Unlocking the Medicinal Mysteries: Preventing Lacunar Stroke with Drug Repurposing
Abstract
:1. Introduction
2. Materials and Methods
2.1. Potential Risk Factors
2.2. Data Sources
2.3. Genetic Variants
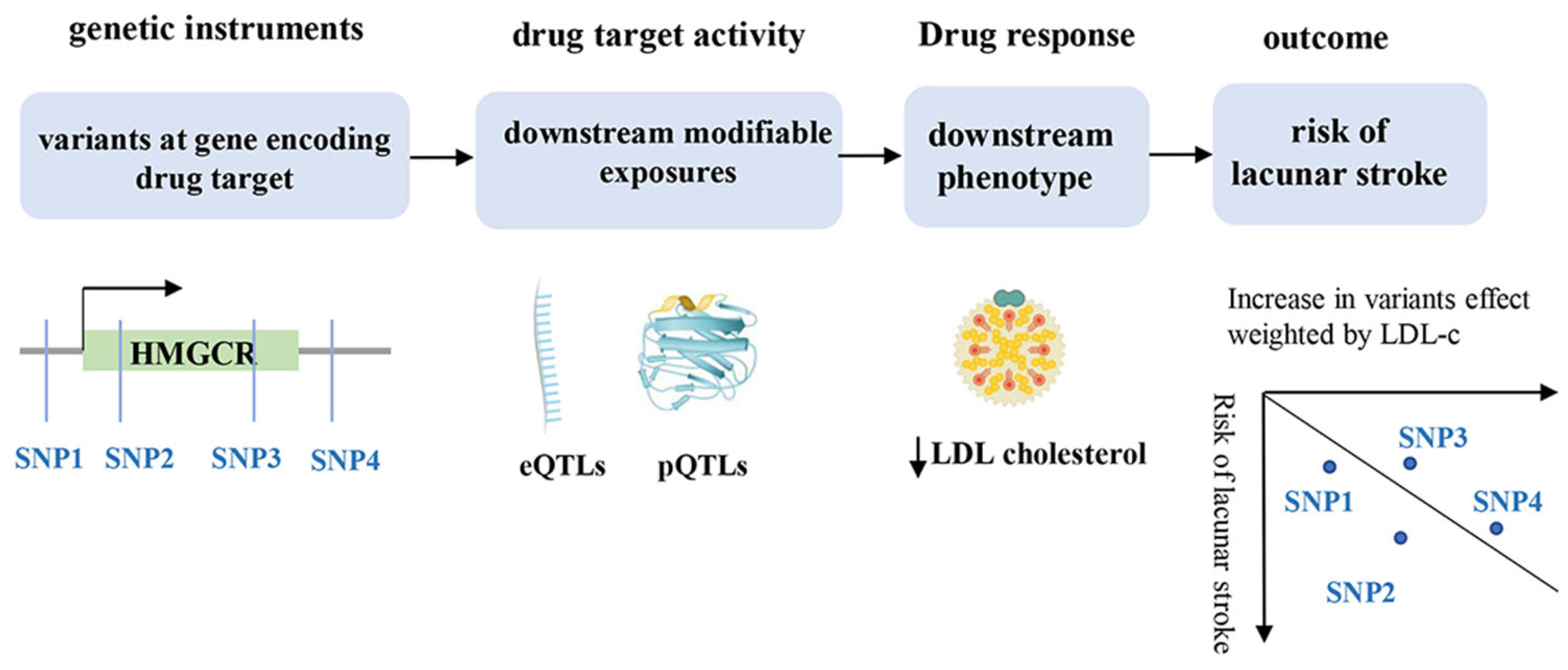
2.4. Mendelian Randomization Analysis
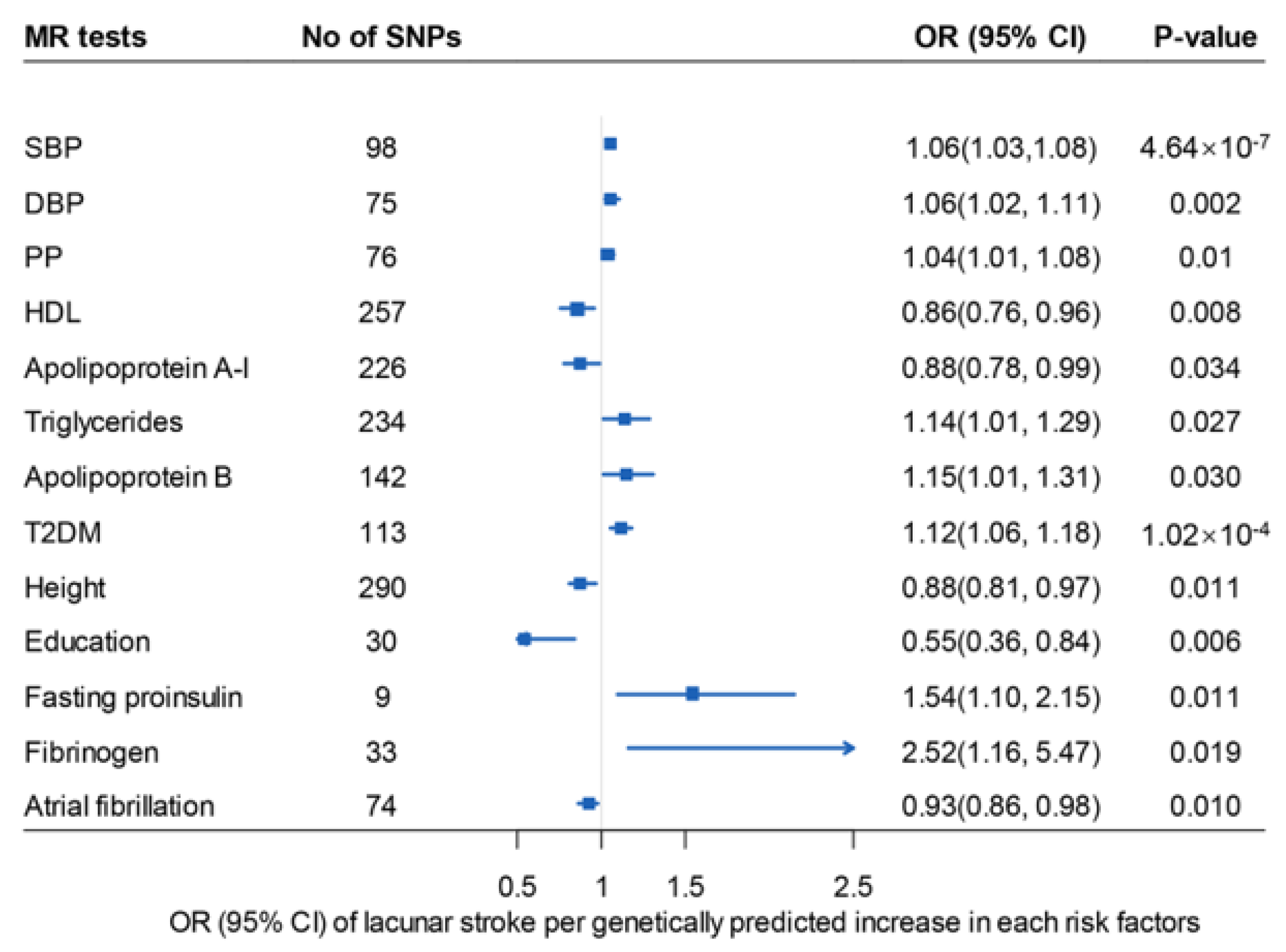
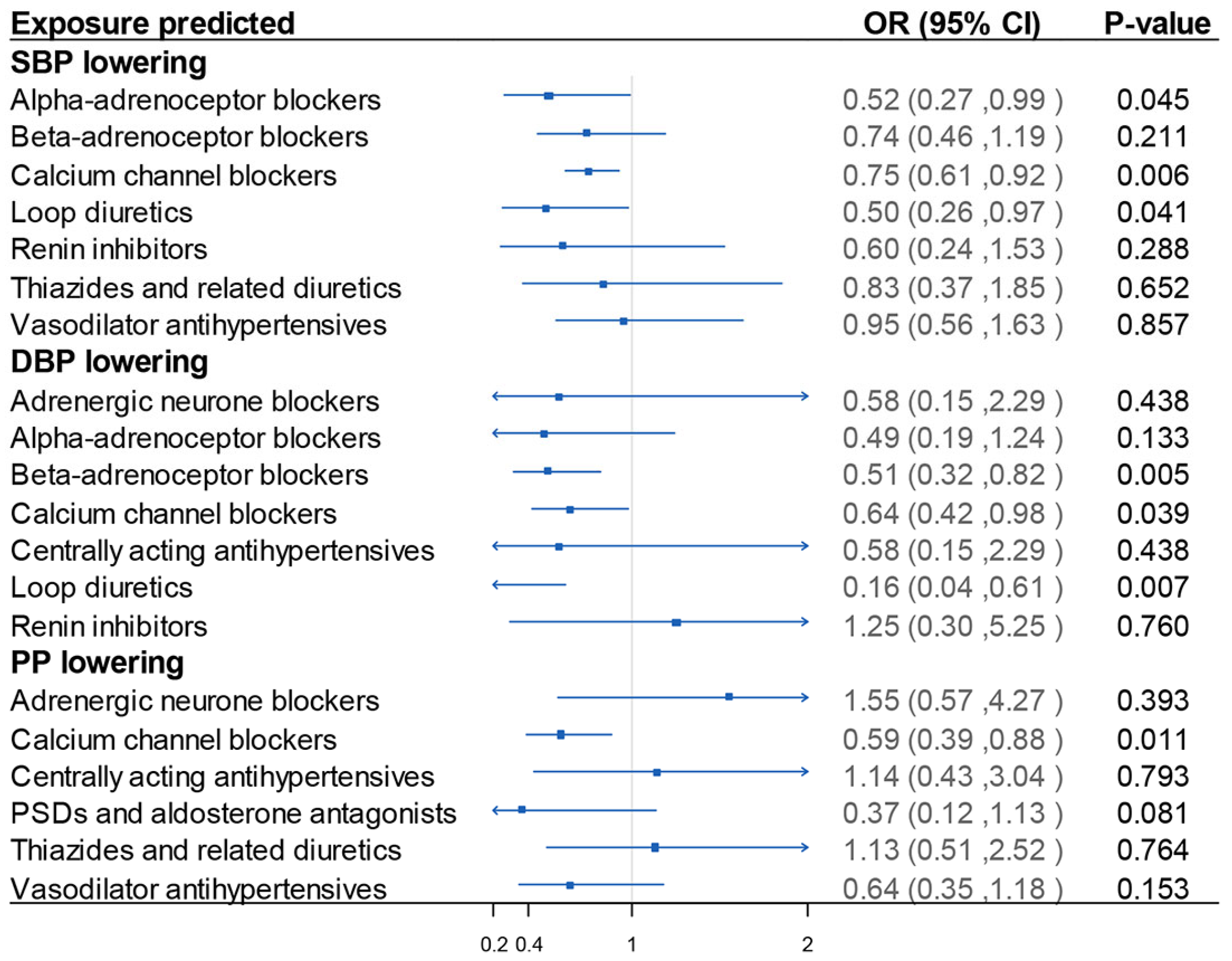
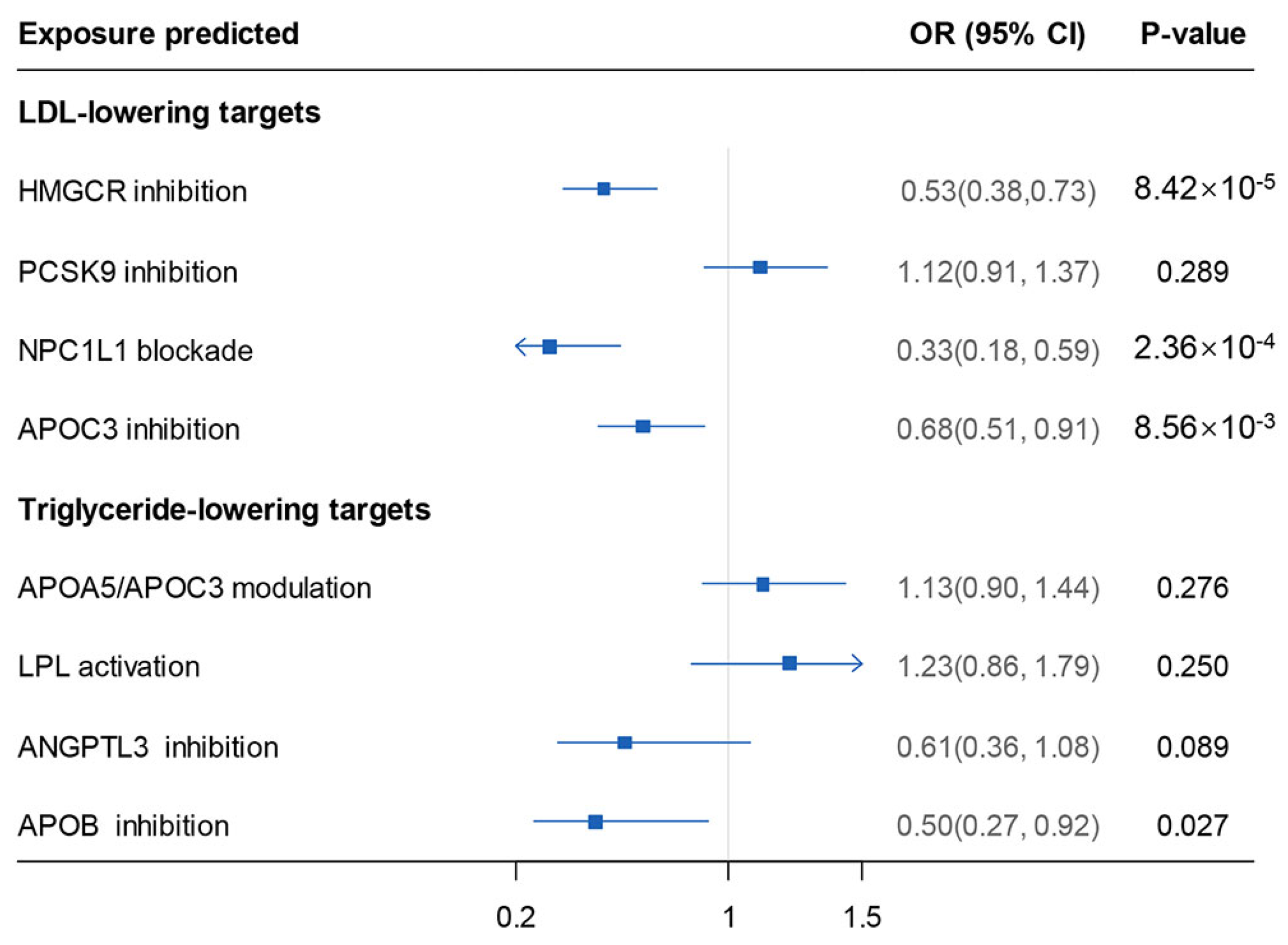
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Pantoni, L. Cerebral small vessel disease: From pathogenesis and clinical characteristics to therapeutic challenges. Lancet Neurol. 2010, 9, 689–701. [Google Scholar] [CrossRef]
- Traylor, M.; Persyn, E.; Tomppo, L.; Klasson, S.; Abedi, V.; Bakker, M.K.; Torres, N.; Li, L.; Bell, S.; Rutten-Jacobs, L.; et al. Genetic basis of lacunar stroke: A pooled analysis of individual patient data and genome-wide association studies. Lancet Neurol. 2021, 20, 351–361. [Google Scholar] [CrossRef]
- Nelson, M.R.; Tipney, H.; Painter, J.L.; Shen, J.; Nicoletti, P.; Shen, Y.; Floratos, A.; Sham, P.C.; Li, M.J.; Wang, J.; et al. The support of human genetic evidence for approved drug indications. Nat. Genet. 2015, 47, 856–860. [Google Scholar] [CrossRef]
- Davey Smith, G.; Hemani, G. Mendelian randomization: Genetic anchors for causal inference in epidemiological studies. Human. Mol. Genet. 2014, 23, R89–R98. [Google Scholar] [CrossRef]
- Schmidt, A.F.; Finan, C.; Gordillo-Marañón, M.; Asselbergs, F.W.; Freitag, D.F.; Patel, R.S.; Tyl, B.; Chopade, S.; Faraway, R.; Zwierzyna, M.; et al. Genetic drug target validation using Mendelian randomisation. Nat. Commun. 2020, 11, 3255. [Google Scholar] [CrossRef]
- Swerdlow, D.I.; Holmes, M.V.; Kuchenbaecker, K.B.; Engmann, J.E.; Shah, T.; Sofat, R.; Guo, Y.; Chung, C.; Peasey, A.; Pfister, R.; et al. The interleukin-6 receptor as a target for prevention of coronary heart disease: A mendelian randomisation analysis. Lancet 2012, 379, 1214–1224. [Google Scholar] [CrossRef]
- Swerdlow, D.I.; Preiss, D.; Kuchenbaecker, K.B.; Holmes, M.V.; Engmann, J.E.; Shah, T.; Sofat, R.; Stender, S.; Johnson, P.C.; Scott, R.A.; et al. HMG-coenzyme A reductase inhibition, type 2 diabetes, and bodyweight: Evidence from genetic analysis and randomised trials. Lancet 2015, 385, 351–361. [Google Scholar] [CrossRef]
- Ashburn, T.T.; Thor, K.B. Drug repositioning: Identifying and developing new uses for existing drugs. Nat. Rev. Drug Discov. 2004, 3, 673–683. [Google Scholar] [CrossRef]
- Acosta, J.N.; Szejko, N.; Falcone, G.J. Mendelian Randomization in Stroke: A Powerful Approach to Causal Inference and Drug Target Validation. Front. Genet. 2021, 12, 683082. [Google Scholar] [CrossRef]
- Hemani, G.; Zheng, J.; Elsworth, B.; Wade, K.H.; Haberland, V.; Baird, D.; Laurin, C.; Burgess, S.; Bowden, J.; Langdon, R.; et al. The MR-Base platform supports systematic causal inference across the human phenome. eLife 2018, 7, e34408. [Google Scholar] [CrossRef]
- Kilarski, L.L.; Rutten-Jacobs, L.C.; Bevan, S.; Baker, R.; Hassan, A.; Hughes, D.A.; Markus, H.S. Prevalence of CADASIL and Fabry Disease in a Cohort of MRI Defined Younger Onset Lacunar Stroke. PLoS ONE 2015, 10, e0136352. [Google Scholar] [CrossRef]
- Adams, H.P., Jr.; Bendixen, B.H.; Kappelle, L.J.; Biller, J.; Love, B.B.; Gordon, D.L.; Marsh, E.E., 3rd. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in Acute Stroke Treatment. Stroke 1993, 24, 35–41. [Google Scholar] [CrossRef]
- Bellenguez, C.; Bevan, S.; Gschwendtner, A.; Spencer, C.C.; Burgess, A.I.; Pirinen, M.; Jackson, C.A.; Traylor, M.; Strange, A.; Su, Z.; et al. Genome-wide association study identifies a variant in HDAC9 associated with large vessel ischemic stroke. Nat. Genet. 2012, 44, 328–333. [Google Scholar] [CrossRef]
- NINDS Stroke Genetics Network (SiGN); International Stroke Genetics Consortium (ISGC). Loci associated with ischaemic stroke and its subtypes (SiGN): A genome-wide association study. Lancet Neurol. 2016, 15, 174–184. [Google Scholar] [CrossRef]
- Pierce, B.L.; Ahsan, H.; Vanderweele, T.J. Power and instrument strength requirements for Mendelian randomization studies using multiple genetic variants. Int. J. Epidemiol. 2011, 40, 740–752. [Google Scholar] [CrossRef]
- Bowden, J.; Del Greco, M.F.; Minelli, C.; Davey Smith, G.; Sheehan, N.A.; Thompson, J.R. Assessing the suitability of summary data for two-sample Mendelian randomization analyses using MR-Egger regression: The role of the I2 statistic. Int. J. Epidemiol. 2016, 45, 1961–1974. [Google Scholar] [CrossRef]
- Sanderson, E.; Davey Smith, G.; Windmeijer, F.; Bowden, J. An examination of multivariable Mendelian randomization in the single-sample and two-sample summary data settings. Int. J. Epidemiol. 2019, 48, 713–727. [Google Scholar] [CrossRef]
- Sanderson, E. Multivariable Mendelian Randomization and Mediation. Cold Spring Harb. Perspect. Med. 2021, 11, a038984. [Google Scholar] [CrossRef]
- Evangelou, E.; Warren, H.R.; Mosen-Ansorena, D.; Mifsud, B.; Pazoki, R.; Gao, H.; Ntritsos, G.; Dimou, N.; Cabrera, C.P.; Karaman, I.; et al. Genetic analysis of over 1 million people identifies 535 new loci associated with blood pressure traits. Nat. Genet. 2018, 50, 1412–1425. [Google Scholar] [CrossRef]
- Yu, Z.; Zhang, L.; Zhang, G.; Xia, K.; Yang, Q.; Huang, T.; Fan, D. Lipids, Apolipoproteins, Statins and ICH: A Mendelian Randomization Study. Ann. Neurol. 2022, 92, 390–399. [Google Scholar] [CrossRef]
- Gill, D.; Georgakis, M.K.; Koskeridis, F.; Jiang, L.; Feng, Q.; Wei, W.Q.; Theodoratou, E.; Elliott, P.; Denny, J.C.; Malik, R.; et al. Use of Genetic Variants Related to Antihypertensive Drugs to Inform on Efficacy and Side Effects. Circulation 2019, 140, 270–279. [Google Scholar] [CrossRef]
- Walker, V.M.; Kehoe, P.G.; Martin, R.M.; Davies, N.M. Repurposing antihypertensive drugs for the prevention of Alzheimer’s disease: A Mendelian randomization study. Int. J. Epidemiol. 2020, 49, 1132–1140. [Google Scholar] [CrossRef]
- Levin, M.G.; Klarin, D.; Walker, V.M.; Gill, D.; Lynch, J.; Hellwege, J.N.; Keaton, J.M.; Lee, K.M.; Assimes, T.L.; Natarajan, P.; et al. Association Between Genetic Variation in Blood Pressure and Increased Lifetime Risk of Peripheral Artery Disease. Arterioscler. Thromb. Vasc. Biol. 2021, 41, 2027–2034. [Google Scholar] [CrossRef]
- Ference, B.A.; Ray, K.K.; Catapano, A.L.; Ference, T.B.; Burgess, S.; Neff, D.R.; Oliver-Williams, C.; Wood, A.M.; Butterworth, A.S.; Di Angelantonio, E.; et al. Mendelian Randomization Study of ACLY and Cardiovascular Disease. N. Engl. J. Med. 2019, 380, 1033–1042. [Google Scholar] [CrossRef]
- Do, R.; Willer, C.J.; Schmidt, E.M.; Sengupta, S.; Gao, C.; Peloso, G.M.; Gustafsson, S.; Kanoni, S.; Ganna, A.; Chen, J.; et al. Common variants associated with plasma triglycerides and risk for coronary artery disease. Nat. Genet. 2013, 45, 1345–1352. [Google Scholar] [CrossRef]
- Daghlas, I.; Karhunen, V.; Ray, D.; Zuber, V.; Burgess, S.; Tsao, P.S.; Lynch, J.A.; Lee, K.M.; Voight, B.F.; Chang, K.M.; et al. Genetic Evidence for Repurposing of GLP1R (Glucagon-Like Peptide-1 Receptor) Agonists to Prevent Heart Failure. J. Am. Heart Assoc. 2021, 10, e020331. [Google Scholar] [CrossRef]
- Vujkovic, M.; Keaton, J.M.; Lynch, J.A.; Miller, D.R.; Zhou, J.; Tcheandjieu, C.; Huffman, J.E.; Assimes, T.L.; Lorenz, K.; Zhu, X.; et al. Discovery of 318 new risk loci for type 2 diabetes and related vascular outcomes among 1.4 million participants in a multi-ancestry meta-analysis. Nat. Genet. 2020, 52, 680–691. [Google Scholar] [CrossRef]
- Burgess, S.; Davey Smith, G.; Davies, N.M.; Dudbridge, F.; Gill, D.; Glymour, M.M.; Hartwig, F.P.; Kutalik, Z.; Holmes, M.V.; Minelli, C.; et al. Guidelines for performing Mendelian randomization investigations: Update for summer 2023. Wellcome Open. Res. 2019, 4, 186. [Google Scholar] [CrossRef]
- Bowden, J.; Spiller, W.; Del Greco, M.F.; Sheehan, N.; Thompson, J.; Minelli, C.; Davey Smith, G. Improving the visualization, interpretation and analysis of two-sample summary data Mendelian randomization via the Radial plot and Radial regression. Int. J. Epidemiol. 2018, 47, 1264–1278. [Google Scholar] [CrossRef]
- Burgess, S.; Bowden, J.; Fall, T.; Ingelsson, E.; Thompson, S.G. Sensitivity Analyses for Robust Causal Inference from Mendelian Randomization Analyses with Multiple Genetic Variants. Epidemiology 2017, 28, 30–42. [Google Scholar] [CrossRef]
- Bowden, J.; Davey Smith, G.; Burgess, S. Mendelian randomization with invalid instruments: Effect estimation and bias detection through Egger regression. Int. J. Epidemiol. 2015, 44, 512–525. [Google Scholar] [CrossRef]
- Bowden, J.; Davey Smith, G.; Haycock, P.C.; Burgess, S. Consistent Estimation in Mendelian Randomization with Some Invalid Instruments Using a Weighted Median Estimator. Genet. Epidemiol. 2016, 40, 304–314. [Google Scholar] [CrossRef]
- Sanderson, E.; Spiller, W.; Bowden, J. Testing and correcting for weak and pleiotropic instruments in two-sample multivariable Mendelian randomization. Stat. Med. 2021, 40, 5434–5452. [Google Scholar] [CrossRef]
- Rees, J.M.B.; Wood, A.M.; Burgess, S. Extending the MR-Egger method for multivariable Mendelian randomization to correct for both measured and unmeasured pleiotropy. Stat. Med. 2017, 36, 4705–4718. [Google Scholar] [CrossRef]
- Zheng, J.; Brion, M.J.; Kemp, J.P.; Warrington, N.M.; Borges, M.C.; Hemani, G.; Richardson, T.G.; Rasheed, H.; Qiao, Z.; Haycock, P.; et al. The Effect of Plasma Lipids and Lipid-Lowering Interventions on Bone Mineral Density: A Mendelian Randomization Study. J. Bone Miner. Res. 2020, 35, 1224–1235. [Google Scholar] [CrossRef]
- Williams, D.M.; Finan, C.; Schmidt, A.F.; Burgess, S.; Hingorani, A.D. Lipid lowering and Alzheimer disease risk: A mendelian randomization study. Ann. Neurol. 2020, 87, 30–39. [Google Scholar] [CrossRef]
- Grant, A.J.; Burgess, S. Pleiotropy robust methods for multivariable Mendelian randomization. Stat. Med. 2021, 40, 5813–5830. [Google Scholar] [CrossRef]
- Rasooly, D.; Patel, C.J. Conducting a Reproducible Mendelian Randomization Analysis Using the R Analytic Statistical Environment. Curr. Protoc. Hum. Genet. 2019, 101, e82. [Google Scholar] [CrossRef]
- Daghlas, I.; Gill, D. Mendelian randomization as a tool to inform drug development using human genetics. Camb. Prism. Precis. Med. 2023, 1, e16. [Google Scholar] [CrossRef]
- Pushpakom, S.; Iorio, F.; Eyers, P.A.; Escott, K.J.; Hopper, S.; Wells, A.; Doig, A.; Guilliams, T.; Latimer, J.; McNamee, C.; et al. Drug repurposing: Progress, challenges and recommendations. Nat. Rev. Drug. Discov. 2019, 18, 41–58. [Google Scholar] [CrossRef]
- Georgakis, M.K.; Gill, D.; Webb, A.J.S.; Evangelou, E.; Elliott, P.; Sudlow, C.L.M.; Dehghan, A.; Malik, R.; Tzoulaki, I.; Dichgans, M. Genetically determined blood pressure, antihypertensive drug classes, and risk of stroke subtypes. Neurology 2020, 95, e353–e361. [Google Scholar] [CrossRef] [PubMed]
- Rothwell, P.M.; Howard, S.C.; Dolan, E.; O’Brien, E.; Dobson, J.E.; Dahlöf, B.; Poulter, N.R.; Sever, P.S. Effects of beta blockers and calcium-channel blockers on within-individual variability in blood pressure and risk of stroke. Lancet. Neurol. 2010, 9, 469–480. [Google Scholar] [CrossRef] [PubMed]
- Webb, A.J.; Fischer, U.; Mehta, Z.; Rothwell, P.M. Effects of antihypertensive-drug class on interindividual variation in blood pressure and risk of stroke: A systematic review and meta-analysis. Lancet 2010, 375, 906–915. [Google Scholar] [CrossRef] [PubMed]
- Hindy, G.; Engström, G.; Larsson, S.C.; Traylor, M.; Markus, H.S.; Melander, O.; Orho-Melander, M. Role of Blood Lipids in the Development of Ischemic Stroke and its Subtypes. Stroke 2018, 49, 820–827. [Google Scholar] [CrossRef]
- Rothwell, P.M.; Howard, S.C.; Power, D.A.; Gutnikov, S.A.; Algra, A.; van Gijn, J.; Clark, T.G.; Murphy, M.F.; Warlow, C.P. Fibrinogen concentration and risk of ischemic stroke and acute coronary events in 5113 patients with transient ischemic attack and minor ischemic stroke. Stroke 2004, 35, 2300–2305. [Google Scholar] [CrossRef]
- Martiskainen, M.; Pohjasvaara, T.; Mikkelsson, J.; Mäntylä, R.; Kunnas, T.; Laippala, P.; Ilveskoski, E.; Kaste, M.; Karhunen, P.J.; Erkinjuntti, T. Fibrinogen gene promoter -455 A allele as a risk factor for lacunar stroke. Stroke 2003, 34, 886–891. [Google Scholar] [CrossRef]
- Onodera, O.; Uemura, M.; Ando, S.; Hayashi, H.; Kanazawa, M. Rethinking Lacunar Stroke: Beyond Fisher’s Curse. Brain Nerve 2021, 73, 991–998. [Google Scholar] [CrossRef]
- Palmer, A.K.; Tchkonia, T.; LeBrasseur, N.K.; Chini, E.N.; Xu, M.; Kirkland, J.L. Cellular Senescence in Type 2 Diabetes: A Therapeutic Opportunity. Diabetes 2015, 64, 2289–2298. [Google Scholar] [CrossRef]
- Satny, M.; Hubacek, J.A.; Vrablik, M. Statins and Inflammation. Curr. Atheroscler. Rep. 2021, 23, 80. [Google Scholar] [CrossRef]
- Caturano, A.; D’Angelo, M.; Mormone, A.; Russo, V.; Mollica, M.P.; Salvatore, T.; Galiero, R.; Rinaldi, L.; Vetrano, E.; Marfella, R.; et al. Oxidative Stress in Type 2 Diabetes: Impacts from Pathogenesis to Lifestyle Modifications. Curr. Issues Mol. Biol. 2023, 45, 6651–6666. [Google Scholar] [CrossRef]
- Ng, Y.L.; Salim, C.K.; Chu, J.J.H. Drug repurposing for COVID-19: Approaches, challenges and promising candidates. Pharmacol. Ther. 2021, 228, 107930. [Google Scholar] [CrossRef]
- Smith, G.D.; Ebrahim, S. Mendelian randomization: Prospects, potentials, and limitations. Int. J. Epidemiol. 2004, 33, 30–42. [Google Scholar] [CrossRef] [PubMed]
- Pierce, B.L.; Burgess, S. Efficient design for Mendelian randomization studies: Subsample and 2-sample instrumental variable estimators. Am. J. Epidemiol. 2013, 178, 1177–1184. [Google Scholar] [CrossRef] [PubMed]
- VanderWeele, T.J.; Tchetgen Tchetgen, E.J.; Cornelis, M.; Kraft, P. Methodological challenges in mendelian randomization. Epidemiology 2014, 25, 427–435. [Google Scholar] [CrossRef] [PubMed]
- Ma, T.; Tian, X.; Zhang, B.; Li, M.; Wang, Y.; Yang, C.; Wu, J.; Wei, X.; Qu, Q.; Yu, Y.; et al. Low-dose metformin targets the lysosomal AMPK pathway through PEN2. Nature 2022, 603, 159–165. [Google Scholar] [CrossRef] [PubMed]
- Hemani, G.; Bowden, J.; Davey Smith, G. Evaluating the potential role of pleiotropy in Mendelian randomization studies. Hum. Mol. Genet. 2018, 27, R195–R208. [Google Scholar] [CrossRef] [PubMed]
- Bonkhoff, A.K.; Grefkes, C. Precision medicine in stroke: Towards personalized outcome predictions using artificial intelligence. Brain 2021, 145, 457–475. [Google Scholar] [CrossRef]
- Emdin, C.A.; Khera, A.V.; Kathiresan, S. Mendelian Randomization. JAMA 2017, 318, 1925–1926. [Google Scholar] [CrossRef]
- Gill, D.; Vujkovic, M. The Potential of Genetic Data for Prioritizing Drug Repurposing Efforts. Neurology 2022, 99, 267–268. [Google Scholar] [CrossRef]
- Georgakis, M.K.; Malik, R.; Gill, D.; Franceschini, N.; Sudlow, C.L.M.; Dichgans, M. Interleukin-6 Signaling Effects on Ischemic Stroke and Other Cardiovascular Outcomes: A Mendelian Randomization Study. Circ. Genom. Precis. Med. 2020, 13, e002872. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, L.; Wang, F.; Xia, K.; Yu, Z.; Fu, Y.; Huang, T.; Fan, D. Unlocking the Medicinal Mysteries: Preventing Lacunar Stroke with Drug Repurposing. Biomedicines 2024, 12, 17. https://doi.org/10.3390/biomedicines12010017
Zhang L, Wang F, Xia K, Yu Z, Fu Y, Huang T, Fan D. Unlocking the Medicinal Mysteries: Preventing Lacunar Stroke with Drug Repurposing. Biomedicines. 2024; 12(1):17. https://doi.org/10.3390/biomedicines12010017
Chicago/Turabian StyleZhang, Linjing, Fan Wang, Kailin Xia, Zhou Yu, Yu Fu, Tao Huang, and Dongsheng Fan. 2024. "Unlocking the Medicinal Mysteries: Preventing Lacunar Stroke with Drug Repurposing" Biomedicines 12, no. 1: 17. https://doi.org/10.3390/biomedicines12010017
APA StyleZhang, L., Wang, F., Xia, K., Yu, Z., Fu, Y., Huang, T., & Fan, D. (2024). Unlocking the Medicinal Mysteries: Preventing Lacunar Stroke with Drug Repurposing. Biomedicines, 12(1), 17. https://doi.org/10.3390/biomedicines12010017