1. Introduction
Chorea-acanthocytosis (ChAc) is a rare neurodegenerative disease characterized by spiculated red blood cells (acanthocytosis) and Huntington’s-disease-like neuropsychiatric symptoms. Although loss-of-function mutations of the vacuolar protein sorting 13 homolog A (
VPS13A) gene—which produces the 360 kDa protein, also known as chorein—have been identified as causative, the detailed molecular pathology remains to be elucidated [
1,
2,
3]. Chorein has been implicated in a variety of intracellular phenomena. These include interactions with membrane cytoskeletal proteins [
4], regulation of autophagy [
5,
6], influences on lipid transport and mitochondrial morphology [
7,
8], mitochondrial trafficking [
9], and plasma membrane phospholipid scrambling [
10].
We established a murine ChAc model (ChAc
Del/Del) by using gene-targeting techniques to delete exons 60–61 of
Vps13a, which are homologous to the human disease mutation [
11]. These ChAc
Del/Del mice showed various phenotypes depending on their strain [
11]. Notably, we found male infertility in the 129S6/SvEv strain, and our previous study revealed the immobility and dysmorphic mitochondria of spermatozoa in these mice [
12]. Spermatozoa derived from ChAc
Del/Del mice constitute suitable samples for investigating the molecular pathology of ChAc because of their readily apparent phenotypes.
In this study, we undertook a series of targeted experiments to explore specific aspects of chorein’s role in spermatogenesis. Firstly, we examined the impact of chorein deficiency on the mitochondrial structure within sperm cells during the process of spermatogenesis, utilizing transmission electron microscopy (TEM) to reveal mitochondrial dysmorphology. Secondly, we conducted a proteomic analysis of chorein co-immunoprecipitated (co-IP) proteins in murine sperm lysates to detect chorein-interacting proteins. This was followed by Western blotting and immunohistochemical analysis of murine sperm and testes based on the proteomics results. We also performed metabolomic analyses on sperm from ChAcDel/Del and wild-type (WT) mice to investigate enzymes involved in energy metabolism. Additionally, building on our preliminary research with human embryonic kidney 293 (HEK293) cells, which indicated an association between VPS13A knockdown and ferroptosis, we specifically stained for lipid peroxidation within the testes during the process of spermatogenesis, using an anti-malondialdehyde antibody to identify increased oxidative stress, particularly during the advanced stages of spermatogenesis.
2. Materials and Methods
2.1. Animals
This study was conducted in accordance with the Guidelines for Animal Experimentation (MD14017) and Gene Recombination Experiments (24054) of the Graduate School of Medical and Dental Sciences, Kagoshima University, Japan.
We previously generated mice harboring deletion of exons 60–61 in
Vps13a [
11]. In this study, we used an inbred strain, 129S6/SvEv (Taconic Labs, Hudson, NY, USA); 129S6/SvEv-
Vps13atm1asan/
Vps13atm1asan (ChAc
Del/Del) was established previously [
11].
2.2. TEM Analysis
TEM analysis was conducted as previously described [
12]. Briefly, murine testes and epididymides were removed and fixed with 2% paraformaldehyde (PFA) and 2% glutaraldehyde (GA). The samples were then postfixed with 2% osmium tetroxide (OsO
4) and embedded in resin (Quetol-812; Nisshin EM Co., Tokyo, Japan). The polymerized resins were divided into ultrathin sections and stained with 2% uranyl acetate and lead stain solution (Sigma-Aldrich Co., Tokyo, Japan). The sections were observed using a transmission electron microscope (JEM-1400Plus; JEOL Ltd., Tokyo, Japan). Images were taken with a CCD camera (EM-14830RUBY2; JEOL Ltd., Tokyo, Japan).
2.3. Co-Immunoprecipitation Analyses
Co-IP and reverse co-IP assays were performed using Dynabeads Protein G (Thermo Fisher Scientific, Rockford, IL, USA), according to the manufacturer’s instructions. Anti-chorein antibody, anti-IDH3A protein antibody, rabbit IgG (NB810, Novus Biologicals, Centennial, CO, USA), and goat IgG (sc2028, Santa Cruz biotechnology, Inc., Dallas, TX, USA) were used for the Dynabeads–antibody complex. Epididymal sperm from eight wild-type mice were washed with phosphate-buffered saline (PBS) and centrifuged (10,000× g for 10 min). Spermatozoa were solubilized in 1% NP-40 with 1× protease inhibitor cocktail (M-PER; Roche Diagnostics, Indianapolis, IN, USA) and 0.5 mM phenylmethylsulfonyl fluoride at 4 °C for 60 min. A total of 500 µg of the soluble fractions of the spermatozoa lysates (input) was incubated for 10 min at room temperature with the Dynabeads–antibody complex. Then, the beads were washed three times with lysis buffer before elution. The protein samples were eluted with 1× NuPAGE LDS Sample Buffer (Life Technologies Corporation, Carlsbad, CA, USA) and 2.5% 2-mercaptoethanol. The eluates were analyzed via proteomics and/or immunoblot analysis. Co-IP and reverse co-IP assays of proteins extracted from HEK293 cells were also performed in the same manner.
2.4. Proteomic Analysis
The eluates of chorein co-IP and IgG co-IP (negative control) were boiled in 60 µL of 1.5× LDS buffer for 15 min. Half of each sample was processed by SDS-PAGE using a NuPAGE 10% Bis-Tris Gel with the MES buffer system (Life Technologies Corporation, Carlsbad, CA, USA). The mobility region was excised into 10 equally sized segments, and in-gel digestion with trypsin was performed. Half of each digestate was analyzed by nano-liquid chromatography coupled with tandem mass spectrometry (LC-MS/MS), using a Waters NanoAcquity HPLC system interfaced with a Fusion Lumos mass spectrometer (Thermo Fisher Scientific, Rockford, IL, USA). Peptides were loaded on a trapping column and eluted over a 75 µm analytical column at 350 nL/min; both columns were packed with Luna C18 resin (Phenomenex, Torrance, CA, USA). The mass spectrometer was operated in data-dependent mode, with the Orbitrap operating at 70,000 FWHM and 17,500 FWHM for MS and MS/MS, respectively. The fifteen most abundant ions were selected for MS/MS. The instrument was run with a 3 s cycle for MS and MS/MS, with advanced peak determination (APD) enabled. A total of 5 h of instrument time was employed per sample. Data were sought using a local copy of Mascot (Matrix Science Inc., Boston, MA, USA). Mascot DAT files were parsed into Scaffold version 5.3.0 (Proteome Software Inc., Portland, OR, USA) for validation and filtering and to create a non-redundant list for each sample. Data were filtered using 1% protein and peptide FDR and required at least two unique peptides per protein. Protein mass spectrometry was carried out at MS Bioworks LLC., Ann Arbor, MI, USA.
The identified proteins were screened based on the spectral count (SpC). The spectral counting method is a relative quantification method comparing the SpC of each protein across LC-MS/MS runs. We selected proteins with at least SpC > 4 in the chorein co-IP samples and detected those with a fourfold or greater increase compared to the negative control sample based on dividing the SpC.
2.5. Metabolomic Analysis
Epididymal spermatozoa from five WT and five ChAc
Del/Del mice were individually analyzed for intracellular metabolites. The spermatozoa were suspended in PBS and collected by centrifugation (500×
g and 4 °C for 5 min). The cells were then treated with 800 µL of methanol and vortexed for 30 s. Next, the cell extract was treated with 550 µL of Milli-Q water containing internal standards (H3304-1002 [
13], Human Metabolome Technologies, Tsuruoka, Japan) and vortexed for 30 s. The extract was obtained and centrifuged at 2300×
g and 4 °C for 5 min, and then, 800 µL of the upper aqueous layer was centrifugally filtered through a Millipore 5 kDa cutoff filter at 9100×
g and 4 °C for 120 min to remove proteins. The filtrate was centrifugally concentrated and resuspended in 25 µL of Milli-Q water for capillary electrophoresis time-of-flight mass spectrometry analysis. Metabolomic analysis was carried out through a facility service at Human Metabolome Technologies. For the metabolites that were able to determine the standard deviation for both groups, the Welch’s
t-test was performed for statistical analysis.
2.6. Antibodies
Antibodies against the following proteins were purchased: chorein, NBP1-85641 (Novus Biologicals, Centennial, CO, USA); phosphoglycerate kinase 2 (PGK2), 13686-1-AP (Proteintech Group, Rosemont, IL, USA); lactate dehydrogenase C (LDHC), PA5-18779 and acetyl-CoA acetyltransferase 1 (ACAT1), PA5-34676 (Thermo Fisher Scientific, Rockford, IL, USA); isocitrate dehydrogenase 3 subunit alpha (IDH3A), ab118278, annexin A1 (ANXA1), ab214486 and outer dense fiber protein 1 (ODF1), ab197029 (Abcam, Cambridge, UK); translocase of outer membrane 20 kDa subunit (TOM20), sc-11415 (Santa Cruz Biotechnologies, Dallas, TX, USA); alpha-tubulin, 11H10 (Cell Signaling Technology, Danvers, MA, USA); malondialdehyde (MDA), MA5-27559 (Thermo Fisher Scientific).
2.7. Western Blotting Analysis
In addition to eluates of the co-IPs, lysates of testes and epididymal sperm from five wild-type and five ChAc
Del/Del mice were individually solubilized with RIPA buffer (Thermo Fisher Scientific, Rockford, IL, USA) and analyzed by Western blotting. The proteins were denatured with NuPAGE LDS Sample Buffer (Life Technologies Corporation), separated on appropriate gels and transferred to polyvinylidene difluoride membranes (Merck KGaA, Darmstadt, Germany). The amount of protein sample was visualized using the MemCode Reversible Protein Stain Kit (Thermo Fisher Scientific). Membranes were blocked for 1 h at room temperature with 3% non-fat dried milk in PBS containing 0.1% Tween-20, and then, they were incubated with primary antibodies (chorein, PGK2, LDHC, ACAT1, IDH3A, ANXA1, ODF1, TOM20, and alpha-tubulin) and the appropriate secondary antibodies. Proteins were visualized using ECL Prime Western Blotting Detection Reagent (GE Healthcare, Buckinghamshire, UK), and images were recorded with a digital analyzer (FUSION-SOLO.7S.WL; Vilber-Lourmat, Marne-la-Vallée, France). ImageJ software version 1.52a (U.S. National Institutes of Health, Bethesda, ML, USA) was used for the densitometric analysis of protein bands. The results were standardized to alpha-tubulin. The Mann–Whitney U test was performed for statistical analysis using EZR in R Commander version 1.27 [
14].
2.8. Immunohistochemistry and Immunocytochemistry
Immunohistochemical analysis was performed as previously described in [
12]. Briefly, the testes were removed and fixed in 4% paraformaldehyde. The tissues were frozen and cut into 25 µm sections, which were mounted on slide glasses. Suspended spermatozoa were treated with 4% paraformaldehyde and washed in 10 mM glycine in PBS, and then, they were mounted on glass slides and air-dried. The tissues and cells were treated with the appropriate blocking buffer and incubated with primary antibodies (chorein, PGK2, LDHC, and IDH3A) and the appropriate secondary antibodies. The immunoreactions were visualized using the avidin-biotinylated enzyme complex method (VECTASTAIN ABC kit, Vector Laboratories, Burlingame, CA, USA) or the immunofluorescence technique using secondary antibodies (Alexa Fluor 555 Donkey anti-goat IgG (H + L) and/or Alexa Fluor 480 donkey anti-rabbit IgG (H + L), Invitrogen, Eugene, OR, USA). For immunofluorescence, the coverslips were washed and mounted with Vectashield medium containing DAPI (Vector Laboratories) and then viewed with a BZ-X 710 fluorescence microscope system (Keyence, Osaka, Japan).
4. Discussion
The phenotypes of ChAc
Del/Del mice do not completely mimic human ChAc [
11]. Some of the predominant symptoms of ChAc, such as involuntary orofacial movement, dystonia, psychotic symptoms, etc., could not be clearly detected in ChAc
Del/Del mice. Recently, male infertility has been reported in human ChAc patients [
15]. In this situation, male infertility as a clear-cut phenotype in ChAc
Del/Del mice would provide an ideal target to understand chorein’s function at the molecular level. We previously reported asthenozoospermia with dysmorphic mitochondria in the spermatozoa of ChAc
Del/Del mice [
12]. However, the morphological abnormalities of the mitochondria during spermatogenesis, along with the detailed molecular pathology, remain poorly understood in ChAc
Del/Del mice.
In the present study, we performed TEM analysis of the seminiferous tubules in ChAc
Del/Del mice to detect morphological abnormalities of the mitochondria during spermatogenesis. We observed distinctive mitochondrial dysmorphology in the late stages of the mitochondrial sheath development process in ChAc
Del/Del mice, as illustrated in
Figure 1. Similarities in electron density within the mitochondrial matrix of sperm from the seminiferous tubules of both wild-type and ChAc
Del/Del mice suggest that chorein deficiency may not significantly alter the basic mitochondrial structure during the early stages of spermatogenesis. However, the notable differences observed in sperm from the epididymides of ChAc
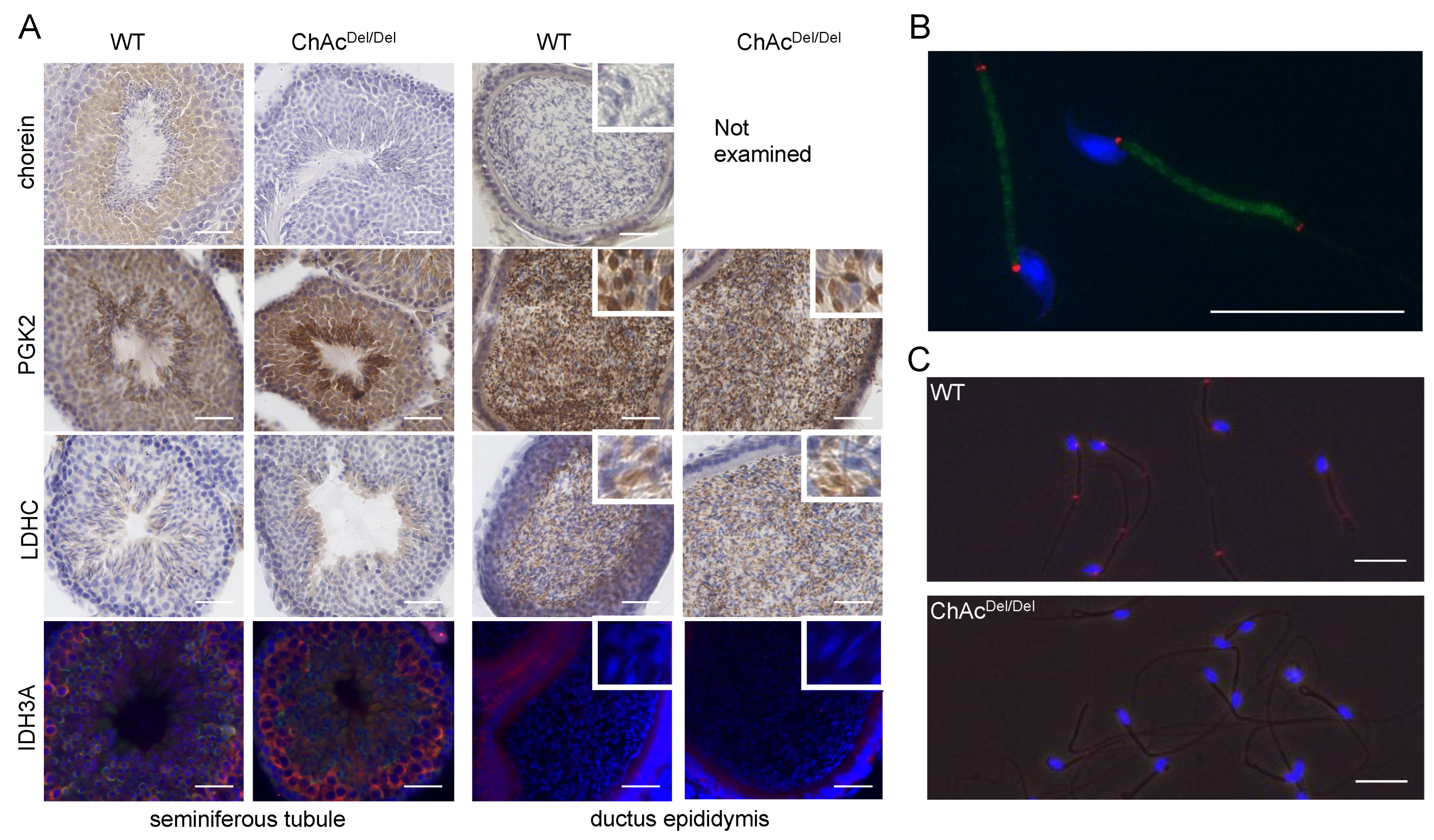
Del/Del mice—particularly the destruction of cristae structures—indicate a progressive deterioration of mitochondrial integrity in the absence of chorein. Additionally, chorein immunoreaction was homogeneously and granularly detected in the cytoplasm of spermatids (
Figure 5A), aligning with and underscoring the critical role of chorein in maintaining mitochondrial structure and function during the later stages of sperm development. These observations raise intriguing questions about the specific mechanisms by which chorein deficiency leads to mitochondrial dysmorphology and subsequent sperm dysfunction. The progressive nature of mitochondrial deterioration observed during spermatogenesis suggests that chorein may play a more crucial role in the later stages of spermatogenesis, particularly in the maturation and maintenance of sperm cells.
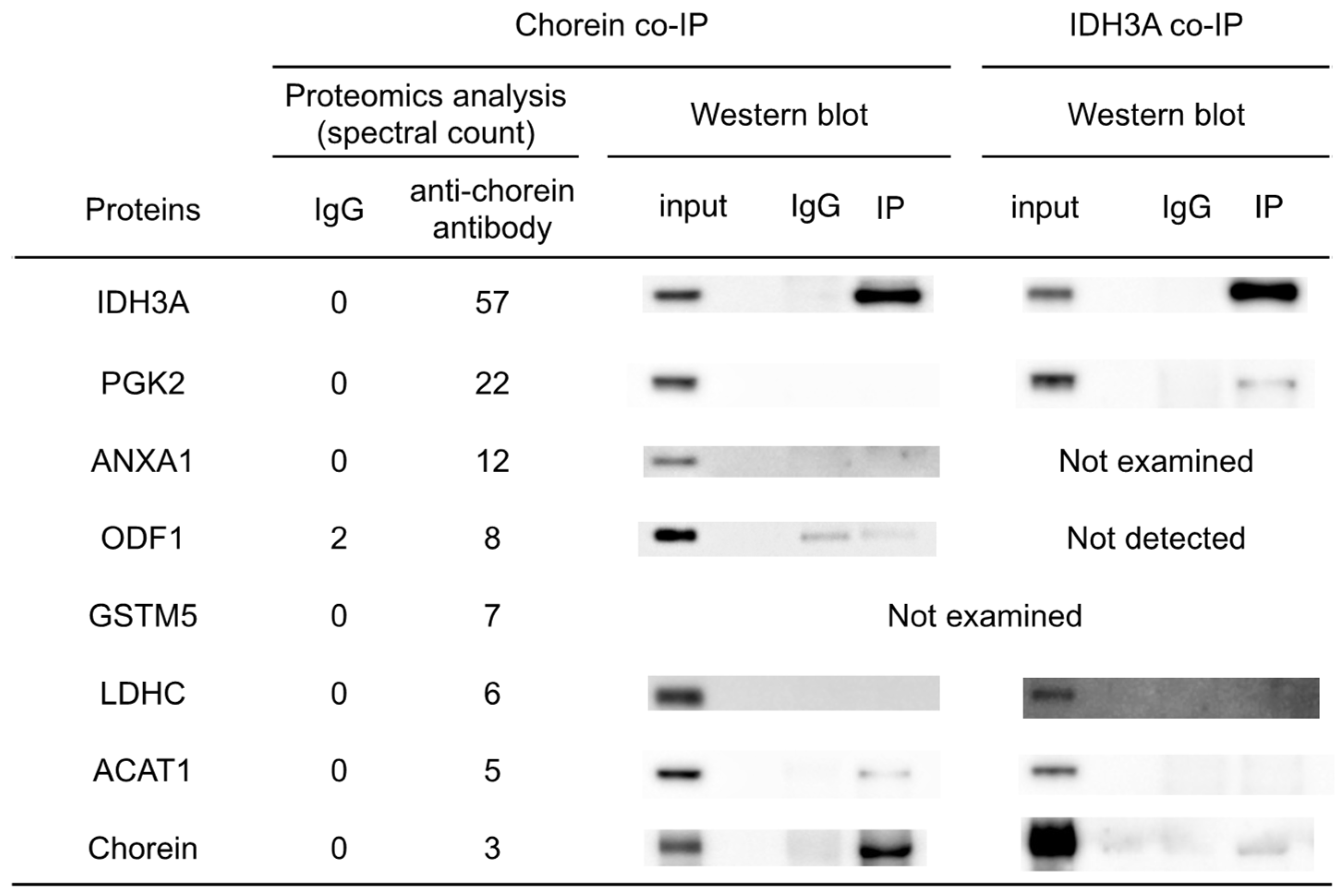
Co-IP and subsequent proteomic analyses were conducted to identify chorein-interacting proteins in mouse sperm. The results and reverse co-IP revealed chorein–IDH3A interaction (
Figure 2). The IDH3A protein is the alpha subunit of NAD (+)-dependent isocitrate dehydrogenase, which is a TCA-cycle enzyme localized in the mitochondria. It was shown that oxidative stress induced by 6-hydroxydopamine (6OHDA) or rotenone can alter
IDH3A and high-temperature-requirement protein A2 (
HTRA2/OMI) (a mitochondrial protease) gene expression [
16]. A recent study on flies reported that
idh3a knockout caused dysmorphic mitochondria that lacked cristae in
idh3a-knockout flies [
17]. These studies suggest that IDH3A may play a crucial role in mitochondrial quality control. Disorganized inner membrane structures of mitochondria were observed in the sperm of ChAc
Del/Del mice [
12]. However, to elucidate the details of the relationships between chorein, IDH3A, and dysmorphic mitochondria, further investigations are needed. Another study showed retinal degeneration and mitochondrial dysfunction in
Idh3a-mutant mice [
18], but no other detectable abnormal phenotypes were observed. The sperm mobility and morphology of
Idh3a-mutant mice remain unknown.
Immunocytochemical analysis revealed that IDH3A was ubiquitously distributed in spermatogonia and disappeared in spermatids or spermatozoa packed in the ductus epididymis (
Figure 5A). IDH3A immunoreaction returned in the spermatozoa and was localized at both ends of the midpiece (
Figure 5B) in WT mice. However, there was no immunoreaction of IDH3A in the majority of ChAc
Del/Del spermatozoa (
Figure 3C), which we previously reported as immotile. These findings suggest that IDH3A may be involved in murine sperm motility.
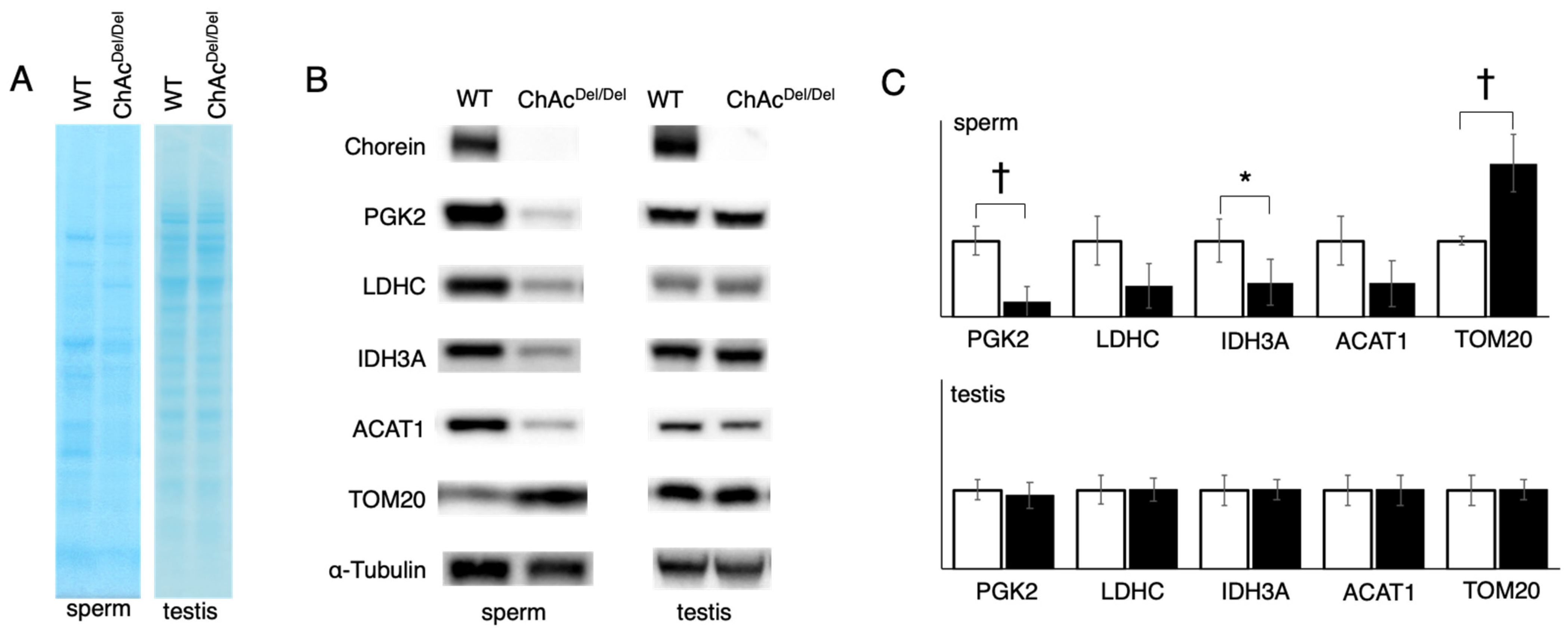
In our study, we observed that protein staining in sperm exhibited different patterns between WT and ChAc
Del/Del mice (
Figure 3A), suggesting alterations in protein expression or localization during spermatogenesis. This is particularly notable because testis lysates from both groups displayed similar staining patterns. The disparity in sperm protein staining patterns, in contrast to the uniformity observed in testis lysates, indicates specific disruptions in the later stages of spermatogenesis in ChAc
Del/Del mice. These disruptions could be associated with the morphological abnormalities in mitochondria identified during these stages by TEM analysis. In addition, the Western blotting analysis revealed a decrease in band density for IDH3A and ACAT1, whereas an increase was noted for TOM20 in ChAc
Del/Del mice (
Figure 3B). This contrast in protein levels—particularly the increase in TOM20, which is a mitochondrial outer membrane protein—suggests a compensatory response to the mitochondrial dysmorphology observed in these mice. Notably, such differences were not observed in the testis lysates of either group, indicating that these changes likely occur during the later stages of spermatogenesis. The alterations in protein levels, especially the increase in TOM20 in sperm, are consistent with the morphological abnormalities in mitochondria detected during the later stages of spermatogenesis in ChAc
Del/Del mice, as revealed by TEM analysis. The decrease in IDH3A and ACAT1 could be indicative of impaired mitochondrial function, while the increase in TOM20 might reflect an attempt by the cell to maintain mitochondrial integrity. This is particularly relevant given the role of TOM20 in protein import into the mitochondria, suggesting a potential adaptive mechanism to counteract mitochondrial damage. The disparity in protein expression patterns in sperm, as compared to the testes, underscores the specificity of these molecular changes to the spermatogenic process, particularly in the maturation phase of spermatozoa. Given the crucial role of mitochondria in energy production and cellular metabolism within sperm, such alterations could have significant implications for sperm motility and functionality. This is supported by the previously reported poor motility in ChAc
Del/Del spermatozoa [
12] and provides a molecular basis for the observed sperm dysfunction.
Co-IP analysis suggested that IDH3A interacts with PGK2 (
Figure 2), which is a glycolytic enzyme specifically expressed in sperm. Regarding the chorein–PGK2 interaction, the results of the proteomic analyses and Western blotting were inconsistent. This suggests that both chorein and PGK2 interact with IDH3A but that chorein and PGK2 may interact indirectly.
PGK2 and LDHC, another sperm-specific glycolytic enzyme, are known as essential proteins for sperm motility and male fertility in mice [
19,
20]. The reductions in those enzymes observed in the sperm of ChAc
Del/Del mice were consistent with sperm immobility. Although the results of immunohistochemical analysis and Western blotting of testis lysates suggested that the PGK2 and LDHC proteins were expressed in similar patterns in the sperm of both WT and ChAc
Del/Del mice (
Figure 5A), Western blotting of sperm lysates after washing in PBS and centrifugation showed reduced immunoreaction of those proteins in ChAc
Del/Del mice relative to WT mice (
Figure 2). PGK2 and LDHC were enriched in the cytoplasmic droplet, a cytoplasm-containing structure attached to epididymal sperm. Yuan et al. reported that cytoplasmic droplets play essential roles in sperm maturation, including mitochondrial activation in the epididymis [
21]. They also showed that the function of the cytoplasmic droplet is abrogated by removing it from sperm through mechanical stimulation. We thus hypothesized that the sperm of ChAc
Del/Del mice might have a fragile plasma membrane and that their cytoplasmic droplets might be easily removed by mechanical stimulation, consequently leading to impaired sperm motility.
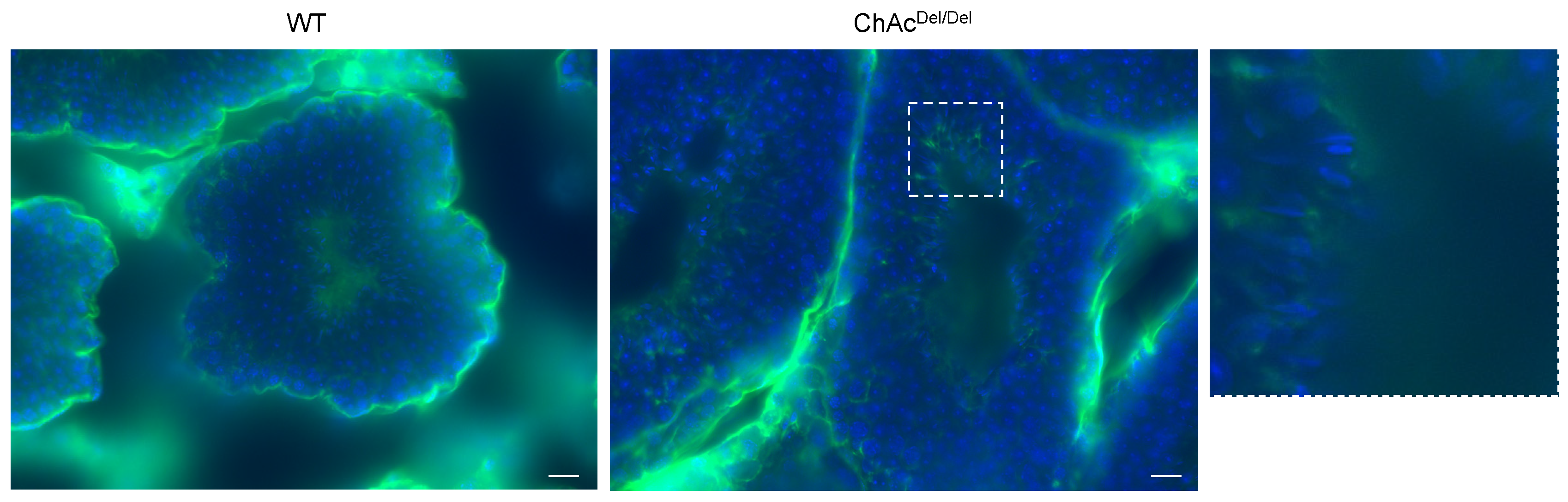
Building on our preliminary research in human embryonic kidney 293 (HEK293) cells, which indicated an association between
VPS13A knockdown and ferroptosis, in the present study, we performed immunocytochemistry of MDA to stain for lipid peroxidation within the testes during spermatogenesis. Interestingly, in the final stage of spermatogenesis, spermatozoa of ChAc
Del/Del mice showed increased staining in the region encompassing the midpiece of the sperm (
Figure 6). This suggests that ferroptosis may occur in ChAc
Del/Del mice during the final stages of spermatogenesis. Activating lipid peroxidation through ferroptosis possibly causes fragile mitochondrial dysmorphological changes and plasma membrane changes in the late stages of spermatogenesis. These may explain the previously described abnormalities observed in the late stages of the spermatogenesis of ChAc
Del/Del mice.
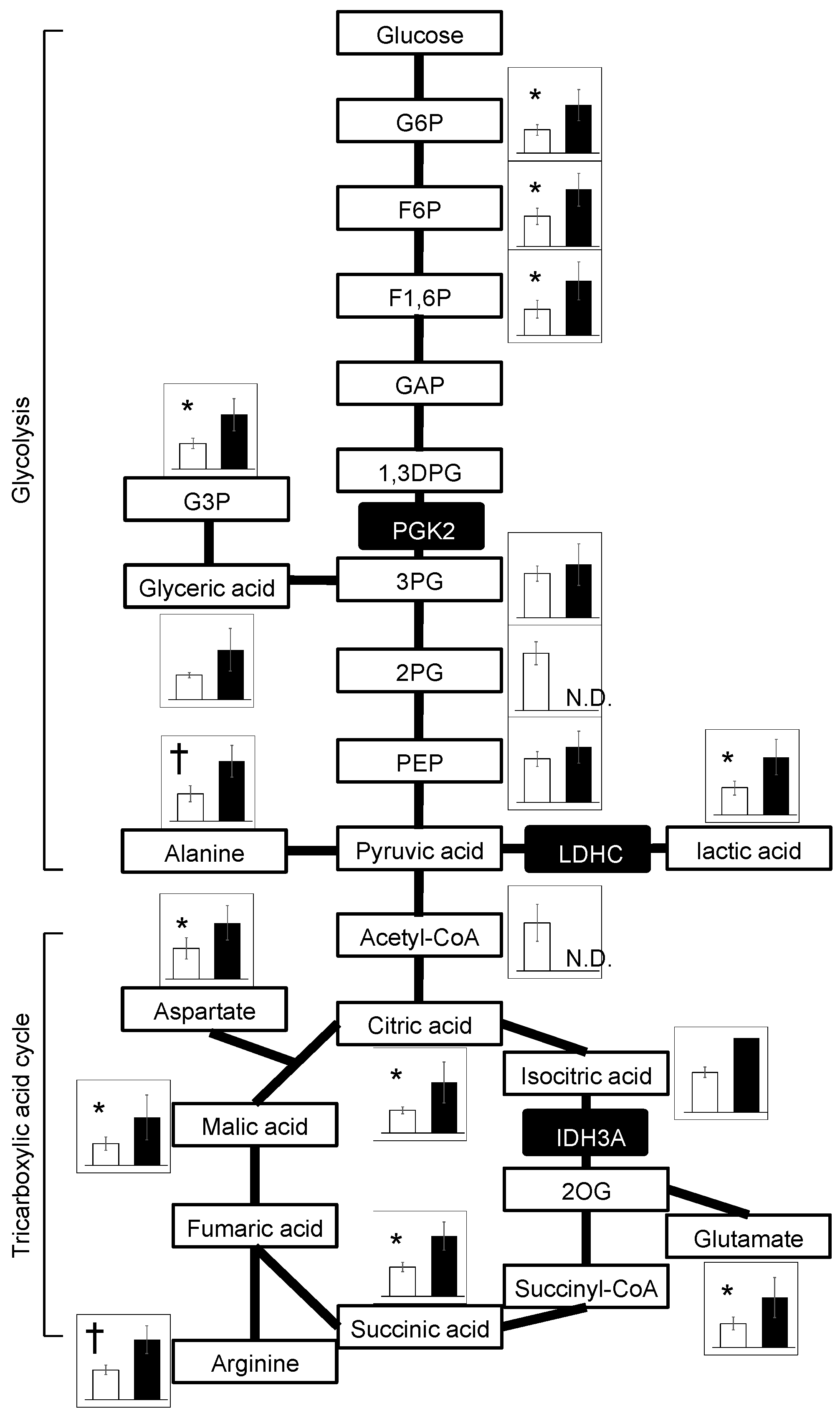
Metabolomic analyses detected widely increased metabolites of the glycolysis and TCA cycles in ChAc
Del/Del mice relative to WT mice (
Figure 4), aligning with the commonly reported metabolomic profiles in mitochondrial disease patients and animal models [
22]. In contrast, a metabolomic study using human sperm cells from idiopathic asthenozoospermia patients showed a decrease in these metabolites [
23]. It was also reported that
Idh3a-knockout flies showed decreased levels of TCA-cycle substrates [
17]. Concerning energy metabolism, the results should be interpreted with caution because the samples were epididymal spermatozoa. Since mitochondria are inactive in epididymal spermatozoa, glycolytic enzymes that are abundant in the cytoplasmic droplet have been shown to play an important role in ATP production [
21]. Spermatozoa from ChAc
Del/Del mice have intact cytoplasmic droplets when stored in the epididymis, suggesting that they are capable of ATP production. This is consistent with the previous study that showed there is no difference in the amount of ATP in epididymal spermatozoa of WT and ChAc
Del/Del mice [
12]. However, as previously mentioned in the discussion, there is a potential vulnerability of cytoplasmic droplets in ChAc
Del/Del mouse sperm to mechanical stimulation. Additionally, the impact of these metabolic changes on sperm function in ChAc
Del/Del mice has not been fully elucidated. These require further investigation into the mechanisms underlying sperm dysfunction in this model.
Because we focused on glycolytic enzymes and TCA-cycle enzymes in the present study, we did not expend substantial effort on ACAT1, ANXA1, GSTM5, and ODF1. Chorein–ACAT1 interaction was suggested by chorein co-IP analysis (
Figure 2). ACAT1, a protein localized in the mitochondria, plays a pivotal role in fatty acid metabolism and energy production. This enzyme catalyzes the reversible formation of acetoacetyl-CoA from two molecules of acetyl-CoA. ACAT1’s involvement extends to the processing of acetyl-CoA produced during beta-oxidation, a critical pathway for energy production. Furthermore, the acetoacetyl-CoA generated by ACAT1 is utilized in the TCA cycle, which is essential for cellular energy supply. The reduction in ACAT1 in the sperm of ChAc
Del/Del mice, as indicated by our findings, may contribute to a complex metabolic scenario. While ACAT1 impairment primarily impacts fatty acid metabolism, this could indirectly influence the TCA cycle. Decreased beta-oxidation activity might necessitate enhanced glycolysis and TCA-cycle activity to meet energy demands, possibly explaining the increased levels of TCA-cycle metabolites observed. This interplay illustrates the intricate balance of cellular metabolic pathways and highlights the need for an integrated understanding of how various metabolic alterations, including those involving ACAT1, impact overall cellular function, especially in the context of chorein-deficient sperm. Future studies delving deeper into these metabolic networks will be essential to unravel the detailed pathological changes and their broader implications.
In conclusion, our findings highlight the critical role of chorein in maintaining mitochondrial integrity during sperm development and underscore the complexities of mitochondrial regulation in spermatogenesis. The observed protein expression changes in ChAcDel/Del mice, particularly the increase in TOM20 and the decreases in IDH3A and ACAT1, offer insights into the molecular mechanisms underlying the mitochondrial dysmorphology and asthenozoospermia in these mice. Furthermore, the increased lipid peroxidation and staining patterns that we observed suggest the involvement of ferroptosis, particularly in the advanced stages of spermatogenesis. This potential association between ferroptosis and the observed mitochondrial and cellular abnormalities in ChAcDel/Del mice provides a new perspective on the molecular pathology of ChAc underlying these defects and opens up avenues for further research into the role of ferroptosis in spermatogenesis and male infertility. Further studies are required to elucidate the precise molecular pathways through which chorein deficiency leads to ferroptosis and its impact on sperm development. This exploration could potentially expand our understanding of male reproductive health.