2326. https://doi.org/10.3390/biomedicines12102326
Kardaras G, Boariu M, Varlamov V, Vintila C, Boia S, Belova A, Rusu D, Machoy M, Solomon SM, Stratul S-I. Three-Dimensional Planimetry Assessment of Dental Plaque-Covered Area Reduction after Rinsing with 0.2% Sodium Hypochlorite Solution as Part of a Guided Biofilm Therapy® Protocol—Pilot Longitudinal Study. Biomedicines. 2024; 12(10):2326. https://doi.org/10.3390/biomedicines12102326
Chicago/Turabian StyleKardaras, Georgios, Marius Boariu, Vadym Varlamov, Claudiu Vintila, Simina Boia, Alla Belova, Darian Rusu, Monika Machoy, Sorina Mihaela Solomon, and Stefan-Ioan Stratul. 2024. "Three-Dimensional Planimetry Assessment of Dental Plaque-Covered Area Reduction after Rinsing with 0.2% Sodium Hypochlorite Solution as Part of a Guided Biofilm Therapy® Protocol—Pilot Longitudinal Study" Biomedicines 12, no. 10: 2326. https://doi.org/10.3390/biomedicines12102326
APA StyleKardaras, G., Boariu, M., Varlamov, V., Vintila, C., Boia, S., Belova, A., Rusu, D., Machoy, M., Solomon, S. M., & Stratul, S.-I. (2024). Three-Dimensional Planimetry Assessment of Dental Plaque-Covered Area Reduction after Rinsing with 0.2% Sodium Hypochlorite Solution as Part of a Guided Biofilm Therapy® Protocol—Pilot Longitudinal Study. Biomedicines, 12(10), 2326. https://doi.org/10.3390/biomedicines12102326





