Seasonal Variations in Stroke and a Comparison of the Predictors of Unfavorable Outcomes among Patients with Acute Ischemic Stroke and Cardioembolic Stroke
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population and Data Collection
2.2. Statement of Ethics
2.3. Stroke Severity, Classification, and Clinical Features
2.4. Seasonal Variation and Weekday Identification
2.5. Statistical Analyses
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Adams, H.P., Jr.; Bendixen, B.H.; Kappelle, L.J.; Biller, J.; Love, B.B.; Gordon, D.L.; Marsh, E.E., 3rd. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in Acute Stroke Treatment. Stroke 1993, 24, 35–41. [Google Scholar] [CrossRef]
- White, H.; Boden-Albala, B.; Wang, C.; Elkind, M.S.; Rundek, T.; Wright, C.B.; Sacco, R.L. Ischemic stroke subtype incidence among whites, blacks, and Hispanics: The Northern Manhattan Study. Circulation 2005, 111, 1327–1331. [Google Scholar] [CrossRef]
- Kim, B.J.; Kim, J.S. Ischemic stroke subtype classification: An Asian viewpoint. J. Stroke 2014, 16, 8–17. [Google Scholar] [CrossRef]
- Kamel, H.; Healey, J.S. Cardioembolic stroke. Circ. Res. 2017, 120, 514–526. [Google Scholar] [CrossRef]
- Hsieh, F.I.; Lien, L.M.; Chen, S.T.; Bai, C.H.; Sun, M.C.; Tseng, H.P.; Chen, Y.W.; Chen, C.H.; Jeng, J.S.; Tsai, S.Y.; et al. Get With the Guidelines-Stroke performance indicators: Surveillance of stroke care in the Taiwan Stroke Registry: Get with the Guidelines-Stroke in Taiwan. Circulation 2010, 122, 1116–1123. [Google Scholar] [CrossRef]
- Ntaios, G. Embolic stroke of undetermined source: JACC Review Topic of the Week. J. Am. Coll. Cardiol. 2020, 75, 333–340. [Google Scholar] [CrossRef] [PubMed]
- Lian, H.; Ruan, Y.; Liang, R.; Liu, X.; Fan, Z. Short-term effect of ambient temperature and the risk of stroke: A systematic review and meta-analysis. Int. J. Environ. Res. Public Health 2015, 12, 9068–9088. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhou, Z.; Chen, N.; He, L.; Zhou, M. Seasonal variation in the occurrence of ischemic stroke: A meta-analysis. Environ. Geochem. Health 2019, 41, 2113–2130. [Google Scholar] [CrossRef] [PubMed]
- Kuzmenko, N.V.; Galagudza, M.M. Dependence of seasonal dynamics of hemorrhagic and ischemic strokes on the climate of a region: A meta-analysis. Int. J. Stroke 2022, 17, 226–235. [Google Scholar] [CrossRef] [PubMed]
- Rakers, F.; Schiffner, R.; Rupprecht, S.; Brandstädt, A.; Witte, O.W.; Walther, M.; Schlattmann, P.; Schwab, M. Rapid weather changes are associated with increased ischemic stroke risk: A case-crossover study. Eur. J. Epidemiol. 2016, 31, 137–146. [Google Scholar] [CrossRef]
- Spengos, K.; Vemmos, K.; Tsivgoulis, G.; Manios, E.; Zakopoulos, N.; Mavrikakis, M.; Vassilopoulos, D. Diurnal and seasonal variation of stroke incidence in patients with cardioembolic stroke due to atrial fibrillation. Neuroepidemiology 2003, 22, 204–210. [Google Scholar] [CrossRef]
- Censi, F.; Calcagnini, G.; Mattei, E.; Calò, L.; Curnis, A.; D’Onofrio, A.; Vaccari, D.; Zanotto, G.; Morichelli, L.; Rovai, N.; et al. Seasonal trends in atrial fibrillation episodes and physical activity collected daily with a remote monitoring system for cardiac implantable electronic devices. Int. J. Cardiol. 2017, 234, 48–52. [Google Scholar] [CrossRef] [PubMed]
- Demsar, J.; Curk, T.; Erjavec, A.; Gorup, C.; Hocevar, T.; Milutinovic, M.; Mozina, M.; Polajnar, M.; Toplak, M.; Staric, A.; et al. Orange: Data mining toolbox in Python. J. Mach. Learn Res. 2013, 14, 2349–2353. [Google Scholar]
- Central Weather Administration, Ministry of Transportation and Communications. Available online: https://www.cwa.gov.tw/V8/C/C/Statistics/monthlymean.html (accessed on 24 July 2023).
- Lin, S.K.; Chen, P.Y.; Chen, G.C.; Hsu, P.J.; Hsiao, C.L.; Yang, F.Y.; Liu, C.Y.; Tsou, A. Association of a high neutrophil-to-lymphocyte ratio with hyperdense artery sign and unfavorable short-term outcomes in patients with acute ischemic stroke. J. Inflamm. Res. 2021, 14, 313–324. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.C.; Lim, S.N.; Yang, F.Y.; Lin, S.K. Evaluation of cerebral blood flow in acute ischemic stroke patients with atrial fibrillation: A sonographic study. J. Formos. Med. Assoc. 2017, 116, 287–294. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wasmer, K.; Eckardt, L.; Breithardt, G. Predisposing factors for atrial fibrillation in the elderly. J. Geriatr. Cardiol. 2017, 14, 179–184. [Google Scholar] [CrossRef]
- Go, A.S.; Hylek, E.M.; Phillips, K.A.; Chang, Y.; Henault, L.E.; Selby, J.V.; Singer, D.E. Prevalence of diagnosed atrial fibrillation in adults: National implications for rhythm management and stroke prevention: The AnTicoagulation and Risk Factors in Atrial Fibrillation (ATRIA) Study. JAMA 2001, 285, 2370–2375. [Google Scholar] [CrossRef]
- Zathar, Z.; Karunatilleke, A.; Fawzy, A.M.; Lip, G.Y.H. Atrial fibrillation in older people: Concepts and controversies. Front. Med. 2019, 6, 175. [Google Scholar] [CrossRef]
- Bücke, P.; Henkes, H.; Arnold, G.; Herting, B.; Jüttler, E.; Klötzsch, C.; Lindner, A.; Mauz, U.; Niehaus, L.; Reinhard, M.; et al. Seasonal patterns and associations in the incidence of acute ischemic stroke requiring mechanical thrombectomy. Eur. J. Neurol. 2021, 28, 2229–2237. [Google Scholar] [CrossRef] [PubMed]
- Liao, J.N.; Chao, T.F.; Liu, C.J.; Chen, S.J.; Hung, C.L.; Lin, Y.J.; Chang, S.L.; Lo, L.W.; Hu, Y.F.; Tuan, T.C.; et al. Seasonal variation in the risk of ischemic stroke in patients with atrial fibrillation: A nationwide cohort study. Heart Rhythm 2018, 15, 1611–1616. [Google Scholar] [CrossRef] [PubMed]
- Loomba, R.S. Seasonal variation in paroxysmal atrial fibrillation: A systematic review. J. Atr. Fibrillation 2015, 7, 1201. [Google Scholar]
- Woodhouse, P.R.; Khaw, K.T.; Plummer, M.; Foley, A.; Meade, T.W. Seasonal variations of plasma fibrinogen and factor VII activity in the elderly: Winter infections and death from cardiovascular disease. Lancet 1994, 343, 435–439. [Google Scholar] [CrossRef] [PubMed]
- Angerer, S.; Buttinger, K.; Stummer, H. The weekend effect revisited: Evidence from the Upper Austrian stroke registry. Eur. J. Health Econ. 2019, 20, 729–737. [Google Scholar] [CrossRef] [PubMed]
- Doi, S.; Yoneyama, K.; Yoshida, T.; Kawagoe, Y.; Nakai, M.; Sumita, Y.; Ishibashi, Y.; Izumo, M.; Tanabe, Y.; Harada, T.; et al. Effects of temperature and humidity on cerebrovascular disease hospitalization in a super-aging society. Sci. Rep. 2023, 13, 20602. [Google Scholar] [CrossRef] [PubMed]
- Jolugbo, P.; Ariëns, R.A.S. Thrombus composition and efficacy of thrombolysis and thrombectomy in acute ischemic stroke. Stroke 2021, 52, 1131–1142. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.G.; Zhang, L.Q.; Liao, X.L.; Pan, Y.S.; Shi, Y.Z.; Wang, C.J.; Wang, Y.L.; Liu, L.P.; Zhao, X.Q.; Wang, Y.J.; et al. Unfavorable outcome of thrombolysis in Chinese patients with cardioembolic stroke: A prospective cohort study. CNS. Neurosci. Ther. 2015, 21, 657–661. [Google Scholar] [CrossRef] [PubMed]
- Anticoli, S.; Bravi, M.C.; Perillo, G.; Siniscalchi, A.; Pozzessere, C.; Pezzella, F.R.; Tanzi, P.; Gallelli, L.; Cartoni, D. Effect of cardioembolic etiology on intravenous thrombolysis efficacy for acute ischemic stroke. Curr. Neurovasc. Res. 2016, 13, 193–198. [Google Scholar] [CrossRef] [PubMed]
- Vaclavik, D.; Vilionskis, A.; Jatuzis, D.; Karlinski, M.A.; Gdovinova, Z.; Kõrv, J.; Tsivgoulis, G.; Mikulik, R. Clinical outcome of cardioembolic stroke treated by intravenous thrombolysis. Acta. Neurol. Scand. 2018, 137, 347–355. [Google Scholar] [CrossRef]
- Liu, H.; Liu, K.; Zhang, K.; Zong, C.; Yang, H.; Li, Y.; Li, S.; Wang, X.; Zhao, J.; Xia, Z.; et al. Early neurological deterioration in patients with acute ischemic stroke: A prospective multicenter cohort study. Ther. Adv. Neurol. Disord. 2023, 16, 17562864221147743. [Google Scholar] [CrossRef]
- Siegler, J.E.; Albright, K.C.; George, A.J.; Boehme, A.K.; Gillette, M.A.; Kumar, A.D.; Aswani, M.; Martin-Schild, S. Time to neurological deterioration in ischemic stroke. Med. Student Res. J. 2017, 4, 18–24. [Google Scholar] [CrossRef][Green Version]
- Toyoda, K.; Fujimoto, S.; Kamouchi, M.; Iida, M.; Okada, Y. Acute blood pressure levels and neurological deterioration in different subtypes of ischemic stroke. Stroke 2009, 40, 2585–2588. [Google Scholar] [CrossRef]
- Chung, J.W.; Kim, N.; Kang, J.; Park, S.H.; Kim, W.J.; Ko, Y.; Park, J.H.; Lee, J.S.; Lee, J.; Yang, M.H.; et al. Blood pressure variability and the development of early neurological deterioration following acute ischemic stroke. J. Hypertens. 2015, 33, 2099–2106. [Google Scholar] [CrossRef]
- Sabir Rashid, A.; Huang-Link, Y.; Johnsson, M.; Wetterhäll, S.; Gauffin, H. Predictors of early neurological deterioration and functional outcome in acute ischemic stroke: The Importance of large artery disease, hyperglycemia and inflammatory blood biomarkers. Neuropsychiatr. Dis. Treat. 2022, 18, 1993–2002. [Google Scholar] [CrossRef]
- Yates, S.W. Novel oral anticoagulants for stroke prevention in atrial fibrillation: A focus on the older patient. Int. J. Gen. Med. 2013, 21, 167–180. [Google Scholar] [CrossRef]
- Sonbol, Y.T.; Elgenidy, A.; Awad, A.K.; Elmehrath, A.O.; Kobeissi, H.; Afifi, A.M.; Ghozy, S. Stroke as a cause of death in patients with cancer: A SEER-based study. J. Stroke Cerebrovasc. Dis. 2023, 32, 107154. [Google Scholar] [CrossRef]
- Lun, R.; Roy, D.C.; Hao, Y.; Deka, R.; Huang, W.K.; Navi, B.B.; Siegal, D.M.; Ramsay, T.; Fergusson, D.; Shorr, R.; et al. Incidence of stroke in the first year after diagnosis of cancer—A systematic review and meta-analysis. Front. Neurol. 2022, 13, 966190. [Google Scholar] [CrossRef]
- Chen, Y.; Wright, N.; Guo, Y.; Turnbull, I.; Kartsonaki, C.; Yang, L.; Bian, Z.; Pei, P.; Pan, D.; Zhang, Y.; et al. Mortality and recurrent vascular events after first incident stroke: A 9-year community-based study of 0.5 million Chinese adults. Lancet Glob. Health 2020, 8, e580–e590. [Google Scholar] [CrossRef]
- Soto-Cámara, R.; González-Santos, J.; González-Berna, J.; Trejo-Gabriel-Galán, J.M. Factors associated with a rapid call for assistance for patients with ischemic stroke. Emergencias 2020, 32, 33–39. [Google Scholar]
- Hsiao, C.L.; Su, Y.C.; Yang, F.Y.; Liu, C.Y.; Chiang, H.L.; Chen, G.C.; Hsu, P.J.; Chen, P.Y.; Lin, S.K. Impact of code stroke on thrombolytic therapy in patients with acute ischemic stroke at a secondary referral hospital in Taiwan. J. Chin. Med. Assoc. 2018, 81, 942–948. [Google Scholar] [CrossRef]
- Yiang, G.T.; Chen, Y.H.; Chen, P.Y.; Hsiao, C.L.; Lin, S.K. Rapid identification of patients eligible for direct emergent computed tomography angiography during acute ischemic stroke: The DARE-PACE assessment. Diagnostics 2022, 12, 511. [Google Scholar] [CrossRef]



| Characteristics | All Patients (n = 4040) | Gender | p-Value | |
|---|---|---|---|---|
| Men (n = 2299) | Women (n = 1741) | |||
| Age (years) | 71.9 (62.2–81.5) | 68.5 (59.7–78.8) | 76.4 (66.0–84.4) | <0.001 |
| Onset-to-ED time (min; n = 1866) | 278 (70–633) | 277 (85–642) | 281 (197–625) | 0.759 |
| Systolic blood pressure (mmHg) | 161 (142–185) | 160 (142–182) | 163 (142–188) | 0.009 |
| Heart rate (beats/minute) | 80 (69–92) | 79 (68–91) | 80 (70–92) | 0.008 |
| Hemoglobulin (g/dL) | 13.7 (12.3–15.0) | 14.4 (13.1–15.5) | 12.9 (11.6–14.0) | <0.001 |
| Platelet (×109/L) | 208 (169–254) | 203 (164–247) | 216 (179–265) | <0.001 |
| White blood cells (×103/mL) | 7.60 (6.17–9.50) | 7.70 (6.21–9.56) | 7.50 (6.10–9.35) | 0.036 |
| Neutrophil-to-lymphocyte ratio | 2.90 (2.0–4.8) | 3.0 (2.1–4.8) | 2.9 (1.9–4.8) | 0.340 |
| Glucose (mg/dL) | 138 (112–189) | 138 (113–187) | 138 (112–190) | 0.952 |
| Creatinine (mg/dL) | 1.05 (0.87–1.30) | 1.10 (0.96–1.40) | 0.90 (0.71–1.20) | <0.001 |
| Cholesterol (mg/dL) | 165 (140–193) | 162 (137–189) | 170 (143–200) | <0.001 |
| LDL cholesterol (mg/dL) | 104 (82–128) | 102 (81–128) | 105 (84–129) | 0.095 |
| Triglyceride (mg/dL) | 103 (74–149) | 103 (74–153) | 104 (74–144) | 0.340 |
| Uric acid (mg/dL) | 5.1 (4.1–6.2) | 5.4 (4.4–6.5) | 4.7 (3.8–5.9) | <0.001 |
| Hypertension | 2863 (71) | 1608 (70) | 1255 (72) | 0.138 |
| Diabetes mellitus | 1467 (36) | 818 (36) | 649 (37) | 0.267 |
| Heart disease | 1168 (29) | 618 (27) | 510 (32) | 0.001 |
| Dyslipidemia | 878 (22) | 492 (21) | 386 (22) | 0.557 |
| Prior stroke | 962 (24) | 576 (25) | 386 (22) | 0.033 |
| Current smoker | 853 (21) | 793 (35) | 60 (3) | <0.001 |
| Alcohol consumption | 257 (7) | 252 (11) | 5 (0.3) | <0.001 |
| History of cancer | 275 (7) | 127 (6) | 148 (9) | <0.001 |
| In-hospital complications | 509 (13) | 235 (10) | 274 (16) | <0.001 |
| Neurological deterioration | 425 (11) | 229 (10) | 196 (11) | 0.183 |
| TOAST classification | <0.001 | |||
| Small-artery occlusion | 1799 (44.5) | 1088 (47.3) | 711 (40.9) | |
| Large-artery atherosclerosis | 1288 (31.9) | 726 (31.6) | 562 (32.3) | |
| Cardioembolism | 729 (18.1) | 363 (15.8) | 366 (21.0) | |
| Other determined etiology | 81 (2.0) | 30 (1.3) | 51 (2.9) | |
| Undetermined etiology | 143 (3.5) | 92 (4.0) | 51 (2.9) | |
| Intravenous thrombolytic therapy | 261 (6.5) | 165 (7.2) | 96 (5.5) | 0.033 |
| Endovascular thrombectomy therapy | 99 (2.5) | 59 (2.6) | 40 (2.3) | 0.609 |
| Length of stay (days) | 10 (5–21) | 9 (5–19) | 12 (6–22) | <0.001 |
| Initial NIHSS score | 5 (2–11) | 4 (2–9) | 6 (3–13) | <0.001 |
| Discharge modified Rankin Scale score | 3 (1–4) | 2 (1–4) | 4 (2–4) | <0.001 |
| Discharge modified Rankin Scale score > 2 | 2262 (56) | 1124 (49) | 1138 (65) | <0.001 |
| Death at discharge | 191 (4.7) | 97 (4.2) | 94 (5.4) | 0.081 |
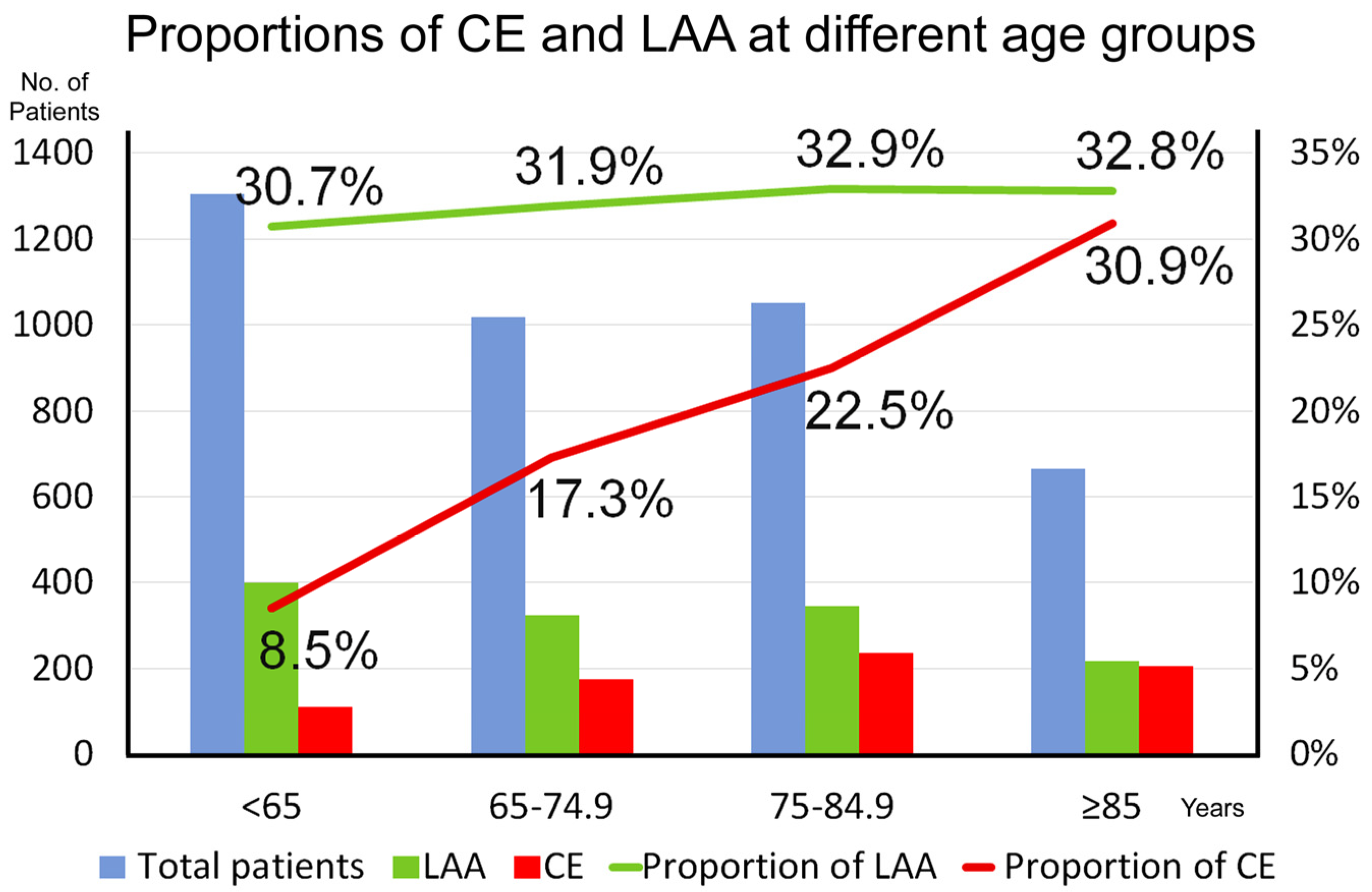
| Characteristics | SAO (n = 1798) | LAA (n = 1288) | CE (n = 729) | OD (n = 81) | UD (n = 143) | p-Value |
|---|---|---|---|---|---|---|
| Age (years) | 69.1 (60.5–79.0) | 72.6 (62.2–81.8) | 78.5 (69.2–85.7) | 60.1 (50.1–78.9) | 69.0 (60.5–80.4) | <0.001 |
| Female sex | 711 (40) | 562 (44) | 366 (50) | 51 (63) | 51 (36) | <0.001 |
| Onset-to-ED (min; n = 1866) | 388 (149–793) | 271 (86–621) | 166 (50–404) | 215 (97–374) | 236 (49–581) | <0.001 |
| Systolic blood pressure (mmHg) | 163 (144–186) | 162 (142–186) | 157 (137–178) | 144 (132–171) | 159 (140–186) | <0.001 |
| Heart rate (Beats/minute) | 78 (68–89) | 80 (69–91) | 87(76–98) | 85(72–98) | 82 (69–93) | <0.001 |
| Hemoglobulin (g/dL) | 13.8 (12.6–15.0) | 13.7 (12.2–15.0) | 13.5 (12.1–15.0) | 12.9 (108–14.4) | 13.8 (12.1–15.2) | <0.001 |
| Platelet (×109/L) | 211 (176–256) | 212 (171–260) | 190 (155–231) | 240 (180–240) | 204 (162–252) | <0.001 |
| White blood cells (×103/mL) | 7.32 (6.05–8.98) | 7.98 (6.49–10.1) | 7.45 (5.92–9.49) | 8.22 (6.65–10.5) | 7.97 (6.15–9.94) | <0.001 |
| Neutrophil-to-lymphocyte ratio | 2.7 (2.0–4.1) | 3.3 (2.1–5.3) | 3.1 (2.0–5.6) | 3.8 (2.6–6.1) | 3.2 (1.8–5.0) | <0.001 |
| Glucose (mg/dL) | 136 (110–196) | 142 (115–199) | 134 (113–169) | 124 (107–173) | 143 (116–175) | <0.001 |
| Creatinine (mg/dL) | 1.00 (0.82–1.30) | 1.10 (0.89–1.40) | 1.10 (0.90–1.40) | 1.00 (0.79–1.30) | 1.00 (0.80–1.40) | <0.001 |
| Cholesterol (mg/dL) | 171 (147–199) | 165 (138–195) | 154 (129–177) | 157 (140–186) | 155 (132–180) | <0.001 |
| LDL cholesterol (mg/dL) | 109 (88–133) | 104 (81–130) | 93 (73–114) | 97 (85–120) | 94 (77–121) | <0.001 |
| Triglyceride (mg/dL) | 116 (80–163) | 104 (75–147) | 82 (60–114) | 99 (74–157) | 100 (76–149) | <0.001 |
| Uric acid (mg/dL) | 5.0 (4.1–6.1) | 5.1 (4.0–6.2) | 5.4 (4.3–6.6) | 4.9 (3.7–6.3) | 5.0 (4.–6.4) | 0.003 |
| Hypertension | 1290 (72) | 923 (72) | 514 (71) | 46 (57) | 90 (63) | 0.014 |
| Diabetes mellitus | 672 (37) | 509 (40) | 221 (30) | 14 (17) | 51 (36) | <0.001 |
| Heart disease | 276 (15) | 281 (22) | 555 (78) | 14 (17) | 42 (30) | <0.001 |
| Dyslipidemia | 474 (26) | 281 (22) | 82 (11) | 15 (19) | 26 (18) | <0.001 |
| Prior stroke | 417 (23) | 318 (25) | 190 (26) | 12 (15) | 25 (17) | 0.035 |
| Current smoker | 419 (23) | 287 (22) | 94 (13) | 11 (14) | 42 (29) | <0.001 |
| Alcohol consumption | 124 (7) | 90 (7) | 35 (5) | 2 (2) | 6 (4) | 0.066 |
| Cancer history | 84 (5) | 97 (8) | 61 (8) | 21 (26) | 12 (8) | <0.001 |
| Intravenous thrombolysis | 56 (3) | 92 (7) | 93 (13) | 4 (5) | 16 (11) | <0.001 |
| Endovascular thrombectomy | 0 (0) | 50 (4) | 44 (7) | 4 (5) | 1 (1) | <0.001 |
| In-hospital complications | 100 (6) | 208 (16) | 169 (23) | 14 (17) | 18 (13) | <0.001 |
| Neurological deterioration | 106 (6) | 192 (15) | 103 (14) | 7 (9) | 17 (12) | <0.001 |
| Length of stay (days) | 8 (5–16) | 13 (6–24) | 14 (7–26) | 12 (7–22) | 8 (5–18) | <0.001 |
| Initial NIHSS score | 3 (2–6) | 7 (3–15) | 10 (4–19) | 6 (2–12) | 5 (2–14) | <0.001 |
| Discharge NIHSS score | 2 (1–4) | 5 (2–13) | 6 (2–18) | 4 (1–11) | 3 (1–10) | <0.001 |
| Discharge mRS score | 2 (1–4) | 4 (2–5) | 4 (2–5) | 3 (2–4) | 4 (1–4) | <0.001 |
| Discharge mRS score > 2 | 762 (42) | 875 (68) | 500 (69) | 47 (58) | 78 (55) | <0.001 |
| Death at discharge | 4 (0.2) | 93 (7) | 73 (10) | 8 (10) | 13 (9) | <0.001 |
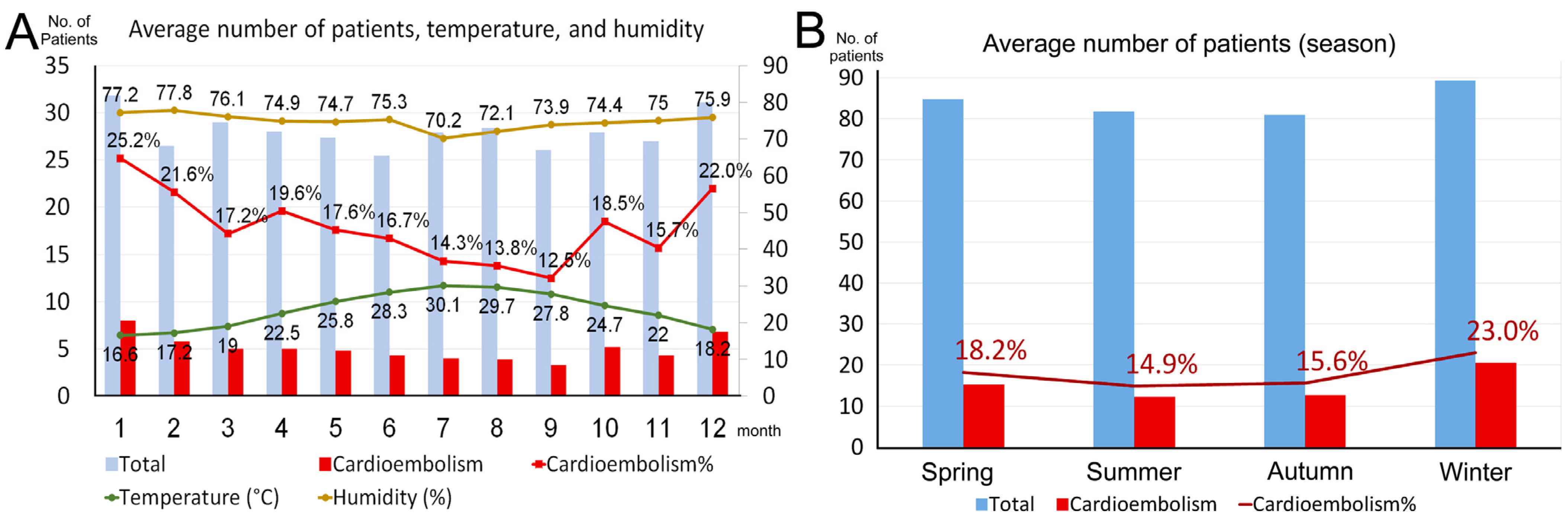
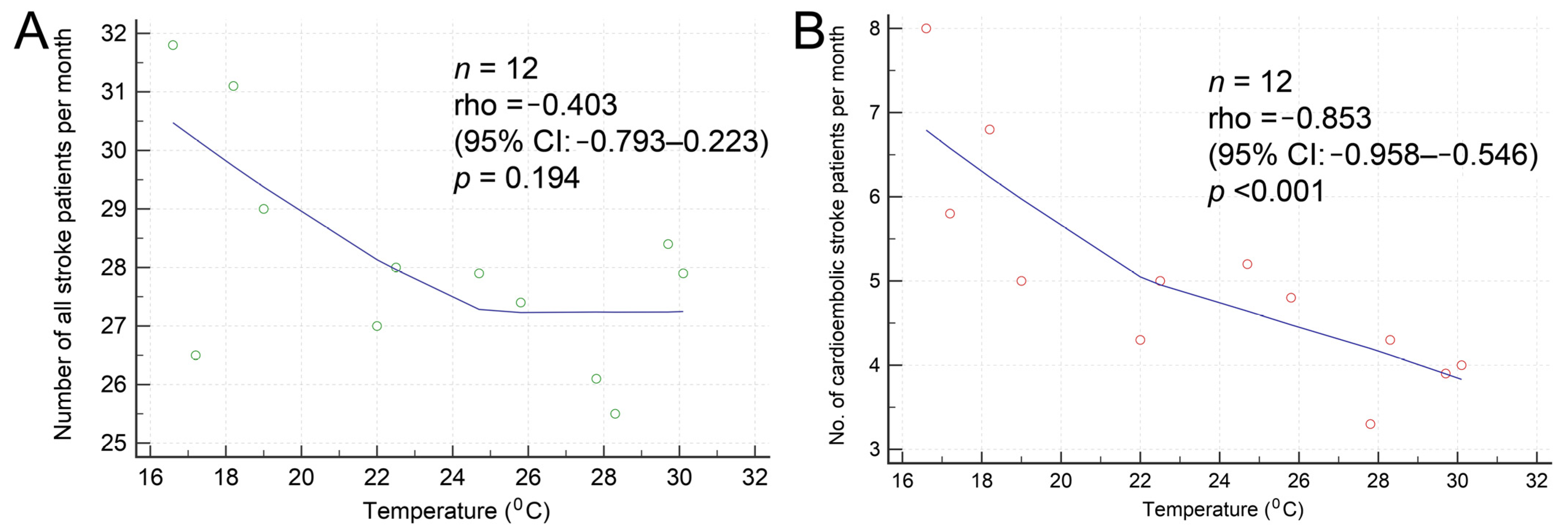
| Characteristics | Spring (n = 1013) | Summer (n = 982) | Autumn (n = 972) | Winter (n = 1073) | p-Value |
|---|---|---|---|---|---|
| Age (years) | 72.5 (63.2–81.3) | 71.1(61.6–81.3) | 71.8 (62.2–81.4) | 72.1 (61.3–81.8) | 0.542 |
| Onset-to-ED (min; n = 1866) | 248 (91–653) | 276 (87–663) | 265 (78–545) | 321 (99–651) | 0.084 |
| Systolic blood pressure (mmHg) | 163 (142–186) | 157 (137–180) | 161 (143–183) | 165 (145–189) | <0.001 |
| Heart rate (Beats/minute) | 80 (70–92) | 79 (68–90) | 79 (67–90) | 82 (70–94) | <0.001 |
| Intravenous thrombolytic therapy | 75 (7.4) | 62 (6.3) | 64 (6.6) | 60 (5.6) | 0.409 |
| Endovascular thrombectomy therapy | 27 (2.7) | 25 (2.6) | 30 (3.1) | 17 (16) | 0.155 |
| In-hospital complications | 139 (14) | 130 (13) | 118 (12) | 122 (11) | 0.365 |
| Neurological deterioration | 107 (11) | 104 (11) | 102 (11) | 112 (10) | 0.999 |
| TOAST classification | <0.001 | ||||
| Small-artery occlusion (n = 1799) | 429 (42.3) | 437 (44.5) | 459 (47.2) | 474 (44.0) | |
| Large-artery atherosclerosis (n = 1288) | 337 (33.3) | 347 (35.3) | 308 (31.7) | 296 (27.6) | |
| Cardioembolism (n = 729) | 184 (18.2) | 146 (14.9) | 152 (15.7) | 207 (23.0) | |
| Other determined etiology (n = 81) | 23 (2.3) | 13 (1.3) | 17 (1.7) | 28 (2.6) | |
| Undetermined etiology (n = 143) | 40 (3.9) | 39 (4.0) | 36 (3.7) | 28 (2.6) | |
| Initial NIHSS score | 5 (3–11) | 5 (2–10) | 5 (2–10) | 5 (2–10) | 0.339 |
| Discharge modified Rankin Scale score | 3 (1–4) | 3 (1–4) | 3 (1–4) | 3 (1–4) | 0.833 |
| Discharge modified Rankin Scale score > 2 | 569 (56) | 547 (56) | 550 (57) | 596 (56) | 0.965 |
| Death | 58 (5.7) | 46 (4.7) | 34 (3.5) | 53 (4.9) | 0.122 |
| Characteristics | Weekdays (n = 2942) | Holidays (n = 1098) | p-Value |
|---|---|---|---|
| Age (years) | 71.9 (62.4–81.4) | 71.4 (61.6–81.9) | 0.755 |
| Onset-to-ED (min; n = 1866) | 281 (94–642) | 272 (78–603) | 0.269 |
| Intravenous thrombolytic therapy | 196 (7) | 65 (6) | 0.429 |
| Endovascular thrombectomy therapy | 75 (3) | 24 (2) | 0.568 |
| In-hospital complications | 369 (13) | 140 (13) | 0.873 |
| Neurological deterioration | 312 (11) | 113 (10) | 0.818 |
| TOAST classification | 0.406 | ||
| Small-vessel occlusion | 1317 (45) | 482 (44) | |
| Large-artery atherosclerosis | 933 (32) | 355 (32) | |
| Cardioembolism | 521 (18) | 208 (19) | |
| Other determined etiology | 66 (2) | 15 (1) | |
| Undetermined etiology | 105 (4) | 38 (3) | |
| Initial NIHSS score | 5 (3–11) | 4 (2–10) | 0.045 |
| Discharge modified Rankin Scale score | 3 (1–4) | 3 (1–4) | 0.031 |
| Discharge modified Rankin Scale score > 2 | 1674 (57) | 588 (54) | 0.059 |
| Death | 143 (5) | 48 (4) | 0.560 |
| All 4040 Patient with Acute Ischemic Stroke | 729 Patients with Cardioembolic Stroke | ||||
|---|---|---|---|---|---|
| Characteristics | Odds Ratio (95% CI) | p-Value | Characteristics | Odds Ratio (95% CI) | p-Value |
| Age > 72 years | 2.594 (2.170–3.102) | <0.001 | Age > 73 years | 2.230 (1.431–3.474) | <0.001 |
| Heart rate > 86 (beats/minute) | 1.203 (1.009–1.435) | 0.039 | Initial NIHSS score > 6 | 9.423 (6.118–14.514) | <0.001 |
| Initial NIHSS score > 5 | 10.067 (8.414–12.045) | <0.001 | Hemoglobin < 12.8 g/dL | 1.804 (1.109–2.934) | 0.018 |
| Hemoglobin < 13.5 g/dL | 1.170 (0.977–1.401) | 0.088 | NLR > 5 | 2.356 (1.427–3.889) | <0.001 |
| Platelet < 183 (×109/L) | 1.128 (0.939–1.356) | 0.198 | Glucose > 125 mg/dL | 1.270 (0.825–1.954) | 0.277 |
| NLR > 3.5 | 1.562 (1.317–1.853) | <0.001 | Triglyceride < 107 mg/dL | 1.206 (0.763–1.906) | 0.422 |
| Glucose > 112 mg/dL | 1.300 (1.079–1.566) | 0.006 | Female gender | 1.008 (0.631–1.610) | 0.974 |
| LDL cholesterol < 88 mg/dL | 0.935 (0.777–1.125) | 0.478 | LDL cholesterol < 88 mg/dL | 0.947 (0.788–1.138) | 0.563 |
| Triglyceride < 110 mg/dL | 1.121 (0.939–1.338) | 0.208 | Diabetes mellitus | 1.413 (0.866–2.305) | 0.167 |
| Female gender | 1.430 (1.184–1.728) | <0.001 | Smoking | 0.989 (0.504–1.940) | 0.974 |
| Diabetes mellitus | 1.396 (1.165–1.672) | <0.001 | Alcohol consumption | 0.781 (0.292–2.087) | 0.621 |
| Heart disease | 0.884 (0.729–1.071) | 0.207 | In-hospital complications | 6.264 (2.380–16.483) | <0.001 |
| Dyslipidemia | 1.006 (0.822–1.233) | 0.951 | Deterioration | 15.689 (4.426–55.615) | <0.001 |
| Prior stroke | 1.583 (1.304–1.922) | <0.001 | |||
| Smoking | 0.817 (0.652–1.023) | 0.078 | |||
| Alcohol consumption | 0.832 (0.580–1.193) | 0.317 | |||
| Cancer history | 1.353 (0.972–1.884) | 0.073 | |||
| In-hospital complications | 7.156 (4.377–11.700) | <0.001 | |||
| Neurological deterioration | 7.475 (5.142–10.867) | <0.001 | |||
| All 4040 Patient with Acute Ischemic Stroke | 729 Patients with Cardioembolic Stroke | ||||
|---|---|---|---|---|---|
| Characteristics | C-Statistics (95% CI) | p-Value a | Characteristics | C-Statistics (95% CI) | p-Value a |
| Initial NIHSS score > 5 | 0.772 (0.759–0.785) | Initial NIHSS score > 6 | 0.788 (0.752–0.825) | ||
| Includes neurological deterioration | 0.796 (0.783–0.809) | <0.001 | Includes neurological deterioration | 0.816 (0.785–0.843) | <0.001 |
| Further includes in-hospital complications | 0.811 (0.799–0.823) | <0.001 | Further includes in-hospital complications | 0.842 (0.813–0.867) | <0.001 |
| Further includes age > 72 years | 0.849 (0.837–0.860) | <0.001 | Further includes NLR > 5 | 0.860 (0.833–0.885) | <0.001 |
| Further includes prior stroke | 0.854 (0.842–0.864) | <0.001 | Further includes age > 73 years | 0.875 (0.849–0.898) | 0.012 |
| Further includes female gender | 0.859 (0.847–0.869) | <0.001 | Further includes hemoglobin < 12.8 g/L | 0.882 (0.856–0.905) | 0.044 |
| Further includes diabetes mellitus | 0.861 (0.850–0.872) | 0.001 | |||
| Further includes NLR > 3.5 | 0.864 (0.853–0.874) | 0.054 | |||
| Further includes glucose > 112 mg/dL | 0.865 (0.854–0.875) | 0.067 | |||
| Further includes heart rate > 86 BPM | 0.865 (0.854–0.876) | 0.467 | |||
| All 4040 Patient with Acute Ischemic Stroke | 729 Patients with Cardioembolic Stroke | ||||
|---|---|---|---|---|---|
| Characteristics | Odds Ratio (95% CI) | p-Value | Characteristics | Odds Ratio (95% CI) | p-Value |
| Age > 77 years | 1.092 (0.763–1.565) | 0.630 | Age > 86 years | 2.123 (1.167–3.862) | 0.014 |
| Heart rate > 87 beats/minute | 1.277 (0.903–1.805) | 0.167 | Heart rate > 79 beats/minute | 1.949 (1.032–3.680) | 0.039 |
| Initial NIHSS score > 10 | 12.810 (7.908–20.750) | <0.001 | Initial NIHSS score > 16 | 5.820 (3.080–10.996) | <0.001 |
| Hemoglobin < 12.2 g/dL | 1.185 (0.812–1.730) | 0.379 | NLR > 6.4 | 2.359 (1.282–4.341) | 0.006 |
| NLR > 6 | 2.247 (1.568–3.220) | <0.001 | Glucose > 159 mg/dL | 1.451 (0.805–2.616) | 0.215 |
| Glucose > 129 mg/dL | 1.865 (1.283–2.711) | 0.001 | Cancer history | 4.216 (1.939–9.168) | <0.001 |
| Creatinine > 1.11 gm/dL | 1.549 (1.090–2.200) | 0.015 | In-hospital complications | 1.591 (0.885–2.862) | 0.121 |
| LDL cholesterol < 87 mg/dL | 1.633 (0.129–2.362) | 0.009 | Neurological deterioration | 5.737 (3.104–10.604) | <0.001 |
| Triglyceride < 91 mg/dL | 1.600 (1.091–2.348) | 0.016 | |||
| Heart disease | 1.660 (1.172–2.353) | 0.004 | |||
| Dyslipidemia | 0.955 (0.561–1.625) | 0.865 | |||
| Cancer history | 2.238 (1.314–3.8147) | 0.003 | |||
| In-hospital complications | 1.790 (1.255–2.553) | 0.001 | |||
| Neurological deterioration | 5.967 (4.133–8.614) | <0.001 | |||
| All 4040 Patient with Acute Ischemic Stroke | 729 Patients with Cardioembolic Stroke | ||||
|---|---|---|---|---|---|
| Characteristics | C-Statistics (95% CI) | p-Value a | Characteristics | C-Statistics (95% CI) | p-Value a |
| Initial NIHSS score > 10 | 0.832 (0.820–0.844) | Initial NIHSS score > 16 | 0.752 (0.719–0.783) | ||
| Includes neurological deterioration | 0.877 (0.867–0.887) | <0.001 | Includes neurological deterioration | 0.806 (0.776–0.835) | <0.001 |
| Further includes NLR > 6.0 | 0.897 (0.887–0.906) | <0.001 | Further includes NLR > 6.4 | 0.835 (0.806–0.862) | 0.007 |
| Further includes in-hospital complications | 0.909 (0.900–0.918) | <0.001 | Further includes cancer history | 0.851 (0.823–0.876) | 0.091 |
| Further includes creatinine > 1.11 mg/dL | 0.916 (0.907–0.927) | 0.003 | Further includes age > 86 years | 0.858 (0.831–0.883) | 0.092 |
| Further includes heart disease | 0.919 (0.910–0.927) | 0.266 | Further includes heart rate > 79 BPM | 0.861 (0.834–0.886) | 1.000 |
| Further includes cancer history | 0.922 (0.913–0.931) | 0.318 | |||
| Further includes triglyceride < 91 mg/dL | 0.923 (0.914–0.931) | 0.421 | |||
| Further includes glucose > 129 mg/dL | 0.923 (0.915–0.931) | 0.818 | |||
| Further includes LDL cholesterol < 87 mg/dL | 0.924 (0.915–0.932) | 0.729 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, P.-Y.; Chang, W.-L.; Hsiao, C.-L.; Lin, S.-K. Seasonal Variations in Stroke and a Comparison of the Predictors of Unfavorable Outcomes among Patients with Acute Ischemic Stroke and Cardioembolic Stroke. Biomedicines 2024, 12, 223. https://doi.org/10.3390/biomedicines12010223
Chen P-Y, Chang W-L, Hsiao C-L, Lin S-K. Seasonal Variations in Stroke and a Comparison of the Predictors of Unfavorable Outcomes among Patients with Acute Ischemic Stroke and Cardioembolic Stroke. Biomedicines. 2024; 12(1):223. https://doi.org/10.3390/biomedicines12010223
Chicago/Turabian StyleChen, Pei-Ya, Wan-Ling Chang, Cheng-Lun Hsiao, and Shinn-Kuang Lin. 2024. "Seasonal Variations in Stroke and a Comparison of the Predictors of Unfavorable Outcomes among Patients with Acute Ischemic Stroke and Cardioembolic Stroke" Biomedicines 12, no. 1: 223. https://doi.org/10.3390/biomedicines12010223
APA StyleChen, P.-Y., Chang, W.-L., Hsiao, C.-L., & Lin, S.-K. (2024). Seasonal Variations in Stroke and a Comparison of the Predictors of Unfavorable Outcomes among Patients with Acute Ischemic Stroke and Cardioembolic Stroke. Biomedicines, 12(1), 223. https://doi.org/10.3390/biomedicines12010223






