Pharmacogenetic Association between Allelic Variants of the Autophagy-Related Genes and Anti-Vascular Endothelial Growth Factor Treatment Response in Neovascular Age-Related Macular Degeneration
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Participants
2.2. Optical Coherence Tomography Study
2.3. DNA Isolation and Genotyping
2.4. Statistical Analysis
3. Results
3.1. Association with Risk of nAMD
3.2. Association between SNPs and OCT Markers
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, J.Q.; Welchowski, T.; Schmid, M.; Mauschitz, M.M.; Holz, F.G.; Finger, R.P. Prevalence and incidence of age-related macular degeneration in Europe: A systematic review and meta-analysis. Br. J. Ophthalmol. 2020, 104, 1077–1084. [Google Scholar] [CrossRef]
- Mitchell, P.; Liew, G.; Gopinath, B.; Wong, T.Y. Age-related macular degeneration. Lancet 2018, 392, 1147–1159. [Google Scholar] [CrossRef]
- Fursova, A.Z.; Derbeneva, A.S.; Vasilyeva, M.A.; Nikulich, I.F.; Tarasov, M.S.; Gamza, Y.A.; Chubar, N.V.; Gusarevitch, O.G.; Dmitrieva, E.I.; Kozhevnikova, O.S.; et al. New findings on pathogenetic mechanisms in the development of age-related macular degeneration. Vestn. Oftalmol. 2022, 138, 120–130. [Google Scholar] [CrossRef]
- Spaide, R.F.; Jaffe, G.J.; Sarraf, D.; Freund, K.B.; Sadda, S.R.; Staurenghi, G.; Waheed, N.K.; Chakravarthy, U.; Rosenfeld, P.J.; Holz, F.G.; et al. Consensus Nomenclature for Reporting Neovascular Age-Related Macular Degeneration Data: Consensus on Neovascular Age-Related Macular Degeneration Nomenclature Study Group. Ophthalmology 2020, 127, 616–636. [Google Scholar] [CrossRef]
- Nguyen, D.D.; Luo, L.J.; Yang, C.J.; Lai, J.Y. Highly Retina-Permeating and Long-Acting Resveratrol/Metformin Nanotherapeutics for Enhanced Treatment of Macular Degeneration. ACS Nano 2023, 17, 168–183. [Google Scholar] [CrossRef]
- Cobos, E.; Recalde, S.; Anter, J.; Hernandez-Sanchez, M.; Barreales, C.; Olavarrieta, L.; Valverde, A.; Suarez-Figueroa, M.; Cruz, F.; Abraldes, M.; et al. Association between CFH, CFB, ARMS2, SERPINF1, VEGFR1 and VEGF polymorphisms and anatomical and functional response to ranibizumab treatment in neovascular age-related macular degeneration. Acta Ophthalmol. 2018, 96, e201–e212. [Google Scholar] [CrossRef]
- Yang, S.; Zhao, J.; Sun, X. Resistance to anti-VEGF therapy in neovascular age-related macular degeneration: A comprehensive review. Drug Des. Dev. Ther. 2016, 10, 1857–1867. [Google Scholar] [CrossRef]
- Lee, A.Y.; Raya, A.K.; Kymes, S.M.; Shiels, A.; Brantley, M.A., Jr. Pharmacogenetics of complement factor H (Y402H) and treatment of exudative age-related macular degeneration with ranibizumab. Br. J. Ophthalmol. 2009, 93, 610–613. [Google Scholar] [CrossRef]
- Hong, N.; Shen, Y.; Yu, C.Y.; Wang, S.Q.; Tong, J.P. Association of the polymorphism Y402H in the CFH gene with response to anti-VEGF treatment in age-related macular degeneration: A systematic review and meta-analysis. Acta Ophthalmol. 2016, 94, 334–345. [Google Scholar] [CrossRef]
- Dedania, V.S.; Grob, S.; Zhang, K.; Bakri, S.J. Pharmacogenomics of response to anti-VEGF therapy in exudative age-related macular degeneration. Retina 2015, 35, 381–391. [Google Scholar] [CrossRef]
- Medina, F.; Motta, A.; Takahashi, W.Y.; Carricondo, P.C.; Motta, M.; Melo, M.B.; Vasconcellos, J. Association of the CFH Y402H Polymorphism with the 1-Year Response of Exudative AMD to Intravitreal Anti-VEGF Treatment in the Brazilian Population. Ophthalmic Res. 2019, 61, 168–173. [Google Scholar] [CrossRef] [PubMed]
- Kozhevnikova, O.S.; Fursova, A.Z.; Derbeneva, A.S.; Nikulich, I.F.; Tarasov, M.S.; Devyatkin, V.A.; Rumyantseva, Y.V.; Telegina, D.V.; Kolosova, N.G. Association between Polymorphisms in CFH, ARMS2, CFI, and C3 Genes and Response to Anti-VEGF Treatment in Neovascular Age-Related Macular Degeneration. Biomedicines 2022, 10, 1658. [Google Scholar] [CrossRef] [PubMed]
- Akiyama, M.; Takahashi, A.; Momozawa, Y.; Arakawa, S.; Miya, F.; Tsunoda, T.; Ashikawa, K.; Oshima, Y.; Yasuda, M.; Yoshida, S.; et al. Genome-wide association study suggests four variants influencing outcomes with ranibizumab therapy in exudative age-related macular degeneration. J. Hum. Genet. 2018, 63, 1083–1091. [Google Scholar] [CrossRef]
- Balikova, I.; Postelmans, L.; Pasteels, B.; Coquelet, P.; Catherine, J.; Efendic, A.; Hosoda, Y.; Miyake, M.; Yamashiro, K.; ANGEL Study Group Members; et al. Genetic biomarkers in the VEGF pathway predicting response to anti-VEGF therapy in age-related macular degeneration. BMJ Open Ophthalmol. 2019, 4, e000273. [Google Scholar] [CrossRef]
- Wu, M.; Xiong, H.; Xu, Y.; Xiong, X.; Zou, H.; Zheng, M.; Wang, X.; Zhou, X. Association between VEGF-A and VEGFR-2 polymorphisms and response to treatment of neovascular AMD with anti-VEGF agents: A meta-analysis. Br. J. Ophthalmol. 2017, 101, 976–984. [Google Scholar] [CrossRef]
- Paterno, J.J.; Koskela, A.; Hyttinen, J.M.T.; Vattulainen, E.; Synowiec, E.; Tuuminen, R.; Watala, C.; Blasiak, J.; Kaarniranta, K. Autophagy Genes for Wet Age-Related Macular Degeneration in a Finnish Case-Control Study. Genes 2020, 11, 1318. [Google Scholar] [CrossRef] [PubMed]
- Chandra, A.; Rick, J.; Yagnik, G.; Aghi, M.K. Autophagy as a mechanism for anti-angiogenic therapy resistance. Semin Cancer Biol. 2020, 66, 75–88. [Google Scholar] [CrossRef]
- Kocak, M.; Ezazi Erdi, S.; Jorba, G.; Maestro, I.; Farres, J.; Kirkin, V.; Martinez, A.; Pless, O. Targeting autophagy in disease: Established and new strategies. Autophagy 2022, 18, 473–495. [Google Scholar] [CrossRef]
- Kozhevnikova, O.S.; Telegina, D.V.; Tyumentsev, M.A.; Kolosova, N.G. Disruptions of Autophagy in the Rat Retina with Age During the Development of Age-Related-Macular-Degeneration-like Retinopathy. Int. J. Mol. Sci. 2019, 20, 4804. [Google Scholar] [CrossRef]
- Blasiak, J.; Pawlowska, E.; Szczepanska, J.; Kaarniranta, K. Interplay between Autophagy and the Ubiquitin-Proteasome System and Its Role in the Pathogenesis of Age-Related Macular Degeneration. Int. J. Mol. Sci. 2019, 20, 210. [Google Scholar] [CrossRef]
- Grosjean, I.; Romeo, B.; Domdom, M.A.; Belaid, A.; D’Andrea, G.; Guillot, N.; Gherardi, R.K.; Gal, J.; Milano, G.; Marquette, C.H.; et al. Autophagopathies: From autophagy gene polymorphisms to precision medicine for human diseases. Autophagy 2022, 18, 2519–2536. [Google Scholar] [CrossRef]
- Fursova, A.Z.; Derbeneva, A.S.; Tarasov, M.S.; Nikulich, I.F.; Devyatkin, V.A.; Telegina, D.V.; Kolosova, N.G.; Kozhevnikova, O.S. Leukocyte telomere length and response to antiangiogenic therapy in patients with neovascular age-related macular degeneration. Adv. Gerontol. 2022, 12, 135–142. [Google Scholar] [CrossRef]
- Solé, X.; Guinó, E.; Valls, J.; Iniesta, R.; Moreno, V. SNPStats: A web tool for the analysis of association studies. Bioinformatics 2006, 22, 1928–1929. [Google Scholar] [CrossRef]
- Kaarniranta, K.; Blasiak, J.; Liton, P.; Boulton, M.; Klionsky, D.J.; Sinha, D. Autophagy in age-related macular degeneration. Autophagy 2023, 19, 388–400. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Yao, Y.; Li, R.; Wu, B.; Lu, H.; Cheng, J.; Liu, Z.; Du, J. Different effects of anti-VEGF drugs (Ranibizumab, Aflibercept, Conbercept) on autophagy and its effect on neovascularization in RF/6A cells. Microvasc. Res. 2021, 138, 104207. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.L.; Jahangiri, A.; De Lay, M.; Aghi, M.K. Hypoxia-induced tumor cell autophagy mediates resistance to anti-angiogenic therapy. Autophagy 2012, 8, 979–981. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Fung, N.S.K.; Lam, W.C.; Lo, A.C.Y. mTOR Signalling Pathway: A Potential Therapeutic Target for Ocular Neurodegenerative Diseases. Antioxidants 2022, 11, 1304. [Google Scholar] [CrossRef]
- Fernandes, S.A.; Demetriades, C. The Multifaceted Role of Nutrient Sensing and mTORC1 Signaling in Physiology and Aging. Front. Aging 2021, 2, 707372. [Google Scholar] [CrossRef]
- Merle, D.A.; Provenzano, F.; Jarboui, M.A.; Kilger, E.; Clark, S.J.; Deleidi, M.; Armento, A.; Ueffing, M. mTOR Inhibition via Rapamycin Treatment Partially Reverts the Deficit in Energy Metabolism Caused by FH Loss in RPE Cells. Antioxidants 2021, 10, 1944. [Google Scholar] [CrossRef]
- Tamargo-Gómez, I.; Fernández, Á.F.; Mariño, G. Pathogenic Single Nucleotide Polymorphisms on Autophagy-Related Genes. Int. J. Mol. Sci. 2020, 21, 8196. [Google Scholar] [CrossRef]
- Nakatogawa, H. Mechanisms governing autophagosome biogenesis. Nat. Rev. Mol. Cell Biol. 2020, 21, 439–458. [Google Scholar]
- Zachari, M.; Ganley, I.G. The Mammalian ULK1 Complex and Autophagy Initiation. Essays Biochem. 2017, 61, 585–596. [Google Scholar] [CrossRef] [PubMed]
- Henckaerts, L.; Cleynen, I.; Brinar, M.; John, J.M.; Van Steen, K.; Rutgeerts, P.; Vermeire, S. Genetic variation in the autophagy gene ULK1 and risk of Crohn’s disease. Inflamm. Bowel Dis. 2011, 17, 1392–1397. [Google Scholar] [PubMed]
- Zhang, R.R.; Liang, L.; Chen, W.W.; Wen, C.; Wan, B.S.; Luo, L.L.; Zhao, Y.L.; Chen, J.; Yue, J. ULK1 polymorphisms confer susceptibility to pulmonary tuberculosis in a Chinese population. Int. J. Tuberc. Lung Dis. 2019, 23, 265–271. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Han, R.; Wang, M.; Li, X.; Yang, X.; Xia, Q.; Liu, R.; Yuan, Y.; Hu, X.; Chen, M.; et al. Association between the autophagy-related gene ULK1 and ankylosing spondylitis susceptibility in the Chinese Han population: A case-control study. Postgrad. Med. J. 2017, 93, 752–757. [Google Scholar]
- Katsuragi, Y.; Ichimura, Y.; Komatsu, M. P62/SQSTM1 Functions as a Signaling Hub and an Autophagy Adaptor. FEBS J. 2015, 82, 4672–4678. [Google Scholar] [CrossRef]
- Johansen, T.; Lamark, T. Selective Autophagy: ATG8 Family Proteins, LIR Motifs and Cargo Receptors. J. Mol. Biol. 2020, 432, 80–103. [Google Scholar] [CrossRef]
- Haack, T.B.; Ignatius, E.; Calvo-Garrido, J.; Iuso, A.; Isohanni, P.; Maffezzini, C.; Lönnqvist, T.; Suomalainen, A.; Gorza, M.; Kremer, L.S.; et al. Absence of the Autophagy Adaptor SQSTM1/p62 Causes Childhood-Onset Neurodegeneration with Ataxia, Dystonia, and Gaze Palsy. Am. J. Hum. Genet. 2016, 99, 735–743. [Google Scholar] [CrossRef]
- Teyssou, E.; Takeda, T.; Lebon, V.; Boillée, S.; Doukouré, B.; Bataillon, G.; Sazdovitch, V.; Cazeneuve, C.; Meininger, V.; Leguern, E.; et al. Mutations in SQSTM1 encoding p62 in amyotrophic lateral sclerosis: Genetics and neuropathology. Acta Neuropathol. 2013, 125, 511–522. [Google Scholar] [CrossRef]
- Le Ber, I.; Camuzat, A.; Guerreiro, R.; Bouya-Ahmed, K.; Bras, J.; Nicolas, G.; Gabelle, A.; Didic, M.; De Septenville, A.; Millecamps, S.; et al. SQSTM1 Mutations in french patients with frontotemporal dementia or frontotemporal dementia with amyotrophic lateral sclerosis. JAMA Neurol. 2013, 70, 1403–1410. [Google Scholar]
- Fursova, A.Z.; Nikulich, I.F.; Dmitrieva, E.I.; Gusarevich, O.G.; Derbeneva, A.S.; Vasilyeva, M.A.; Kozhevnikova, O.S.; Kolosova, N.G. Three-year follow-up study of clinical effectiveness of antiangiogenic therapy for neovascular age-related macular degeneration. Vestn. Oftalmol. 2023, 139, 45–52. [Google Scholar] [CrossRef] [PubMed]
| Gene | SNP | Location/Consequence | Position | Minor Allele | MAF |
|---|---|---|---|---|---|
| ATG5 | rs573775 | Intron variant | chr6:106316991 | A | 0.27 |
| MAP1LC3A | rs73105013 | Intron variant | chr20:34557008 | C | 0.08 |
| MTOR | rs1064261 | Missense variant | chr1:11228701 | G | 0.28 |
| rs1057079 | Synonymous variant | chr1:11145001 | C | 0.26 | |
| rs11121704 | Intron variant | chr1:11233902 | C | 0.28 | |
| rs2295080 | Upstream variant | chr1:11262571 | G | 0.31 | |
| SQSTM1 | rs10277 | 3 Prime UTR Variant | chr5:179837731 | T | 0.48 |
| ULK1 | rs11246867 | Upstream variant | chr12:131893472 | A | 0.06 |
| rs3088051 | 3 Prime UTR Variant | chr12:131922463 | C | 0.3 |
| rsID | Gene Name | Sequence |
|---|---|---|
| rs573775 | ATG5 | Forward 5′-CCCTACCTAGTATGCTCCTC-3′ |
| Reverse 5′-AAAAGCCATGTCCTTATGCC-3′ | ||
| 5′-FAM-CCTCTGGCCCCAGTGAAACAG-BHQ1-3′ | ||
| 5′-VIC-CTCTGGCCCCA[+A]TGAAACAGT-BHQ1-3′ | ||
| rs1064261 | MTOR | Forward 5′-AAGGATTGGGGTTTGAGGTA-3′ |
| Reverse 5′-GACCAGTCACTCTCTCATCA-3′ | ||
| 5′-FAM-CACGTTCCTTAA[+C]GTCATTCGA-BHQ1-3′ | ||
| 5′-VIC-CACGTTCCTTAA[+T]GTCATTCGA-BHQ1-3′ | ||
| rs1057079 | MTOR | Forward 5′-GCAGCCTGTAAGTTCTCAAT-3′ |
| Reverse 5′-CCCAAGGGTTGTTTCTCTTC-3′ | ||
| 5′-FAM-CTCCTGCCATCGCAGTTAATTCA-BHQ1-3′ | ||
| 5′-VIC-CTCCTGCCAT[+T]GCAGTTAATT-BHQ1-3′ | ||
| rs11121704 | MTOR | Forward 5′-TTTTTCCTCATTTTGGGCGA-3′ |
| Reverse 5′-TATCAGTTGCAGGAAAGTGC-3′ | ||
| 5′-FAM-CAGGCACATCATCGCAGATGTTT-BHQ1-3′ | ||
| 5′-VIC-CAGGCACATCATCACAGATGTTTG-BHQ1-3′ | ||
| rs2295080 | MTOR | Forward 5′-TTCCCCGCTGTCCTCTA-3′ |
| Reverse 5′-GCCTGTTTTTCAGTCCATCT-3′ | ||
| 5′-FAM-CCTCAGGGCTGGGAACCC-BHQ1-3′ | ||
| 5′-VIC-CCTCAGGG[+A]TGGGAACCCTC-BHQ1-3′ | ||
| rs73105013 | MAP1LC3A | Forward 5′-CAGCCTTAAAAACAAAAACCCT-3′ |
| Reverse 5′-ATGGAAGGCAGAAAGGGAGA-3′ | ||
| 5′-FAM-CTTATCCCCAG[+T]GTCTTCTGC-BHQ1-3′ | ||
| 5′-VIC-CTTATCCCCAG[+C]GTCTTCTGC-BHQ1-3′ | ||
| rs10277 | SQSTM1 | Forward 5′-GTCCCTCTGAAGAGACCTTG-3′ |
| Reverse 5′-CTGGGAAGGAGCTATGGAG-3′ | ||
| 5′-FAM-AGGACAAAT[+T]GCGCCCAT-BHQ1-3′ | ||
| 5′-VIC-CAGGACAAATCGCGCCCATT-BHQ1-3′ | ||
| rs11246867 | ULK1 | Forward 5′-GTACGGTGAACAGCACTAAC-3′ |
| Reverse 5′-CAGCCAAAAGAGCCCG-3′ | ||
| 5′-FAM-CAGCCAACAG[+C]GATTGCTCT-BHQ1-3′ | ||
| 5′-VIC-CAGCCAACAG[+T]GATTGCTCT-BHQ1-3′ | ||
| rs3088051 | ULK1 | Forward 5′-GGAAGCAGATGAGGGGAATA-3′ |
| Reverse 5′-CTCTCTGCAGATGCCCTC-3′ | ||
| 5′-FAM-CAGTCAGTTT[+T]GATGTCAGCTC-BHQ1-3′ | ||
| 5′-VIC-CAGTCAGTTT[+C]GATGTCAGCTC-BHQ1-3′ |
| SNP | Genotype/Allele | Control | AMD | p-Value, χ2 |
|---|---|---|---|---|
| rs573775 ATG5 | A/A | 24 (8%) | 26 (8%) | p = 0.838, 0.354 |
| G/A | 145 (46%) | 137 (43%) | ||
| G/G | 148 (47%) | 152 (48%) | ||
| MAF | 0.3 | 0.3 | ||
| rs1064261 MTOR | A/A | 150 (47%) | 125 (40%) | p = 0.093, 4.757 |
| A/G | 130 (41%) | 156 (50%) | ||
| G/G | 37 (12%) | 34 (11%) | ||
| MAF | 0.32 | 0.36 | ||
| rs1057079 MTOR | C/C | 28 (9%) | 21 (7%) | p = 0.002, 12.563 |
| T/C | 109 (34%) | 152 (48%) | ||
| T/T | 180 (57%) | 142 (45%) | ||
| MAF | 0.26 | 0.31 | ||
| rs11121704 MTOR | C/C | 28 (9%) | 29 (9%) | p = 0.006, 10.324 |
| T/C | 121 (38%%) | 158 (50%) | ||
| T/T | 168 (53%) | 128 (41%) | ||
| MAF | 0.28 | 0.34 | ||
| rs2295080 MTOR | G/G | 36 (11%) | 31(10%) | p = 0.002, 12.562 |
| T/G | 123 (39%) | 166 (53%) | ||
| T/T | 158 (5%) | 118 (37%) | ||
| MAF | 0.31 | 0.36 | ||
| rs73105013 MAP1LC3A | C/C | 2 (0.6%) | 0 (0%) | p = 0.169, 3.557 |
| T/C | 41 (13%) | 52 (17%) | ||
| T/T | 273 (86%) | 261 (83%) | ||
| MAF | 0.07 | 0.08 | ||
| rs10277 SQSTM1 | C/C | 118 (37%) | 106 (34%) | p = 0.385, 1.913 |
| C/T | 157 (50%) | 156 (50%) | ||
| T/T | 42 (13%) | 53 (17%) | ||
| MAF | 0.38 | 0.42 | ||
| rs11246867 ULK1 | A/A | 2 (0.6%) | 1 (0.3%) | p = 0.574, 1.113 |
| G/A | 33 (10%) | 40 (13%) | ||
| G/G | 282 (89%) | 274 (87%) | ||
| MAF | 0.06 | 0.07 | ||
| rs3088051 ULK1 | C/C | 32 (10%) | 27 (9%) | p = 0.579, 1.096 |
| T/C | 121 (38%) | 132 (42%) | ||
| T/T | 164 (52%) | 156 (50%) | ||
| MAF | 0.29 | 0.3 |
| SNP | Model of Inheritance | OR (95% CI) Adjusted for Sex and Age by Logistic Regression | p-Value | AIC |
|---|---|---|---|---|
| rs1057079 MTOR | Codominant: | 0.0018 | 814.5 | |
| C/T vs. T/T | 1.85 (1.31–2.62) | |||
| C/C vs. T/T | 1.07 (0.57–2.03) | |||
| Dominant: C/T-C/C vs. T/T | 1.70 (1.22–2.36) | 0.0017 | 815.2 | |
| Overdominant: C/T vs. C/C-T/T | 1.83 (1.31–2.56) | 0.0004 | 812.5 | |
| Recessive: C/C vs. C/T-T/T | 0.82 (0.44–1.51) | 0.51 | 824.6 | |
| Additive | 1.34 (1.03–1.74) | 0.028 | 820.2 | |
| rs11121704 MTOR | Codominant: | 0.0067 | 817 | |
| C/T vs. T/T | 1.74 (1.23–2.46) | |||
| C/C vs. T/T | 1.47 (0.81–2.68) | |||
| Dominant: C/T-C/C vs. T/T | 1.69 (1.21–2.35) | 0.0018 | 815.3 | |
| Overdominant: C/T vs. C/C-T/T | 1.63 (1.17–2.27) | 0.0038 | 816.7 | |
| Recessive: C/C vs. C/T-T/T | 1.13 (0.64–2.00) | 0.68 | 824.9 | |
| Additive | 1.40 (1.09–1.81) | 0.0094 | 818.3 | |
| rs2295080 MTOR | Codominant: | 0.0021 | 814.8 | |
| G/T vs. T/T | 1.86 (1.31–2.64) | |||
| G/G vs. T/T | 1.29 (0.73–2.26) | |||
| Dominant: G/T-G/G vs. T/T | 1.74 (1.24–2.42) | 0.0011 | 814.4 | |
| Overdominant: G/T vs. G/G-T/T | 1.77 (1.27–2.47) | 0.0007 | 813.5 | |
| Recessive: G/G vs. G/T-T/T | 0.93 (0.55–1.59) | 0.8 | 825 | |
| Additive | 1.35 (1.05–1.73) | 0.019 | 819.6 |
| Baseline | 3 IVIs | p-Value | |
|---|---|---|---|
| BCVA, letters | 48 ± 22 | 55 ± 21 | <0.001 a |
| CRT, mkm | 316 [271–372] | 246 [218–289] | <0.001 a |
| PED height, mkm | 126 [89–190] | 46 [21–96] | <0.001 a |
| SRF height, mkm | 56 [29–90] | 22 [0–44] | <0.001 a |
| IRF, abs. (%) | 140 (71.4) | 89 (45.4) | <0.001 b |
| SNP | Genotype | Decrease in CRT, µm | p-Value | ||
|---|---|---|---|---|---|
| Me | Q1–Q3 | n | |||
| rs10277 SQSTM1 | C/C | 47 | 24–68 | 63 | 0.001 p C/T–C/C = 0.014 p T/T–C/C = 0.002 |
| C/T | 66 | 30–105 | 100 | ||
| T/T | 93 | 58–122 | 33 | ||
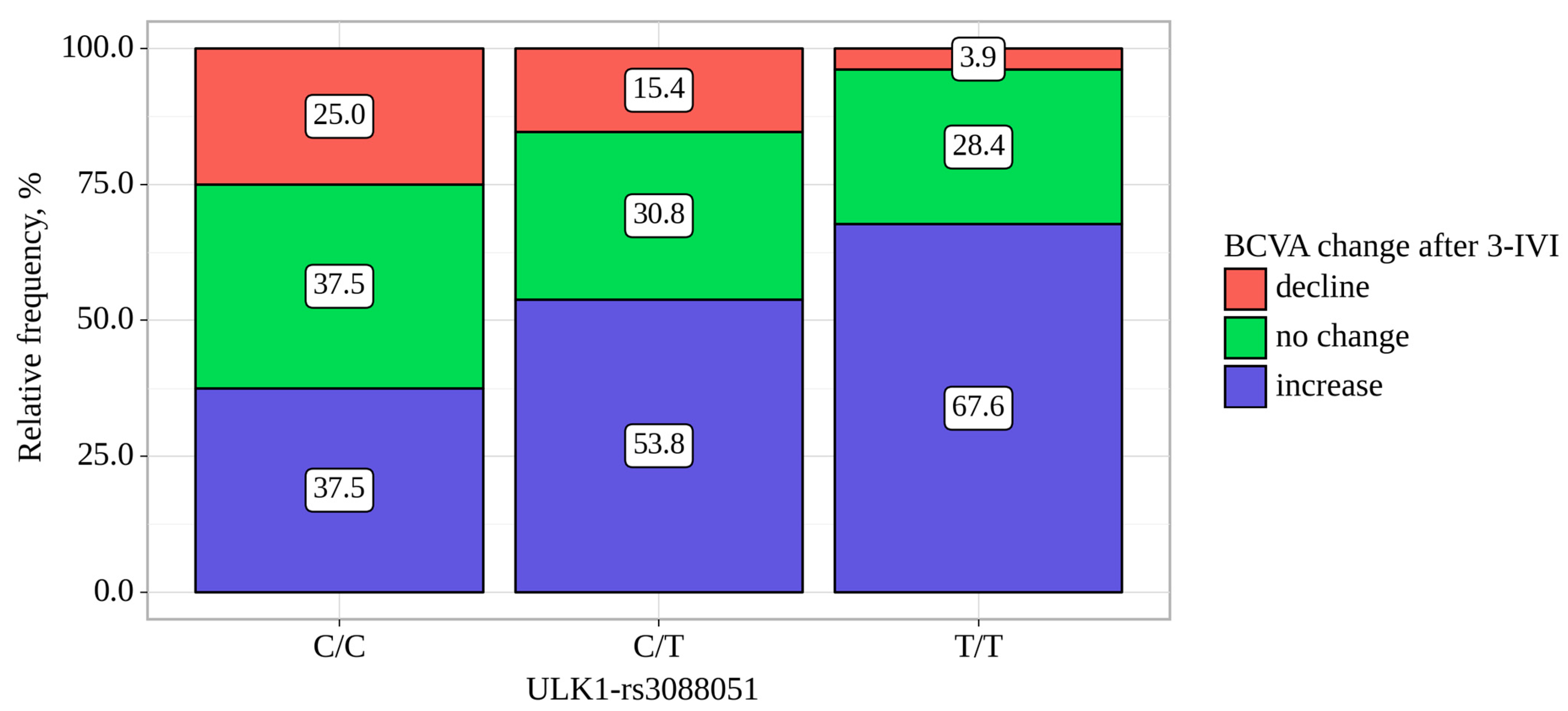
| ULK1-rs3088051 | BCVA Change, abs. (%). | p-Value, Pearson’s Chi-Square | ||
|---|---|---|---|---|
| Decline | no Change | Increase | ||
| C/C | 4 (25.0) | 6 (37.5) | 6 (37.5) | 0.013 p C/C–T/T = 0.010 p C/T–T/T = 0.037 |
| C/T | 12 (15.4) | 24 (30.8) | 42 (53.8) | |
| T/T | 4 (3.9) | 29 (28.4) | 69 (67.6) | |
| ULK1-rs3088051 | BCVA change, letters. | p-value, Kruskal–Wallis | ||
| Me | Q1–Q3 | n | ||
| C/C | 0 | −0–6 | 16 | 0.008 p T/T–C/C = 0.022 |
| C/T | 5 | 0–12 | 78 | |
| T/T | 5 | 0–16 | 102 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kozhevnikova, O.S.; Fursova, A.Z.; Derbeneva, A.S.; Nikulich, I.F.; Devyatkin, V.A.; Kolosova, N.G. Pharmacogenetic Association between Allelic Variants of the Autophagy-Related Genes and Anti-Vascular Endothelial Growth Factor Treatment Response in Neovascular Age-Related Macular Degeneration. Biomedicines 2023, 11, 3079. https://doi.org/10.3390/biomedicines11113079
Kozhevnikova OS, Fursova AZ, Derbeneva AS, Nikulich IF, Devyatkin VA, Kolosova NG. Pharmacogenetic Association between Allelic Variants of the Autophagy-Related Genes and Anti-Vascular Endothelial Growth Factor Treatment Response in Neovascular Age-Related Macular Degeneration. Biomedicines. 2023; 11(11):3079. https://doi.org/10.3390/biomedicines11113079
Chicago/Turabian StyleKozhevnikova, Oyuna S., Anzhella Zh. Fursova, Anna S. Derbeneva, Ida F. Nikulich, Vasiliy A. Devyatkin, and Nataliya G. Kolosova. 2023. "Pharmacogenetic Association between Allelic Variants of the Autophagy-Related Genes and Anti-Vascular Endothelial Growth Factor Treatment Response in Neovascular Age-Related Macular Degeneration" Biomedicines 11, no. 11: 3079. https://doi.org/10.3390/biomedicines11113079
APA StyleKozhevnikova, O. S., Fursova, A. Z., Derbeneva, A. S., Nikulich, I. F., Devyatkin, V. A., & Kolosova, N. G. (2023). Pharmacogenetic Association between Allelic Variants of the Autophagy-Related Genes and Anti-Vascular Endothelial Growth Factor Treatment Response in Neovascular Age-Related Macular Degeneration. Biomedicines, 11(11), 3079. https://doi.org/10.3390/biomedicines11113079






