Autocrine IGF-II-Associated Cancers: From a Rare Paraneoplastic Event to a Hallmark in Malignancy
Abstract
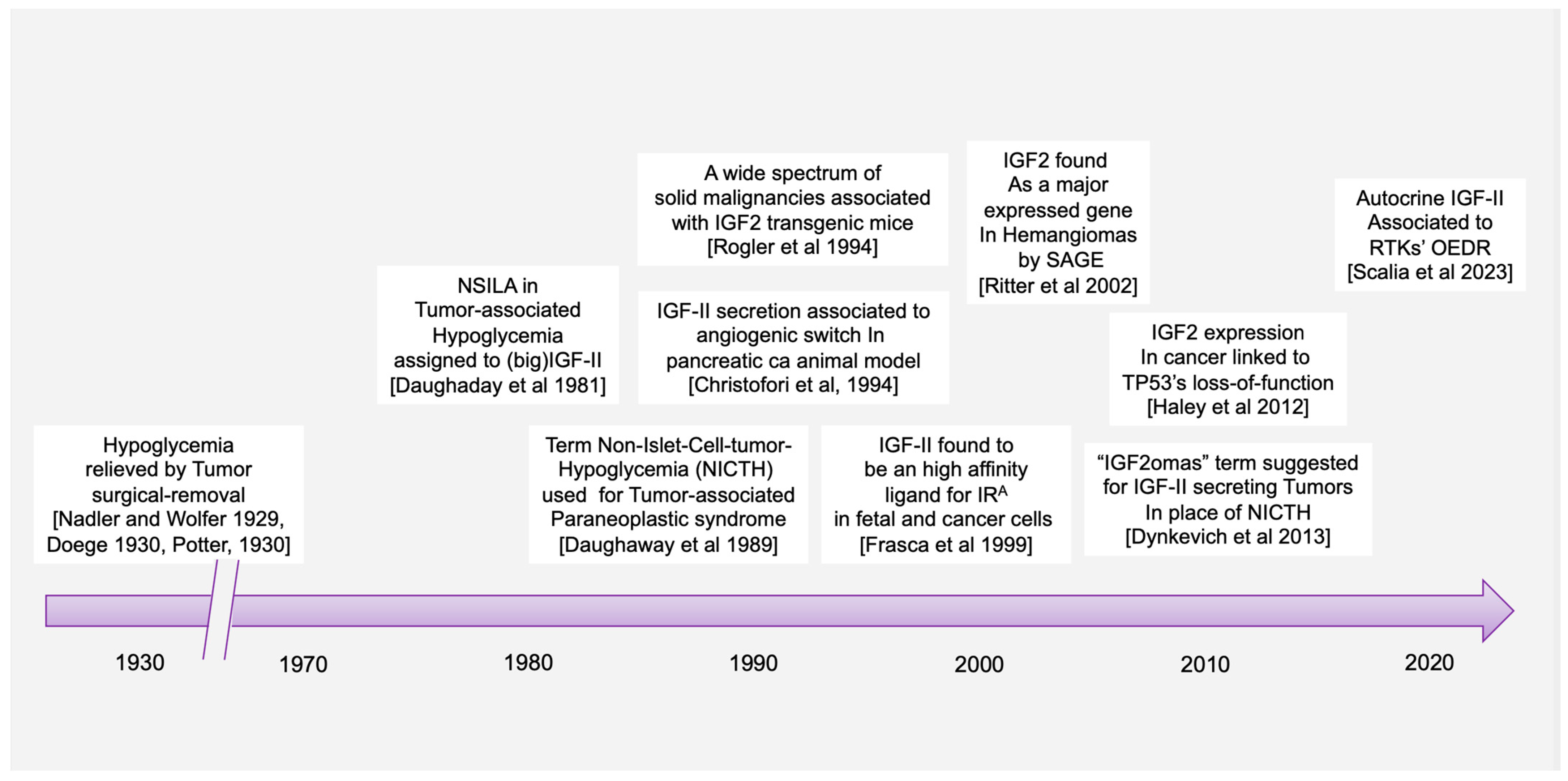
1. Introduction
2. Cancer-Secreted IGF-II and Paraneoplastic Hypoglycemia: Is There Sufficient Evidence Supporting IGF-II as the Key IGF Ligand Involved in Solid Malignancy?
- (a)
- It does not take in consideration the actual in vivo IGFs ligands and receptors co-expression context, which, taken together, supports a specific and independent role for cancer-secreted IGF-II and its autocrine loops;
- (b)
- It does not succeed in explaining the failure of the individual pharmacological blockers of IGF-IR in clinical trials towards meeting the invoked therapeutic advantages suggested by the in vitro and epidemiologic studies;
- (c)
- It has kept excluding alternative hypotheses and proper controls in experimental design which have been suggested by additional evidence available since the late nineties and proving the existence of an IGF-II- Insulin fetal receptor isoform (IRA) axis in mammalian fetal and cancer cells [16], as well as the expression and biological impact of IGF-IR/IR isoform-specific hybrids [25] in the studied cancer models.
3. IGF-II Over-Expression Is a Common Event in Cancer Cell-Lines
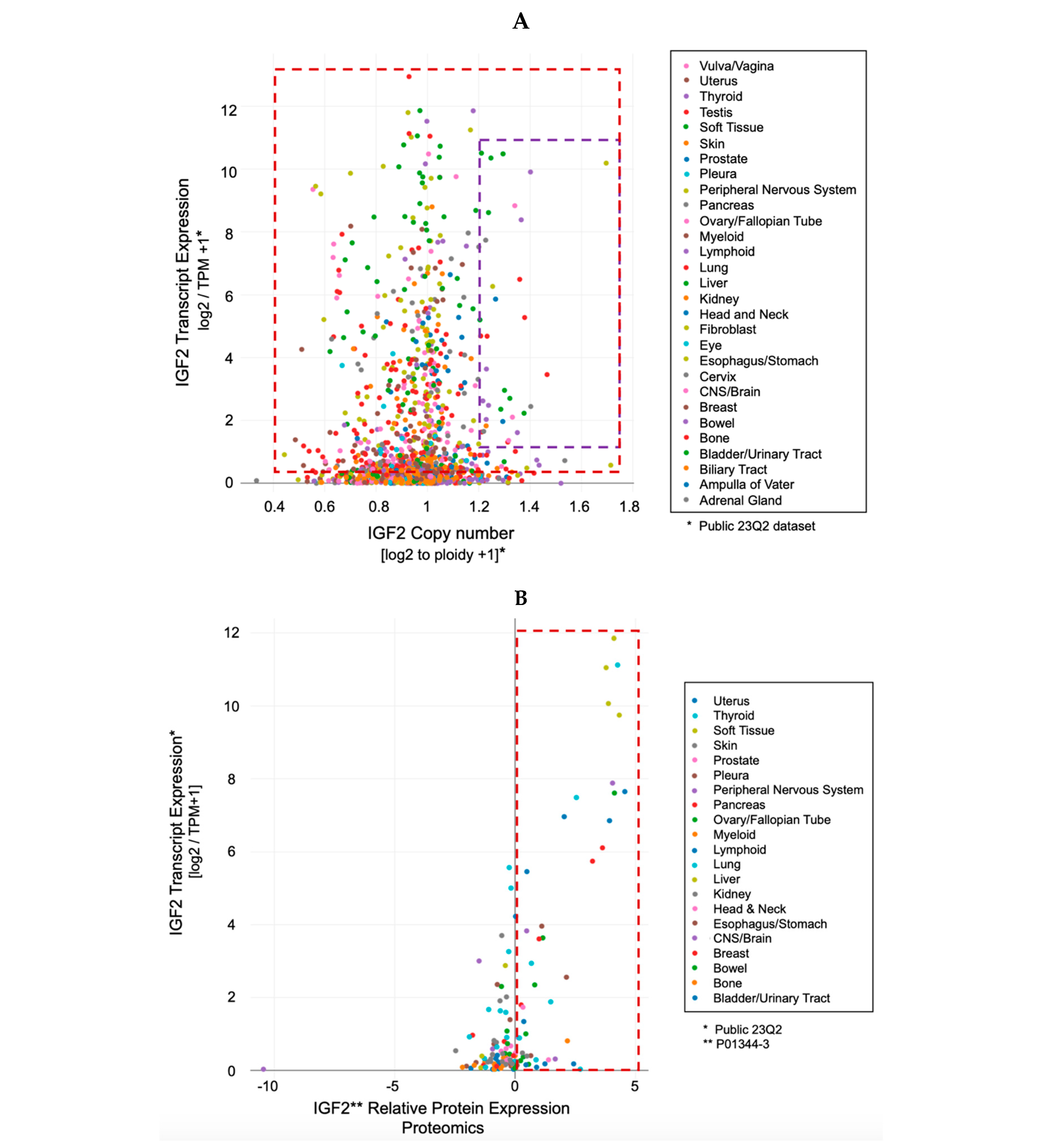
- The IGF-II transcript expression in cancer cells exceeds the expression of normal cells and tissues by a range of 0.1- to 12-fold (Figure 3A–C);
- The IGF-II transcript (mRNA) expression is not commonly associated with gene duplication events (Figure 3A);
- The IGF-II protein expression in human-derived cancer cells exceeds normal cells/tissues by 0.1- to 5-fold (Figure 3B);
- IGF-II gene editing and or transcript silencing negatively affects ~60–65% of cancer cells (Figure 3C).
4. The Role of IGF-II in Cancer Is Not Alternative to IGF-I

5. The IGF-II Cancer Driving Signal Mediating Receptors: An Unexploited Combinatorial Landscape

6. What Makes IGF-II Secretion in Solid Tumors a Hallmark of Malignancy beyond Its Renown Para-Neoplastic Association?
- (A)
- Cancer tissues, irrespective of their embryological tissue of origin (spanning from sarcomas to carcinomas), express a wide-spanning number of IGF-I receptors (IGF-IR), along with a tumorigenic stage-specific (from benign to malignant) increase in fetal insulin receptor isoform variant (IRA). Under such circumstances, cancer cells and tissues will display a variable amount of homogeneous receptors (individual IR and IGF1R) along with an increasing amount of hybrid IGF1R-IR receptors (HRs) directly depending on the increase in the expressed IGF1R (the higher the IGF-IR expression, the higher the amount of HRs) [25].
- (B)
- With the only exception of pancreatic beta-cell benign tumors (insulinomas), which produce an excess of insulin, the vast majority of solid malignancies express IGF-II in higher molecular variants (O-Glycosylated pro-hormone peptides with MW spanning between 15 and 27 KDa [7,74]) which are resistant to extracellular binding and sequestration by physiological IGF binding/scavenging factors, namely IGFBP3 and SpI2-6 [12], improperly referred as IGF-II “receptor” (IGF2R). IGF-II production/secretion has also been shown to be provided by cancer-associated fibroblasts [68], although there is still no evidence that this type of IGF-II belongs to an high-molecular-weight variant. As conveyed in Figure 1 and Table 1, it is worth noting that isolated IGF-I over-expression in cancer cell is a minor event compared to IGF-II over-expression, and it is mostly restricted to its stromal component, supporting its paracrine functions (Figure 4).
- (C)
- IGF-II over-production in cancer is currently demonstrated in the clinical setting in the presence of a diagnosed cancer patient displaying variable glycemic levels, spanning from normal to sub-normal, along with recurrent episodes of hypoglycemic symptoms fully reversible via surgical removal or ablation of the underlying cancer tissue. In these patients, the measured blood IGF-II levels (by ELISA) span from overtly supraphysiologic levels to apparently normal ranges but with a reduced or inverted ratio between IGF-II and IGF-I (normally <10). In all other malignancies not displaying such findings, IGF-II expression could be detected via both traditional histopathologic means in the clinical setting or more accurately (not frequently adopted in the clinical settings) via molecular techniques (namely qRT-PCR and NGS-RNAseq). The inclusion of IGF-II among the high-throughput targets of positional tissue expression panels able to localize and measure specific gene expression patterns within cellular components throughout bioptic tissues will provide a confirmatory tool for both the relative and absolute quantification of the above-cited IGF factors, along with hundreds of other known cancer-driving gene products.
7. Is IGF-II-Secreting Tumor (IGF-IIsT) a Biologically Sounder Acronym for the Role of IGF-II in Cancer Biology?
- (a)
- Any tumor mass, independent of its size or staging, characterized by inner mass hypoxic conditions (e.g., by CT/PET-FDG) and underlying angiogenic switch, preceding any other histopathological feature coupled with an inversion of the circulating detectable IGF-I/IGF-II ratio (with IGF-II > IGF-I);
- (b)
- Any histopathology or molecular biology report of a solid tumor bioptic specimen displaying co-expression of pre-pro-IGF-II (associated with its cancer-secreted high-molecular weight variant) and Insulin receptor fetal isoform (IRA) transcripts;
- (c)
- Any undetected metastatic foci in a previously diagnosed patient or in an apparently normal subject with familiarity for solid cancer in which a circulating tumor cell (CTC) can be isolated and analyzed using available high-sensitivity single-cell applicable methodologies (dPCR).
8. Conclusions and Perspectives
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rinderknecht, E.; Humbel, R.E. Polypeptides with nonsuppressible insulin-like and cell-growth promoting activities in human serum: Isolation, chemical characterization, and some biological properties of forms I and II. Proc. Natl. Acad. Sci. USA 1976, 73, 2365–2369. [Google Scholar] [CrossRef] [PubMed]
- Nadler, W.H.; Wolfer, J.A. Hepatogenic hypoglycemia associated with primary liver cell carcinoma. Arch. Intern. Med. 1929, 44, 700–710. [Google Scholar] [CrossRef]
- Doege, K.W. Fibro-Sarcoma of the Mediastinum. Ann. Surg. 1930, 92, 955–960. [Google Scholar] [PubMed]
- Potter, R.P. Intrathoracic Tumors. Radiology 1930, 14, 60–61. [Google Scholar] [CrossRef]
- Dynkevich, Y.; Rother, K.I.; Whitford, I.; Qureshi, S.; Galiveeti, S.; Szulc, A.L.; Danoff, A.; Breen, T.L.; Kaviani, N.; Shanik, M.H.; et al. Tumors, IGF-2, and hypoglycemia: Insights from the clinic, the laboratory, and the historical archive. Endocr. Rev. 2013, 34, 798–826. [Google Scholar] [CrossRef] [PubMed]
- Daughaday, W.H.; Kapadia, M. Significance of abnormal serum binding of insulin-like growth factor II in the development of hypoglycemia in patients with non-islet-cell tumors. Proc. Natl. Acad. Sci. USA 1989, 86, 6778–6782. [Google Scholar] [CrossRef] [PubMed]
- Daughaday, W.H.; Trivedi, B.; Baxter, R.C. Serum “big insulin-like growth factor II” from patients with tumor hypoglycemia lacks normal E-domain O-linked glycosylation, a possible determinant of normal propeptide processing. Proc. Natl. Acad. Sci. USA 1993, 90, 5823–5827. [Google Scholar] [CrossRef] [PubMed]
- van Doorn, J. Insulin-like growth factor-II and bioactive proteins containing a part of the E-domain of pro-insulin-like growth factor-II. Biofactors 2020, 46, 563–578. [Google Scholar] [CrossRef]
- Duguay, S.J.; Jin, Y.; Stein, J.; Duguay, A.N.; Gardner, P.; Steiner, D.F. Post-translational processing of the insulin-like growth factor-2 precursor. Analysis of O-glycosylation and endoproteolysis. J. Biol. Chem. 1998, 273, 18443–18451. [Google Scholar] [CrossRef]
- Oka, Y.; Rozek, L.M.; Czech, M.P. Direct demonstration of rapid insulin-like growth factor II Receptor internalization and recycling in rat adipocytes. Insulin stimulates 125I-insulin-like growth factor II degradation by modulating the IGF-II receptor recycling process. J. Biol. Chem. 1985, 260, 9435–9442. [Google Scholar] [CrossRef]
- Greenall, S.A.; Bentley, J.D.; Pearce, L.A.; Scoble, J.A.; Sparrow, L.G.; Bartone, N.A.; Xiao, X.; Baxter, R.C.; Cosgrove, L.J.; Adams, T.E. Biochemical characterization of individual human glycosylated pro-insulin-like growth factor (IGF)-II and big-IGF-II isoforms associated with cancer. J. Biol. Chem. 2013, 288, 59–68. [Google Scholar] [CrossRef] [PubMed]
- Scalia, P.; Williams, S.J.; Fujita-Yamaguchi, Y.; Giordano, A. Cell cycle control by the insulin-like growth factor signal: At the crossroad between cell growth and mitotic regulation. Cell Cycle 2023, 22, 1–37. [Google Scholar] [CrossRef] [PubMed]
- Daughaday, W.H.; Trivedi, B.; Kapadia, M. Measurement of insulin-like growth factor II by a specific radioreceptor assay in serum of normal individuals, patients with abnormal growth hormone secretion, and patients with tumor-associated hypoglycemia. J. Clin. Endocrinol. Metab. 1981, 53, 289–294. [Google Scholar] [CrossRef]
- Rogler, C.; Yang, D.; Rossetti, L.; Donohoe, J.; Alt, E.; Chang, C.; Rosenfeld, R.; Neely, K.; Hintz, R. Altered body composition and increased frequency of diverse malignancies in insulin-like growth factor-II transgenic mice. J. Biol. Chem. 1994, 269, 13779–13784. [Google Scholar] [CrossRef]
- Christofori, G.; Naik, P.; Hanahan, D. A second signal supplied by insulin-like growth factor II in oncogene-induced tumorigenesis. Nature 1994, 369, 414–418. [Google Scholar] [CrossRef] [PubMed]
- Frasca, F.; Pandini, G.; Scalia, P.; Sciacca, L.; Mineo, R.; Costantino, A.; Goldfine, I.D.; Belfiore, A.; Vigneri, R. Insulin receptor isoform, A.; a newly recognized, high-affinity insulin-like growth factor II receptor in fetal and cancer cells. Mol. Cell. Biol. 1999, 19, 3278–3288. [Google Scholar] [CrossRef] [PubMed]
- Ritter, M.R.; Dorrell, M.I.; Edmonds, J.; Friedlander, S.F.; Friedlander, M. Insulin-like growth factor 2 and potential regulators of hemangioma growth and involution identified by large-scale expression analysis. Proc. Natl. Acad. Sci. USA 2002, 99, 7455–7460. [Google Scholar] [CrossRef] [PubMed]
- Haley, V.L.; Barnes, D.J.; Sandovici, I.; Constancia, M.; Graham, C.F.; Pezzella, F.; Bühnemann, C.; Carter, E.J.; Hassan, A.B. Igf2 pathway dependency of the Trp53 developmental and tumour phenotypes. EMBO Mol. Med. 2012, 4, 705–718. [Google Scholar] [CrossRef]
- Scalia, P.; Williams, S.J. Over-expression by degradation rescue of RTKs via cancer-secreted autocrine growth factors: A Phospho-degron-driven actionable layer of post-translational regulation? Front. Oncol. 2023, 13, 1278402. [Google Scholar] [CrossRef]
- Sell, C.; Dumenil, G.; Deveaud, C.; Miura, M.; Coppola, D.; DeAngelis, T.; Rubin, R.; Efstratiadis, A.; Baserga, R. Effect of a null mutation of the insulin-like growth factor I receptor gene on growth and transformation of mouse embryo fibroblasts. Mol. Cell. Biol. 1994, 14, 3604–3612. [Google Scholar] [CrossRef]
- DeChiara, T.M.; Efstratiadis, A.; Robertson, E.J. A growth-deficiency phenotype in heterozygous mice carrying an insulin-like growth factor II gene disrupted by targeting. Nature 1990, 345, 78–80. [Google Scholar] [CrossRef] [PubMed]
- Baker, J.; Liu, J.P.; Robertson, E.J.; Efstratiadis, A. Role of insulin-like growth factors in embryonic and postnatal growth. Cell 1993, 75, 73–82. [Google Scholar] [CrossRef] [PubMed]
- Louvi, A.; Accili, D.; Efstratiadis, A. Growth-promoting interaction of IGF-II with the insulin receptor during mouse embryonic development. Dev. Biol. 1997, 189, 33–48. [Google Scholar] [CrossRef] [PubMed]
- Ludwig, T.; Eggenschwiler, J.; Fisher, P.; D’Ercole, A.J.; Davenport, M.L.; Efstratiadis, A. Mouse mutants lacking the type 2 IGF receptor (IGF2R) are rescued from perinatal lethality in Igf2 and Igf1r null backgrounds. Dev. Biol. 1996, 177, 517–535. [Google Scholar] [CrossRef]
- Pandini, G.; Frasca, F.; Mineo, R.; Sciacca, L.; Vigneri, R.; Belfiore, A. Insulin/insulin-like growth factor I hybrid receptors have different biological characteristics depending on the insulin receptor isoform involved. J. Biol. Chem. 2002, 277, 39684–39695. [Google Scholar] [CrossRef]
- Rieger, L.; O’Shea, S.; Godsmark, G.; Stanicka, J.; Kelly, G.; O’Connor, R. IGF-1 receptor activity in the Golgi of migratory cancer cells depends on adhesion-dependent phosphorylation of Tyr(1250) and Tyr(1251). Sci. Signal. 2020, 13, 633. [Google Scholar] [CrossRef]
- Crudden, C.; Girnita, L. The tale of a tail: The secret behind IGF-1R’s oncogenic power. Sci. Signal. 2020, 13, 633. [Google Scholar] [CrossRef]
- Scalia, P.; Williams, S.J.; Fujita-Yamaguchi, Y. Human IGF2 Gene Epigenetic and Transcriptional Regulation: At the Core of Developmental Growth and Tumorigenic Behavior. Biomedicines 2023, 11, 1655. [Google Scholar] [CrossRef]
- Soumerai, T.E.; Cote, G.M.; Goiffon, R.J.; Yerevanian, A.I.; Sy, A.L. Case 20-2023: A 52-Year-Old Man with a Solitary Fibrous Tumor and Hypoglycemia. N. Engl. J. Med. 2023, 388, 2467–2477. [Google Scholar] [CrossRef]
- Feldser, D.; Agani, F.; Iyer, N.V.; Pak, B.; Ferreira, G.; Semenza, G.L. Reciprocal positive regulation of hypoxia-inducible factor 1alpha and insulin-like growth factor 2. Cancer Res. 1999, 59, 3915–3918. [Google Scholar]
- Tsherniak, A.; Vazquez, F.; Montgomery, P.G.; Weir, B.A.; Kryukov, G.; Cowley, G.S.; Gill, S.; Harrington, W.F.; Pantel, S.; Krill-Burger, J.M.; et al. Defining a Cancer Dependency Map. Cell 2017, 170, 564–576.e16. [Google Scholar] [CrossRef] [PubMed]
- Zapf, J.; Walter, H.; Froesch, E.R. Radioimmunological determination of insulinlike growth factors I and II in normal subjects and in patients with growth disorders and extrapancreatic tumor hypoglycemia. J. Clin. Investig. 1981, 68, 1321–1330. [Google Scholar] [CrossRef] [PubMed]
- Folkman, J.; Watson, K.; Ingber, D.; Hanahan, D. Induction of angiogenesis during the transition from hyperplasia to neoplasia. Nature 1989, 339, 58–61. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D. Heritable formation of pancreatic beta-cell tumours in transgenic mice expressing recombinant insulin/simian virus 40 oncogenes. Nature 1985, 315, 115–122. [Google Scholar] [CrossRef] [PubMed]
- Renehan, A.G.; Zwahlen, M.; Minder, C.; O’Dwyer, S.T.; Shalet, S.M.; Egger, M. Insulin-like growth factor (IGF)-I, IGF binding protein-3, and cancer risk: Systematic review and meta-regression analysis. Lancet 2004, 363, 1346–1353. [Google Scholar] [CrossRef] [PubMed]
- Schernhammer, E.S.; Holly, J.M.; Pollak, M.N.; Hankinson, S.E. Circulating levels of insulin-like growth factors, their binding proteins, and breast cancer risk. Cancer Epidemiol. Biomark. Prev. 2005, 14, 699–704. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Katki, H.; Graubard, B.; Pollak, M.; Martin, M.; Tao, Y.; Schoen, R.E.; Church, T.; Hayes, R.B.; Greene, M.H.; et al. Serum IGF1, IGF2 and IGFBP3 and risk of advanced colorectal adenoma. Int. J. Cancer 2012, 131, E105–E113. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Lindström, S.; Schumacher, F.; Stevens, V.L.; Albanes, D.; Berndt, S.; Boeing, H.; Bueno-De-Mesquita, H.B.; Canzian, F.; Chamosa, S.; et al. Insulin-like growth factor pathway genetic polymorphisms, circulating IGF1 and IGFBP3, and prostate cancer survival. J. Natl. Cancer Inst. 2014, 106, dju085. [Google Scholar] [CrossRef]
- Adamo, M.L.; Shao, Z.M.; Lanau, F.; Chen, J.C.; Clemmons, D.R.; Roberts, C.T., Jr.; LeRoith, D.; Fontana, J.A. Insulin-like growth factor-I (IGF-I) and retinoic acid modulation of IGF-binding proteins (IGFBPs): IGFBP-2, -3, and -4 gene expression and protein secretion in a breast cancer cell line. Endocrinology 1992, 131, 1858–1866. [Google Scholar] [CrossRef]
- Milazzo, G.; Giorgino, F.; Damante, G.; Sung, C.; Stampfer, M.R.; Vigneri, R.; Goldfine, I.D.; Belfiore, A. Insulin receptor expression and function in human breast cancer cell lines. Cancer Res. 1992, 52, 3924–3930. [Google Scholar]
- Pandini, G.; Vigneri, R.; Costantino, A.; Frasca, F.; Ippolito, A.; Fujita-Yamaguchi, Y.; Siddle, K.; Goldfine, I.D.; Belfiore, A. Insulin and insulin-like growth factor-I (IGF-I) receptor overexpression in breast cancers leads to insulin/IGF-I hybrid receptor overexpression: Evidence for a second mechanism of IGF-I signaling. Clin. Cancer Res. 1999, 5, 1935–1944. [Google Scholar] [PubMed]
- Samani, A.A.; Yakar, S.; LeRoith, D.; Brodt, P. The role of the IGF system in cancer growth and metastasis: Overview and recent insights. Endocr. Rev. 2007, 28, 20–47. [Google Scholar] [CrossRef] [PubMed]
- Gardner, D.G.; Shoback, D.M.; Greenspan, F.S. Greenspan’s Basic & Clinical Endocrinology, 10th ed.; McGraw-Hill Education LLC: New York, NY, USA, 2017. [Google Scholar]
- Werner, H.; Laron, Z. Insulin-like growth factors and aging: Lessons from Laron syndrome. Front. Endocrinol. 2023, 14, 1291812. [Google Scholar] [CrossRef] [PubMed]
- Doepfner, K.T.; Spertini, O.; Arcaro, A. Autocrine insulin-like growth factor-I signaling promotes growth and survival of human acute myeloid leukemia cells via the phosphoinositide 3-kinase/Akt pathway. Leukemia 2007, 21, 1921–1930. [Google Scholar] [CrossRef] [PubMed]
- Yee, D.; Paik, S.; Lebovic, G.S.; Marcus, R.R.; Favoni, R.E.; Cullen, K.J.; Lippman, M.E.; Rosen, N. Analysis of insulin-like growth factor I gene expression in malignancy: Evidence for a paracrine role in human breast cancer. Mol. Endocrinol. 1989, 3, 509–517. [Google Scholar] [CrossRef]
- Cullen, K.J.; Allison, A.; Martire, I.; Ellis, M.; Singer, C. Insulin-like growth factor expression in breast cancer epithelium and stroma. Breast Cancer Res. Treat. 1992, 22, 21–29. [Google Scholar] [CrossRef] [PubMed]
- Sciacca, L.; Costantino, A.; Pandini, G.; Mineo, R.; Frasca, F.; Scalia, P.; Sbraccia, P.; Goldfine, I.D.; Vigneri, R.; Belfiore, A. Insulin receptor activation by IGF-II in breast cancers: Evidence for a new autocrine/paracrine mechanism. Oncogene 1999, 18, 2471–2479. [Google Scholar] [CrossRef] [PubMed]
- Bond, J.J.; Meka, S.; Baxter, R.C. Binding characteristics of pro-insulin-like growth factor-II from cancer patients: Binary and ternary complex formation with IGF binding proteins-1 to -6. J. Endocrinol. 2000, 165, 253–260. [Google Scholar] [CrossRef]
- Potalitsyn, P.; Mrázková, L.; Selicharová, I.; Tencerová, M.; Ferenčáková, M.; Chrudinová, M.; Turnovská, T.; Brzozowski, A.M.; Marek, A.; Kaminský, J.; et al. Non-glycosylated IGF2 prohormones are more mitogenic than native IGF2. Commun. Biol. 2023, 6, 863. [Google Scholar] [CrossRef]
- Yamada, T.; De Souza, A.T.; Finkelstein, S.; Jirtle, R.L. Loss of the gene encoding mannose 6-phosphate/insulin-like growth factor II receptor is an early event in liver carcinogenesis. Proc. Natl. Acad. Sci. USA 1997, 94, 10351–10355. [Google Scholar] [CrossRef]
- Chappell, S.A.; Walsh, T.; Walker, R.A.; Shaw, J.A. Loss of heterozygosity at the mannose 6-phosphate insulin-like growth factor 2 receptor gene correlates with poor differentiation in early breast carcinomas. Br. J. Cancer 1997, 76, 1558–1561. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hu, C.K.; McCall, S.; Madden, J.; Huang, H.; Clough, R.; Jirtle, R.L.; Anscher, M.S. Loss of heterozygosity of M6P/IGF2R gene is an early event in the development of prostate cancer. Prostate Cancer Prostatic Dis. 2006, 9, 62–67. [Google Scholar] [CrossRef] [PubMed]
- Hankins, G.R.; DHankins, G.R.; De Souza, A.T.; Bentley, R.C.; Patel, M.R.; Marks, J.R.; Iglehart, J.D. M6P/IGF2 receptor: A candidate breast tumor suppressor gene. Oncogene 1996, 12, 2003–2009. [Google Scholar] [PubMed]
- Nissley, S.P.; Rechler, M.M. Somatomedin/insulin-like growth factor tissue receptors. Clin. Endocrinol. Metab. 1984, 13, 43–67. [Google Scholar] [CrossRef] [PubMed]
- Ballard, F.J.; Ross, M.; Upton, F.M.; Francis, G.L. Specific binding of insulin-like growth factors 1 and 2 to the type 1 and type 2 receptors respectively. Biochem. J. 1988, 249, 721–726. [Google Scholar] [CrossRef] [PubMed]
- Grimberg, A.; Coleman, C.M.; Burns, T.F.; Himelstein, B.P.; Koch, C.J.; Cohen, P.; El-Deiry, W.S. p53-Dependent and p53-independent induction of insulin-like growth factor binding protein-3 by deoxyribonucleic acid damage and hypoxia. J. Clin. Endocrinol. Metab. 2005, 90, 3568–3574. [Google Scholar] [CrossRef] [PubMed]
- Gariboldi, M.B.; Ravizza, R.; Monti, E. The IGFR1 inhibitor NVP-AEW541 disrupts a pro-survival and pro-angiogenic IGF-STAT3-HIF1 pathway in human glioblastoma cells. Biochem. Pharmacol. 2010, 80, 455–462. [Google Scholar] [CrossRef]
- Silha, J.V.; Sheppard, P.C.; Mishra, S.; Gui, Y.; Schwartz, J.; Dodd, J.G.; Murphy, L.J. Insulin-like growth factor (IGF) binding protein-3 attenuates prostate tumor growth by IGF-dependent and IGF-independent mechanisms. Endocrinology 2006, 147, 2112–2121. [Google Scholar] [CrossRef]
- Takaoka, M.; Kim, S.-H.; Okawa, T.; Michaylira, C.Z.; Stairs, D.; Johnston, C.; Andl, C.D.; Rhoades, B.; Lee, J.; Klein-Szanto, A.; et al. IGFBP-3 regulates esophageal tumor growth through IGF-dependent and independent mechanisms. Cancer Biol. Ther. 2007, 6, 534–540. [Google Scholar] [CrossRef]
- Takaoka, M.; Harada, H.; Andl, C.D.; Oyama, K.; Naomoto, Y.; Dempsey, K.L.; Klein-Szanto, A.J.; El-Deiry, W.S.; Grimberg, A.; Nakagawa, H. Epidermal growth factor receptor regulates aberrant expression of insulin-like growth factor-binding protein 3. Cancer Res. 2004, 64, 7711–7723. [Google Scholar] [CrossRef]
- Ostrovsky, O.; Makarewich, C.A.; Snapp, E.L.; Argon, Y. An essential role for ATP binding and hydrolysis in the chaperone activity of GRP94 in cells. Proc. Natl. Acad. Sci. USA 2009, 106, 11600–11605. [Google Scholar] [CrossRef]
- Ostrovsky, O.; Ahmed, N.T.; Argon, Y. The chaperone activity of GRP94 toward insulin-like growth factor II is necessary for the stress response to serum deprivation. Mol. Biol. Cell 2009, 20, 1855–1864. [Google Scholar] [CrossRef] [PubMed]
- Argon, Y.; Bresson, S.E.; Marzec, M.T.; Grimberg, A. Glucose-Regulated Protein 94 (GRP94): A Novel Regulator of Insulin-Like Growth Factor Production. Cells 2020, 9, 1844. [Google Scholar] [CrossRef] [PubMed]
- Reddy, R.K.; Dubeau, L.; Kleiner, H.; Parr, T.; Nichols, P.; Ko, B.; Dong, D.; Ko, H.; Mao, C.; DiGiovanni, J.; et al. Cancer-inducible transgene expression by the Grp94 promoter: Spontaneous activation in tumors of various origins and cancer-associated macrophages. Cancer Res. 2002, 62, 7207–7212. [Google Scholar] [PubMed]
- Dejeans, N.; Glorieux, C.; Guenin, S.; Beck, R.; Sid, B.; Rousseau, R.; Bisig, B.; Delvenne, P.; Calderon, P.B.; Verrax, J. Overexpression of GRP94 in breast cancer cells resistant to oxidative stress promotes high levels of cancer cell proliferation and migration: Implications for tumor recurrence. Free Radic. Biol. Med. 2012, 52, 993–1002. [Google Scholar] [CrossRef] [PubMed]
- Bunn, R.C.; Fowlkes, J.L. Insulin-like growth factor binding protein proteolysis. Trends Endocrinol. Metab. 2003, 14, 176–181. [Google Scholar] [CrossRef] [PubMed]
- Singer, C.; Rasmussen, A.; Smith, H.S.; Lippman, M.E.; Lynch, H.T.; Cullen, K.J. Malignant breast epithelium selects for insulin-like growth factor II expression in breast stroma: Evidence for paracrine function. Cancer Res. 1995, 55, 2448–2454. [Google Scholar] [PubMed]
- Ulanet, D.B.; Ludwig, D.L.; Kahn, C.R.; Hanahan, D. Insulin receptor functionally enhances multistage tumor progression and conveys intrinsic resistance to IGF-1R targeted therapy. Proc. Natl. Acad. Sci. USA 2010, 107, 10791–10798. [Google Scholar] [CrossRef] [PubMed]
- Bailyes, E.M.; Nave, B.T.; Soos, M.A.; Orr, S.R.; Hayward, A.C.; Siddle, K. Insulin receptor/IGF-I receptor hybrids are widely distributed in mammalian tissues: Quantification of individual receptor species by selective immunoprecipitation and immunoblotting. Biochem. J. 1997, 327 Pt 1, 209–215. [Google Scholar] [CrossRef]
- Belfiore, A.; Frasca, F.; Pandini, G.; Sciacca, L.; Vigneri, R. Insulin receptor isoforms and insulin receptor/insulin-like growth factor receptor hybrids in physiology and disease. Endocr. Rev. 2009, 30, 586–623. [Google Scholar] [CrossRef]
- Denley, A.; Carroll, J.M.; Brierley, G.V.; Cosgrove, L.; Wallace, J.; Forbes, B.; Roberts, C.T., Jr. Differential activation of insulin receptor substrates 1 and 2 by insulin-like growth factor-activated insulin receptors. Mol. Cell. Biol. 2007, 27, 3569–3577. [Google Scholar] [CrossRef] [PubMed]
- Frasca, F.; Pandini, G.; Vigneri, R.; Goldfine, I.D. Insulin and hybrid insulin/IGF receptors are major regulators of breast cancer cells. Breast Dis. 2003, 17, 73–89. [Google Scholar] [CrossRef] [PubMed]
- Scalia, P.; Pandini, G.; Carnevale, V.; Giordano, A.; Williams, S.J. Identification of a novel EphB4 phosphodegron regulated by the autocrine IGFII/IR(A) axis in malignant mesothelioma. Oncogene 2019, 38, 5987–6001. [Google Scholar] [CrossRef] [PubMed]
- Morcavallo, A.; Gaspari, M.; Pandini, G.; Palummo, A.; Cuda, G.; Larsen, M.R.; Vigneri, R.; Belfiore, A. Research resource: New and diverse substrates for the insulin receptor isoform A revealed by quantitative proteomics after stimulation with IGF-II or insulin. Mol. Endocrinol. 2011, 25, 1456–1468. [Google Scholar] [CrossRef]
- Scalia, P.; Giordano, A.; Williams, S.J. The IGF-II-Insulin Receptor Isoform-A Autocrine Signal in Cancer: Actionable Perspectives. Cancers 2020, 12, 366. [Google Scholar] [CrossRef] [PubMed]
- Valdés, A.; Lewitt, M.; Wiss, E.; Ramström, M.; Strage, E.M. Development of a Parallel Reaction Monitoring-MS Method To Quantify IGF Proteins in Dogs and a Case of Nonislet Cell Tumor Hypoglycemia. J. Proteome Res. 2019, 18, 18–29. [Google Scholar] [CrossRef]
- Slaaby, R.; Schäffer, L.; Lautrup-Larsen, I.; Andersen, A.S.; Shaw, A.C.; Mathiasen, I.S.; Brandt, J. Hybrid receptors formed by insulin receptor (IR) and insulin-like growth factor I receptor (IGF-IR) have low insulin and high IGF-1 affinity irrespective of the IR splice variant. J. Biol. Chem. 2006, 281, 25869–25874. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Li, B.; Wang, X.; Leri, A.; Jana, K.P.; Liu, Y.; Kajstura, J.; Baserga, R.; Anversa, P. Overexpression of insulin-like growth factor-1 in mice protects from myocyte death after infarction, attenuating ventricular dilation, wall stress, and cardiac hypertrophy. J. Clin. Investig. 1997, 100, 1991–1999. [Google Scholar] [CrossRef]
- Peruzzi, F.; Prisco, M.; Dews, M.; Salomoni, P.; Grassilli, E.; Romano, G.; Calabretta, B.; Baserga, R. Multiple signaling pathways of the insulin-like growth factor 1 receptor in protection from apoptosis. Mol. Cell. Biol. 1999, 19, 7203–7215. [Google Scholar] [CrossRef]
- Morrione, A.; Valentinis, B.; Xu, S.-Q.; Yumet, G.; Louvi, A.; Efstratiadis, A.; Baserga, R. Insulin-like growth factor II stimulates cell proliferation through the insulin receptor. Proc. Natl. Acad. Sci. USA 1997, 94, 3777–3782. [Google Scholar] [CrossRef]
- Lau, M.M.; Stewart, C.E.; Liu, Z.; Bhatt, H.; Rotwein, P.; Stewart, C.L. Loss of the imprinted IGF2/cation-independent mannose 6-phosphate receptor results in fetal overgrowth and perinatal lethality. Genes Dev. 1994, 8, 2953–2963. [Google Scholar] [CrossRef] [PubMed]
- Belfiore, A.; Rapicavoli, R.V.; Le Moli, R.; Lappano, R.; Morrione, A.; De Francesco, E.M.; Vella, V. IGF2: A Role in Metastasis and Tumor Evasion from Immune Surveillance? Biomedicines 2023, 11, 229. [Google Scholar] [CrossRef] [PubMed]
- Baciuchka, M.; Remacle-Bonnet, M.; Garrouste, F.; Favre, R.; Sastre, B.; Pommier, G. Insulin-like growth factor (IGF)-binding protein-3 (IGFBP-3) proteolysis in patients with colorectal cancer: Possible association with the metastatic potential of the tumor. Int. J. Cancer 1998, 79, 460–467. [Google Scholar] [CrossRef]
- Hata, T.; Tsuruta, Y.; Takamori, S.; Shishikura, Y. Non-islet cell tumor hypoglycemia at the second recurrence of malignant solitary fibrous tumor in the retroperitoneum and pelvis: A case report. Case Rep. Oncol. 2012, 5, 420–427. [Google Scholar] [CrossRef] [PubMed]
- Yamakawa-Yokota, F.; Ozaki, N.; Okajima, A.; Nishio, H.; Nagasaka, T.; Oiso, Y. Retroperitoneal solitary fibrous tumor-induced hypoglycemia associated with high molecular weight insulin-like growth factor II. Clin. Med. Res. 2010, 8, 159–162. [Google Scholar] [CrossRef] [PubMed]
- Shotliff, K.P.; Allen, A.; Nussey, S.S. Ovarian carcinoma producing hypoglycaemia. Postgrad. Med. J. 1998, 74, 117–118. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Crowley, M.T.; Lonergan, E.; O’Callaghan, P.; Joyce, C.M.; Morita, M.; Conlon, N.; O’halloran, D.J. IGF-2 mediated hypoglycemia and the paradox of an apparently benign lesion: A case report & review of the literature. BMC Endocr. Disord. 2022, 22, 262. [Google Scholar] [CrossRef]
- Silzle, T.; Randolph, G.J.; Kreutz, M.; Kunz-Schughart, L.A. The fibroblast: Sentinel cell and local immune modulator in tumor tissue. Int. J. Cancer 2004, 108, 173–180. [Google Scholar] [CrossRef]
- Brunner, N.; Moser, C.; Clarke, R.; Cullen, K. IGF-I and IGF-II expression in human breast cancer xenografts: Relationship to hormone independence. Breast Cancer Res. Treat. 1992, 22, 39–45. [Google Scholar] [CrossRef]
- Levinovitz, A.; Norstedt, G. Developmental and steroid hormonal regulation of insulin-like growth factor II expression. Mol. Endocrinol. 1989, 3, 797–804. [Google Scholar] [CrossRef]
- Lee, C.; Jia, Z.; Rahmatpanah, F.; Zhang, Q.; Zi, X.; McClelland, M.; Mercola, D. Role of the adjacent stroma cells in prostate cancer development and progression: Synergy between TGF-beta and IGF signaling. BioMed Res. Int. 2014, 2014, 502093. [Google Scholar] [CrossRef] [PubMed]
- Chu, T.; Khine, A.A.; Wu, N.Y.; Chen, P.; Chu, S.; Lee, M.; Huang, H. Insulin-like growth factor (IGF) and hepatocyte growth factor (HGF) in follicular fluid cooperatively promote the oncogenesis of high-grade serous carcinoma from fallopian tube epithelial cells: Dissection of the molecular effects. Mol. Carcinog. 2023, 62, 1417–1427. [Google Scholar] [CrossRef] [PubMed]



| Cancer Associated Hypoglycemia | Reports of Secreted Autocrine/Paracrine Growth Factor | Reporting Elevated Plasma Growth Factor | Reports of Elevated IGF Gene Transcripts Level in Underlying Tumor | Cancer Case Report (1972) | Hypoglycemia Case Reports | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| IGF-I | IGF-II | IGF-I | IGF-II | IGF-I | IGF-II | IGF-1 | IGF-2 | IGF-I | IGF-II | ||
| Cancer associated hypoglycemia | Total cancer associated hypoglycemia cases = 1949 | 18 | 171 | 66 | 24 | 1 | 2 | 1690 | 1690 | ||
| Protein expressing/Secreted IGF | 18 | 171 | 1644 * | 3830 | 301 | 201 | 312 | 136 | 136 | 1657 | |
| Reporting elevated plasma IGF | 66 | 24 | 322 | 201 | 893 | 892 | 172 | 16 | 22 | 64 | |
| Cancer (case report) | 1656 | 1656 | 980 | 1644 | 22 | 48 | 5 | 2 | 623,826 | 623,826 | |
| 7616 | |||||||||||
| Feature | Ligand | Effect(s) | Efficiency | Biological/Clinical context | Reference(s) |
|---|---|---|---|---|---|
| Binding to IGF-IR | IGF-I | cell growth, pro-mitotic, anti-apoptotic | +++ | Extracellular/Tumor microenvironment | Li et al., 1997 [79] Peruzzi et al., 1999 [80] |
| IGF-II | cell growth, pro-mitotic, anti-apoptotic | ++ | Potalitsyn et al., 2023 [50] | ||
| Big-IGF-II | cell growth, anti-apoptotic pro-tumorigenic | ++ | Potalitsyn et al., 2023 [50] | ||
| Binding to IRA (*) | IGF-I | Negligible at physiological levels | −/+ | Extracellular/Tumor microenvironment | Frasca et al., 1999, Sciacca et al., 1999 [16,48] |
| IGF-II | IGF-I-like (**) plus pro-angiogenic and pro-invasive | ++ | Frasca et al., 1999, Morrione et al, 1998, Louvi et al., 1998, [16,23,81] | ||
| Big-IGF-II | IGF-I-like (**) plus events linked to malignant switch | ++ | Greenhall et al., 2013, Ulanet et al., 2010, Scalia et al., 2019, Potalitsyn et al., 2023 [11,50,69,74] | ||
| Binding to SpI2-6/IGF2R | IGF-I | n/d | − | Extracellular/Tumor microenvironment | Nissley et al., 1984 [55] Bond et al., 2000 [49] |
| IGF-II | Overgrowth rescue, IGF-II degradation | +++ | Lau et al., 1994 [82], Oka et al., 1985 [10] | ||
| Big-IGF-II | Maintenance of (big)IGF-II Extracellular bioavailability | −/+ | Greenhall et al., 2013 [11] Potalitsyn et al., 2023 [50] | ||
| Binding to IGFBP-3 | IGF-I | Decrease bioavailability, IGF-independent TBD | +++ | Extracellular/Tumor microenvironment | Grimberg et al., 2005, Silha et al., 2006, Takayoka et al., 2007 [57,59,60] |
| IGF-II | Decrease bioavailability (***), IGF independent (TBD) | ++/+++ | Potsalitsyn et al., 2023 [50] | ||
| Big-IGF-II | Maintained bioavailability | −/+ | Potsalitsyn et al., 2023 [50] | ||
| Source and effects in Cancer | IGF-I | Tropic, survival, Pro-tumorigenic (TBD) | Stroma | normal tissue, solid malignancies | Yee et al., 1989, Cullen et al., 1992, [46,47] |
| IGF-II | IGF-I-like (**), pro-tumorigenic, immune-evasion | Stroma | solid malignancies | Rogler et al., 1994, [14], Cullen et al., 1992, Yee et al., 1989, [46,47] Belfiore et al., 2023 [83] | |
| Big-IGF-II | Pro-tumorigenic, events linked to malignant switch | Cancer cell | solid malignancies, paraneoplastic hypoglycemia | Doughaday et al., 1989, Christofori et al., 1994 Dynkevich et al., 2013 [5,6,15] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Scalia, P.; Marino, I.R.; Asero, S.; Pandini, G.; Grimberg, A.; El-Deiry, W.S.; Williams, S.J. Autocrine IGF-II-Associated Cancers: From a Rare Paraneoplastic Event to a Hallmark in Malignancy. Biomedicines 2024, 12, 40. https://doi.org/10.3390/biomedicines12010040
Scalia P, Marino IR, Asero S, Pandini G, Grimberg A, El-Deiry WS, Williams SJ. Autocrine IGF-II-Associated Cancers: From a Rare Paraneoplastic Event to a Hallmark in Malignancy. Biomedicines. 2024; 12(1):40. https://doi.org/10.3390/biomedicines12010040
Chicago/Turabian StyleScalia, Pierluigi, Ignazio R. Marino, Salvatore Asero, Giuseppe Pandini, Adda Grimberg, Wafik S. El-Deiry, and Stephen J. Williams. 2024. "Autocrine IGF-II-Associated Cancers: From a Rare Paraneoplastic Event to a Hallmark in Malignancy" Biomedicines 12, no. 1: 40. https://doi.org/10.3390/biomedicines12010040
APA StyleScalia, P., Marino, I. R., Asero, S., Pandini, G., Grimberg, A., El-Deiry, W. S., & Williams, S. J. (2024). Autocrine IGF-II-Associated Cancers: From a Rare Paraneoplastic Event to a Hallmark in Malignancy. Biomedicines, 12(1), 40. https://doi.org/10.3390/biomedicines12010040







