In Utero Origins of Acute Leukemia in Children
Abstract
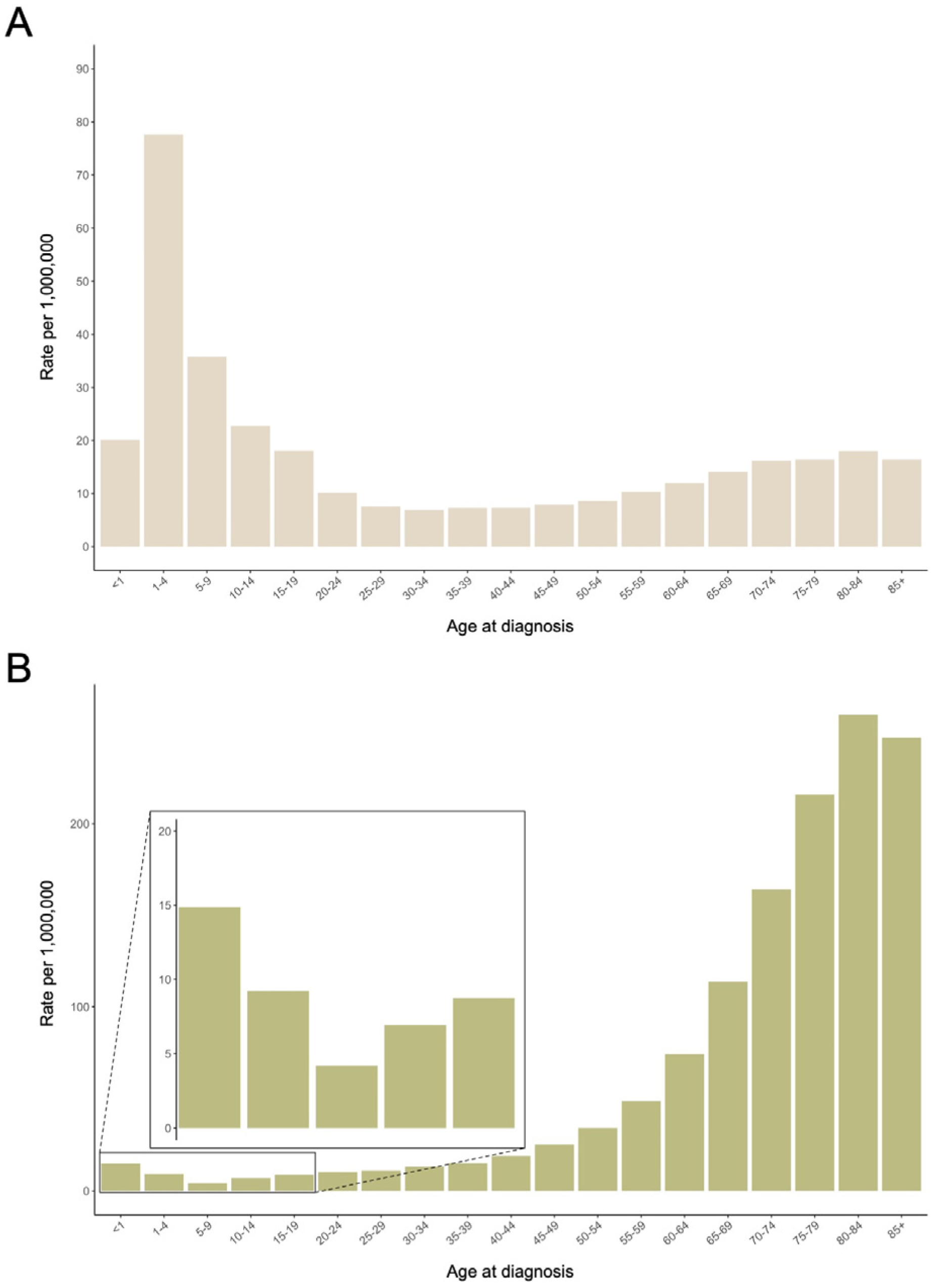
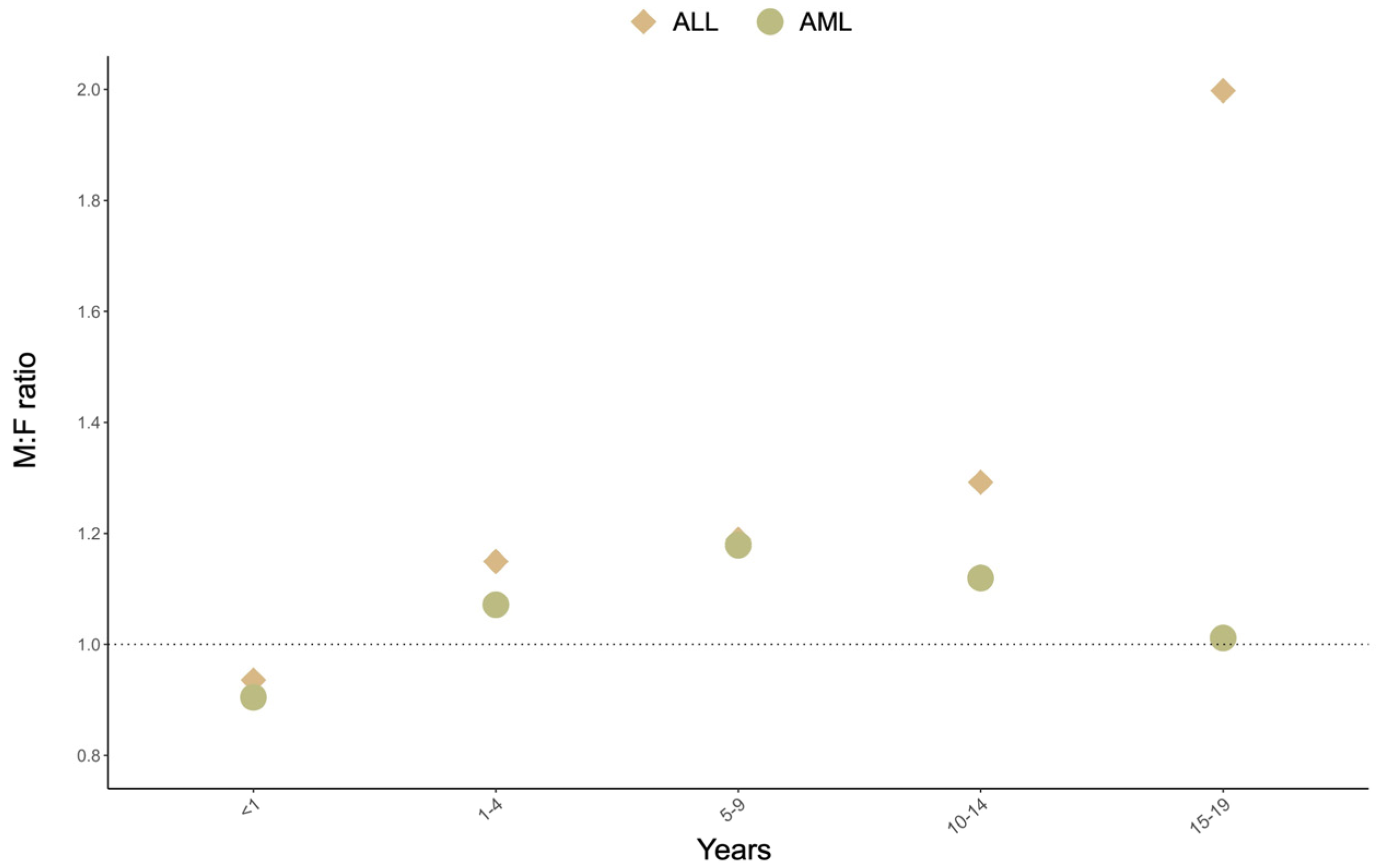
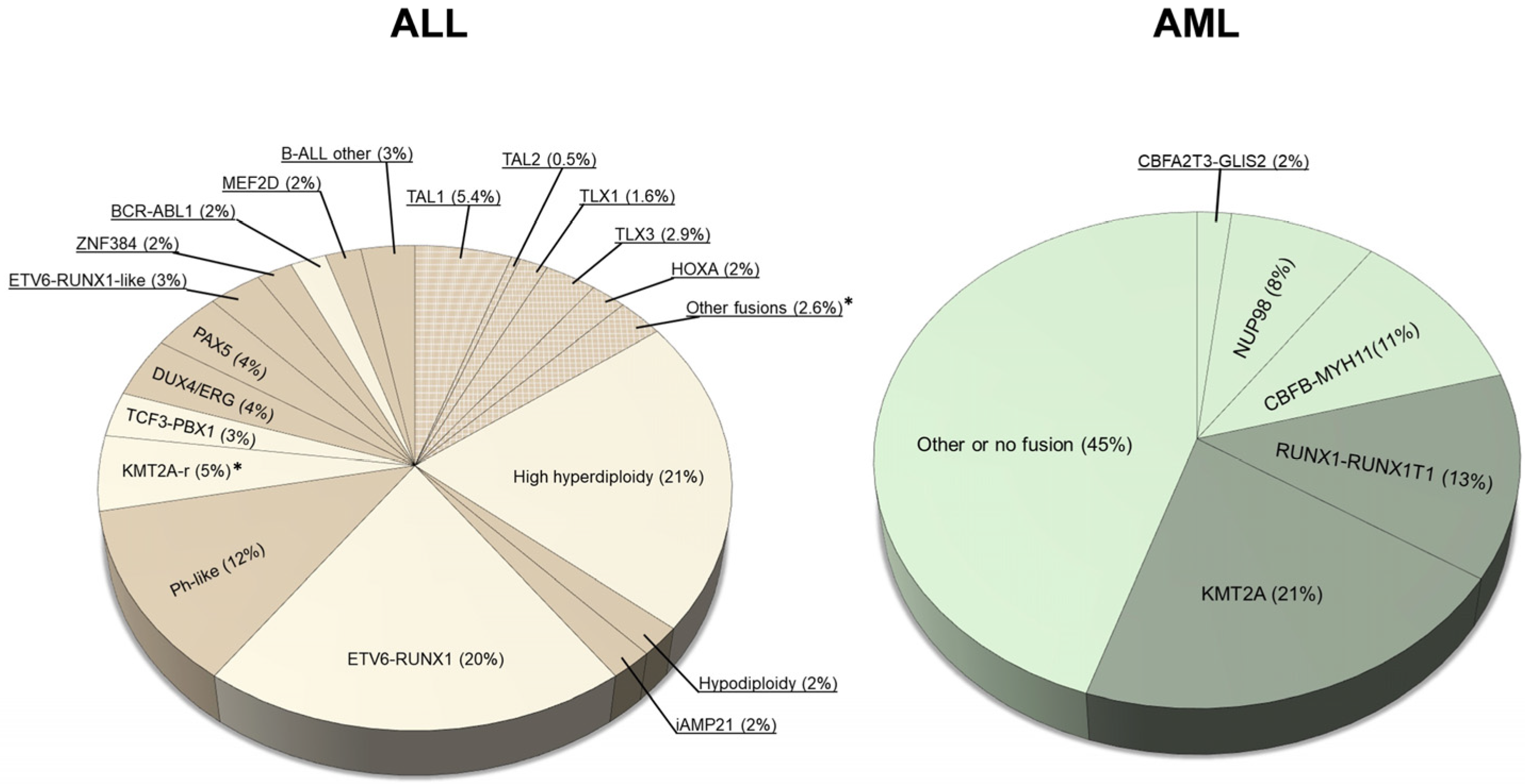
1. Descriptive Epidemiology of Childhood Acute Leukemias
2. Evidence for an In Utero Origin of Acute Leukemias in Brief
3. Twin Studies
4. Backtracking Studies
5. Prenatal Risk Factors for Acute Leukemia in Childhood
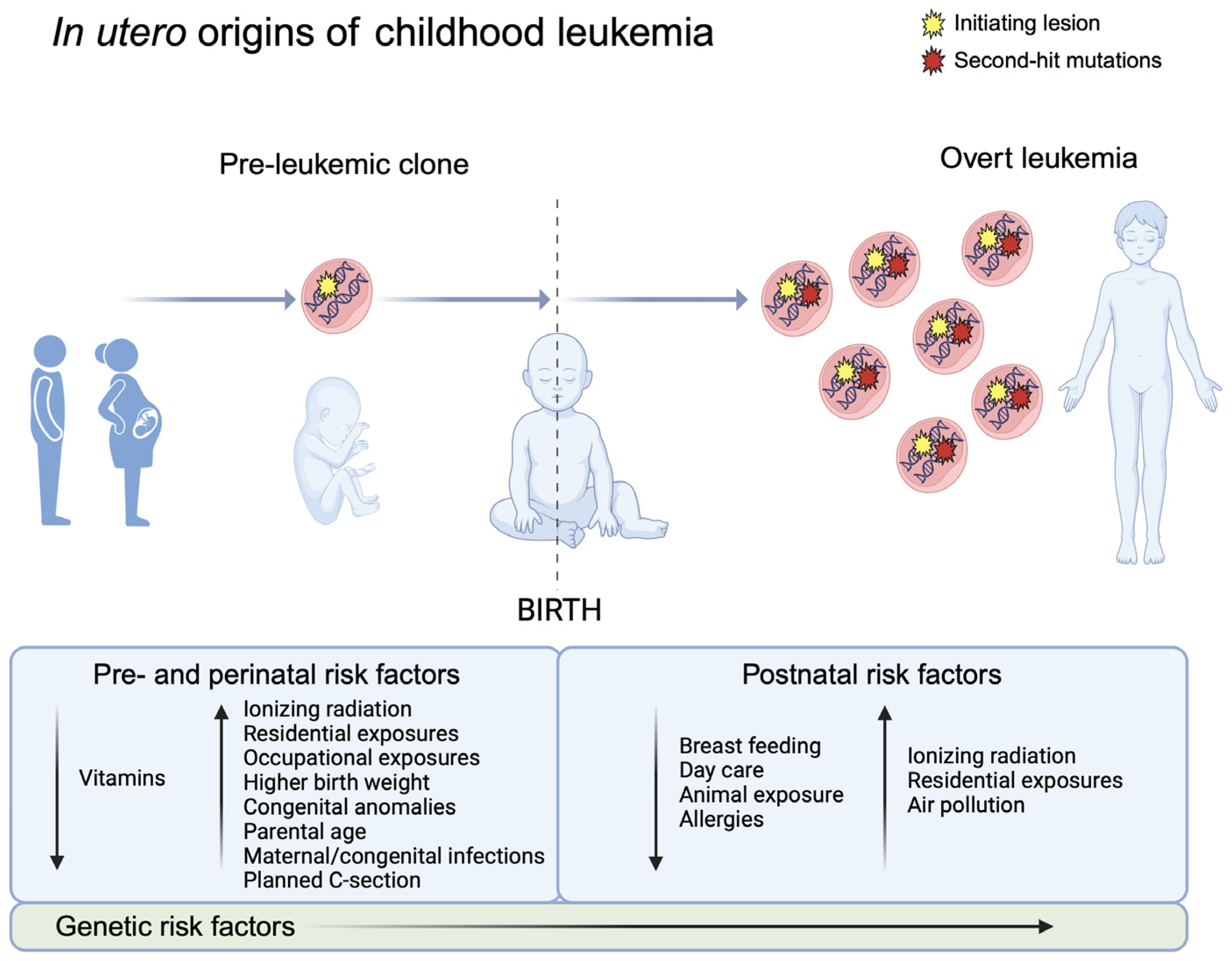
5.1. Pathologies of Pregnancy
5.2. Maternal Exposures
5.3. Perinatal Characteristics
6. Opportunities for Prevention
7. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- SEER*Explorer. Available online: https://seer.cancer.gov/statistics-network/explorer/ (accessed on 20 September 2023).
- Spector, L.G.; DeWan, A.T.; Pankratz, N.D.; Turcotte, L.M.; Yang, J.J.; Scheurer, M.E. RE: Racial and ethnic differences in socioeconomic position and risk of childhood acute lymphoblastic leukemia. Am. J. Epidemiol. 2019, 188, 1192–1193. [Google Scholar] [CrossRef] [PubMed]
- Feng, Q.; de Smith, A.J.; Vergara-Lluri, M.; Muskens, I.S.; McKean-Cowdin, R.; Kogan, S.; Brynes, R.; Wiemels, J.L. Trends in Acute Lymphoblastic Leukemia Incidence in the United States by Race/Ethnicity from 2000 to 2016. Am. J. Epidemiol. 2021, 190, 519–527. [Google Scholar] [CrossRef] [PubMed]
- Moore, K.J.; Hubbard, A.K.; Williams, L.A.; Spector, L.G. Childhood Cancer Incidence among Specific Asian and Pacific Islander Populations in the United States. Int. J. Cancer 2020, 147, 3339–3348. [Google Scholar] [CrossRef] [PubMed]
- Katz, A.J.; Chia, V.M.; Schoonen, W.M.; Kelsh, M.A. Acute Lymphoblastic Leukemia: An Assessment of International Incidence, Survival, and Disease Burden. Cancer Causes Control 2015, 26, 1627–1642. [Google Scholar] [CrossRef] [PubMed]
- Steliarova-Foucher, E.; Colombet, M.; Ries, L.A.G.; Moreno, F.; Dolya, A.; Bray, F.; Hesseling, P.; Shin, H.Y.; Stiller, C.A.; Bouzbid, S.; et al. IICC-3 contributors International Incidence of Childhood Cancer, 2001–2010: A Population-Based Registry Study. Lancet Oncol. 2017, 18, 719–731. [Google Scholar] [CrossRef]
- Miranda-Filho, A.; Piñeros, M.; Ferlay, J.; Soerjomataram, I.; Monnereau, A.; Bray, F. Epidemiological Patterns of Leukaemia in 184 Countries: A Population-Based Study. Lancet Haematol. 2018, 5, e14–e24. [Google Scholar] [CrossRef]
- Jackson, T.R.; Ling, R.E.; Roy, A. The Origin of B-Cells: Human Fetal B Cell Development and Implications for the Pathogenesis of Childhood Acute Lymphoblastic Leukemia. Front. Immunol. 2021, 12, 637975. [Google Scholar] [CrossRef]
- Robertson, M.; De Jong, G.; Mansvelt, E. Prenatal Diagnosis of Congenital Leukemia in a Fetus at 25 Weeks’ Gestation with Down Syndrome: Case Report and Review of the Literature. Ultrasound Obstet. Gynecol. 2003, 21, 486–489. [Google Scholar] [CrossRef]
- Greaves, M.F.; Maia, A.T.; Wiemels, J.L.; Ford, A.M. Leukemia in Twins: Lessons in Natural History. Blood 2003, 102, 2321–2333. [Google Scholar] [CrossRef]
- Macmahon, B.; Levy, M.A. Prenatal Origin of Childhood Leukemia. Evidence from Twins. N. Engl. J. Med. 1964, 270, 1082–1085. [Google Scholar] [CrossRef]
- Clarkson, B.; Boyse, E. Possible Explanation of the High Concordance for Acute Leukæmia in Monozygotic Twins. Lancet 1971, 297, 699–701. [Google Scholar] [CrossRef] [PubMed]
- Wolman, I.J. Parallel Responses to Chemotherapy in Identical Twin Infants with Concordant Leukemia. J. Pediatr. 1962, 60, 91–95. [Google Scholar] [CrossRef] [PubMed]
- Lewi, L.; Deprest, J.; Hecher, K. The Vascular Anastomoses in Monochorionic Twin Pregnancies and Their Clinical Consequences. Am. J. Obstet. Gynecol. 2013, 208, 19–30. [Google Scholar] [CrossRef]
- Ford, A.M.; Ridge, S.A.; Cabrera, M.E.; Mahmoud, H.; Steel, C.M.; Chan, L.C.; Greaves, M. In Utero Rearrangements in the Trithorax-Related Oncogene in Infant Leukaemias. Nature 1993, 363, 358–360. [Google Scholar] [CrossRef] [PubMed]
- Ford, A.M.; Bennett, C.A.; Price, C.M.; Bruin, M.C.; Van Wering, E.R.; Greaves, M. Fetal Origins of the TEL-AML1 Fusion Gene in Identical Twins with Leukemia. Proc. Natl. Acad. Sci. USA 1998, 95, 4584–4588. [Google Scholar] [CrossRef] [PubMed]
- Wiemels, J.L.; Ford, A.M.; Van Wering, E.R.; Postma, A.; Greaves, M. Protracted and Variable Latency of Acute Lymphoblastic Leukemia after TEL-AML1 Gene Fusion in Utero. Blood 1999, 94, 1057–1062. [Google Scholar] [CrossRef] [PubMed]
- Hong, D.; Gupta, R.; Ancliff, P.; Atzberger, A.; Brown, J.; Soneji, S.; Green, J.; Colman, S.; Piacibello, W.; Buckle, V.; et al. Initiating and Cancer-Propagating Cells in TEL-AML1-Associated Childhood Leukemia. Science 2008, 319, 336–339. [Google Scholar] [CrossRef]
- Cazzaniga, G.; van Delft, F.W.; Lo Nigro, L.; Ford, A.M.; Score, J.; Iacobucci, I.; Mirabile, E.; Taj, M.; Colman, S.M.; Biondi, A.; et al. Developmental Origins and Impact of BCR-ABL1 Fusion and IKZF1 Deletions in Monozygotic Twins with Ph+ Acute Lymphoblastic Leukemia. Blood 2011, 118, 5559–5564. [Google Scholar] [CrossRef]
- Bueno, C.; Tejedor, J.R.; Bashford-Rogers, R.; González-Silva, L.; Valdés-Mas, R.; Agraz-Doblás, A.; Díaz de la Guardia, R.; Ribera, J.; Zamora, L.; Bilhou-Nabera, C.; et al. Natural History and Cell of Origin of TCF3-ZNF384 and PTPN11 Mutations in Monozygotic Twins with Concordant BCP-ALL. Blood 2019, 134, 900–905. [Google Scholar] [CrossRef]
- Maia, A.T.; van der Velden, V.H.J.; Harrison, C.J.; Szczepanski, T.; Williams, M.D.; Griffiths, M.J.; van Dongen, J.J.M.; Greaves, M.F. Prenatal Origin of Hyperdiploid Acute Lymphoblastic Leukemia in Identical Twins. Leukemia 2003, 17, 2202–2206. [Google Scholar] [CrossRef][Green Version]
- Bateman, C.M.; Alpar, D.; Ford, A.M.; Colman, S.M.; Wren, D.; Morgan, M.; Kearney, L.; Greaves, M. Evolutionary Trajectories of Hyperdiploid ALL in Monozygotic Twins. Leukemia 2015, 29, 58–65. [Google Scholar] [CrossRef] [PubMed]
- Megonigal, M.D.; Rappaport, E.F.; Jones, D.H.; Williams, T.M.; Lovett, B.D.; Kelly, K.M.; Lerou, P.H.; Moulton, T.; Budarf, M.L.; Felix, C.A. t(11;22)(q23;q11.2) In Acute Myeloid Leukemia of Infant Twins Fuses MLL with hCDCrel, a Cell Division Cycle Gene in the Genomic Region of Deletion in DiGeorge and Velocardiofacial Syndromes. Proc. Natl. Acad. Sci. USA 1998, 95, 6413–6418. [Google Scholar] [CrossRef] [PubMed]
- Bateman, C.M.; Colman, S.M.; Chaplin, T.; Young, B.D.; Eden, T.O.; Bhakta, M.; Gratias, E.J.; van Wering, E.R.; Cazzaniga, G.; Harrison, C.J.; et al. Acquisition of Genome-Wide Copy Number Alterations in Monozygotic Twins with Acute Lymphoblastic Leukemia. Blood 2010, 115, 3553–3558. [Google Scholar] [CrossRef] [PubMed]
- Davidow, K.; Mumanachit, S.; Mangum, D.S. The Two-Hit Hypothesis in Practice: Monozygotic Twins with Simultaneous Hyperdiploid Acute Lymphoblastic Leukemia. Pediatr. Blood Cancer 2022, 69, e29885. [Google Scholar] [CrossRef] [PubMed]
- Greaves, M. A Causal Mechanism for Childhood Acute Lymphoblastic Leukaemia. Nat. Rev. Cancer 2018, 18, 471–484. [Google Scholar] [CrossRef]
- Ford, A.M.; Colman, S.; Greaves, M. Covert Pre-Leukaemic Clones in Healthy Co-Twins of Patients with Childhood Acute Lymphoblastic Leukaemia. Leukemia 2023, 37, 47–52. [Google Scholar] [CrossRef] [PubMed]
- Debeljak, M.; Kitanovski, L.; Pajič, T.; Jazbec, J. Concordant Acute Myeloblastic Leukemia in Monozygotic Twins with Germline and Shared Somatic Mutations in the Gene for CCAAT-Enhancer-Binding Protein α with 13 Years Difference at Onset. Haematologica 2013, 98, e73–e74. [Google Scholar] [CrossRef]
- Dameshek, W.; Savitz, H.A.; Arbor, B. Chronic lymphatic leukemia in twin brothers aged fifty-six. J. Am. Med. Assoc. 1929, 92, 1348–1349. [Google Scholar] [CrossRef]
- Sousos, N.; Ní Leathlobhair, M.; Simoglou Karali, C.; Louka, E.; Bienz, N.; Royston, D.; Clark, S.-A.; Hamblin, A.; Howard, K.; Mathews, V.; et al. In Utero Origin of Myelofibrosis Presenting in Adult Monozygotic Twins. Nat. Med. 2022, 28, 1207–1211. [Google Scholar] [CrossRef]
- Pui, C.-H.; Nichols, K.E.; Yang, J.J. Somatic and Germline Genomics in Paediatric Acute Lymphoblastic Leukaemia. Nat. Rev. Clin. Oncol. 2019, 16, 227–240. [Google Scholar] [CrossRef]
- Huang, B.J.; Smith, J.L.; Farrar, J.E.; Wang, Y.-C.; Umeda, M.; Ries, R.E.; Leonti, A.R.; Crowgey, E.; Furlan, S.N.; Tarlock, K.; et al. Integrated Stem Cell Signature and Cytomolecular Risk Determination in Pediatric Acute Myeloid Leukemia. Nat. Commun. 2022, 13, 5487. [Google Scholar] [CrossRef] [PubMed]
- Gale, K.B.; Ford, A.M.; Repp, R.; Borkhardt, A.; Keller, C.; Eden, O.B.; Greaves, M.F. Backtracking Leukemia to Birth: Identification of Clonotypic Gene Fusion Sequences in Neonatal Blood Spots. Proc. Natl. Acad. Sci. USA 1997, 94, 13950–13954. [Google Scholar] [CrossRef] [PubMed]
- Jones, L.K.; Neat, M.J.; van Delft, F.W.; Mitchell, M.P.; Adamaki, M.; Stoneham, S.J.; Patel, N.; Saha, V. Cryptic Rearrangement Involving MLL and AF10 Occurring in Utero. Leukemia 2003, 17, 1667–1669. [Google Scholar] [CrossRef] [PubMed]
- Wiemels, J.L.; Cazzaniga, G.; Daniotti, M.; Eden, O.B.; Addison, G.M.; Masera, G.; Saha, V.; Biondi, A.; Greaves, M.F. Prenatal Origin of Acute Lymphoblastic Leukaemia in Children. Lancet 1999, 354, 1499–1503. [Google Scholar] [CrossRef] [PubMed]
- Wiemels, J.L.; Xiao, Z.; Buffler, P.A.; Maia, A.T.; Ma, X.; Dicks, B.M.; Smith, M.T.; Zhang, L.; Feusner, J.; Wiencke, J.; et al. In Utero Origin of t(8;21) AML1-ETO Translocations in Childhood Acute Myeloid Leukemia. Blood 2002, 99, 3801–3805. [Google Scholar] [CrossRef] [PubMed]
- Yagi, T.; Hibi, S.; Tabata, Y.; Kuriyama, K.; Teramura, T.; Hashida, T.; Shimizu, Y.; Takimoto, T.; Todo, S.; Sawada, T.; et al. Detection of Clonotypic IGH and TCR Rearrangements in the Neonatal Blood Spots of Infants and Children with B-Cell Precursor Acute Lymphoblastic Leukemia. Blood 2000, 96, 264–268. [Google Scholar] [CrossRef]
- Panzer-Grümayer, E.R.; Fasching, K.; Panzer, S.; Hettinger, K.; Schmitt, K.; Stöckler-Ipsiroglu, S.; Haas, O.A. Nondisjunction of Chromosomes Leading to Hyperdiploid Childhood B-Cell Precursor Acute Lymphoblastic Leukemia Is an Early Event during Leukemogenesis. Blood 2002, 100, 347–349. [Google Scholar] [CrossRef][Green Version]
- Gruhn, B.; Taub, J.W.; Ge, Y.; Beck, J.F.; Zell, R.; Häfer, R.; Hermann, F.H.; Debatin, K.-M.; Steinbach, D. Prenatal Origin of Childhood Acute Lymphoblastic Leukemia, Association with Birth Weight and Hyperdiploidy. Leukemia 2008, 22, 1692–1697. [Google Scholar] [CrossRef]
- Taub, J.W.; Konrad, M.A.; Ge, Y.; Naber, J.M.; Scott, J.S.; Matherly, L.H.; Ravindranath, Y. High Frequency of Leukemic Clones in Newborn Screening Blood Samples of Children with B-Precursor Acute Lymphoblastic Leukemia. Blood 2002, 99, 2992–2996. [Google Scholar] [CrossRef]
- Hein, D.; Dreisig, K.; Metzler, M.; Izraeli, S.; Schmiegelow, K.; Borkhardt, A.; Fischer, U. The Preleukemic TCF3-PBX1 Gene Fusion Can Be Generated in Utero and Is Present in ≈0.6% of Healthy Newborns. Blood 2019, 134, 1355–1358. [Google Scholar] [CrossRef]
- Wiemels, J.L.; Leonard, B.C.; Wang, Y.; Segal, M.R.; Hunger, S.P.; Smith, M.T.; Crouse, V.; Ma, X.; Buffler, P.A.; Pine, S.R. Site-Specific Translocation and Evidence of Postnatal Origin of the t(1;19) E2A-PBX1 Fusion in Childhood Acute Lymphoblastic Leukemia. Proc. Natl. Acad. Sci. USA 2002, 99, 15101–15106. [Google Scholar] [CrossRef] [PubMed]
- Den Boer, M.L.; van Slegtenhorst, M.; De Menezes, R.X.; Cheok, M.H.; Buijs-Gladdines, J.G.C.A.M.; Peters, S.T.C.J.M.; Van Zutven, L.J.C.M.; Beverloo, H.B.; Van der Spek, P.J.; Escherich, G.; et al. A Subtype of Childhood Acute Lymphoblastic Leukaemia with Poor Treatment Outcome: A Genome-Wide Classification Study. Lancet Oncol. 2009, 10, 125–134. [Google Scholar] [CrossRef] [PubMed]
- Mullighan, C.G.; Su, X.; Zhang, J.; Radtke, I.; Phillips, L.A.A.; Miller, C.B.; Ma, J.; Liu, W.; Cheng, C.; Schulman, B.A.; et al. Deletion of IKZF1 and Prognosis in Acute Lymphoblastic Leukemia. N. Engl. J. Med. 2009, 360, 470–480. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; McCastlain, K.; Yoshihara, H.; Xu, B.; Chang, Y.; Churchman, M.L.; Wu, G.; Li, Y.; Wei, L.; Iacobucci, I.; et al. Deregulation of DUX4 and ERG in Acute Lymphoblastic Leukemia. Nat. Genet. 2016, 48, 1481–1489. [Google Scholar] [CrossRef] [PubMed]
- Yasuda, T.; Tsuzuki, S.; Kawazu, M.; Hayakawa, F.; Kojima, S.; Ueno, T.; Imoto, N.; Kohsaka, S.; Kunita, A.; Doi, K.; et al. Corrigendum: Recurrent DUX4 Fusions in B Cell Acute Lymphoblastic Leukemia of Adolescents and Young Adults. Nat. Genet. 2016, 48, 1591. [Google Scholar] [CrossRef] [PubMed]
- Hirabayashi, S.; Ohki, K.; Nakabayashi, K.; Ichikawa, H.; Momozawa, Y.; Okamura, K.; Yaguchi, A.; Terada, K.; Saito, Y.; Yoshimi, A.; et al. ZNF384-Related Fusion Genes Define a Subgroup of Childhood B-Cell Precursor Acute Lymphoblastic Leukemia with a Characteristic Immunotype. Haematologica 2017, 102, 118–129. [Google Scholar] [CrossRef]
- Gu, Z.; Churchman, M.; Roberts, K.; Li, Y.; Liu, Y.; Harvey, R.C.; McCastlain, K.; Reshmi, S.C.; Payne-Turner, D.; Iacobucci, I.; et al. Genomic Analyses Identify Recurrent MEF2D Fusions in Acute Lymphoblastic Leukaemia. Nat. Commun. 2016, 7, 13331. [Google Scholar] [CrossRef]
- Wiemels, J.L.; Hofmann, J.; Kang, M.; Selzer, R.; Green, R.; Zhou, M.; Zhong, S.; Zhang, L.; Smith, M.T.; Marsit, C.; et al. Chromosome 12p Deletions in TEL-AML1 Childhood Acute Lymphoblastic Leukemia Are Associated with Retrotransposon Elements and Occur Postnatally. Cancer Res. 2008, 68, 9935–9944. [Google Scholar] [CrossRef]
- Wiemels, J.L.; Kang, M.; Chang, J.S.; Zheng, L.; Kouyoumji, C.; Zhang, L.; Smith, M.T.; Scelo, G.; Metayer, C.; Buffler, P.; et al. Backtracking RAS Mutations in High Hyperdiploid Childhood Acute Lymphoblastic Leukemia. Blood Cells Mol. Dis. 2010, 45, 186–191. [Google Scholar] [CrossRef]
- Chang, P.; Kang, M.; Xiao, A.; Chang, J.; Feusner, J.; Buffler, P.; Wiemels, J. FLT3 Mutation Incidence and Timing of Origin in a Population Case Series of Pediatric Leukemia. BMC Cancer 2010, 10, 513. [Google Scholar] [CrossRef]
- Marcotte, E.L.; Spector, L.G.; Mendes-de-Almeida, D.P.; Nelson, H.H. The Prenatal Origin of Childhood Leukemia: Potential Applications for Epidemiology and Newborn Screening. Front. Pediatr. 2021, 9, 639479. [Google Scholar] [CrossRef] [PubMed]
- Hein, D.; Borkhardt, A.; Fischer, U. Insights into the Prenatal Origin of Childhood Acute Lymphoblastic Leukemia. Cancer Metastasis Rev. 2020, 39, 161–171. [Google Scholar] [CrossRef] [PubMed]
- Wagenblast, E.; Araújo, J.; Gan, O.I.; Cutting, S.K.; Murison, A.; Krivdova, G.; Azkanaz, M.; McLeod, J.L.; Smith, S.A.; Gratton, B.A.; et al. Mapping the Cellular Origin and Early Evolution of Leukemia in Down Syndrome. Science 2021, 373, eabf6202. [Google Scholar] [CrossRef] [PubMed]
- Hasle, H. Pattern of Malignant Disorders in Individuals with Down’s Syndrome. Lancet Oncol. 2001, 2, 429–436. [Google Scholar] [CrossRef]
- Roberts, I.; Alford, K.; Hall, G.; Juban, G.; Richmond, H.; Norton, A.; Vallance, G.; Perkins, K.; Marchi, E.; McGowan, S.; et al. GATA1-Mutant Clones Are Frequent and Often Unsuspected in Babies with Down Syndrome: Identification of a Population at Risk of Leukemia. Blood 2013, 122, 3908–3917. [Google Scholar] [CrossRef]
- Wechsler, J.; Greene, M.; McDevitt, M.A.; Anastasi, J.; Karp, J.E.; Le Beau, M.M.; Crispino, J.D. Acquired Mutations in GATA1 in the Megakaryoblastic Leukemia of Down Syndrome. Nat. Genet. 2002, 32, 148–152. [Google Scholar] [CrossRef]
- Labuhn, M.; Perkins, K.; Matzk, S.; Varghese, L.; Garnett, C.; Papaemmanuil, E.; Metzner, M.; Kennedy, A.; Amstislavskiy, V.; Risch, T.; et al. Mechanisms of Progression of Myeloid Preleukemia to Transformed Myeloid Leukemia in Children with Down Syndrome. Cancer Cell 2019, 36, 340. [Google Scholar] [CrossRef]
- Cazzola, A.; Cazzaniga, G.; Biondi, A.; Meneveri, R.; Brunelli, S.; Azzoni, E. Prenatal Origin of Pediatric Leukemia: Lessons From Hematopoietic Development. Front. Cell Dev. Biol. 2020, 8, 618164. [Google Scholar] [CrossRef]
- Lupo, P.J.; Spector, L.G. Cancer Progress and Priorities: Childhood Cancer. Cancer Epidemiol. Biomark. Prev. 2020, 29, 1081–1094. [Google Scholar] [CrossRef]
- Marley, A.R.; Domingues, A.; Ghosh, T.; Turcotte, L.M.; Spector, L.G. Maternal Body Mass Index, Diabetes, and Gestational Weight Gain and Risk for Pediatric Cancer in Offspring: A Systematic Review and Meta-Analysis. JNCI Cancer Spectr. 2022, 6, pkac020. [Google Scholar] [CrossRef]
- He, J.-R.; Ramakrishnan, R.; Hirst, J.E.; Bonaventure, A.; Francis, S.S.; Paltiel, O.; Håberg, S.E.; Lemeshow, S.; Olsen, S.; Tikellis, G.; et al. Maternal Infection in Pregnancy and Childhood Leukemia: A Systematic Review and Meta-Analysis. J. Pediatr. 2020, 217, 98–109.e8. [Google Scholar] [CrossRef] [PubMed]
- He, J.-R.; Hirst, J.E.; Tikellis, G.; Phillips, G.S.; Ramakrishnan, R.; Paltiel, O.; Ponsonby, A.-L.; Klebanoff, M.; Olsen, J.; Murphy, M.F.G.; et al. Common Maternal Infections during Pregnancy and Childhood Leukaemia in the Offspring: Findings from Six International Birth Cohorts. Int. J. Epidemiol. 2022, 51, 769–777. [Google Scholar] [CrossRef] [PubMed]
- Wakeford, R.; Bithell, J.F. A Review of the Types of Childhood Cancer Associated with a Medical X-Ray Examination of the Pregnant Mother. Int. J. Radiat. Biol. 2021, 97, 571–592. [Google Scholar] [CrossRef] [PubMed]
- Yoon, I.; Slesinger, T.L. Radiation Exposure in Pregnancy. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Bailey, H.D.; Fritschi, L.; Infante-Rivard, C.; Glass, D.C.; Miligi, L.; Dockerty, J.D.; Lightfoot, T.; Clavel, J.; Roman, E.; Spector, L.G.; et al. Parental Occupational Pesticide Exposure and the Risk of Childhood Leukemia in the Offspring: Findings from the Childhood Leukemia International Consortium. Int. J. Cancer 2014, 135, 2157–2172. [Google Scholar] [CrossRef] [PubMed]
- Bailey, H.D.; Fritschi, L.; Metayer, C.; Infante-Rivard, C.; Magnani, C.; Petridou, E.; Roman, E.; Spector, L.G.; Kaatsch, P.; Clavel, J.; et al. Parental Occupational Paint Exposure and Risk of Childhood Leukemia in the Offspring: Findings from the Childhood Leukemia International Consortium. Cancer Causes Control 2014, 25, 1351–1367. [Google Scholar] [CrossRef] [PubMed]
- Bailey, H.D.; Infante-Rivard, C.; Metayer, C.; Clavel, J.; Lightfoot, T.; Kaatsch, P.; Roman, E.; Magnani, C.; Spector, L.G.; Th Petridou, E.; et al. Home Pesticide Exposures and Risk of Childhood Leukemia: Findings from the Childhood Leukemia International Consortium. Int. J. Cancer 2015, 137, 2644–2663. [Google Scholar] [CrossRef] [PubMed]
- Metayer, C.; Milne, E.; Dockerty, J.D.; Clavel, J.; Pombo-de-Oliveira, M.S.; Wesseling, C.; Spector, L.G.; Schüz, J.; Petridou, E.; Ezzat, S.; et al. Maternal Supplementation with Folic Acid and Other Vitamins and Risk of Leukemia in Offspring: A Childhood Leukemia International Consortium Study. Epidemiology 2014, 25, 811–822. [Google Scholar] [CrossRef]
- Bunin, G.R.; Gyllstrom, M.E.; Brown, J.E.; Kahn, E.B.; Kushi, L.H. Recall of Diet during a Past Pregnancy. Am. J. Epidemiol. 2001, 154, 1136–1142. [Google Scholar] [CrossRef]
- Blanco-Lopez, J.; Iguacel, I.; Pisanu, S.; Almeida, C.C.B.; Steliarova-Foucher, E.; Sierens, C.; Gunter, M.J.; Ladas, E.J.; Barr, R.D.; Van Herck, K.; et al. Role of Maternal Diet in the Risk of Childhood Acute Leukemia: A Systematic Review and Meta-Analysis. Int. J. Environ. Res. Public Health 2023, 20, 5428. [Google Scholar] [CrossRef]
- Chunxia, D.; Meifang, W.; Jianhua, Z.; Ruijuan, Z.; Xiue, L.; Zhuanzhen, Z.; Linhua, Y. Tobacco Smoke Exposure and the Risk of Childhood Acute Lymphoblastic Leukemia and Acute Myeloid Leukemia: A Meta-Analysis. Medicine 2019, 98, e16454. [Google Scholar] [CrossRef]
- Karalexi, M.A.; Dessypris, N.; Thomopoulos, T.P.; Ntouvelis, E.; Kantzanou, M.; Diamantaras, A.-A.; Moschovi, M.; Baka, M.; Hatzipantelis, E.; Kourti, M.; et al. Parental Alcohol Consumption and Risk of Leukemia in the Offspring: A Systematic Review and Meta-Analysis. Eur. J. Cancer Prev. 2017, 26, 433–441. [Google Scholar] [CrossRef] [PubMed]
- Hill, A.B. The environment and disease: Association or causation? J. R. Soc. Med. 1965, 58, 295–300. [Google Scholar] [CrossRef]
- Ross, J.A. Birth Weight and Childhood Leukemia: Time to Tackle Bigger Lessons. Pediatr. Blood Cancer 2012, 58, 1–2. [Google Scholar] [CrossRef]
- Che, H.; Long, D.; Sun, Q.; Wang, L.; Li, Y. Birth Weight and Subsequent Risk of Total Leukemia and Acute Leukemia: A Systematic Review and Meta-Analysis. Front. Pediatr. 2021, 9, 722471. [Google Scholar] [CrossRef] [PubMed]
- Hoang, T.T.; Schraw, J.M.; Peckham-Gregory, E.C.; Scheurer, M.E.; Lupo, P.J. Fetal Growth and Pediatric Cancer: A Pan-Cancer Analysis in 7000 Cases and 37,000 Controls. Int. J. Cancer 2024, 154, 41–52. [Google Scholar] [CrossRef] [PubMed]
- Milne, E.; Greenop, K.R.; Metayer, C.; Schüz, J.; Petridou, E.; Pombo-de-Oliveira, M.S.; Infante-Rivard, C.; Roman, E.; Dockerty, J.D.; Spector, L.G.; et al. Fetal Growth and Childhood Acute Lymphoblastic Leukemia: Findings from the Childhood Leukemia International Consortium. Int. J. Cancer 2013, 133, 2968–2979. [Google Scholar] [CrossRef]
- O’Neill, K.A.; Bunch, K.J.; Vincent, T.J.; Spector, L.G.; Moorman, A.V.; Murphy, M.F.G. Immunophenotype and Cytogenetic Characteristics in the Relationship between Birth Weight and Childhood Leukemia. Pediatr. Blood Cancer 2012, 58, 7–11. [Google Scholar] [CrossRef] [PubMed]
- Petridou, E.T.; Georgakis, M.K.; Erdmann, F.; Ma, X.; Heck, J.E.; Auvinen, A.; Mueller, B.A.; Spector, L.G.; Roman, E.; Metayer, C.; et al. Advanced Parental Age as Risk Factor for Childhood Acute Lymphoblastic Leukemia: Results from Studies of the Childhood Leukemia International Consortium. Eur. J. Epidemiol. 2018, 33, 965–976. [Google Scholar] [CrossRef]
- Panagopoulou, P.; Skalkidou, A.; Marcotte, E.; Erdmann, F.; Ma, X.; Heck, J.E.; Auvinen, A.; Mueller, B.A.; Spector, L.G.; Roman, E.; et al. Parental Age and the Risk of Childhood Acute Myeloid Leukemia: Results from the Childhood Leukemia International Consortium. Cancer Epidemiol. 2019, 59, 158–165. [Google Scholar] [CrossRef]
- Mohiuddin, M.; Kooy, R.F.; Pearson, C.E. De Novo Mutations, Genetic Mosaicism and Human Disease. Front. Genet. 2022, 13, 983668. [Google Scholar] [CrossRef]
- Rashed, W.M.; Marcotte, E.L.; Spector, L.G. Germline De Novo Mutations as a Cause of Childhood Cancer. JCO Precis. Oncol. 2022, 6, e2100505. [Google Scholar] [CrossRef]
- Schmidt, L.; Sobotka, T.; Bentzen, J.G.; Nyboe Andersen, A.; ESHRE Reproduction and Society Task Force. Demographic and Medical Consequences of the Postponement of Parenthood. Hum. Reprod. Update 2012, 18, 29–43. [Google Scholar] [CrossRef]
- Lupo, P.J.; Chambers, T.M.; Mueller, B.A.; Clavel, J.; Dockerty, J.D.; Doody, D.R.; Erdmann, F.; Ezzat, S.; Filippini, T.; Hansen, J.; et al. Nonchromosomal Birth Defects and Risk of Childhood Acute Leukemia: An Assessment in 15,000 Leukemia Cases and 46,000 Controls from the Childhood Cancer and Leukemia International Consortium. Int. J. Cancer 2024, 154, 434–447. [Google Scholar] [CrossRef]
- Lupo, P.J.; Schraw, J.M.; Desrosiers, T.A.; Nembhard, W.N.; Langlois, P.H.; Canfield, M.A.; Copeland, G.; Meyer, R.E.; Brown, A.L.; Chambers, T.M.; et al. Association Between Birth Defects and Cancer Risk Among Children and Adolescents in a Population-Based Assessment of 10 Million Live Births. JAMA Oncol. 2019, 5, 1150–1158. [Google Scholar] [CrossRef]
- Ríos-Covian, D.; Langella, P.; Martín, R. From Short- to Long-Term Effects of C-Section Delivery on Microbiome Establishment and Host Health. Microorganisms 2021, 9, 2122. [Google Scholar] [CrossRef]
- Shao, Y.; Forster, S.C.; Tsaliki, E.; Vervier, K.; Strang, A.; Simpson, N.; Kumar, N.; Stares, M.D.; Rodger, A.; Brocklehurst, P.; et al. Stunted Microbiota and Opportunistic Pathogen Colonization in Caesarean-Section Birth. Nature 2019, 574, 117–121. [Google Scholar] [CrossRef]
- Marcotte, E.L.; Thomopoulos, T.P.; Infante-Rivard, C.; Clavel, J.; Petridou, E.T.; Schüz, J.; Ezzat, S.; Dockerty, J.D.; Metayer, C.; Magnani, C.; et al. Caesarean Delivery and Risk of Childhood Leukaemia: A Pooled Analysis from the Childhood Leukemia International Consortium (CLIC). Lancet Haematol. 2016, 3, e176–e185. [Google Scholar] [CrossRef]
- Martinez, L.D.; Glynn, L.M.; Sandman, C.A.; Wing, D.A.; Davis, E.P. Cesarean Delivery and Infant Cortisol Regulation. Psychoneuroendocrinology 2020, 122, 104862. [Google Scholar] [CrossRef]
- Inaba, H.; Pui, C.-H. Glucocorticoid Use in Acute Lymphoblastic Leukaemia. Lancet Oncol. 2010, 11, 1096–1106. [Google Scholar] [CrossRef] [PubMed]
- Schraw, J.M.; Bailey, H.D.; Bonaventure, A.; Mora, A.M.; Roman, E.; Mueller, B.A.; Clavel, J.; Petridou, E.T.; Karalexi, M.; Ntzani, E.; et al. Infant Feeding Practices and Childhood Acute Leukemia: Findings from the Childhood Cancer & Leukemia International Consortium. Int. J. Cancer 2022, 151, 1013–1023. [Google Scholar] [PubMed]
- Greaves, M.; Cazzaniga, V.; Ford, A. Can We Prevent Childhood Leukaemia? Leukemia 2021, 35, 1258–1264. [Google Scholar] [CrossRef]
- Peppas, I.; Ford, A.M.; Furness, C.L.; Greaves, M.F. Gut Microbiome Immaturity and Childhood Acute Lymphoblastic Leukaemia. Nat. Rev. Cancer 2023, 23, 565–576. [Google Scholar] [CrossRef]
- Rüchel, N.; Jepsen, V.H.; Hein, D.; Fischer, U.; Borkhardt, A.; Gössling, K.L. In Utero Development and Immunosurveillance of B Cell Acute Lymphoblastic Leukemia. Curr. Treat. Options Oncol. 2022, 23, 543–561. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Smith, A.J.; Spector, L.G. In Utero Origins of Acute Leukemia in Children. Biomedicines 2024, 12, 236. https://doi.org/10.3390/biomedicines12010236
de Smith AJ, Spector LG. In Utero Origins of Acute Leukemia in Children. Biomedicines. 2024; 12(1):236. https://doi.org/10.3390/biomedicines12010236
Chicago/Turabian Stylede Smith, Adam J., and Logan G. Spector. 2024. "In Utero Origins of Acute Leukemia in Children" Biomedicines 12, no. 1: 236. https://doi.org/10.3390/biomedicines12010236
APA Stylede Smith, A. J., & Spector, L. G. (2024). In Utero Origins of Acute Leukemia in Children. Biomedicines, 12(1), 236. https://doi.org/10.3390/biomedicines12010236





