Abstract
We investigated the association of proton pump inhibitor (PPI) use with the risk of stroke and ischemic heart disease (IHD). The Korean National Health Insurance Service-Health Screening cohort from 2002 to 2003, the participants of which were followed up until 2019, was used. In study I, 45,905 participants who were diagnosed with stroke were matched with 91,810 control I participants. The history of PPI medication was examined. In study II, 40,928 participants who were diagnosed with IHD were matched with 81,856 control II participants. In both study I and study II, the previous history of PPI medication was examined. A propensity score overlap-weighted multivariable logistic regression analysis was conducted to estimate the overlap-weighted odds ratios (ORs) of PPI use for stroke (study I) and IHD (study II). Current PPI use was linked with higher odds for stroke in study I. The odds for stroke were higher in groups with a longer duration of PPI use (OR = 0.96 [95% CI = 0.92–1.00] < 1.55 [1.50–1.61] < 1.62 [1.57–1.68] for < 30 days, 30 to 180 days, and ≥180 days of PPI use). Previous PPI use was linked with higher odds for IHD in study II. The odds for stroke were higher in groups with a longer duration of PPI use (OR = 1.13 [95% CI = 1.08–1.18] < 2.12 [2.04–2.21] < 2.60 [2.51–2.69] for <30 days, 30 to 180 days, and ≥180 days of PPI use). Current PPI medication is associated with a high risk of stroke and IHD. A longer duration of PPI medication was related to a higher risk of stroke and IHD. However, a prior history of PPI medication was not linked with a high risk of stroke or IHD.
1. Introduction
Proton pump inhibitors (PPIs) are one of the most commonly used medicines with widespread indications for gastroesophageal reflux disease (GERD), peptic ulcers, and other indications to repress gastric acid-related disorders. PPIs act by inhibiting 70–80% of the active potassium pumps that reside in the apical membrane of gastric parietal cells, diminishing acid synthesis from the stomach [1]. Novel gastric pumps are synthesized continuously, which takes approximately 36–96 h. Thus, the effect of a PPI on gastric acidity lasts approximately 12 h when it is prescribed as a one-daily regimen. Thus, PPIs have been considered to have fewer side effects and demand repetitive medication. Indeed, PPIs showed an excellent safety profile, and less than 1% of patients complained of minor adverse symptoms such as headache, nausea, and abdominal discomfort [2]. The effectiveness for acid control and safety of drugs have paved the way to the wide use of PPIs. However, the expanding indication for the preventive management of gastroduodenal ulcers in patients without any risks, for instance, in nonintensive care units, steroid therapy alone, and antiplatelet or anticoagulant therapy without any risk for gastric ulcers, has imposed concerns of inadvertent complications from PPI overuse [3].
Many previous studies have examined the potential adverse effects of PPIs. The prolonged suppression of acid can lead to an increased gastric pH, hypochlorhydria, and achlorhydria, which increase the risk of congenital malformations in pregnant women and accelerate the metaplastic transformation of gastric polyps, carcinoids, and carcinomas [4].
In addition, PPIs were supposed to suppress the renal tubular proton pump, in addition to gastric pumps, which increase the risk of chronic renal disease [5]. Other disease risks, such as osteoporosis-related fractures, dementia, and liver disease, have also been reported by diverse researchers. However, there has also been some criticism of the noncausal association between PPI use and its proposed adverse effects [6].
There has been supporting evidence on the additional risk of cardiovascular diseases associated with PPI use [7]. PPIs are known to be a competitive inhibitor of CYP2C19, which metabolizes the clopidogrel prodrug to its active form [8]. Thus, the combinational use of PPIs with antiplatelets was suggested to elevate the risk of cardiac complications [9]. In addition, by inhibiting dimethylarginine dimethylaminohydrolase (DDAH), PPIs have been suggested to impair endothelial function [10]. However, the impact of PPIs on cardiovascular diseases has been controversial [11,12]. A meta-analysis estimated that there was no relationship between PPI use and cardiovascular disease in randomized controlled studies (risk ratio = 0.89, 95% confidence intervals [CI] = 0.34–2.33, p = 0.85).
We aimed to investigate the risk of cardiovascular disease, especially stroke and ischemic heart disease (IHD), related to PPI use. The incidence of stroke and IHD was counted following PPI medication to estimate the effect of PPIs on the new incidence of these diseases. The comorbidities and demographic and lifestyle factors were collected and matching and adjusting were conducted to alleviate the potential confounding effects from these variables.
2. Materials and Methods
2.1. Exposure (Proton Pump Inhibitors)
Proton pump inhibitor (PPI) use was defined as the prescription and duration during the 1 year before the index date. In this study, PPI duration was categorized into four categories: non-user, <30 days, 30 to 180 days, and ≥180 days.
2.2. Outcome (Cardiovascular Diseases; CVDs)
Stroke and IHD were selected based on ICD-10 codes (I60-I69 for stroke and I20-I25 for IHD). We only included participants who were hospitalized ≥2 days or who died because of each disease, as described in our previous studies [13,14].
2.3. Participant Selection
The detailed description of the Korean National Health Insurance Service-Health Screening (NHIS-HealS) cohort data (2002–2003, follow-up until 2019) was described elsewhere [15,16,17].
2.3.1. Study I
Among 514,866 participants (2002–2019), the participants with stroke were identified (n = 49,725). The participants without history of stroke were randomly selected to collect the control I participants (n = 465,141). The participants who were diagnosed with stroke in 2002 were removed from the study population (n = 970). Stroke participants who did not have records of BMI, blood pressure, fasting blood glucose, and total cholesterol (n = 44) were excluded. Control I participants who were diagnosed with stroke or IHD at least once (n = 142,593) were excluded. The participants with stroke were 1:2 matched with control I participants. The date of initial diagnosis of stroke was defined as the index date. As a result, 2806 stroke participants and 230,738 control I participants were excluded. Ultimately, 45,905 stroke participants were 1:2 matched with 91,810 control I participants (Figure 1a).
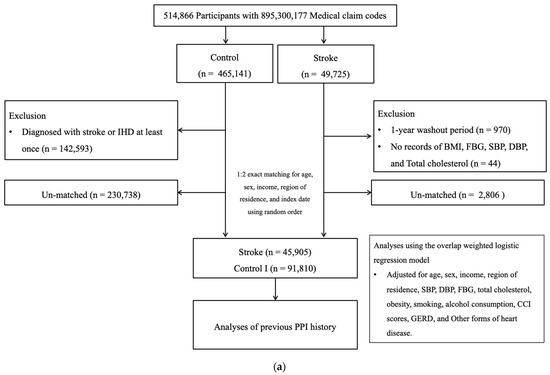
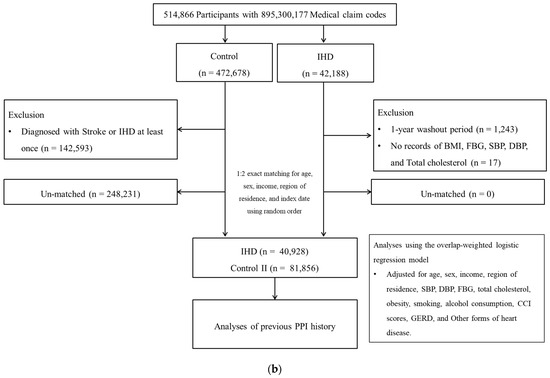
Figure 1.
(a) A schematic illustration of the participant selection process that was used in the present study I. Of a total of 514,866 participants, 45,905 stroke participants were matched with 91,810 control participants for age, sex, income, and region of residence. (b) A schematic illustration of the participant selection process that was used in the present study II. Of a total of 514,866 participants, 40,928 IHD participants were matched with 81,856 control II participants for age, sex, income, and region of residence.
2.3.2. Study II
IHD participants were selected from 514,866 participants with 895,300,177 medical claim codes from 2002 to 2019 (n = 42,188). The control II group was included if participants were not defined as having IHD from 2002 to 2019 (n = 472,678). To select IHD participants who were diagnosed for the first time, IHD participants diagnosed in 2002 were excluded (washout periods, n = 1243). IHD participants who did not have records of BMI, blood pressure, fasting blood glucose, and total cholesterol (n = 17) were excluded. Control II participants who were diagnosed with stroke or IHD at least once (n = 142,593) were excluded. IHD participants were 1:2 matched with control II participants. The date of initial diagnosis of IHD was defined as the index date. To sum up, 40,928 IHD participants were 1:2 matched with 81,856 control II participants (Figure 1b).
2.4. Covariates
Age groups were divided into 5-year intervals: 40–44, 45–49…, and 85+ years old (total of 10 age groups). The level of income, region of residence, histories of tobacco smoking and alcohol consumption, obesity group based on BMI (body mass index, kg/m2), systolic blood pressure (SBP, mmHg), diastolic blood pressure (DBP, mmHg), fasting blood glucose (FBG, mg/dL), and total cholesterol (mg/dL) were collected and adjusted in the analyses [18,19].
The Charlson Comorbidity Index (CCI) was counted, except for cerebrovascular disease, congestive heart failure, and acute myocardial infarction, and adjusted in this study.
The number of patients diagnosed with GERD (ICD-10 code: K21, treated ≥2 times and prescribed a PPI for ≥2 weeks) for 1 year prior to the index date was additionally assessed.
Regarding CVDs, other forms of heart disease (ICD-10 codes: I30-I52) were further evaluated.
2.5. Statistical Analyses
We conducted propensity score overlap weighting analyses to reflect the covariate balance and effective sample size [20,21]. The propensity score (PS) was applied and overlap weighting was calculated [22,23]. The variables of study and control groups were compared using the standardized difference.
To analyze the overlap-weighted odds ratios (ORs) of prescription dates of proton pump inhibitors for stroke and IHD, a propensity score overlap-weighted multivariable logistic regression analysis was used. In these analyses, crude and overlap-weighted models were used.
The 95% confidence interval (CI) was calculated. Subgroup analyses were conducted.
Two-tailed analyses were performed, and significance was defined as p values less than 0.05. SAS version 9.4 (SAS Institute Inc., Cary, NC, USA) was used for statistical analyses.
3. Results
A total of 12.36% (5197/45,905) of stroke patients and 20.09% (16,893/91,810) of control I participants were non-PPI users (Table S1). There were differences in the distributions of obesity groups, smoking status, alcohol consumption, blood pressure, fasting blood glucose, total cholesterol, CCI score, history of GERD, and other forms of heart disease between the stroke and control I groups. Thus, all these variables were included in the PS overlap weight adjustment. After the PS overlap weight adjustment, 13.21% of stroke patients and 18.00% of control I participants were non-PPI users (sd = 0.22, Table 1).

Table 1.
General characteristics of participants after propensity score overlap weighting adjustment.
The ≥180 days of PPI use was associated with 2.13-fold higher odds for stroke in the crude model (95% CI = 2.06–2.21, p < 0.001, Table 2). In the overlap-weighted model, the odds for stroke were 1.62-fold higher in the ≥180 days of PPI users (95% CI = 1.57–1.68, p < 0.001). The odds for stroke were higher when the duration of PPI use was longer (OR = 0.96 [95% CI = 0.92–1.00] < 1.55 [1.50–1.61] < 1.62 [1.57–1.68] for <30 days, 30 to 180 days, and ≥180 days of PPI use).

Table 2.
Crude and overlap propensity score-weighted odd ratios of proton pump inhibitor (ref: non-user) for stroke.
Secondary analyses according to age, sex, income, region of residence, obesity, smoking status, alcohol consumption, blood pressure, fasting blood glucose, total cholesterol, CCI scores, GERD, and other forms of heart disease demonstrated consistently higher odds for stroke in PPI users (Figure 2a).
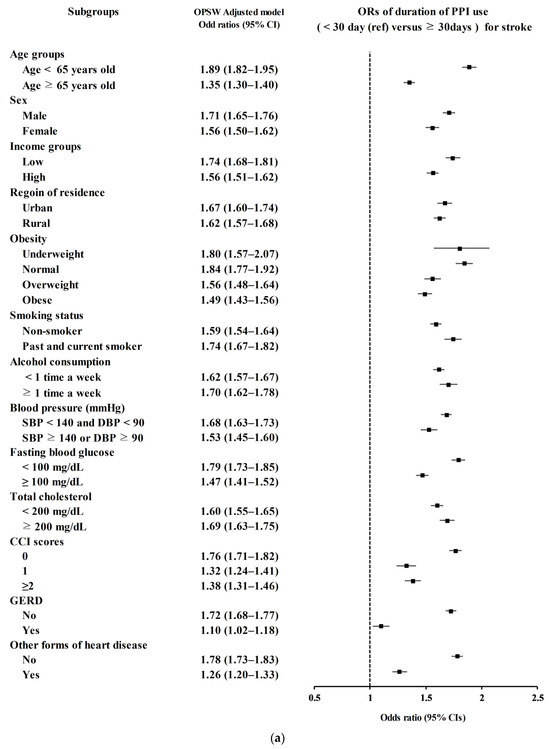
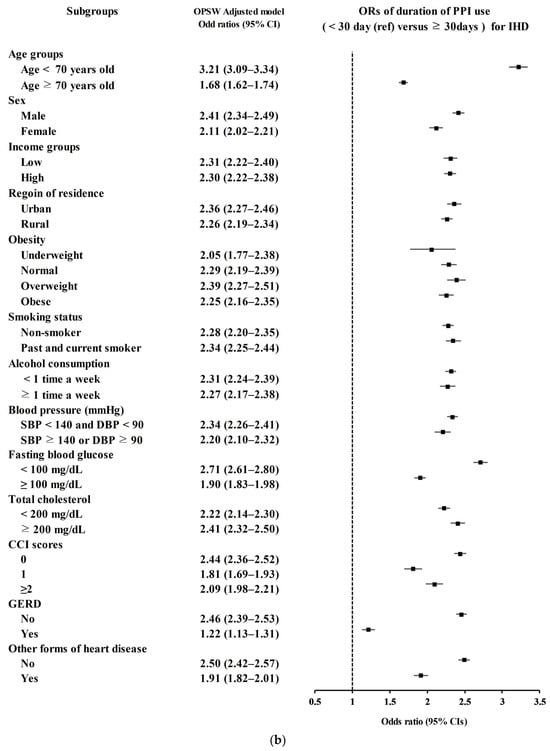
Figure 2.
(a) Overlap propensity score-weighted (OPSW) odds ratios (OR) of PPI for stroke. (b) Overlap propensity score-weighted (OPSW) odds ratios (OR) of PPI for ischemic heart disease (IHD).
In total, 9.27% (3795/40,928) of IHD patients and 20.67% (16,917/81,856) of control II participants were non-PPI users (sd = 0.52, Table S1). There were differences in the distributions of obesity groups, smoking status, alcohol consumption, blood pressure, fasting blood glucose, total cholesterol, CCI score, history of GERD, and other forms of heart disease between the IHD and control II groups. Thus, all these variables were included in the PS overlap weight adjustment. After the PS overlap weight adjustment, 10.05% of IHD patients and 18.57% of control II participants were non-PPI users (sd = 0.37, Table 1).
The ≥ 180 days of PPI use was associated with 3.41-fold higher odds for IHD in the crude model (95% CI = 3.28–3.55, p < 0.001, Table 3). In the overlap-weighted model, the odds for IHD were 2.60-fold higher in the ≥180 days of PPI users (95% CI = 2.51–2.69, p < 0.001). The odds for IHD were higher when the duration of PPI use was longer (OR = 1.13 [95% CI = 1.08–1.18] < 2.12 [2.04–2.21] < 2.60 [2.51–2.69] for <30 days, 30 to 180 days, and ≥180 days of PPI use).

Table 3.
Crude and overlap propensity score-weighted odd ratios of proton pump inhibitor (ref: non-user) for IHD.
Secondary analyses according to age, sex, income, region of residence, obesity, smoking status, alcohol consumption, blood pressure, fasting blood glucose, total cholesterol, CCI scores, GERD, and other forms of heart disease demonstrated consistently higher odds for IHD in PPI users (Figure 2b).
4. Discussion
PPI use was related to an increased incidence of stroke and IHD in this study. The risks of stroke and IHC were predicted to be higher in patients with a longer duration of PPI use.
Therefore, it can be presumed that PPI use can be associated with an increased incidence of stroke and IHD in past PPI users with a dose-response relationship. Past PPI users may show an increased incidence of stroke and IHD because they may have higher risk characteristics, such as chest tightness, which can lead to mistakenly identifying the symptoms of IHD as gastric reflux symptoms. In summary, the potential impacts of PPIs on stroke and IHD can be mixed with the possible harmful effects of PPIs and misusage of PPIs in inappropriate indications. Although the odds of PPI use for the incidence of stroke and IHD were high in the current study, we cannot conclude the direct adverse effects of PPIs on the cardiovascular system.
The adverse effects of PPIs on the cardiovascular system have been actively discussed by many researchers with some conflicting results [24]. A number of previous studies have suggested a high risk of stroke and IHD in PPI users. A review study using ChatGPT estimated that most observational studies indicated a positive relationship between PPI use and major cardiovascular events [24]. A nationwide study in Denmark demonstrated a 1.13 times higher risk of ischemic stroke in PPI users (95% CI = 1.09–1.19) [25]. They also reported an increased risk of myocardial infarction in PPI users (hazard ratio = 1.31, 95% CI = 1.23–1.39). The increased risk of stroke and myocardial infarction was higher in long-term and high-dose PPI users. Moreover, a prospective study using the UK Biobank estimated that the risk of stroke was 1.16-fold higher in PPI users than in non-users (95% CI = 1.06–1.27) [26]. They also conducted a meta-analysis that consolidated their finding with a 1.22-fold higher risk of stroke (95% CI = 1.00–1.50). However, a self-controlled case series study reported no increased risk of myocardial infarction in short-term PPI users compared to H2 receptor antagonist users [27]. They suggested potential bias, such as confounding effects and protopathic bias, in studies on the risk of PPIs.
PPIs can exert negative effects on the cardiovascular system by a number of plausible mechanisms. PPIs were suggested to impair endothelial function by inhibiting endothelial nitric oxide synthase and the absorption of vitamin C and B12, and increasing the risk of metabolic syndrome. PPIs are known to interfere with DDAH activity. The decreased DDAH activity increases the plasma level of asymmetric dimethylarginine (ADMA), which competitively inhibits nitric oxide (NO) synthesis [10]. Because NO is a crucial vasoprotective molecule that represses cell proliferation, platelet aggregation, and the interaction between endothelial cells and leukocytes, reduced NO synthesis can result in cardiovascular compromise [10]. In addition, PPIs were suggested to induce vitamin B12 deficiency by increasing homocysteine levels and elevating ADMA levels [28]. Moreover, it was reported that PPIs can increase the risk of metabolic syndrome and diabetes [29,30].
However, short-term PPI use was less linked with an elevated incidence of stroke or IHD in the present study. It can be supposed that the impact of PPI on stroke and IHD may not last for a long time. The half-life of PPI elimination was estimated to be approximately one hour [31]. In an animal study, the half-life for the recovery of proton pump activity following PPI use was predicted to be approximately 15 h after PPI use [31]. In addition, the symptoms of GERD can be similar or indistinguishable from those of cardiovascular disease. The burning sensation in the epigastric area and chest pain are common extraesophageal symptoms in patients with GERD [32]. Thus, patients with subclinical or mild symptoms of cardiovascular disease can be prescribed PPIs depending on their symptoms and delay the diagnosis of cardiovascular diseases, which can result in protopathic bias [33]. Moreover, PPIs are often prescribed along with other medicines, such as corticosteroids, anticoagulants, and nonsteroidal anti-inflammatory agents, to prevent medication-associated ulcers [34]. Thus, the high comorbidities and concurrent medication in high-risk patients for stroke and IHD can increase the rate of PPI use in these populations. Although we adjusted a considerable number of variables in the analyses, this possible reverse causality cannot be completely excluded.
This study was based on a large, nationwide cohort, through which we can achieve a large control population to alleviate the risk of selection bias. Because all Koreans must register with the national health insurance system, which is regularly monitored by statisticians hired by the Korean government, there was little concern about missing or duplicated data. However, because the current study was based on health claim codes, undiagnosed or misdiagnosed participants can be missed in the analysis. In addition, the accuracy of the diagnosis of stroke and IHD was objective and reliable because it was determined by physicians, and the severity and management of diseases were heterogeneous in this study. For PPI use, this study used prescription data. Thus, the compliance of patients with PPI prescriptions can influence the analytic results of the present study. Moreover, concurrent medication including aspirin was not considered in this study. Although this study investigated the incidence of stroke and IHD after PPI medication, the temporal relation between PPI use and stroke or IHD cannot be concluded. Because the study population of the current study was Korean, the results may be different in other ethnic or regional populations.
5. Conclusions
Previous PPI use was related to a higher risk of stroke and IHD. A longer duration of PPI use was associated with a higher risk of stroke and IHD than short-term PPI use.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/biomedicines12010170/s1, Table S1: General characteristics of participants before propensity score overlap weighting adjustment.
Author Contributions
H.G.C. designed the study; S.J., M.J.K., J.H.K., J.-H.K. and H.G.C. analyzed the data; S.Y.K., S.J., M.J.K., J.H.K., J.-H.K., Y.K. and H.G.C. drafted and revised the paper; S.J. and H.G.C. drew the figures. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported in part by a research grant (RS-2023-00208042) from the National Research Foundation (NRF) grant funded by the Korean government (MSIT).
Institutional Review Board Statement
The ethics committee of Hallym University (2019-10-23) permitted this study following the guidelines and regulations.
Informed Consent Statement
Written informed consent was waived by the Institutional Review Board. Patient consent was waived by the Hallym University.
Data Availability Statement
Data are contained within the article and Supplementary Material.
Conflicts of Interest
Author Hyo Geun Choi was employed by the company Mdanalytics. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The company Mdanalytics had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- Schnoll-Sussman, F.; Niec, R.; Katz, P.O. Proton Pump Inhibitors: The Good, Bad, and Ugly. Gastrointest. Endosc. Clin. N. Am. 2020, 30, 239–251. [Google Scholar] [CrossRef] [PubMed]
- Katz, P.O.; Gerson, L.B.; Vela, M.F. Guidelines for the diagnosis and management of gastroesophageal reflux disease. Am. J. Gastroenterol. 2013, 108, 308–328. [Google Scholar] [CrossRef] [PubMed]
- Savarino, V.; Marabotto, E.; Zentilin, P.; Furnari, M.; Bodini, G.; De Maria, C.; Pellegatta, G.; Coppo, C.; Savarino, E. Proton pump inhibitors: Use and misuse in the clinical setting. Expert Rev. Clin. Pharmacol. 2018, 11, 1123–1134. [Google Scholar] [CrossRef]
- Koyyada, A. Long-term use of proton pump inhibitors as a risk factor for various adverse manifestations. Therapie 2021, 76, 13–21. [Google Scholar] [CrossRef] [PubMed]
- Fossmark, R.; Martinsen, T.C.; Waldum, H.L. Adverse Effects of Proton Pump Inhibitors-Evidence and Plausibility. Int. J. Mol. Sci. 2019, 20, 5203. [Google Scholar] [CrossRef]
- Malfertheiner, P.; Kandulski, A.; Venerito, M. Proton-pump inhibitors: Understanding the complications and risks. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 697–710. [Google Scholar] [CrossRef] [PubMed]
- Ariel, H.; Cooke, J.P. Cardiovascular Risk of Proton Pump Inhibitors. Methodist. Debakey Cardiovasc. J. 2019, 15, 214–219. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.; Tong, J.; Kuang, X.; Chen, W.; Liu, Z. Influence of proton pump inhibitors on clinical outcomes in coronary heart disease patients receiving aspirin and clopidogrel: A meta-analysis. Medicine 2018, 97, e9638. [Google Scholar] [CrossRef]
- Cardoso, R.N.; Benjo, A.M.; DiNicolantonio, J.J.; Garcia, D.C.; Macedo, F.Y.B.; El-Hayek, G.; Nadkarni, G.N.; Gili, S.; Iannaccone, M.; Konstantinidis, I.; et al. Incidence of cardiovascular events and gastrointestinal bleeding in patients receiving clopidogrel with and without proton pump inhibitors: An updated meta-analysis. Open Heart 2015, 2, e000248. [Google Scholar] [CrossRef]
- Ghebremariam, Y.T.; LePendu, P.; Lee, J.C.; Erlanson, D.A.; Slaviero, A.; Shah, N.H.; Leiper, J.; Cooke, J.P. Unexpected effect of proton pump inhibitors: Elevation of the cardiovascular risk factor asymmetric dimethylarginine. Circulation 2013, 128, 845–853. [Google Scholar] [CrossRef]
- Batchelor, R.; Kumar, R.; Gilmartin-Thomas, J.F.M.; Hopper, I.; Kemp, W.; Liew, D. Systematic review with meta-analysis: Risk of adverse cardiovascular events with proton pump inhibitors independent of clopidogrel. Aliment. Pharmacol. Ther. 2018, 48, 780–796. [Google Scholar] [CrossRef] [PubMed]
- Shiraev, T.P.; Bullen, A. Proton Pump Inhibitors and Cardiovascular Events: A Systematic Review. Heart Lung Circ. 2018, 27, 443–450. [Google Scholar] [CrossRef]
- Choi, S.; Kim, K.; Kim, S.M.; Lee, G.; Jeong, S.-M.; Park, S.Y.; Kim, Y.-Y.; Son, J.S.; Yun, J.-M.; Park, S.M. Association of Obesity or Weight Change with Coronary Heart Disease Among Young Adults in South Korea. JAMA Intern. Med. 2018, 178, 1060–1068. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.; Park, S.M.; Lee, K. Weight gain after smoking cessation does not modify its protective effect on myocardial infarction and stroke: Evidence from a cohort study of men. Eur. Heart J. 2018, 39, 1523–1531. [Google Scholar] [CrossRef]
- Service, W.N.H.I.; National Health Insurance Service. National Health Insurance Sharing Service 2019. Available online: https://nhiss.nhis.or.kr/bd/ab/bdaba002cv.do (accessed on 1 December 2019).
- Seong, S.C.; Kim, Y.-Y.; Park, S.K.; Khang, Y.H.; Kim, H.C.; Park, J.H.; Kang, H.-J.; Do, C.-H.; Song, J.-S.; Lee, E.-J.; et al. Cohort profile: The National Health Insurance Service-National Health Screening Cohort (NHIS-HEALS) in Korea. BMJ Open. 2017, 7, e016640. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Lee, J.S.; Park, S.H.; Shin, S.A.; Kim, K. Cohort Profile: The National Health Insurance Service-National Sample Cohort (NHIS-NSC), South Korea. Int. J. Epidemiol. 2017, 46, e15. [Google Scholar] [CrossRef]
- Kim, S.Y.; Min, C.; Oh, D.J.; Choi, H.G. Bidirectional Association Between GERD and Asthma: Two Longitudinal Follow-Up Studies Using a National Sample Cohort. J. Allergy Clin. Immunol. Pract. 2020, 8, 1005–1013.e9. [Google Scholar] [CrossRef]
- Kim, S.Y.; Oh, D.J.; Park, B.; Choi, H.G. Bell’s palsy and obesity, alcohol consumption and smoking: A nested case-control study using a national health screening cohort. Sci. Rep. 2020, 10, 4248. [Google Scholar] [CrossRef]
- Quan, H.; Li, B.; Couris, C.M.; Fushimi, K.; Graham, P.; Hider, P.; Januel, J.-M.; Sundararajan, V. Updating and validating the Charlson comorbidity index and score for risk adjustment in hospital discharge abstracts using data from 6 countries. Am. J. Epidemiol. 2011, 173, 676–682. [Google Scholar] [CrossRef]
- Quan, H.; Sundararajan, V.; Halfon, P.; Fong, A.; Burnand, B.; Luthi, J.-C.; Saunders, L.D.; Beck, C.A.; Feasby, T.E.; Ghali, W.A. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med. Care. 2005, 43, 1130–1139. [Google Scholar] [CrossRef]
- Li, F.; Thomas, L.E.; Li, F. Addressing Extreme Propensity Scores via the Overlap Weights. Am. J. Epidemiol. 2019, 188, 250–257. [Google Scholar] [CrossRef] [PubMed]
- Thomas, L.E.; Li, F.; Pencina, M.J. Overlap Weighting: A Propensity Score Method That Mimics Attributes of a Randomized Clinical Trial. JAMA 2020, 323, 2417–2418. [Google Scholar] [CrossRef] [PubMed]
- Teperikidis, E.; Boulmpou, A.; Potoupni, V.; Kundu, S.; Singh, B.; Papadopoulos, C. Does the long-term administration of proton pump inhibitors increase the risk of adverse cardiovascular outcomes? A ChatGPT powered umbrella review. Acta Cardiol. 2023, 78, 980–988. [Google Scholar] [CrossRef] [PubMed]
- Sehested, T.S.G.; Gerds, T.A.; Fosbøl, E.L.; Hansen, P.W.; Charlot, M.G.; Carlson, N.; Hlatky, M.A.; Torp-Pedersen, C.; Gislason, G.H. Long-term use of proton pump inhibitors, dose-response relationship and associated risk of ischemic stroke and myocardial infarction. J. Intern. Med. 2018, 283, 268–281. [Google Scholar] [CrossRef]
- Yang, M.; He, Q.; Gao, F.; Nirantharakumar, K.; Veenith, T.; Qin, X.; Page, A.T.; Wong, M.C.S.; Huang, J.; Kuo, Z.C.; et al. Regular use of proton-pump inhibitors and risk of stroke: A population-based cohort study and meta-analysis of randomized-controlled trials. BMC Med. 2021, 19, 316. [Google Scholar] [CrossRef] [PubMed]
- Chui, C.S.L.; Cheung, K.S.; Brown, J.P.; Douglas, I.J.; Wong, I.C.K.; Chan, E.W.; Wong, A.Y.S. Proton pump inhibitors and myocardial infarction: An application of active comparators in a self-controlled case series. Int. J. Epidemiol. 2023, 52, 899–907. [Google Scholar] [CrossRef]
- Lam, J.R.; Schneider, J.L.; Zhao, W.; Corley, D.A. Proton pump inhibitor and histamine 2 receptor antagonist use and vitamin B12 deficiency. JAMA 2013, 310, 2435–2442. [Google Scholar] [CrossRef]
- Imperatore, N.; Tortora, R.; Testa, A.; Gerbino, N.; Caporaso, N.; Rispo, A. Proton pump inhibitors as risk factor for metabolic syndrome and hepatic steatosis in coeliac disease patients on gluten-free diet. J. Gastroenterol. 2018, 53, 507–516. [Google Scholar] [CrossRef]
- Yuan, J.; He, Q.; Nguyen, L.H.; Wong, M.C.S.; Huang, J.; Yu, Y.; Xia, B.; Tang, Y.; He, Y.; Zhang, C. Regular use of proton pump inhibitors and risk of type 2 diabetes: Results from three prospective cohort studies. Gut 2021, 70, 1070–1077. [Google Scholar] [CrossRef]
- Shin, J.M.; Kim, N. Pharmacokinetics and pharmacodynamics of the proton pump inhibitors. J. Neurogastroenterol. Motil. 2013, 19, 25–35. [Google Scholar] [CrossRef]
- Min, Y.W.; Lim, S.W.; Lee, J.H.; Lee, H.L.; Lee, O.Y.; Park, J.M.; Choi, M.-G.; Rhee, P.-L. Prevalence of Extraesophageal Symptoms in Patients with Gastroesophageal Reflux Disease: A Multicenter Questionnaire-based Study in Korea. J. Neurogastroenterol. Motil. 2014, 20, 87–93. [Google Scholar] [CrossRef] [PubMed]
- Horwitz, R.I.; Feinstein, A.R. The problem of “protopathic bias” in case-control studies. Am. J. Med. 1980, 68, 255–258. [Google Scholar] [CrossRef] [PubMed]
- Shanika, L.G.T.; Reynolds, A.; Pattison, S.; Braund, R. Proton pump inhibitor use: Systematic review of global trends and practices. Eur. J. Clin. Pharmacol. 2023, 79, 1159–1172. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).